Abstract
As the predominant aetiological agent of the common cold, human rhinovirus (HRV) is the leading cause of human infectious disease. Early studies showed that a monovalent formalin-inactivated HRV vaccine can be protective, and virus-neutralizing antibodies (nAb) correlated with protection. However, co-circulation of many HRV types discouraged further vaccine efforts. Here, we test the hypothesis that increasing virus input titres in polyvalent inactivated HRV vaccine may result in broad nAb responses. We show that serum nAb against many rhinovirus types can be induced by polyvalent, inactivated HRVs plus alhydrogel (alum) adjuvant. Using formulations up to 25-valent in mice and 50-valent in rhesus macaques, HRV vaccine immunogenicity was related to sufficient quantity of input antigens, and valency was not a major factor for potency or breadth of the response. Thus, we have generated a vaccine capable of inducing nAb responses to numerous and diverse HRV types.
 Existence of 150–170 serologically distinct human rhinoviruses (HRV) has hampered vaccine development for this human pathogen. Here, the authors show that a prime-boost regimen with an inactivated 50-valent HRV vaccine induces neutralizing antibody responses to diverse HRV serotypes in rhesus macaques.
Existence of 150–170 serologically distinct human rhinoviruses (HRV) has hampered vaccine development for this human pathogen. Here, the authors show that a prime-boost regimen with an inactivated 50-valent HRV vaccine induces neutralizing antibody responses to diverse HRV serotypes in rhesus macaques.
HRV causes respiratory illness in billions of people annually, a socio-economic burden1. HRV also causes pneumonia hospitalizations in children and adults, and exacerbations of asthma and chronic obstructive pulmonary disease (COPD)2. HRV was found to be the second leading cause of community-acquired pneumonia requiring hospitalization in US children, second only to respiratory syncytial virus, and the most common pathogen associated with pneumonia hospitalization in US adults3,4. A vaccine for HRV could alleviate serious disease in asthma and COPD, reduce pneumonia hospitalizations and have widespread benefits for society on the whole. Decades ago, researchers identified inactivated HRV as a protective vaccine, defined virus-neutralizing antibodies (nAb) as a correlate of protection, and estimated duration of immunity5,6,7,8,9,10,11. Trials with monovalent HRV vaccine demonstrated that protection from homologous challenge and disease can be achieved with formalin-inactivated virus given intramuscularly (i.m.) or intranasally8,10,11. Humoral immunity to heterologous virus types was not observed, though cross-reactive CD8 T cells can promote clearance12,13. Limited cross-neutralizing antibodies can be induced by hyper-immunization in animals14,15. The possibility of a vaccine composed of 50, 100 or more distinct HRV antigens has been viewed as formidable or impossible2,16,17. There are two main challenges, generating a broad immune response and the feasibility of composing such a vaccine. The Ab repertoire is theoretically immense, and most vaccines in clinical use are thought to work via a polyclonal Ab response. Deep-sequencing of human Ab genes following vaccination against influenza virus found thousands of Ab lineages18,19. Whole pathogen and polyvalent vaccines carrying natural immunogens take advantage of this capacity. Valency has increased for pneumococcal and human papillomavirus virus vaccines in recent years. Given the significance of HRV, we tested polyvalent HRV vaccines.
There are three species of HRV, A, B and C. Sequencing methods define 83 A types, 32 B types and 55 C types20,21. It is thought there are 150–170 serologically distinct HRV types. HRV A and C are associated with asthma exacerbations and with more acute disease than HRV B22,23. HRV C was discovered in 2006 and 2007 (refs 24, 25, 26, 27) and recently cultured in cells28,29. Here, we focused on HRV A, the most prevalent species. There are no permissive animal challenge models of HRV virus replication, but mice and cotton rats can recapitulate aspects of HRV pathogenesis30,31. The best efficacy model is human challenge. In monovalent vaccine trials, formalin-inactivated HRV-13 was validated before clinical testing by assessing induction of nAb in guinea pigs, and a reciprocal serum nAb titre of 23 resulting from four doses of a 1:125 dilution of the vaccine correlated with vaccine efficacy in humans9. Although the nAb titre required for protection is not defined, early studies established inactivated HRV as protective in humans, and immunogenicity in animals informed clinical testing.
Here, we show that polyvalent inactivated HRV vaccines generate broad nAb responses in a BALB/c mouse model (up to 25-valent) as well as a rhesus macaque (RM) model (up to 50-valent). Immunogenicity of polyvalent inactivated HRV vaccines is not affected by increasing valency, and it relates to antigen dose. A highly polyvalent strategy may facilitate practical HRV vaccine development.
Results
Immunogenicity of 10-valent HRV vaccine in mice
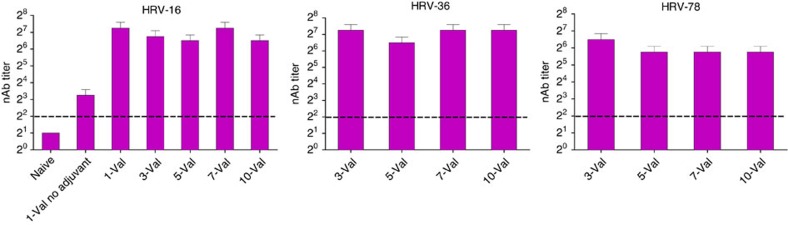
We first used BALB/c mice to test immunogenicity. We propagated HRVs in H1-HeLa cells and inactivated infectivity using formalin. Sera from naive mice had no detectable nAb against HRV-16 (Fig. 1). Alum adjuvant enhanced the nAb response induced by i.m. inactivated HRV-16 (Fig. 1). There was no effect of valency (comparing 1-, 3-, 5-, 7- and 10-valent) on the nAb response induced by inactivated HRV-16 or to the 3 types in the 3-valent vaccine (HRV-16, HRV-36 and HRV-78; Fig. 1). The 50% tissue culture infectious dose (TCID50) titres of the input viruses before inactivation (inactivated-TCID50) are provided in Supplementary Table 1. Original antigenic sin can occur when sequential exposure to related virus variants results in biased immunity to the type encountered first32. In bivalent HRV-immune mice, we observed modest original antigenic sin following prime vaccination with 10-valent inactivated HRV, and boost vaccination partially alleviated the effect (Supplementary Fig. 1), similar to influenza virus32. Collectively, these results prompted us to explore more fully the nAb response to polyvalent HRV vaccine.
Figure 1. Immunogenicity of inactivated HRV is not affected by increasing valency from one to ten.
Mice were vaccinated i.m. with 1-valent inactivated HRV-16 with or without alum adjuvant (5 mice per group) or with 3-valent, 5-valent, 7-valent or 10-valent inactivated HRV with alum (20 mice per group). HRV types and inactivated-TCID50 doses are specified in Supplementary Table 1. Sera were collected 18 days after vaccination and pooled for each group. Serum nAb titres were measured against HRV-16, HRV-36 and HRV-78. The dashed line represents limit of detection (LOD). Error bars show 95% confidence interval. Data depict three independent experiments combined.
Sufficient input HRV titre determines immunogenicity
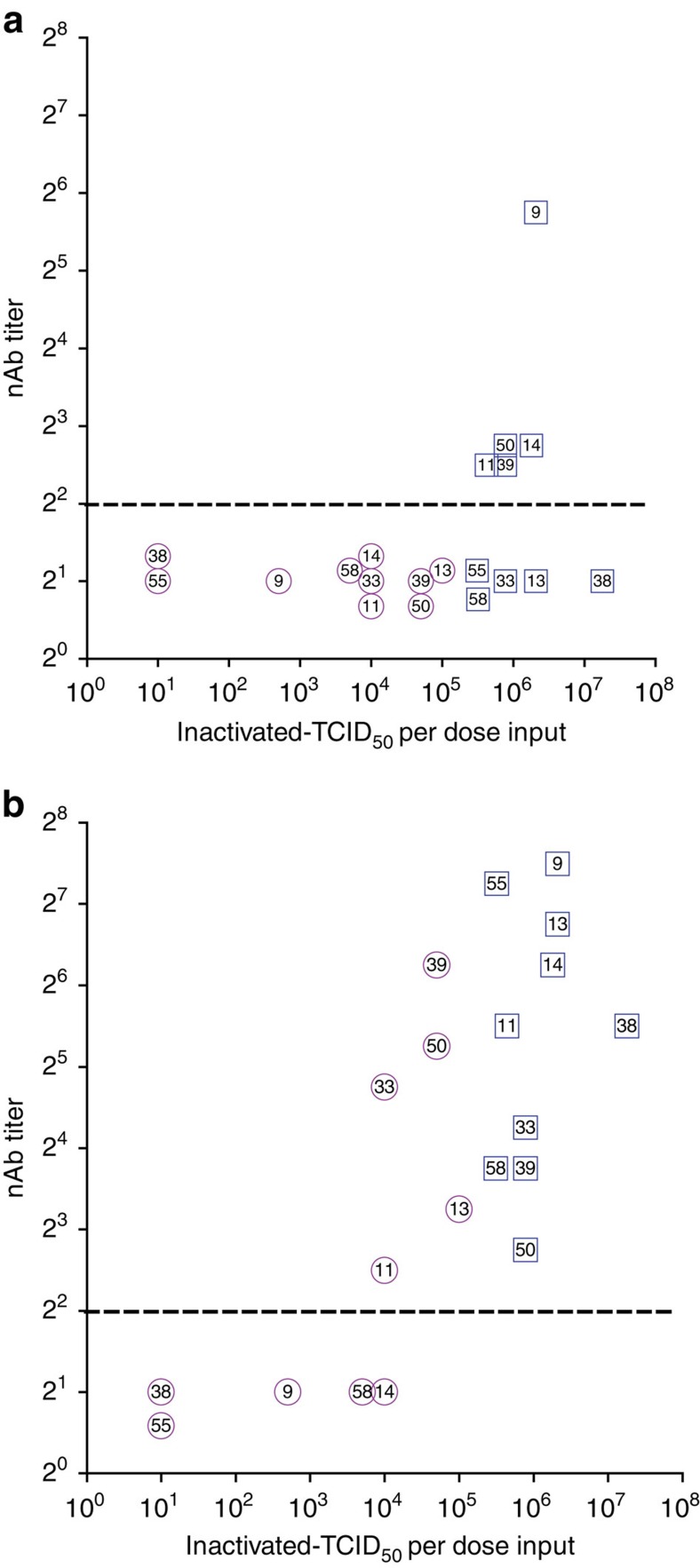
In 1975, it was reported that two different 10-valent inactivated HRV preparations induced nAb titres to only 30–40% of the input virus types in recipient subjects33. However, the input titres of viruses before inactivation ranged from 101.5 to 105.5 TCID50 per ml, and these were then diluted 10-fold to generate 10-valent 1.0 ml doses given i.m. as prime and boost with no adjuvant33. We hypothesized that low input antigen doses are responsible for poor nAb responses to 10-valent inactivated HRV. We reconstituted the 1975 10-valent vaccine, as closely as possible with available HRV types, over a 101 to 105 inactivated-TCID50 per vaccine dose, and we compared it with a 10-valent vaccine of the same types with input titres ranging from>105 to>107 inactivated-TCID50 per dose. The reconstituted 1975 vaccine resulted in no detectable nAb after prime vaccination and, following boost vaccination, nAb to the five types that had the highest input titres (Fig. 2). The high titre vaccine resulted in nAb to 5 of 10 types after prime vaccination and all 10 types after the boost (Fig. 2). Following the boost vaccinations, there appeared to be a 104 inactivated-TCID50 per vaccine dose threshold for the induction of nAb in this model (Fig. 2b). Above this titre, there was no correlation between input load and nAb induction.
Figure 2. Immunogenicity of inactivated polyvalent HRV is related to dose.
Mice (2 groups, 20 per group) were vaccinated with 10-valent HRV vaccine consisting of low inactivated-TCID50 per dose input titres (x-axis), similar to the 1975 Hamory et al. study33, plus alum (magenta open circles) or with 10-valent HRV vaccine with high inactivated-TCID50 per dose input titres plus alum (blue open squares). Sera were collected 18 days after prime (a) and 18 days after boost (b), pooled for each group and nAb titres (y-axis) were measured against the indicated types in the vaccines. The dashed line represents LOD. Undetectable nAb were assigned LOD/2, and some symbols below LOD were nudged for visualization. Three independent experiments using low input titres showed similar results. There was a statistically significant association between input TCID50 virus titre and a detectable nAb response following prime (P=0.01) and boost (P=0.03) vaccination (Fisher's exact test).
Immunogenicity of 25-valent HRV vaccine in mice
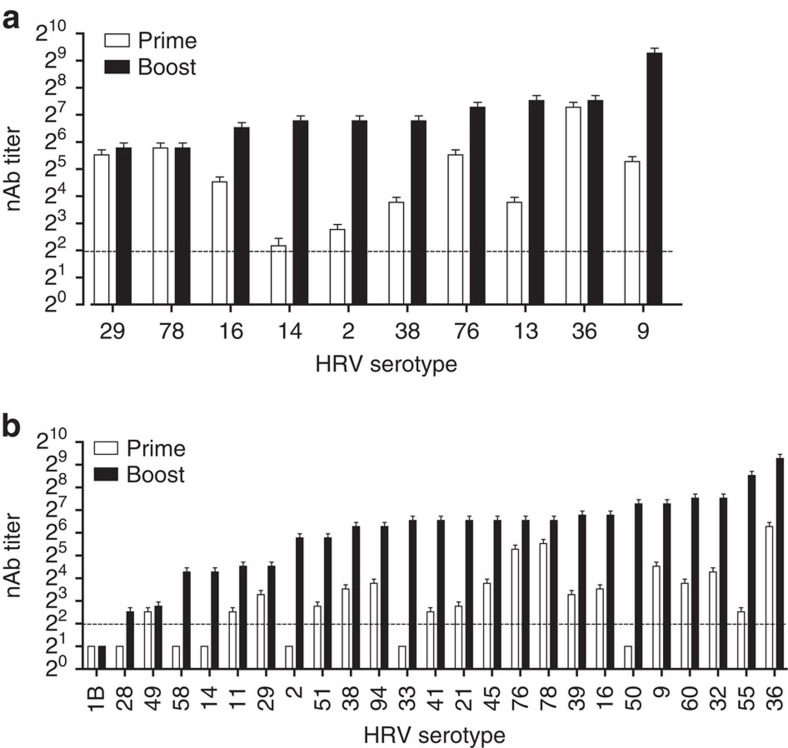
Injectable vaccines used in people are commonly given in a 0.5 ml dose. In our facility, the highest allowable i.m. vaccine volume in mice was 0.1 ml. We tested a 25-valent per 0.1 ml HRV vaccine in mice as a scalable prototype. The 25-valent inactivated HRV vaccine had a 7.4-fold lower average inactivated-TCID50 per type per dose than the 10-valent composition (Supplementary Table 2) to accommodate the volume adjustment. The 10-valent inactivated HRV vaccine induced nAb to 100% of input types following the prime and the boost (Fig. 3a). The nAb induced by 10-valent inactivated HRV were persisting at 203 days post-boost (Supplementary Fig. 2). We used two different sets of virus stocks for the prime and boost 25-valent vaccinations in these experiments (Supplementary Table 2). The reason is that in the interim between the prime and boost vaccinations, we obtained higher titre virus stocks of eleven of the input types (HRV-32, -49, -58, -55, -41, -33, -39, -50, -1B, -21 and -60). The higher titre stocks of these eleven were used in the boost vaccination. The 25-valent inactivated HRV prime vaccination induced nAb to 18 of 25 (72%) virus types, and the 25-valent boost resulted in nAb against 24 of the 25 types (96%; Fig. 3b). The average nAb titre resulting from prime and boost was 27 for 10-valent and 26.8 for 25-valent. These data demonstrate broad neutralization of diverse HRV types with a straightforward vaccine approach.
Figure 3. Broad nAb responses against 10-valent and 25-valent inactivated HRV in mice.
The inactivated-TCID50 input titres per dose are specified in Supplementary Table 2. (a) 20 mice were vaccinated then boosted at 50 days with 10-valent HRV. (b) 30 mice were vaccinated then boosted at 50 days with 25-valent HRV. Sera were collected at day 18 (prime) and day 68 (boost). nAb levels against the indicated types in the vaccines were measured in pooled sera. Error bars depict 95% confidence interval. Data shown represent one of three (10-valent) or two (25-valent) experiments with similar results. The dashed line represents LOD. Undetectable nAb were assigned LOD/2.
Immunogenicity of 25- and 50-valent HRV in RMs
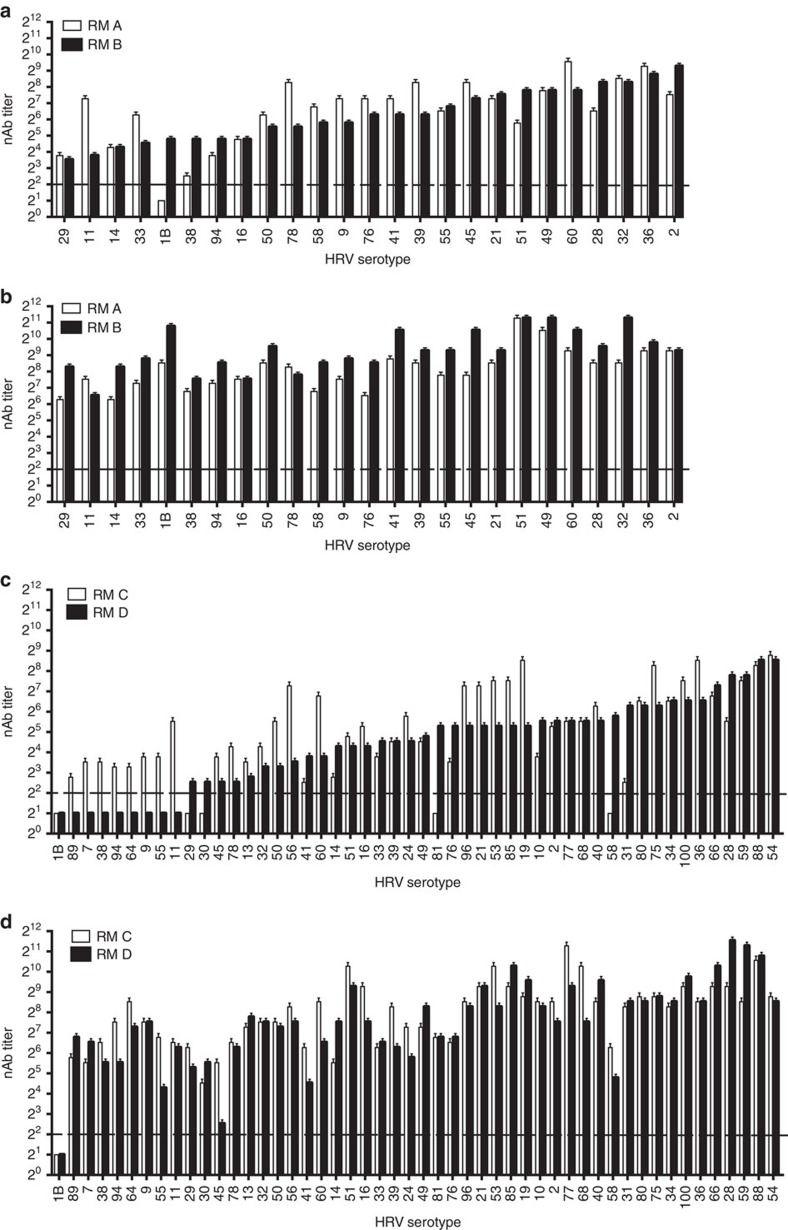
To increase vaccine valency, we chose RMs and a 1.0 ml i.m. vaccine volume. Two RMs were vaccinated with 25-valent inactivated HRV, and two RMs were vaccinated with 50-valent inactivated HRV. Pre-immune sera in RM A and RM B had no detectable nAb against the 25 HRV types included in the 25-valent vaccine. The inactivated-TCID50 titres per dose were higher in RMs than in mice (Supplementary Table 3). The 25-valent vaccine induced nAb to 96% (RM A) and 100% (RM B) of input viruses following the prime vaccination (Fig. 4a). The 50-valent vaccine induced nAb to 90% (RM C) and 82% (RM D) of input viruses following the prime vaccination (Fig. 4c). The breadth of nAb following prime vaccination in RM was superior to what we observed in mice, which may have been due to animal species differences and/or higher inactivated-TCID50 input titres in the RM vaccines. Following boost vaccination, there were serum nAb titres against 100% of the types in 25-valent HRV-vaccinated RMs (Fig. 4b) and 98% (49 out of 50) of the virus types in 50-valent HRV-vaccinated RMs (Fig. 4d). The average nAb titre resulting from prime and boost in RMs was 29.3 for 25-valent and 28.6 for 50-valent. The nAb responses were type-specific, not cross-neutralizing, because there were minimal nAbs induced by the 25-valent vaccine against 10 non-vaccine types (Supplementary Fig. 3). The nAb response to 50-valent inactivated HRV vaccine was broad and potent in RMs.
Figure 4. Broad nAb responses against 25-valent and 50-valent inactivated HRV in RMs.
The inactivated-TCID50 input titres per dose are specified in Supplementary Table 3. Two RMs (RM A and RM B) were vaccinated i.m. with 25-valent HRV and alum (a,b), and two RMs (RM C and RM D) were vaccinated i.m. with 50-valent HRV and alum (c,d). nAb titres against input virus types were measured in individual serum samples collected at day 18 (a,c). The RM received an identical boost vaccination at day 28, and sera were collected at day 46 for determining nAb titres post-boost vaccination (b,d). Error bars depict 95% confidence interval. The dashed line represents LOD. Undetectable nAb were assigned LOD/2.
HRV yield in WI-38 cells and semi-purification by HPLC
The HRV stocks used in our vaccinations were produced in H1-HeLa cells, a good substrate for HRV replication but not suitable for vaccine manufacturing. We compared the infectious yield of 10 HRV types in H1-HeLa and WI-38, which can be qualified for vaccine production. Adequate yields were obtained from WI-38 cells (Supplementary Fig. 4). Injectable vaccines require defined purity. As proof of principle, we semi-purified three HRV types by high-performance liquid chromatography and found uncompromised immunogenicity of trivalent inactivated semi-purified HRV in mice (Supplementary Fig. 5).
Discussion
In this study, we demonstrate that polyvalent inactivated HRV vaccine expanded to a 50-valent composition, with alum adjuvant, is immunogenic against approximately one-third of circulating HRV types. Although fifty HRV types is a minority, these data provide proof of principle for a highly polyvalent vaccine approach. Forty years ago, the prospects for a polyvalent HRV vaccine were dour for good reasons17. However, progress in technology34 and advancement of more complex vaccines renders impediments to a polyvalent HRV vaccine manageable. Scale-up of HRV vaccines may be facilitated by related vaccine production processes and new cost-saving manufacturing technologies35,36,37. Inactivated HRV has a positive history of clinical efficacy6,8,9.
On the basis of our results and doses of early immunogenic HRV vaccines6,8, we estimate 104–5 inactivated-TCID50 per type per dose will be useful. Therefore, HRV stock titres≥107 TCID50 per ml are required for a potential 83-valent HRV A formulation in a 0.5 ml dose containing alum adjuvant. In future studies, we hope to produce a comprehensive 83-valent HRV A vaccine. A limitation of our study is that we have not included HRV C antigens, which will be important for HRV vaccine development, especially for paediatric populations. Ongoing efforts are aimed at propagation of HRV C strains to high titre in cell lines and development of serology assays. Ultimately, our approach may lead to vaccines for rhinovirus-mediated diseases including asthma and COPD exacerbations and the common cold. Advancing valency may be applicable to vaccines for other antigenically variable pathogens.
Methods
Cell lines and viruses
H1-HeLa (CRL-1958) and WI-38 (CCL-75) cells were obtained from the American Type Culture Collection (ATCC) and cultured in minimal essential media with Richter's modification and no phenol red (MEM) (Thermo Fisher) supplemented with 10% fetal bovine serum. The cell lines were not authenticated but are not commonly misidentified (International Cell Line Authentication Committee). We tested HeLa-H1 cells using the LookOut Mycoplasma detection kit (Sigma), and these were mycoplasma negative. HRV-7 (VR-1601), HRV-9 (VR-1745), HRV-11 (VR-1567), HRV-13 (VR-286), HRV-14 (VR-284), HRV-16 (VR-283), HRV-19 (V4-1129), HRV-24 (VR-1134), HRV-29 (VR-1809), HRV-30 (VR-1140), HRV-31 (VR-1795), HRV-32 (VR-329), HRV-36 (VR-509), HRV-38 (VR-511), HRV-40 (VR-341), HRV-41 (VR-1151), HRV-49 (VR-1644), HRV-53 (VR-1163), HRV-56 (VR-1166), HRV-58 (VR-1168), HRV-59 (VR-1169), HRV-60 (VR-1473), HRV-64 (VR-1174), HRV-66 (VR-1176), HRV-68 (VR-1178), HRV-75 (VR-1185), HRV-76 (VR-1186), HRV-77 (VR-1187), HRV-78 (VR-1188), HRV-80 (VR-1190), HRV-81 (VR-1191), HRV-85 (VR-1195), HRV-88 (VR-1198), HRV-89 (VR-1199), HRV-96 (VR-1296), and HRV-100 (VR-1300) prototype strains were purchased from ATCC. HRV-1B, HRV-10, HRV-21, HRV-28, HRV-33, HRV-34, HRV-39, HRV-45, HRV-50, HRV-51, HRV-54, HRV-55 and HRV-94 strains were obtained from the Centers for Disease Control and Prevention. The HRVs in the study are species A, with the exception of HRV-14 (B), and represent A species broadly20,21.
HRV propagation and titration
HRV stocks were generated in H1-HeLa cells. Approximately 0.5 ml of HRV was inoculated onto subconfluent H1-HeLa monolayer cells in a T-182 flask. After adsorption for 1 h at room temperature with rocking, 50 ml of HRV infection medium (MEM supplemented with 2% FBS, 20 mM HEPES, 10 mM MgCl2, 1 × non-essential amino acids (Gibco catalog 11140-050)) was added and the infection was allowed to proceed at 32 °C in a 5% CO2 humidified incubator until the monolayer appeared to be completely involved with cytopathic effect (CPE), 1 to 3 days post-infection. The cells were scraped, and the cells and medium (∼50 ml) were transferred to two pre-chilled 50 ml conical polypropylene tubes and kept on ice while each suspension was sonicated using a Sonic Dismembrator Model 500 (Fisher Scientific) equipped with a ½-inch diameter horn disrupter and 1/4-inch diameter tapered microtip secured on a ring stand. Sonication was performed by an operator in a closed room with ear protection, at 10% amplitude, 1 s on/1 s off intervals and 1 pulse per 1 ml of material. Sonication yielded higher titres than freeze–thaw. The suspension was clarified by centrifugation at 931g for 10 min. The supernatant was transferred to cryovials, snap-frozen in liquid nitrogen and stored at −80 °C. For comparing HRV yield in H1-HeLa and WI-38 cells, T-75 flasks of subconfluent cells were infected at a multiplicity of infection of 0.1 TCID50 per cell, and 20 ml of culture medium were discarded before scraping the cells in the remaining 5 ml followed by sonication. For all stocks, TCID50 ml−1 titres were determined by infecting subconfluent H1-HeLa cells in 96-well plates with serially diluted samples, staining the cells 6 days post-infection with 0.1% crystal violet/20% methanol, scoring wells for CPE and calculating the end point titre using the Reed and Muench method38.
HRV purification
HRV stock was collected from H1-HeLa cell monolayers as described above and clarified by brief centrifugation at low speed to remove large cellular debris (931g, 10 min, 4 °C). To remove excess albumin from the crude virus stock by affinity chromatography, the supernatant was loaded onto a HiTrap Blue HP column (GE Healthcare) using an ÄKTA Purifier system (GE Healthcare) according to the manufacturer specifications. Flowthrough was subsequently loaded through a HiTrap Capto Core 700 column (GE Healthcare) to refine the virus prep by size exclusion chromatography. The flowthrough from the HiTrap Blue HP and the HiTrap Capto Core 700 was captured using the ÄKTA Purifier system (GE Healthcare) with a 20 mM sodium phosphate buffer (pH 7.0). Flowthrough from size exclusion chromatography was dialyzed overnight with 0.1 M Tris-HCl buffer (pH 8.0), then loaded onto a HiTrap Q XL column (GE Healthcare) and separated into fractions by ion exchange chromatography. Virus-containing fractions were eluted using the ÄKTA Purifier system (GE Healthcare) with a 0.1 M Tris-HCl buffer (pH 8.0) and a sodium chloride gradient. Fractions showing high absorption peaks at 280 nM were collected and analysed for viral titre by TCID50 end point dilution assay, and fraction purity visualized on a 10% SDS-PAGE gel by silver stain (Thermo Fisher Scientific; Supplementary Fig. 6 and Supplementary Table 4). Fractions of HRV-16, HRV-36 and HRV-78 of high virus titre and purity were combined for formalin inactivation as described below.
Mice and RMs
All experiments involving animals were performed at Emory University and the Yerkes National Primate Research Center in accordance with guidelines established by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals. Animal facilities at Emory University and the Yerkes Center are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The Institutional Animal Care and Use Committee (IACUC) of Emory University approved these studies. Pathogen-free, 6–7-week female BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were randomly assigned to groups based on the sequential selection from an inventory, and investigators were not blinded to outcome assessment. No statistical methods were used in predetermining sample sizes.
Young adult (3–5 kg, 2–4 years of age, 2 females and 2 males) Indian RMs (Macaca mulatta; RM) were maintained according to NIH guidelines at the Yerkes National Primate Research Center. Handling and movement of RMs was performed by qualified personnel who have received specific training in safe and humane methods of animal handling at the Yerkes National Primate Research Center. The initial exclusion criterion was pre-existing nAb against HRV. The studies were performed in strict accordance with US Department of Agriculture regulations and the recommendations in the Guide for Care and Use of Laboratory Animals of the NIH. The RMs were allocated in an un-blinded manner to two vaccine groups (25-valent and 50-valent), one male and one female per group. No statistical methods were used in predetermining sample sizes.
Vaccination
Before immunization, all HRV types were inactivated by addition of 0.025% formalin followed by incubation with stirring for 72 h at 37 °C, as previously described for HRV vaccine33. Complete inactivation of infectivity was confirmed by end point TCID50 titration in H1-HeLa cells. Formalin inactivation by this method resulted in greater immunogenicity in mice than alternative inactivation by β-propiolactone, suggesting formalin inactivation preserved antigenic determinants. Mice were vaccinated i.m. with inactivated HRV strains mixed with 100 μg of Alhydrogel adjuvant 2% (aluminium hydroxide wet gel suspension, alum; Sigma catalog A8222 or Invivogen catalog vac-alu) according to instructions of the manufacturers. The total volume per mouse was 100 μl, administered in 50 μl per thigh. Mice were given a second identical vaccination (boost) at the time indicated in figure legends. RMs were vaccinated i.m. with inactivated HRV strains mixed with 500 μg of Alhydrogel adjuvant 2%. The total volume per RM was 1 ml, administered in one leg. RMs were boosted with an identical vaccination at four weeks.
Serum collection
In mice, peripheral blood was collected into microcentrifuge tubes from the submandibular vein. Samples were incubated at room temperature for 20 min to clot. The tubes were centrifuged 7,500g for 10 min to separate serum. The serum samples were pooled from mice of each group and stored at −80 °C until used. Phlebotomy involving RMs was performed under either ketamine (10 mg kg−1) or Telazol (4 mg kg−1) anaesthesia on fasting animals. Following anaesthesia with ketamine or Telazol, the animals were bled from the femoral vein. Yerkes blood collection guidelines were followed and no more than 10 ml kg−1 per 28 days of blood was collected. After collecting blood in serum separating tube, samples were incubated at room temperature for 30 min. The tubes were centrifuged at 2,500g for 15 min to separate serum. The serum samples from individual RM were stored at −80 °C until used.
Serum neutralization assay
H1-HeLa cells were seeded in 96-well plates to attain 80–90% confluence in 24 h. Due to the limited volume of mouse serum per bleed and the number of nAb assays required, we pooled mouse sera. After separating serum from whole blood, equal volumes of sera per mouse were pooled from each group, before heat inactivation. Levels of nAb against the indicated HRV types in the vaccines used in mice were measured in pooled sera. Heat-inactivated (56 °C, 30 min) serum samples were twofold serially diluted in MEM and added to 500 TCID50 ml−1 HRV of each type to be tested, in an equal volume. The virus and serum mixtures were incubated at 37 °C for 1 h. Then, 50 μl of the serum–virus mixture was transferred onto H1-HeLa cell monolayers in 96-well plates in triplicate, and plates were spinoculated at 2,095g for 30 min at 4 °C. For each type, a no-serum control was added to test the input 500 TCID50. We tested pooled HRV-16 anti-sera against HRV-16 in each assay as a standard. After spinoculation, 150 μl of HRV infection medium were added to each well. The 96-well plates were incubated for 6 days at 32 °C and 5% CO2 and then stained with crystal violet as described above. Wells were scored for the presence or absence of CPE. Neutralizing antibody end point titres and 95% confidence intervals were determined by the method of Reed and Muench38, as previously described for HRV14,38. The 95% confidence interval indicates variability of three technical replicates within a single nAb experiment. An example of the nAb assay and titre calculation is provided in Supplementary Fig. 7.
Data availability
Primary data that support the findings of this study are available from the corresponding author on request.
Additional information
How to cite this article: Lee, S. et al. A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques. Nat. Commun. 7:12838 doi: 10.1038/ncomms12838 (2016).
Supplementary Material
Supplementary Figures 1-7, Supplementary Tables 1-4 and Supplementary References
Acknowledgments
We are indebted to the Yerkes veterinary personnel for providing technical assistance. We thank the Children's Healthcare of Atlanta and Emory University Pediatrics Biostatistics Core for assistance with statistical analyses. We thank Max Cooper (Emory University) and Joshy Jacob (Emory University) for helpful discussions. This study was supported by a pilot grant from the Emory+Children's Center for Childhood Infections and Vaccines (CCIV) to M.L.M and in part by Department of Health and Human Services, National Institutes of Health grants 1R01AI087798 and 1U19AI095227 to M.L.M. This work is dedicated to ‘A.R.'.
Footnotes
M.L.M. co-founded and serves as Chief Scientific Officer for Meissa Vaccines, Inc. S.L., M.T.N. and M.L.M are co-inventors in a patent application (PCT/US2016/037658) describing the rhinovirus vaccine reported in this paper. The vaccine technology has been optioned to Meissa Vaccines, Inc. by Emory University. The remaining authors declare no competing financial interests.
Author contributions S.L., M.T.N., M.G.C., J.B.J., E.A.S., A.E.K. and R.M.-L. performed experiments. K.R., Y.A.B., J.E.G. and P.S. provided reagents and advice. X.L. and D.D.E. provided rhinovirus types. S.L., M.T.N. and M.L.M. designed the experiments, analysed data and wrote the paper. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Gwaltney J. M. Jr, Hendley J. O., Simon G. & Jordan W. S. Jr Rhinovirus infections in an industrial population. I. The occurrence of illness. N. Engl. J. Med. 275, 1261–1268 (1966). [DOI] [PubMed] [Google Scholar]
- Glanville N. & Johnston S. L. Challenges in developing a cross-serotype rhinovirus vaccine. Curr. Opin. Virol. 11, 83–88 (2015). [DOI] [PubMed] [Google Scholar]
- Jain S. et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 373, 415–427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay W. S., al-Nakib W., Higgins P. G. & Tyrrell D. A. The time course of the humoral immune response to rhinovirus infection. Epidemiol. Infect. 103, 659–669 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett J. E., Bynoe M. L. & Tyrrell D. A. Some attempts to produce an experimental vaccine with rhinoviruses. Br. Med. J. 1, 34–36 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A. et al. Effect of neutralizing antibody on experimental rhinovirus infection. JAMA. 186, 578–584 (1963). [DOI] [PubMed] [Google Scholar]
- Perkins J. C. et al. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am. J. Epidemiol. 90, 319–326 (1969). [DOI] [PubMed] [Google Scholar]
- Perkins J. C. et al. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am. J. Epidemiol. 90, 519–526 (1969). [DOI] [PubMed] [Google Scholar]
- Buscho R. F., Perkins J. C., Knopf H. L., Kapikian A. Z. & Chanock R. M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J. Immunol. 108, 169–177 (1972). [PubMed] [Google Scholar]
- Mitchison D. A. Prevention of colds by vaccination against a rhinovirus: a report by the scientific committee on common cold vaccines. Br. Med. J. 1, 1344–1349 (1965). [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Draper C., Stons P. B. & Tyrrell D. A. Absence of heterologous antibody responses in human volunteers after rhinovirus vaccination. (Brief report). Arch. Gesamte. Virusforsch. 28, 89–92 (1969). [DOI] [PubMed] [Google Scholar]
- Glanville N. et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog. 9, e1003669 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M. K., Fox J. P. & Kenny G. E. Antigenic groupings of 90 rhinovirus serotypes. Infect. Immun. 37, 642–647 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean G. R. et al. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral. Res. 95, 193–201 (2012). [DOI] [PubMed] [Google Scholar]
- Couch R. B. The common cold: control? J. Infect. Dis. 150, 167–173 (1984). [DOI] [PubMed] [Google Scholar]
- Fox J. P. Is a rhinovirus vaccine possible? Am. J. Epidemiol. 103, 345–354 (1976). [DOI] [PubMed] [Google Scholar]
- Crowe J. E. Jr Universal flu vaccines: primum non nocere. Sci. Transl. Med. 5, 200fs234 (2013). [DOI] [PubMed] [Google Scholar]
- Vollmers C., Sit R. V., Weinstein J. A., Dekker C. L. & Quake S. R. Genetic measurement of memory B-cell recall using antibody repertoire sequencing. Proc. Natl Acad. Sci. USA 110, 13463–13468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C. L., Knowles N. J. & Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 94, 1791–1806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324, 55–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. M. et al. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care. Med. 186, 886–891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K. et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 123, 98–104 e101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden K. E., McErlean P., Nissen M. D., Sloots T. P. & Mackay I. M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 78, 1232–1240 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler A. et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 196, 817–825 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson D. et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 194, 1398–1402 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick N. et al. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J. Infect. Dis. 196, 1754–1760 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov Y. A. et al. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat. Med. 17, 627–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov Y. A. et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl Acad. Sci. USA 112, 5485–5490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett N. W. et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 14, 199–204 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J. C. et al. Prophylactic antibody treatment and intramuscular immunization reduce infectious human rhinovirus 16 load in the lower respiratory tract of challenged cotton rats. Trials Vaccinol. 3, 52–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Davis W. G., Sambhara S. & Jacob J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl Acad. Sci. USA 109, 13751–13756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamory B. H., Hamparian V. V., Conant R. M. & Gwaltney J. M. Jr Human responses to two decavalent rhinovirus vaccines. J. Infect. Dis. 132, 623–629 (1975). [DOI] [PubMed] [Google Scholar]
- Racaniello V. R. & Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science 214, 916–919 (1981). [DOI] [PubMed] [Google Scholar]
- Farid S. S., Washbrook J. & Titchener-Hooker N. J. Decision-support tool for assessing biomanufacturing strategies under uncertainty: stainless steel versus disposable equipment for clinical trial material preparation. Biotechnol. Prog. 21, 486–497 (2005). [DOI] [PubMed] [Google Scholar]
- Thomassen Y. E. et al. Next generation inactivated polio vaccine manufacturing to support post polio-eradication biosafety goals. PLoS ONE 8, e83374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen Y. E. et al. Scale-down of the inactivated polio vaccine production process. Biotechnol. Bioeng. 110, 1354–1365 (2013). [DOI] [PubMed] [Google Scholar]
- Reed L. J. & Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27, 493–497 (1938). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7, Supplementary Tables 1-4 and Supplementary References
Data Availability Statement
Primary data that support the findings of this study are available from the corresponding author on request.