Abstract
Background
Non-invasive ventilation (NIV) is an effective form of treatment in patients with obesity hypoventilation syndrome (OHS) who have concomitant severe obstructive sleep apnoea (OSA). However, there is a paucity of evidence on the efficacy of NIV in patients with OHS without severe OSA. We performed a multicentre randomised clinical trial to determine the comparative efficacy of NIV versus lifestyle modification (control group) using daytime arterial carbon dioxide tension (PaCO2) as the main outcome measure.
Methods
Between May 2009 and December 2014 we sequentially screened patients with OHS without severe OSA. Participants were randomised to NIV versus lifestyle modification and were followed for 2 months. Arterial blood gas parameters, clinical symptoms, health-related quality of life assessments, polysomnography, spirometry, 6-min walk distance test, blood pressure measurements and healthcare resource utilisation were evaluated. Statistical analysis was performed using intention-to-treat analysis.
Results
A total of 365 patients were screened of whom 58 were excluded. Severe OSA was present in 221 and the remaining 86 patients without severe OSA were randomised. NIV led to a significantly larger improvement in PaCO2 of −6 (95% CI −7.7 to −4.2) mm Hg versus −2.8 (95% CI −4.3 to −1.3) mm Hg, (p<0.001) and serum bicarbonate of −3.4 (95% CI −4.5 to −2.3) versus −1 (95% CI −1.7 to −0.2 95% CI) mmol/L (p<0.001). PaCO2 change adjusted for NIV compliance did not further improve the inter-group statistical significance. Sleepiness, some health-related quality of life assessments and polysomnographic parameters improved significantly more with NIV than with lifestyle modification. Additionally, there was a tendency towards lower healthcare resource utilisation in the NIV group.
Conclusions
NIV is more effective than lifestyle modification in improving daytime PaCO2, sleepiness and polysomnographic parameters. Long-term prospective studies are necessary to determine whether NIV reduces healthcare resource utilisation, cardiovascular events and mortality.
Trial registration number
NCT01405976; results.
Keywords: Sleep apnoea, Non invasive ventilation
Key messages.
What is the key question?
Non-invasive ventilation (NIV) is an effective form of treatment in patients with obesity hypoventilation syndrome (OHS) who have concomitant severe obstructive sleep apnoea (OSA), but there is a paucity of evidence on the efficacy of NIV in patients with OHS without severe OSA.
What is the bottom line?
NIV was more effective than lifestyle modification in improving daytime PaCO2, sleepiness and polysomnographic parameters.
Why read on?
This randomised study demonstrates the efficacy of NIV in this small subgroup of patients with OHS without severe OSA where central hypoventilation is the main mechanism leading to daytime hypercapnia.
Introduction
Obesity hypoventilation syndrome (OHS) is characterised by obesity and chronic hypercapnic respiratory failure in the absence of neuromuscular, metabolic, lung or chest wall diseases.1 Although the majority of patients with OHS have concomitant severe obstructive sleep apnoea (OSA), nocturnal hypoventilation may be the only respiratory sleep disorder present.2 In fact, in the largest clinical trial of OHS, we recently reported that 73% had severe OSA.3
The prevalence of OHS in the general population is unknown, but it has been estimated to be 0.3–0.4%.4 Given the global obesity epidemic, the prevalence of OHS may be on the rise. It is important for clinicians to promptly diagnose and adequately treat OHS because, when left untreated, it is associated with significantly worse cardiovascular morbidity, mortality and healthcare resource utilisation compared with eucapnic OSA5 6 and eucapnic obese patients.5–13
The aetiology of daytime hypercapnia in OHS is complex and not fully understood, but the progressive accumulation of CO2 caused by repetitive obstructive events (particularly with short inter-event periods14 and non-apnoeic hypoventilation, mainly rapid eye movement sleep hypoventilation) seems to be an important contributor.15 Conceptually, continuous positive airway pressure (CPAP) is not an effective treatment for patients with OHS who are mostly hypoventilators without significant OSA.16 Non-invasive ventilation (NIV), most commonly in the form of bilevel positive airway pressure, can treat both apnoeic and non-apnoeic nocturnal hypoventilation. In this phenotype of patients with OHS who have a clear predominance of severe OSA, both CPAP and NIV have been shown to decrease daytime arterial carbon dioxide tension (PaCO2) in clinical series7 10 16–20 and in three randomised controlled trials.3 21 22 However, in the largest trial there were some respiratory functional advantages in favour of NIV.3
The role of NIV in improving daytime hypercapnia in patients with OHS who do not have concomitant severe OSA has only been examined in three small case series of 22, 13 and 6 patients.23–25 Since most clinical series and clinical trials have focused on OHS patients with severe OSA, there is a gap of knowledge on how to most effectively treat patients with OHS who do not have concomitant OSA.
To that end, we performed a multicentre randomised clinical trial with two open parallel groups to compare the efficacy of NIV versus lifestyle modification (control group) in patients with OHS without severe OSA using PaCO2 at 2 months as the primary outcome variable.
Methods
Study design
This study was designed as a multicentre randomised clinical trial with two open parallel groups. The present study is part of the ‘Pickwick’ project.26 This project has two parallel randomised clinical trials based on the presence or absence of severe OSA (polysomnographic apnoea–hypopnoea index (AHI) ≥30) with identical methodology (see online supplementary data).
thoraxjnl-2016-208501supp.pdf (710.5KB, pdf)
Patients
Between May 2009 and March 2013 we sequentially screened patients between 15 and 80 years of age who were referred for pulmonary consultation due to suspected OHS or OSA at 16 tertiary hospitals in Spain with substantial experience with NIV and CPAP therapy (see online supplementary data). In March 2013 recruitment and randomisation of OHS patients with severe OSA was completed and from April 2013 to December 2014 we continued to sequentially include only patients with OHS who did not have severe OSA. OHS was defined as obesity with a body mass index (BMI) ≥30 kg/m2, stable hypercapnic respiratory failure (daytime awake PaCO2 ≥45 mm Hg, pH ≥7.35 and no clinical worsening during the two previous months) and no significant chronic obstructive pulmonary disease (COPD) (forced expiratory volume in 1 s (FEV1) >70% of predicted when FEV1/forced vital capacity (FVC) <70, neuromuscular, chest wall or metabolic disease. Other inclusion criteria were an absence of narcolepsy or restless leg syndrome and a correctly executed 30 min NIV treatment test (see online supplementary data). The exclusion criteria were as follows: (1) a psycho-physical inability to complete questionnaires; (2) severe chronic debilitating illness; (3) severe chronic nasal obstruction; and (4) a lack of informed consent.
Ethics
The study was approved by the ethics committees of the 16 centres, and written informed consent was obtained from all patients.
Randomisation
Conventional polysomnography (PSG) and analysis of arterial blood gases (ABGs) were performed in enrolled participants. Eligible patients were randomised by an electronic database (simple randomisation) into the NIV or control group.
Control group: lifestyle modification
The lifestyle modification consisted of a 1000 calorie diet and the maintenance of correct sleep hygiene and habits (avoiding supine position during sleep; maintaining regular sleep habits and exercise; not consuming sedatives, stimulants or alcohol; not smoking tobacco; and avoiding heavy meals within 4 hours before bedtime). Oxygen therapy was added if the daytime arterial oxygen tension (PaO2) was <55 mm Hg27 with the necessary flow to maintain waking arterial oxygen saturation (SaO2) between 88% and 92% or PaO2 ≥55 mm Hg for at least 17 hours/day. In patients requiring oxygen a new ABG analysis was performed after 20 min of oxygen treatment. If PaCO2 increased to ≥5 mm Hg or if the pH reached <7.35, the oxygen treatment was stopped.
NIV group
In addition to lifestyle modification and oxygen (if required), patients were instructed to use NIV treatment during the entire sleep period. The ventilator mode was set at bilevel pressure with assured volume (ie, volume targeted pressure support). While the patient was awake, the expiratory positive airway pressure (EPAP) was initially set between 4 and 8 cm H2O and the inspiratory positive airway pressure (IPAP) was set between 18 and 22 cm H2O (EPAP included). The pressures were adjusted to obtain normal oxygen saturation, if possible, as measured by pulse oximetry and patient tolerance. The backup respiratory rate was initially adjusted to 12–15 breaths/min (close to the spontaneous respiratory rate, if possible) and the target volume was set at between 5 and 6 mL/kg of actual weight, allowing for an increase in the maximum pressure over the previously minimum IPAP, if necessary. A check of mechanical ventilation phases (trigger, pressurisation and ending) was also performed to avoid asynchronies and to refine the setting. After 30 min of continuous use with patient adaptation and an adequate patient–ventilator interaction, an ABG analysis was performed. The PaCO2 result was used to adjust the ventilator parameters. The final adjustment was performed by means of conventional PSG, with an increase in EPAP for obstructive apnoeas and an increase in IPAP for hypopnoeas, flow limitation, snoring or non-apnoeic hypoventilation, with the goal of achieving normalisation of oxygen saturation or the maximal pressure tolerated was reached. No changes were made in the assured volume during this nocturnal titration (see online supplementary data for the ventilator and mask types employed).
Procedures and outcomes
Patients were evaluated on three occasions: at baseline, after the first month and after 2 months. At baseline and after 2 months we assessed the primary outcome (PaCO2) by means of ABG analysis while breathing room air (see online supplementary data). We also assessed the following secondary outcomes: other ABG parameters; anthropometric data; clinical symptoms (classified into four levels of intensity); dyspnoea on the Medical Research Council scale; sleepiness on the Epworth sleepiness scale (ESS); health-related quality-of-life (HRQL) tests using the Functional Outcomes of Sleep Questionnaire (FOSQ), the Medical Outcome Survey Short Form 36 (SF 36) and the visual analogue well-being scale (VAWS);28 29 blood pressure measurements; PSG; spirometry; 6-min walk distance (6-MWD) test; healthcare resource utilisation; dropouts and their causes; and compliance using an hourly counter. In the first month we encouraged treatment compliance, performed an ABG analysis and made any necessary changes to the oxygen therapy or NIV settings.
Dropouts were defined as patients who decided to leave the study voluntarily or for one of the following medical reasons: (1) pH <7.33 at the first month evaluation; (2) hospital admission requiring NIV treatment for more than 5 days, conventional mechanical ventilation for more than 3 days or pH <7.33 while breathing room air at hospital discharge; or (3) death. We considered adequate compliance with NIV treatment as daily use for ≥4 hours.
PSG
We performed PSG at baseline for titration (only for the NIV group) and after 2 months of treatment (with the home settings of the NIV). Oxygen treatment was not applied during any PSG. We used standard protocols to perform the PSG and analyse the results (see online supplementary data). A valid PSG recording required at least 3 hours of sleep time. In cases of an invalid recording, the test was repeated one additional time.
Statistical analysis
The sample size was calculated based on a previous study in which the mean PaCO2 in patients with OHS treated with NIV was 45±5 mm Hg.23 We estimated the sample size required to detect average differences of 2.5 mm Hg between groups using a mean comparison of a sample versus a theoretical value method. For a SD of 5 and power of 0.8, with a two-sided significance level of 0.05, the estimated sample size was 34 patients per group. When it was adjusted to a dropout rate of 20%, the estimated sample size was 43 patients per group, or 86 in total.
Intention-to-treat analysis was performed. Missing values for the primary and secondary outcomes (dropouts included) were imputed following a multiple imputation method with iterative multivariable regression because the missing data had characteristics compatible with a missing at random pattern. Intra-group changes in the continuous variables from baseline to 2 months were assessed using paired t-tests. The observed effects in the two arms of the study (inter-group differences) were compared using unpaired t-tests. When the overall comparison was statistically significant (p<0.05), comparisons of groups were performed by analysis of covariance (ANCOVA), taking into account the baseline values of the variable analysed such as age, gender, BMI and AHI (henceforth ‘basic adjustment’). An additional logistic regression was performed for the change in the primary outcome with the basic adjustment, weight change and NIV compliance (more or less than 4 hours of use per day). Secondary categorical variables were compared between baseline and 2 months using the χ2 test. Data management, statistical analyses and the imputation were performed using SPSS software (IBM SPSS Statistics V.22.0; IBM Corp, Armonk, New York, USA).
Results
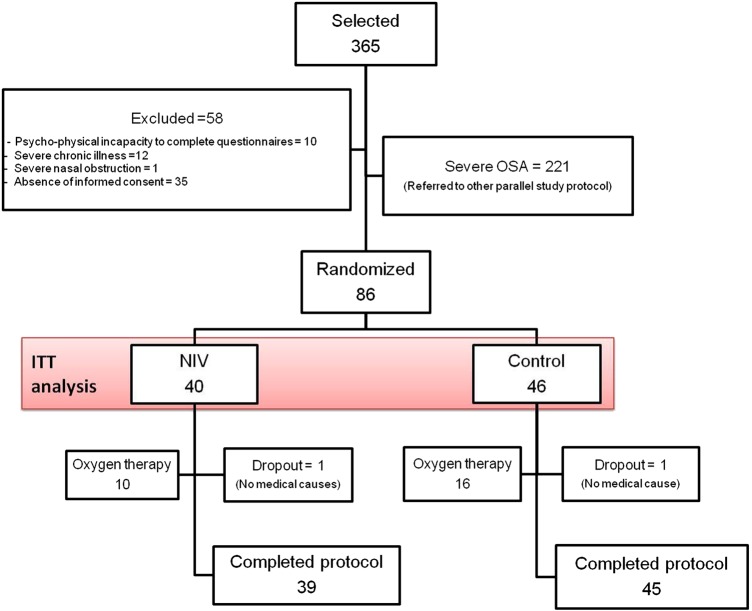
Of the 365 patients who met the inclusion criteria, 58 were excluded, 221 had severe OSA and 86 were randomised (figure 1). Only one patient in each group was a dropout due to non-medical causes.
Figure 1.
Flow chart of the study protocol. Of the 365 selected patients, 58 were excluded, 221 had severe OSA and 86 were randomised. A dropout in the control group was due to hospital admission requiring NIV treatment for more than 5 days. OSA, obstructive sleep apnoea; ITT, intention to treat; NIV, non-invasive ventilation.
Table 1 summarises the baseline demographic data. The median age of the group was 68 years with a BMI of 40 kg/m2 and with a clear female predominance. The rates of comorbidities were high, especially for hypertension, type 2 diabetes, dyslipidaemia and cardiovascular conditions. Patients assigned to the NIV group had a lower frequency of dyslipidaemia and a higher frequency of chronic heart failure than patients assigned to the control group. Table 2 presents the baseline values and the changes with treatment in the primary and secondary outcomes. PaCO2 and serum bicarbonate levels improved with both treatments. The improvements were significantly greater in the NIV group, both in the unadjusted and adjusted inter-group comparisons (table 2 and figure 2). Additionally, PaO2 improved more with NIV without reaching inter-group statistical significance. Although the 6-MWD test results improved more with NIV treatment, it did not reach statistical significance in intra- or inter-group comparisons.
Table 1.
Anthropometric characteristics, alcohol and smoking habits and comorbidities
| NIV (N=40) |
Control (N=46) |
All (N=86) |
|
|---|---|---|---|
| Gender, male, % | 25 | 17 | 21 |
| Age, years, median (IQR) | 67 (12) | 69 (15) | 68 (14) |
| BMI, kg/m2, mean (SD) | 40 (6.3) | 40 (5.6) | 40 (5.9) |
| Neck circumference, cm, median (IQR) | 42 (7) | 42 (5) | 42 (5.8) |
| Waist circumference, cm, mean (SD) | 123 (15) | 119 (12) | 121 (14) |
| Waist/hip ratio, mean (SD) | 0.97 (0.1) | 0.95 (0.08) | 0.96 (0.09) |
| Active drinker, % | 13 | 9 | 11 |
| Alcohol, g, median (IQR) | 30 (12) | 23 (30) | 30 (20) |
| Active smoker, % | 7.5 | 15 | 12 |
| Pack years, median (IQR) | 35 (18) | 40 (26) | 38 (21) |
| COPD, %* | 5.0 | 6.5 | 6.0 |
| Hypertension, % | 80 | 80 | 80 |
| Drug number, median (IQR) | 2 (1) | 1 (1) | 1 (1) |
| Diabetes, % | 35 | 41 | 38 |
| Dyslipidaemia, % | 30 | 54 | 43 |
| Ischaemic heart disease, % | 11 | 8.7 | 9.5 |
| Arrhythmia, % | 16 | 7 | 11 |
| Chronic heart failure, % | 37 | 13 | 24 |
| Stroke, % | 8.1 | 8.9 | 8.5 |
| Leg arteriopathy, % | 14 | 15 | 15 |
| Pulmonary hypertension, % | 18 | 11 | 14 |
*Defined as FEV1 >70% of predicted when FEV1/FVC <70. BMI, body mass index; NIV, non-invasive ventilation.
Table 2.
Baseline measurements and changes with treatment related to the primary and secondary outcomes of pulmonary function and blood pressure measures
| Baseline, mean (SD)/median (IQR) | Intra-group differences, mean (95% CI) | p Value of inter-group differences§ | ||||
|---|---|---|---|---|---|---|
| NIV | Control | NIV | Control | Unadjusted | Adjusted | |
| PaCO2, mm Hg | 49 (4.0) | 49 (3.5) | −6 (−7.7 to −4.2)‡ | −2.8 (−4.3 to −1.3)‡ | 0.006 | 0.019 |
| Serum bicarbonate, mmol/L | 30 (4.1) | 29 (3.8) | −3.4 (−4.5 to −2.3)‡ | −1 (−1.7 to −0.2)* | 0.000 | 0.004 |
| pH | 7.400 (0.040) | 7.400 (0.030) | 0.005 (−0.005 to 0.157) | 0.031 (−0.008 to 0.147) | NS | – |
| PaO2, mm Hg | 64 (10) | 67 (10) | 4.6 (0.5 to 8.8)* | 1.4 (−2.6 to 5.5) | NS | – |
| FEV1, % | 72 (16) | 80 (20) | 1.8 (−2.7 to 6.4) | 1.9 (−1.2 to 5.1) | NS | – |
| FVC, % | 75 (21) | 82 (20) | 4.7 (−4.2 to 14) | 2.9 (−0.5 to 6.3) | NS | – |
| 6-MWD, m | 309 (105) | 349 (105) | 29 (−16 to 74) | −7.2 (−25 to 11) | NS | – |
| Systolic BP, mm Hg | 136 (18) | 136 (15) | −4.2 (−11 to 2.5) | −4.3 (−10 to 1.7) | NS | – |
| Diastolic BP, mm Hg | 80 (16) | 80 (18) | 0.5 (−5.3 to 6.2) | −1.2 (−5.4 to 2.9) | NS | – |
Median (IQR) values are shown in italic.
Bold type indicates statistical significance.
p Values of intra-group differences (2 months − baseline): *p<0.05; †p<0.01; ‡p<0.001. §p Values of inter-group differences unadjusted or adjusted by basic adjustment (baseline values of the variable analysed and age, gender, BMI and AHI).
6-MWD, 6 min walk distance; AHI, apnoea–hypopnoea index; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; NIV, non-invasive ventilation.
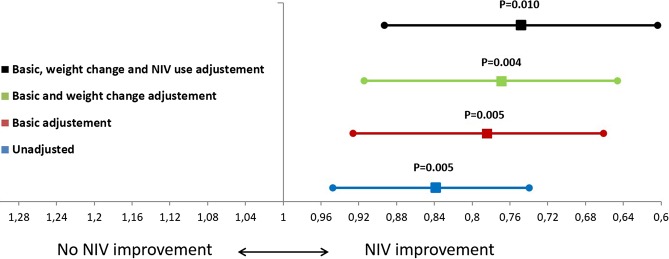
Figure 2.
Inter-group changes in arterial carbon dioxide tension (PaCO2) (means and 95% of CIs) adjusted according to basic adjustments (baseline PaCO2, age, sex, body mass index and apnoea–hypopnoea index), weight change and non-invasive ventilation (NIV) use (more or less than 4 hours/night).
Figure 2 shows the unadjusted and adjusted OR and 95% CIs of the PaCO2 change between the NIV and control groups. Adjustments for weight change and NIV compliance did not substantially modify the inter-group statistical significances. Table 3 presents the baseline values and the changes in the ESS scores, HRQL results and weight with treatments. Although ESS scores improved in both groups, the degree of improvement was significantly larger with NIV. Additionally, significant improvements were observed in the mental component of SF-36 and VAWS questionnaires for the NIV group in the intra-group and inter-group comparisons although, in the adjusted analysis, only the mental component of SF-36 remained statistically significant.
Table 3.
Baseline values and changes in the ESS score, health-related quality of life test results and weight
| Baseline, mean (SD) | Intra-group differences, mean (95% CI) | p Value of inter-group differences | ||||
|---|---|---|---|---|---|---|
| NIV | Control | NIV | Control | Unadjusted | Adjusted | |
| ESS | 7.7 (5.5) | 8.5 (4.2) | −2.9 (−4.1 to −1.7)‡ | −1.2 (−2.2 to −0.2)* | 0.038 | 0.021 |
| FOSQ | 72 (22) | 75 (19) | 4.4 (−1.7 to 10.5) | −2.7 (−8.1 to 3.1) | NS | – |
| SF 36-Physical | 35 (10) | 37 (8) | 3.1 (−0.4 to 6.6) | 0.9 (−1.3 to 3.2) | NS | – |
| SF 36-Mental | 41 (12) | 43 (11) | 4.1 (0 to 8.3)† | −0.9 (−3.7 to 1.8) | 0.038 | 0.035 |
| VAWS | 45 (25) | 63 (22) | 18 (8.4 to 27)† | 1.8 (−4.7 to 8.3) | 0.006 | – |
| Weight, kg | 102 (19) | 100 (17) | 0.7 (−2.5 to 3.9) | −1.6 (−3.1 to −0.2)* | NS | – |
Bold type indicates statistical significance.
p Values of intra-group differences (2 months − baseline): *p<0.05; †p<0.01; ‡p<0.001.
p Values of inter-group differences unadjusted or adjusted by basic adjustment (baseline values of the variable analysed and age, gender, BMI and AHI).
AHI, apnoea–hypopnoea index; BMI, body mass index; ESS, Epworth sleepiness scale; FOSQ, Functional Outcomes of Sleep Questionnaire; NIV, non-invasive ventilation; SF 36, Medical Outcome Survey Short Form 36; VAWS, visual analogue well-being scale.
Figure 3 shows the clinical symptoms at baseline and after 2 months. NIV led to statistically significant improvements in unrefreshing sleep and tiredness compared with controls. Table 4 presents the baseline values and the changes in polysomnographic parameters. As expected, NIV significantly improved AHI, arousal and desaturation indexes, mean oxygen saturation and total sleep time (TST) below 90% oxygen saturation in unadjusted and adjusted intergroup comparisons.
Figure 3.
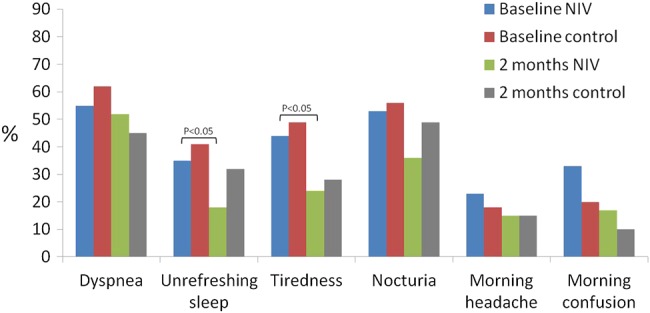
Changes in the percentages of clinical symptoms in the two groups. Non-invasive ventilation (NIV) achieved more important improvement than the control group, with statistical intra-group differences for unrefreshing sleep and tiredness.
Table 4.
Baseline values and changes in polysomnographic parameters
| Baseline, mean (SD)/median (IQR) | Intra-group differences, mean (95% CI) | p Value of inter-group differences | ||||
|---|---|---|---|---|---|---|
| NIV | Control | NIV | Control | Unadjusted | Adjusted | |
| TST, hours | 5.3 (1.7) | 5.4 (1.5) | −0.15 (−1 to 0.6) | 0.1 (−0.5 to 0.7) | NS | – |
| Sleep efficiency | 77 (29) | 79 (24) | −5.4 (−15 to 4.6) | 3.5 (−5.1 to 12) | NS | – |
| % Non-REM light sleep | 67 (27) | 63 (24) | −6.2 (−15 to 2.3) | −4 (−12 to 3.7) | NS | – |
| % Non-REM deep sleep | 18 (17) | 23 (18) | 4.6 (−3.2 to 12) | 1.6 (−6.4 to 9.6) | NS | – |
| % REM sleep | 8.7 (8.9) | 13 (15) | 5.6 (−0.9 to 12) | 3.3 (−3 to 9.6) | NS | – |
| Arousal index | 25 (20) | 22 (18) | −10 (−14 to −6.2)‡ | −0.3 (−2.9 to 2.3) | 0.000 | 0.000 |
| AHI | 14 (8.9) | 15 (7.8) | −11 (−15 to −7.1)‡ | 0.1 (−2.7 to 2.8) | 0.000 | 0.000 |
| DI | 18 (27) | 19 (16) | −19 (−25 to −12)‡ | −0.4 (−4.6:3.8) | 0.000 | 0.000 |
| Mean SaO2 | 88 (8) | 87 (5) | 6 (4.3 to 7.7)‡ | 0.4 (−0.5 to 1.4) | 0.000 | 0.000 |
| %TST <90 | 82 (58) | 69 (79) | −36 (−46 to −25)‡ | −5.7 (−15 to 2) | 0.000 | 0.002 |
Median (IQR) values are shown in italic.
Bold type indicates statistical significance.
p Values of intra-group differences (2 months − baseline): ‡p<0.001.
p Values of inter-group differences unadjusted or adjusted by basic adjustment (baseline values of the variable analysed and age, gender, BMI and AHI).
AHI, apnoea–hypopnoea index; BMI, body mass index; DI, desaturation index; %TST <90, percentage of TST below 90% of oxygen saturation; NIV, non-invasive ventilation; REM, rapid eye movement; SaO2, arterial oxygen saturation; TST, total sleep time.
Table 5 shows the setting for NIV and oxygen, NIV compliance and use of healthcare resource utilisation during the follow-up period. Similar percentages of patients received supplemental oxygen in both groups (NIV 25% and control 35%). The mean backup respiratory rate was 15 breaths/min, the mean IPAP was 18.2 cm H2O and the mean EPAP was 7.1 cm H2O. The mean daily NIV use was 6±2.7 hours. NIV was more effective than lifestyle changes in reducing emergency room visits (NIV 0.05±0.22, control 0.23±0.52), but without reaching statistical significance in the adjusted analysis.
Table 5.
Therapy settings, compliance and use of hospital resources
| Baseline | p Value | |||
|---|---|---|---|---|
| NIV | Control | Unadjusted | Adjusted* | |
| Oxygen therapy, % | 25 | 35 | NS | – |
| Oxygen flow, L/min, mean (SD) | 1.8 (09) | 1.4 (0.4) | NS | – |
| Pressures, cm H2O, mean (SD) | – | – | ||
| IPAP | 18.2 (3.4) | – | ||
| EPAP | 7.1 (1.8) | – | ||
| Respiratory rate, mean (SD) | 15 (3) | – | – | |
| Mask, % | – | – | ||
| Nasal | 18 | – | ||
| Full-face | 82 | – | ||
| Compliance, hours/day, mean (SD) | 6 (2.7) | – | – | – |
| Emergency room visit, mean (SD) | 0.05 (0.22) | 0.23 (0.52) | 0.04 | NS |
| Hospital admission rate, mean (SD) | 0 | 0.05 (0.21) | NS | – |
| Hospital days, mean (SD) | 0 | 0.65 (3) | NS | – |
Bold type indicates statistical significance.
*Adjusted by basic adjustment (baseline values of the variable analysed and age, gender, BMI and AHI).
AHI, apnoea–hypopnoea index; BMI, body mass index; EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; NIV, non-invasive ventilation.
Online supplementary figure E2 presents the PaCO2 at baseline and at 1 and 2 months. Significant differences were observed between baseline and 1-month values for the NIV group, but there were no differences between 1-month and 2-month values. Additional details regarding the polysomnographic titration, assured volume mode, dyspnoea and other clinical symptoms, HRQL tests, transcutaneous PCO2, supplemental oxygen therapy and types of ventilators used are provided in the online supplementary data.
Discussion
This study is the only randomised clinical trial to date comparing two alternative treatment strategies in patients with OHS who do not have concomitant severe OSA. The main results can be summarised as follows: (1) PaCO2 and serum bicarbonate improved significantly more in the NIV group than in the control group (ie, lifestyle changes); (2) the improvement in PaCO2 with NIV treatment was partially independent of NIV compliance, although overall compliance with NIV was high in our cohort (mean 6 hours/night); and (3) AHI and nocturnal oxygenation improved significantly with NIV treatment relative to the control group. Our study is clinically relevant because the few randomised clinical trials performed to date in patients with OHS have focused on the phenotype that has concomitant severe OSA. Our randomised clinical trial has provided clear evidence that NIV is superior to lifestyle changes in this phenotype of patients with OHS and severe OSA. Our present findings are in line with three prior small clinical series of patients with OHS without severe OSA treated with NIV.23–25 Moreover, the degree of improvement in daytime PaCO2 observed in our study was similar to prior studies that enrolled mostly patients with OHS and concomitant severe OSA,7 10 16–19 21 22 including our recently reported largest clinical trial of OHS patients with severe OSA.3
Although there is no clear evidence as to what short-term outcome variable is a reliable predictor of long-term outcomes,7 13 18 we believe our choice of PaCO2 as the primary outcome for this medium-term study is reasonable since PaCO2 is a marker of the severity of hypercapnic respiratory failure and it has been related to mortality in OHS.12 18
Adequate compliance seems necessary to maximise the beneficial effects of NIV therapy. However, in our study the PaCO2 change adjusted for compliance with NIV (>4 hours/day) did not modify the inter-group statistical significance. This is in contrast to the findings in our recently published parallel randomised clinical trial that included patients with OHS with severe OSA.3 One possible explanation is that, in the present study, the mean compliance was slightly higher leading to a ceiling effect (6±2.7 vs 5.3±2.3 hours/day). Moreover, in our study patients with OHS without severe OSA were phenotypically different from patients with OHS with severe OSA.3 Those without severe OSA were older, predominantly female, less obese, less hypersomnolent, with a higher prevalence of hypertension and cardiovascular morbidity, lower exercise tolerance and higher rates of hospital admissions. Importantly, patients with OHS without severe OSA did not have worse gas exchange during wakefulness than patients with severe OSA.30 One might therefore surmise that, in addition to the PaCO2 level, there are other important predictors of outcomes based on the phenotype of OHS. Further investigations on OHS phenotypes, ‘dose-response’ of NIV treatment and long-term outcome are necessary.
Several studies have reported an improvement in various measures of respiratory function in patients with OHS treated with NIV,17 18 21 24 31 although these findings are not universal.10 11 17 In contrast to our recently published parallel randomised clinical trial that included only patients with OHS with severe OSA,3 in the present study we did not observe a significant improvement in spirometry and 6-MWD tests in patients treated with NIV.3 The patients with OHS with severe OSA were more obese, and it is plausible that NIV was more effective in reducing microatelectasis leading to improvement in lung volume. In contrast to patients with OHS with severe OSA,30 those without severe OSA have a higher burden of cardiovascular morbidity which may in part explain the lack of improvement in 6-MWD with NIV therapy.
Although by design we excluded patients with severe OSA, some degree of OSA was present in our patients (mean AHI=14) and NIV significantly reduced the AHI and improved other polysomnographic parameters of sleep-disordered breathing such as arousal index, oxygen desaturation index and TST <90% SaO2 compared with the control group. This finding is similar observations in prior studies which predominantly included patients with OHS with severe OSA,17 21 suggesting a similar improvement in central hypoventilation in both OHS phenotypes. In contrast to prior studies, we did not find any improvement in the percentage of sleep stages, suggesting that the improvement in sleep quality with NIV may be dependent on the presence of severe OSA.
Observational studies have reported a reduction in long-term healthcare resource utilisation in patients with OHS treated with NIV.16 19 In our study we found a clear trend towards a reduction in healthcare resource utilisation with NIV treatment after only 2 months of follow-up, although this degree of improvement did not remain statistically significant in adjusted analysis. A longer period of follow-up is necessary to better assess outcomes such as healthcare resource utilisation.
Our study has several limitations. Given the multiple comparisons of secondary outcomes, those with p values close to the statistical significance limit should be interpreted with caution and taken as hypothesis-generating. With regard to the threshold of OSA severity, we strategically opted to exclude patients with severe OSA (AHI ≥30). Although we acknowledge that most of our patients had some degree of mild to moderate OSA (ie, AHI 5–29), we believe that CPAP is more effective in improving hypercapnia in patients with OHS with severe OSA. Moreover, from a pragmatic standpoint, enrolling patients with OHS with minimal to no OSA would have been challenging since the majority of patients with OHS do have some degree of OSA. Although we recognise that our spirometric criteria may have allowed the inclusion of some patients with mild COPD, we believe that this degree of mild obstructive defect on spirometry is less likely to be a significant contributor to the development of chronic respiratory failure and hypercapnia, particularly with an FEV1 >70% of predicted. Moreover, the low prevalence of COPD (6%) should have minimally impacted our results.
In summary, in the specific OHS phenotype without severe OSA, NIV was more effective than lifestyle modification in improving daytime PaCO2, sleepiness and polysomnographic parameters of sleep-disordered breathing. Long-term randomised controlled trials are necessary to demonstrate if NIV decreases healthcare resource utilisation, cardiovascular events and mortality and whether these outcomes are different between phenotypes of OHS based on OSA severity.
Acknowledgments
We are indebted to Verónica Rodríguez for her assistance in the translation of the manuscript and to Vanessa Iglesias for her technical assistance.
Footnotes
Contributors: Substantial contributions to study conception and design, acquisition of data, or analysis and interpretation of data: JFM, JC, MLA, EO, MFT, MG, SL-M, JMM, SM, TD-C, EC, CE, JT, NG-M, AM, PDL, SJC, OR, JD, CS, MM, FR, AR, CC, JMB, RG, AS-R, SG, MB, EG-L and M-AS-Q. Drafting the article or revising the article critically for important intellectual content: JFM, BM, JC, JMM, SM, EC, EM, JT, NG-M, and PDL. Final approval of the version to be published: JFM, BM and EM. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: JFM. JFM has full access to all data from the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: Funding was provided by Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) PI050402, Spanish Respiratory Foundation 2005 (FEPAR) and Air Liquide Spain. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics approval was obtained from the ethics committees of the 16 centres.
Provenance and peer review: Not commissioned; externally peer reviewed.
Disclaimer: Addition to this paper, we have written 4 other papers based on this dataset. One published on the results of the first phase in OHS patients with severe sleep apnoea3 and one describing the methodology.26 Another paper on the baseline cardiovascular risk (without any treatment) according to the sleep apnoea severity in total population (OHS patients with and without severe sleep apnoea) is in press (Epub ahead of print).30 Finally, a paper on the influence of supplemental oxygen therapy (the effect of supplemental oxygen in obesity hypoventilation syndrome) has been submitted to the Journal of Clinical Sleep Medicine.
References
- 1.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008;5:218–25. 10.1513/pats.200708-122MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R, Chaouat A, Schinkewitch P, et al. . The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001;120:369–76. 10.1378/chest.120.2.369 [DOI] [PubMed] [Google Scholar]
- 3.Masa JF, Corral J, Alonso ML, et al. , Spanish Sleep Network. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick study. Am J Respir Crit Care Med 2015;192:86–95. 10.1164/rccm.201410-1900OC [DOI] [PubMed] [Google Scholar]
- 4.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347–62. [PubMed] [Google Scholar]
- 5.Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. . Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS ONE 2015;10:e0117808 10.1371/journal.pone.0117808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basoglu OK, Tasbakan MS. Comparison of clinical characteristics in patients with obesity hypoventilation syndrome and obese obstructive sleep apnea syndrome: a case-control study. Clin Respir J 2014;8:167–74. 10.1111/crj.12054 [DOI] [PubMed] [Google Scholar]
- 7.Priou P, Hamel J-F, Person C, et al. . Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest 2010;138:84–90. 10.1378/chest.09-2472 [DOI] [PubMed] [Google Scholar]
- 8.Berg G, Delaive K, Manfreda J, et al. . The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001;120:377–83. 10.1378/chest.120.2.377 [DOI] [PubMed] [Google Scholar]
- 9.Jennum P, Kjellberg J. Health, social and economical consequences of sleep-disordered breathing: a controlled national study. Thorax 2011;66:560–6. 10.1136/thx.2010.143958 [DOI] [PubMed] [Google Scholar]
- 10.Pérez de Llano LA, Golpe R, Ortiz Piquer M, et al. . Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest 2005;128:587–94. 10.1378/chest.128.2.587 [DOI] [PubMed] [Google Scholar]
- 11.Ojeda Castillejo E, de Lucas Ramos P, López Martin S, et al. . noninvasive mechanical ventilation in patients with obesity hypoventilation syndrome. Long-term outcome and prognostic factors. Arch Bronconeumol 2015;51:61–8. 10.1016/j.arbres.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Nowbar S, Burkart KM, Gonzales R, et al. . Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004;116:1–7. 10.1016/j.amjmed.2003.08.022 [DOI] [PubMed] [Google Scholar]
- 13.Borel JC, Burel B, Tamisier R, et al. . Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS ONE 2013;8:e52006 10.1371/journal.pone.0052006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayappa I, Berger KI, Norman RG, et al. . Hypercapnia and ventilatory periodicity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2002;166:1112–15. 10.1164/rccm.200203-212OC [DOI] [PubMed] [Google Scholar]
- 15.Masa JF, Kryger M. Restrictive Lung Disorders. In: Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. 5th edn. Philadelphia: Editorial Saunders, 2008:1308–17. [Google Scholar]
- 16.Berger KI, Ayappa I, Chatr-Amontri B, et al. . Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001;120:1231–8. 10.1378/chest.120.4.1231 [DOI] [PubMed] [Google Scholar]
- 17.Chouri-Pontarollo N, Borel J-C, Tamisier R, et al. . Impaired objective daytime vigilance in obesity-hypoventilation syndrome: impact of noninvasive ventilation. Chest 2007;131:148–55. 10.1378/chest.06-1159 [DOI] [PubMed] [Google Scholar]
- 18.Budweiser S, Riedl SG, Jörres RA, et al. . Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med 2007;261:375–83. 10.1111/j.1365-2796.2007.01765.x [DOI] [PubMed] [Google Scholar]
- 19.Janssens J-P, Derivaz S, Breitenstein E, et al. . Changing patterns in long-term noninvasive ventilation: a 7-year prospective study in the Geneva Lake area. Chest 2003;123:67–79. 10.1378/chest.123.1.67 [DOI] [PubMed] [Google Scholar]
- 20.Salord N, Mayos M, Miralda RM, et al. . Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea. Respirology 2013;18:1135–42. 10.1111/resp.12131 [DOI] [PubMed] [Google Scholar]
- 21.Borel JC, Tamisier R, Gonzalez-Bermejo J, et al. . Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomised controlled trial. Chest 2012;141:692–702. 10.1378/chest.10-2531 [DOI] [PubMed] [Google Scholar]
- 22.Piper AJ, Wang D, Yee BJ, et al. . Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008;63:395–401. 10.1136/thx.2007.081315 [DOI] [PubMed] [Google Scholar]
- 23.Masa JF, Celli BR, Riesco JA, et al. . The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest 2001;119:1102–7. 10.1378/chest.119.4.1102 [DOI] [PubMed] [Google Scholar]
- 24.de Lucas-Ramos P, de Miguel-Díez J, Santacruz-Siminiani A, et al. . Benefits at 1 year of nocturnal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Respir Med 2004;98:961–7. 10.1016/j.rmed.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Redolfi S, Corda L, La Piana G, et al. . Long-term non-invasive ventilation increases chemosensitivity and leptin in obesity-hypoventilation syndrome. Respir Med 2007;101:1191–5. 10.1016/j.rmed.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 26.López-Jiménez MJ, Masa JF, Corral J, et al. , Grupo cooperativo. Mid- and long-term efficacy of non-invasive ventilation in obesity hypoventilation syndrome: the Pickwick's study. Arch Bronconeumol 2016;52:158–65. 10.1016/j.arbres.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 27.Ortega Ruiz F, Díaz Lobato S, Galdiz Iturri JB, et al. , SEPAR. Continuous home oxygen therapy. Arch Bronconeumol 2014;50:185–200. 10.1016/j.arbres.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 28.Masa JF, Jiménez A, Durán J, et al. . Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med 2004;170:1218–24. 10.1164/rccm.200312-1787OC [DOI] [PubMed] [Google Scholar]
- 29.Masa JF, Jiménez A, Durán J, et al. , Spanish Group of Breathing Sleep Disorders. Visual analogical well-being scale for sleep apnea patients: validity and responsiveness: a test for clinical practice. Sleep Breath 2011;15:549–59. 10.1007/s11325-010-0399-3 [DOI] [PubMed] [Google Scholar]
- 30.Masa JF, Corral J, Romero A, et al. , Spanish Sleep Network. Protective cardiovascular effect of sleep apnea severity in obesity hypoventilation syndrome. Chest 2016;150:68–79.. 10.1016/j.chest.2016.02.647 [DOI] [PubMed] [Google Scholar]
- 31.Murphy PB, Davidson C, Hind MD, et al. . Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax 2012;67:727–34. 10.1136/thoraxjnl-2011-201081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2016-208501supp.pdf (710.5KB, pdf)