Abstract
Objective
The objective of this study was to assess the efficacy, safety and tolerability of vonoprazan, a novel potassium-competitive acid blocker, as a component of Helicobacter pylori eradication therapy.
Design
A randomised, double-blind, multicentre, parallel-group study was conducted to verify the non-inferiority of vonoprazan 20 mg to lansoprazole 30 mg as part of first-line triple therapy (with amoxicillin 750 mg and clarithromycin 200 or 400 mg) in H pylori-positive patients with gastric or duodenal ulcer history. The first 50 patients failing first-line therapy with good compliance also received second-line vonoprazan-based triple therapy (with amoxicillin 750 mg and metronidazole 250 mg) as an open-label treatment.
Results
Of the 650 subjects randomly allocated to either first-line triple therapy, 641 subjects completed first-line therapy and 50 subjects completed second-line therapy. The first-line eradication rate (primary end point) was 92.6% (95% CI 89.2% to 95.2%) with vonoprazan versus 75.9% (95% CI 70.9% to 80.5%) with lansoprazole, with the difference being 16.7% (95% CI 11.2% to 22.1%) in favour of vonoprazan, thus confirming the non-inferiority of vonoprazan (p<0.0001). The second-line eradication rate (secondary end point) was also high (98.0%; 95% CI 89.4% to 99.9%) in those who received second-line therapy (n=50). Both first-line triple therapies were well tolerated with no notable differences. Second-line triple therapy was also well tolerated.
Conclusion
Vonoprazan is effective as part of first-line triple therapy and as part of second-line triple therapy in H pylori-positive patients with a history of gastric or duodenal ulcer.
Trial registration number
Keywords: HELICOBACTER PYLORI INFECTION, HELICOBACTER PYLORI - TREATMENT, CLINICAL TRIALS, ANTIBIOTIC THERAPY
Significance of this study.
What is already known on this subject?
Helicobacter pylori, which are shown to be present in approximately 50% of the adult population, are associated with a wide array of GI diseases, including peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer, thus placing an enormous burden on the healthcare resources.
Proton pump inhibitor (PPI)-based triple therapy has remained the mainstay of therapy for H pylori eradication. However, the H pylori eradication rate with PPI-based triple therapy has fallen from >90% in the 1990s to current levels of <70% partly due to the increasing resistance to the antimicrobials used, suggesting the need for new options and approaches for H pylori eradication.
What are the new findings?
Vonoprazan, a novel potassium-competitive acid blocker, has been shown in this phase III randomised, double-blind study to be non-inferior to the PPI lansoprazole (H pylori eradication rate: vonoprazan, 92.6%; lansoprazole, 75.9%) as a component of first-line triple therapy with amoxicillin and clarithromycin for H pylori eradication.
Vonoprazan has also been shown to be highly effective as a component of second-line triple therapy with amoxicillin and metronidazole in patients failing vonoprazan-based or lansoprazole-based first-line triple therapy (H pylori eradication rate, 98.0%)
How might it impact on clinical practice in the foreseeable future?
Given the increasing resistance to clarithromycin and/or metronidazole and the declining rates of clinical response to PPI-based triple therapy that have become a globally compelling issue, vonoprazan may represent a novel option as a component of triple therapy for H pylori eradication.
Vonoprazan-based triple therapy may be more effective for H pylori eradication than sequential, quadruple or long-term therapy.
Introduction
Helicobacter pylori are shown to be present in approximately 50% of the adult population and associated with a variety of upper GI diseases, including chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer,1–3 which place an enormous cost burden on healthcare resources due to their high prevalence and chronic nature.4
For H pylori eradication, the most widely prescribed regimen comprises triple therapy with a proton pump inhibitor (PPI) plus amoxicillin (AMX) and clarithromycin (CLR) or metronidazole (MTZ), which is recommended by most consensus guidelines.5–8 However, the H pylori eradication rate with triple therapy has fallen from >90% in the 1990s to current levels of <70%, which may be accounted for in part by the increasing resistance of H pylori to CLR and/or MTZ.7–11 In Japan, a 5-year nationwide surveillance programme conducted in 2002 reported an increasing prevalence of CLR-resistant strains of H pylori,12 suggesting that this is a major factor contributing to the decline in H pylori eradication rates with triple therapy.13
Vonoprazan is a novel oral potassium-competitive acid blocker which, like PPIs, inhibits gastric H+, K+-ATPase, an enzyme that catalyses the final step in the gastric acid secretion pathway.14 However, unlike the PPIs, vonoprazan inhibits the enzyme in a K+-competitive and reversible manner.15 Furthermore, vonoprazan (pKa 9.4) is shown to accumulate in parietal cells16 17 with its acid-inhibitory effect largely unaffected by ambient pH. In preclinical studies, vonoprazan produced more potent and sustained acid-inhibitory effects as well as greater increases in gastric pH than lansoprazole as it accumulated in high concentrations and became slowly cleared from gastric glands.14–17 In healthy volunteers, single doses of vonoprazan (1–120 mg) were well tolerated and produced rapid, profound and dose-related 24 h acid-inhibitory effects,18 with these effects maintained with multiple dosing (10–40 mg/day) over 7 days.19 Again, in a phase II dose-ranging study, vonoprazan (5–40 mg/day) produced healing rates comparable with those of lansoprazole (30 mg/day) in subjects with endoscopically confirmed erosive esophagitis over an 8-week period.20
Given its stronger acid-inhibitory effects, vonoprazan is thus expected to be at least as effective as PPIs as part of H pylori eradication regimens. This study was therefore conducted to determine whether triple therapy with vonoprazan (in combination with AMX and CLR) was as effective as one of the approved first-line triple therapies (lansoprazole/AMX/CLR) for H pylori eradication.
Methods
Study design
This was a phase III, randomised, double-blind, multicentre, parallel-group comparative study designed to verify the non-inferiority of vonoprazan/AMX/CLR (hereafter vonoprazan-based first-line triple therapy) to lansoprazole/AMX/CLR (hereafter lansoprazole-based first-line triple therapy) as first-line triple-therapy for H pylori-positive patients with gastric or duodenal ulcer history. Both first-line triple therapies were evaluated for safety, and second-line triple therapy with vonoprazan/AMX/MTZ (hereafter vonoprazan-based second-line triple therapy) as an open-label treatment was evaluated for efficacy and safety in the first 50 subjects who failed their allocated vonoprazan-based or lansoprazole-based first-line triple therapy.
The study was conducted at 46 sites in Japan between February 2012 and June 2013 in accordance with the Declaration of Helsinki, the ICH Harmonized Tripartite Guideline for Good Clinical Practice and all Japanese regulatory requirements and was registered at ClinicalTrials.gov with the identifier NCT01505127. The protocol was approved by the institutional review board at each study site.
Subjects
Male or female H pylori-positive patients aged ≥20 years with gastric or duodenal ulcer history were eligible for inclusion in the study. The presence of H pylori was confirmed by one or more of the following methodologies before treatment: the rapid urease test, culture, the 13C-urea breath test and/or the stool H pylori antigen test. The main exclusion criteria included acute upper GI bleeding, active gastric or duodenal ulcers, acute gastric or duodenal mucosal lesions, previous H pylori eradication therapy, surgery which might affect gastric acid secretion (upper GI resection or vagotomy), Zollinger–Ellison syndrome or other gastric acid hypersecretion disorders, serious neurological, cardiovascular, pulmonary, hepatic, renal, metabolic, GI, urological, endocrinological or haematological disorders, need for surgery, history of drug (including alcohol) abuse, history of malignancy and female subjects who were pregnant or lactating. Any sexually active female of childbearing potential was required to use adequate contraceptive measures. All subjects provided written informed consent prior to study participation.
Treatment
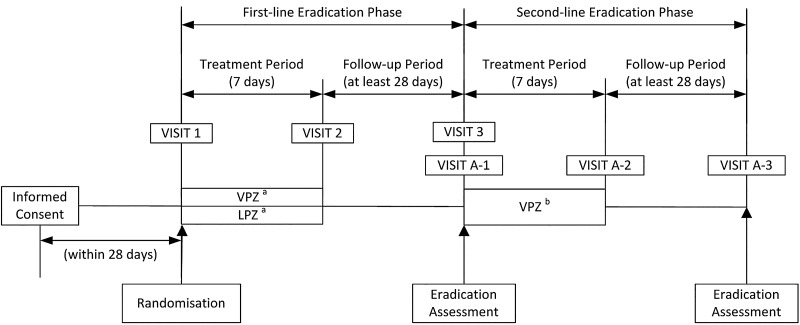
Subjects were randomised in a 1:1:1:1 ratio to receive first-line triple therapy with vonoprazan 20 mg or lansoprazole 30 mg in combination with AMX 750 mg plus CLR 200 or 400 mg. The four treatments were compared in the analyses as two treatment groups (the vonoprazan group vs the lansoprazole group), where subjects randomly allocated to either of the two approved doses of CLR (200 and 400 mg) available in Japan were combined, because the CLR dosage has been shown not to affect the H pylori eradication rate.21 Independent randomisation personnel generated the randomisation table and managed the randomisation process. A double-dummy method involving matched vonoprazan placebo tablets and lansoprazole placebo capsules was employed to ensure that the double-blind conditions were maintained in the study to avoid potential bias. The first 50 subjects who failed their allocated vonoprazan-based or lansoprazole-based first-line triple therapy but who also had good compliance received second-line triple therapy with vonoprazan 20 mg (in combination with AMX 750 mg and MTZ 250 mg) as an open-label treatment. All treatments were administered orally twice daily for 7 days and the subjects were then followed up for an additional ≥4 weeks and evaluated for H pylori status (figure 1).
Figure 1.
Flow diagram showing the study design. aAs part of triple therapy in combination with AMX and CLR. bAs part of triple therapy in combination with AMX and MTZ. AMX, amoxicillin; CLR, clarithromycin; LPZ, lansoprazole; MTZ, metronidazole; VPZ, vonoprazan.
Treatment duration and antimicrobial dosages were determined according to the approved indication in Japan for triple therapy involving PPIs for first-line and second-line H pylori eradication.
Procedures
At the start of the study, the demographics and characteristics of the subjects were recorded, including medication history, concomitant medications and pretreatment adverse events. Subject eligibility was also confirmed. In addition, physical examinations, vital signs assessments, clinical laboratory tests (haematology, serum chemistry and urinalysis), electrocardiogram and endoscopy were performed to determine the status of gastric or duodenal ulceration; antimicrobial susceptibility testing (standard agar plate dilution method) was also conducted to investigate levels of resistance of H pylori to the antimicrobial drugs being administered.
At the end of the first-line eradication therapy, physical examinations, vital signs assessments, electrocardiogram, clinical laboratory tests and cytochrome P450 (CYP) 2C19 genotyping tests were performed. H pylori eradication was determined by 13C-urea breath tests with UBIT 100 mg tablets (Otsuka Pharmaceutical Co., Ltd.) using a cut-off of 2.5%. Treatment-emergent adverse events (TEAEs) and concomitant medications were monitored throughout the study. All TEAEs including their severity and causality, as well as those leading to study drug discontinuation and serious TEAEs were descriptively summarised and categorised in terms of System Organ Class and Preferred Term by treatment group in the safety analysis set (all subjects who received at least one dose of the study drug). Treatment compliance was assessed using patient diaries. All assessments except for the CYP2C19 genotyping test were also performed in those receiving second-line eradication therapy.
The primary end point for the study was the first-line H pylori eradication rate. The secondary end point was the second-line H pylori eradication rate among those who failed first-line eradication therapy. Given that the acid-inhibitory effect of lansoprazole is affected by CYP2C19,22 and the success of eradication therapy is affected by the susceptibility of H pylori to the antimicrobials used,23 pre-planned subgroup analyses were performed by CYP2C19 genotype and by minimum inhibitory concentration (MIC) of CLR and AMX to compare the relative effects of vonoprazan and lansoprazole.
Sample size, statistical analyses and interim analysis
While no data on H pylori eradication rates with first-line triple therapy with vonoprazan were available at the time that this study was started, preclinical testing demonstrated that vonoprazan produced more potent acid-inhibitory effects than lansoprazole,15–17 suggesting that vonoprazan-based first-line triple therapy should be as effective as lansoprazole-based first-line triple therapy for H pylori eradication. Indeed, it was agreed during consultation with the regulatory authorities that this study, whose primary objective was to demonstrate non-inferiority between the two treatments, was sufficient to support the new drug application for vonoprazan treatment for H pylori eradication in Japan. The sample size was therefore calculated relative to that in a previous phase III study of lansoprazole-based triple therapy21 conducted by Takeda Pharmaceutical Company Ltd. To ensure an eradication rate of 90%, the protocol-defined sample size was determined as 200 subjects per treatment group (the vonoprazan group and the lansoprazole group) to have a >90% power to detect the non-inferiority of vonoprazan to lansoprazole with a non-inferiority margin of 10% using the Farrington and Manning test.24 Taking into account possible dropouts (approximately 10% of subjects) after randomisation, a total of 220 subjects were targeted for recruitment for each treatment group. However, at the time that the study was initiated, it was clear that the H pylori eradication rates with first-line triple therapy were declining, particularly due to the increasing prevalence of CLR-resistant strains in Japan, which had risen to around 30%.8 11 Consequently, to ensure that the statistical power of the study was maintained, a blinded interim analysis was included in the protocol to ascertain the pooled first-line H pylori eradication rate with 13C-urea breath tests performed in approximately 200 consecutive subjects before unblinding of the treatment. According to the interim estimate of the first-line H pylori eradication rate, 81.6%, the target number of subjects required for the primary end point was determined to be 318 per treatment group, and the target number of subjects to be randomised was increased to 324 per treatment group (allowing for a 2% dropout rate). The sample size was recalculated in a blinded fashion and was thought unlikely to impact on the type I error rate.25 With an assumed pooled eradication rate of 90% and the original sample size of 440, it was estimated that there would be at least 44 subjects failing their allocated vonoprazan-based or lansoprazole-based first-line triple therapy. Thus, in the protocol, 50 subjects were targeted for the second-line eradication phase.
For the primary and secondary end points, frequency, point estimates and two-sided 95% CIs were calculated by treatment group for the full analysis set (FAS; all randomised subjects who received at least one dose of the study drug). The non-inferiority of vonoprazan-based first-line triple therapy to lansoprazole-based first-line triple therapy was evaluated for the primary end point using the Farrington and Manning test with a non-inferiority margin of 10%.24 A χ2 test (with Yates's continuity correction) of the difference between treatments was also performed as a post hoc analysis for the primary end point including subgroup analyses. A multivariate logistic regression analysis was also performed. The dependent variable was H pylori eradication rate, and the independent variables were age, gender, CLR dose, CYP2C19 genotype, CLR resistance and AMX resistance.
Results
Baseline characteristics
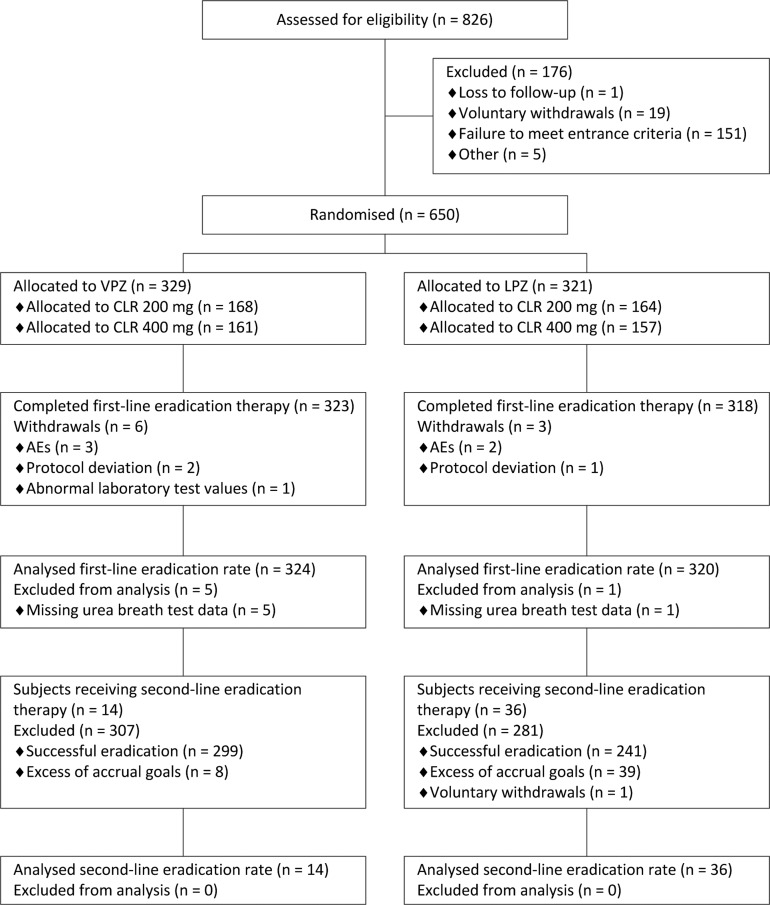
Of the 826 subjects who gave written informed consent, 650 eligible subjects were randomly allocated to receive triple therapy with vonoprazan (n=329) or lansoprazole (n=321) (figure 2). A total of 641 subjects completed first-line eradication triple therapy. Of all subjects failing first-line therapy, the first consecutive 50 subjects received and completed second-line triple therapy (figure 2).
Figure 2.
CONSORT flow diagram showing progression through trial. AE, adverse event; CLR, clarithromycin; LPZ, lansoprazole; VPZ, vonoprazan.
Demographic and other baseline characteristics in the vonoprazan and lansoprazole groups are summarised in table 1. No major differences were found between the treatment groups. Treatment compliance was high (>98%) during first-line and second-line eradication therapies.
Table 1.
Demographic and baseline characteristics (randomised set)
| First-line triple therapy | Second-line triple therapy | ||
|---|---|---|---|
| Characteristic | VPZ/AMX/CLR (n=329) | LPZ/AMX/CLR (n=321)* | VPZ/AMX/MTZ (n=50)† |
| Age (years) | 55.2±12.3 | 53.9±12.9 | 53.0±11.9 |
| Gender | |||
| Male | 196 (59.6) | 194 (60.4) | 25 (50.0) |
| Female | 133 (40.4) | 127 (39.6) | 25 (50.0) |
| Height (cm) | 163.5±8.8 | 164.8±9.1 | 162.4±9.4 |
| Weight (kg) | 62.2±12.4 | 61.5±11.5 | 60.7±11.2 |
| AMX susceptibility | |||
| Susceptible (MIC≤0.03 μg/mL) | 237 (72.0) | 228 (71.3) | 29 (59.2) |
| Resistant (MIC>0.03 μg/mL) | 72 (21.9) | 73 (22.8) | 20 (40.8) |
| Not applicable | 20 (6.1) | 19 (5.9) | 0 (0.0) |
| CLR susceptibility | |||
| Susceptible (MIC≤0.25 μg/mL) | 203 (61.7) | 178 (55.6) | 6 (12.2) |
| Intermediate (MIC=0.5 μg/mL) | 6 (1.8) | 8 (2.5) | 1 (2.0) |
| Resistant (MIC≥1 μg/mL) | 100 (30.4) | 115 (35.9) | 42 (85.7) |
| Not applicable | 20 (6.1) | 19 (5.9) | 0 (0.0) |
| MTZ susceptibility | |||
| Susceptible (MIC<8 μg/mL) | 283 (86.0) | 276 (86.3) | 45 (91.8) |
| Resistant (MIC≥8 μg/mL) | 26 (7.9) | 25 (7.8) | 4 (8.2) |
| Not applicable | 20 (6.1) | 19 (5.9) | 0 (0.0) |
| CLR dose | |||
| 200 mg twice daily | 168 (51.1) | 164 (51.1) | 24 (48.0) |
| 400 mg twice daily | 161 (48.9) | 157 (48.9) | 26 (52.0) |
| CYP2C19 genotype test | |||
| Extensive metabolisers | 274 (83.3) | 273 (85.0) | 43 (86.0) |
| Poor metabolisers | 55 (16.7) | 48 (15.0) | 7 (14.0) |
Data are expressed as mean±SD or as number of subjects with percentage in parentheses.
*Missing antimicrobial susceptibility testing data (n=1).
†Missing antimicrobial susceptibility testing data (n=1).
AMX, amoxicillin; CLR, clarithromycin; CYP, cytochrome P450; LPZ, lansoprazole; MIC, minimum inhibitory concentration; MTZ, metronidazole; VPZ, vonoprazan.
Efficacy
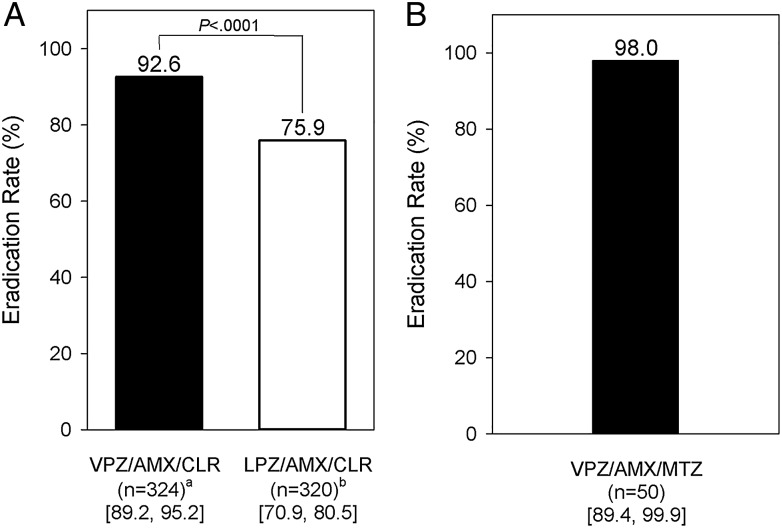
The first-line eradication rate (primary end point) was 92.6% in the vonoprazan group vs 75.9% in the lansoprazole group (figure 3), with the difference between the treatment groups (vonoprazan—lansoprazole) being 16.7% (95% CI 11.2% to 22.1%). Thus, vonoprazan was shown to be non-inferior to lansoprazole in the FAS with a non-inferiority margin of 10% (p<0.0001). The 95% CI suggested a statistically significant difference in favour of vonoprazan. A post hoc statistical test was also performed and the result (p<0.0001) corroborated the findings drawn from the 95% CI in the primary analysis. The second-line eradication rate (secondary end point) was also high (98.0%) (figure 3).
Figure 3.
Helicobacter pylori eradication rates (full analysis set) in (A) first-line triple therapy and (B) second-line triple therapy (95% CIs shown in brackets) are shown. aMissing urea breath test data (n=5); bmissing urea breath test data (n=1); p values for both non-inferiority and superiority tests are also provided. AMX, amoxicillin; CLR, clarithromycin; LPZ, lansoprazole; MTZ, metronidazole; VPZ, vonoprazan.
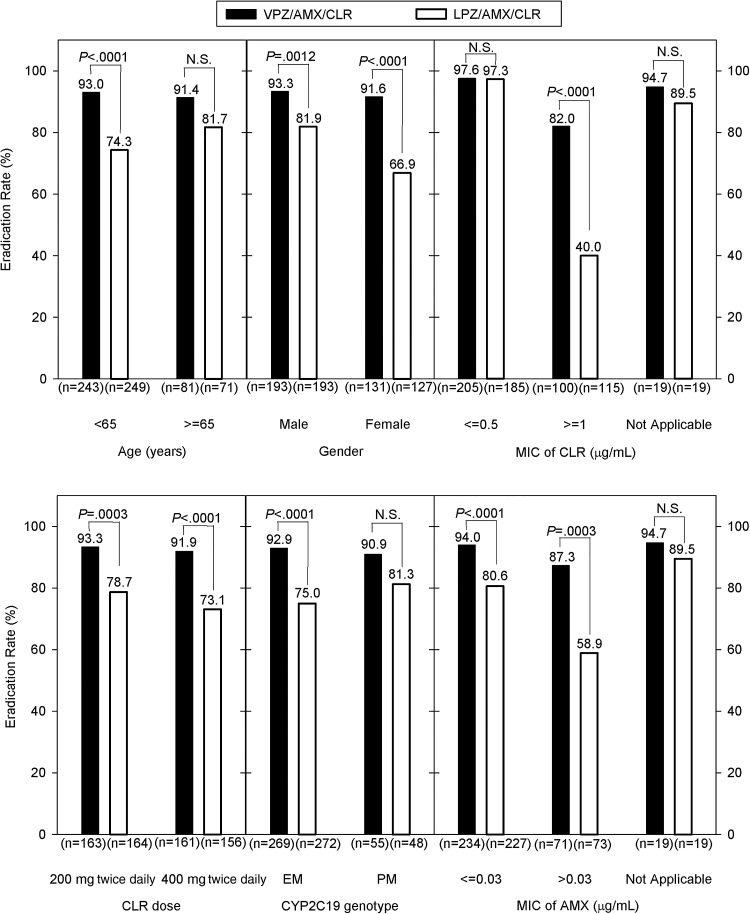
Post hoc analyses performed without adjustment for multiplicity demonstrated that first-line eradication rates were significantly higher with vonoprazan than those with lansoprazole in the following subgroups: those aged <65 years; men; women; those who received CLR 200 mg; those who received CLR 400 mg; CYP2C19 extensive metabolisers; those infected with AMX-susceptible strains (MIC ≤0.03 μg/mL); those infected with AMX-resistant strains (MIC >0.03 μg/mL) and those infected with CLR-resistant strains (MIC ≥1 μg/mL)26 (figure 4). The H pylori eradication rate was lower in those patients infected with CLR-resistant strains compared with those infected with CLR-susceptible or CLR-intermediate strains (MIC ≤0.5 μg/mL) in both groups.26 However, the eradication rate was significantly higher with vonoprazan compared with lansoprazole in those patients infected with CLR-resistant strains (82.0% vs 40.0%; p<0.0001; see also online supplementary table S1).
Figure 4.
First-line Helicobacter pylori eradication rates in various subgroups (full analysis set) are shown. Six randomised subjects in whom the urea breath test had not been performed were excluded from these analyses; p values for superiority tests are provided. AMX, amoxicillin; CLR, clarithromycin; EM, extensive metabolisers; LPZ, lansoprazole; MIC, minimum inhibitory concentration; N.S., not significant; PM, poor metabolisers; VPZ, vonoprazan.
Subgroup analysis of eradication rates (%) by bacterial susceptibility to CLR (MIC) 4 weeks after completion of first-line eradication therapy
gutjnl-2015-311304supp_table.pdf (257KB, pdf)
Additionally, multivariate logistic regression analysis showed that gender, CLR dose and CYP2C19 genotype had no significant effect on the eradication rate; CLR resistance has a significant negative impact (OR, 0.04; 95% CI 0.02 to 0.07) and AMX resistance had a slight but significant negative impact (OR, 0.49; 95% CI 0.27 to 0.89) on the eradication rate, while advancing age had a slight but significant positive impact (OR, 2.26; 95% CI 1.18 to 4.34) on the eradication rate.
Safety
During the first-line eradication phase, the overall incidence of TEAEs was 34.0% in the vonoprazan group compared with 41.1% in the lansoprazole group (20.4% vs 24.6% for drug-related TEAEs, respectively). The incidence of TEAEs, drug-related TEAEs, TEAEs leading to study drug discontinuation and serious TEAEs were comparable between the treatment groups (table 2). TEAEs occurring in >2% of subjects were diarrhoea, nasopharyngitis and dysgeusia (table 3). The incidence of dysgeusia was higher for those receiving high-dose CLR (data not shown). No marked differences were observed in TEAEs between vonoprazan-based and lansoprazole-based first-line triple therapies. Four and two serious TEAEs were reported in those receiving vonoprazan-based and lansoprazole-based first-line triple therapies, respectively. Three and two subjects discontinued treatment due to TEAEs among those receiving vonoprazan-based and lansoprazole-based first-line triple therapies, respectively.
Table 2.
Summary of TEAEs in first-line VPZ-based and LPZ-based triple therapy (in combination with AMX and CLR) and in second-line VPZ-based triple-therapy (in combination with AMX and MTZ) (safety analysis set)
| First-line triple therapy | Second-line triple therapy | |||||
|---|---|---|---|---|---|---|
| VPZ/AMX/CLR (n=329) | LPZ/AMX/CLR (n=321) | VPZ/AMX/MTZ (n=50) | ||||
| Events | Subjects | Events | Subjects | Events | Subjects | |
| TEAEs | 153 | 112 (34.0) | 178 | 132 (41.1) | 26 | 15 (30.0) |
| Related | 85 | 67 (20.4) | 93 | 79 (24.6) | 11 | 8 (16.0) |
| Not related | 68 | 45 (13.7) | 85 | 53 (16.5) | 15 | 7 (14.0) |
| Mild | 145 | 104 (31.6) | 168 | 124 (38.6) | 22 | 14 (28.0) |
| Moderate | 7 | 7 (2.1) | 8 | 6 (1.9) | 2 | 0 (0.0) |
| Severe | 1 | 1 (0.3) | 2 | 2 (0.6) | 2 | 1 (2.0) |
| Leading to study drug discontinuation | 3 | 3 (0.9) | 2 | 2 (0.6) | 0 | 0 (0.0) |
| Serious TEAEs | 4 | 4 (1.2) | 2 | 2 (0.6) | 2 | 1 (2.0) |
| Related | 1 | 1 (0.3) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Not related | 3 | 3 (0.9) | 2 | 2 (0.6) | 2 | 1 (2.0) |
| Deaths | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) |
Data are expressed as number of events or as number of subjects with percentage in parentheses.
AMX, amoxicillin; CLR, clarithromycin; LPZ, lansoprazole; MTZ, metronidazole; TEAE, treatment-emergent adverse event; VPZ, vonoprazan.
Table 3.
TEAEs occurring in >2% of subjects during first-line VPZ-based and LPZ-based triple-therapy (in combination with AMX and CLR) and during second-line VPZ-based triple therapy (in combination with AMX and MTZ) (safety analysis set)
| TEAEs occurring in >2% of subjects during first-line triple therapy | ||
|---|---|---|
| Preferred term* | VPZ/AMX/CLR (n=329) | LPZ/AMX/CLR (n=321) |
| Diarrhoea | 41 (12.5) | 49 (15.3) |
| Nasopharyngitis | 18 (5.5) | 15 (4.7) |
| Dysgeusia | 13 (4.0) | 10 (3.1) |
| TEAEs occurring in >2% of subjects during second-line triple therapy | ||
| Preferred term* | VPZ/AMX/MTZ (n=50) | |
| Diarrhoea | 2 (4.0) | |
| Flatulence | 2 (4.0) | |
| Nasopharyngitis | 2 (4.0) | |
| Alanine aminotransferase increased | 2 (4.0) | |
| Aspartate aminotransferase increased | 2 (4.0) | |
Data are expressed as number of subjects with percentage in parentheses.
*MedDRA (V.16.0).
AMX, amoxicillin; CLR, clarithromycin; LPZ, lansoprazole; MTZ, metronidazole; TEAE, treatment-emergent adverse event; VPZ, vonoprazan.
During second-line eradication phase, the overall incidence of TEAEs was 30.0% (16.0% for drug-related TEAEs) (table 2). TEAEs occurring in >2% of subjects were diarrhoea, flatulence, nasopharyngitis, increased alanine aminotransferase and increased aspartate aminotransferase (table 3). Two serious TEAEs were reported but no TEAEs led to discontinuation of treatment.
No significant changes were observed in mean laboratory test values, vital signs or electrocardiogram findings during the study. In both first-line and second-line eradication phases, serum gastrin levels increased after administration of the study drug, with these increases being significantly greater for those receiving vonoprazan-based first-line triple therapy. However, the gastrin levels returned to pre-administration levels after completion of either first-line triple therapy or second-line triple therapy (see online supplementary figure S1A,B).
Time course of arithmetic mean serum gastrin levels (Safety Analysis Set) during A) first-line triple therapy and B) second-line triple therapy
gutjnl-2015-311304supp_figure.pdf (106.3KB, pdf)
Discussion
Study results demonstrated the non-inferiority of vonoprazan-based first-line triple therapy to lansoprazole-based first-line triple therapy with a non-inferiority margin of 10% (p<0.0001). Furthermore, the eradication rate was shown to be 16.7% higher with vonoprazan-based first-line triple therapy than with lansoprazole-based first-line triple therapy and the 95% CI at the lower limit showed that the eradication rate was 11.2% better with vonoprazan-based first-line triple therapy, suggesting a statistically significant difference in favour of vonoprazan. A high eradication rate was also observed with vonoprazan-based second-line triple therapy (98.0%).
In post hoc analyses, H pylori eradication rates were significantly higher among CYP2C19 extensive metabolisers and those infected with CLR-resistant strains who received vonoprazan-based first-line triple therapy compared with those who received lansoprazole-based first-line triple therapy (p<0.0001).
All treatments were well tolerated, with 641 of the 650 subjects completing eradication triple therapy and with three and two subjects discontinuing vonoprazan-based and lansoprazole-based first-line triple therapies due to TEAEs, respectively. The safety outcomes of this study were consistent with those of an earlier phase II study20 and no new safety signals were identified.
The relatively large sample size of this study allowed exploratory analysis of factors which could impact clinical outcome. However, the subgroup results should be interpreted as hypothesis-generating, given the smaller size of some subgroups and the exploratory nature of the subgroup analyses.
Moreover, the study had some potential limitations. First, individuals with active or bleeding ulcers were excluded and therefore the study results cannot be generalised to these patients, even though PPI-based triple therapy has been shown to produce comparable eradication of H pylori in patients with active ulcer and those with ulcer scars alike.27 Also, as no H pylori efficacy data for vonoprazan were available when the study was planned and H pylori eradication rates with triple therapy were known to be declining, there was some loss of precision when determining the original sample size. However, a pre-planned blinded interim analysis was included in the protocol to ensure that a sufficient number of subjects were recruited in the study to achieve the desired statistical power, so that the efficacy of the two regimens could be accurately compared. Additionally, the post hoc statistical analysis testing for the superiority of vonoprazan compared with lansoprazole was not pre-planned. However, the pre-planned calculation of 95% CIs in the primary analysis suggested a statistically significant difference in favour of vonoprazan, thus corroborating the post hoc analysis testing. Furthermore, the observed sizable clinical treatment difference in the eradication rate favouring vonoprazan, together with consistently favourable outcomes across a number of subgroups with vonoprazan, also provides evidence for the hypothesis of greater efficacy of vonoprazan compared with lansoprazole for the treatment of H pylori eradication.
CLR resistance is becoming a global clinical concern for H pylori eradication, especially in Japan where the drug has been available for the longest time.8 10 As a consequence, the loss of efficacy of traditional PPI-based triple therapy has become a compelling issue, and a number of treatment approaches have been advocated to help improve H pylori eradication.6 Eradication rates were reported to be higher with high-dose PPI-based triple therapy or with 10-day to 14-day treatment regimens.6 Due to the importance of this issue, a potentially important post hoc finding in our study was that the H pylori eradication rate among those infected with CLR-resistant strains was significantly higher with vonoprazan-based first-line triple therapy compared with lansoprazole-based first-line triple therapy (p<0.0001) and also appears to be higher than the eradication rate reported with increased dose or length of treatment with PPI-based therapy. Nevertheless, the efficacy of vonoprazan shown in CLR-resistant strains should be viewed cautiously, given the cross-study nature of the analyses. Additionally, the relatively lighter mean body weight of the Japanese subjects may have favourably affected the study results. A high second-line eradication rate was another important finding in our study, though the sample size was small.
While the mechanism for a high eradication rate with vonoprazan observed in those infected with CLR-resistant strains remains unclear, it may be accounted for in part by the potential synergy between vonoprazan and the antimicrobials used. H pylori is more susceptible to antimicrobials when it restores its replicative capability at a pH higher than 6.10 Vonoprazan has a highly potent gastric acid-inhibitory effect as it accumulates in high concentrations and becomes slowly cleared from gastric glands,14–19 and consequently potentially provides an environment in which antimicrobials can have greater efficacy. In addition, co-administration of omeprazole, a PPI, has been shown to increase the chemical stability of AMX and CLR in gastric juice, thus preventing the antimicrobials, which are fragile at lower pH levels, from degradation.28 29
The International Agency for Research on Cancer Working Group recommend that all countries explore the possibility of introducing population-based H pylori screening and treatment programmes, adjusted to local healthcare environments and needs. This is based on findings from randomised clinical trials, which demonstrated the effectiveness of H pylori eradication in preventing gastric cancer, which is considered a global health problem, as well as on health-economic models which have shown that H pylori screening and treatment may prove to be cost-effective.30
Given the increasing resistance to antimicrobials and the declining clinical response rates observed with current treatments, vonoprazan-based triple therapy may represent a new treatment option for H pylori-positive patients and has the potential to reduce gastric cancer.
In conclusion, vonoprazan-based triple therapy was shown to be effective as first-line and second-line treatments, and was well tolerated. As a promising new treatment option, vonoprazan may also be considered for additional therapeutic approaches, such as sequential, quadruple and long-term therapy for H pylori eradication.
Acknowledgments
The authors would like to thank Richard Jenkins from Takeda Development Centre, Europe, Göran Hasselgren and Fiona Steinkamp of Takeda Pharmaceuticals International GmbH for reviewing the manuscript. The authors thank Steve Clissold, PhD, ContentEdNet and Hiroaki Itoh, Interface for editorial assistance.
Footnotes
Contributors: All authors were involved in the study concept and design. AN was responsible for the study protocol. KM was the medical expert overseeing the study. MA was responsible for coordinating the investigators. All authors contributed to writing and reviewing the various drafts of the manuscript, having the right to access the full dataset required for publication purposes, and approved the final version of the article.
Funding: This study was funded in full by Takeda Pharmaceutical Company Ltd. Takeda Pharmaceutical Company Ltd. contributed to the study design, data collection and interpretation, writing, reviewing and approval of the publication in cooperation with all authors.
Competing interests: KM and MA are independent medical consultants providing expert advice to Takeda Pharmaceutical Company Ltd. YS, MS, NF and AN are employees of Takeda Pharmaceutical Company Ltd.
Ethics approval: Written informed consent was obtained from all study subjects, and the study protocol was approved by the Institutional Review Board at each study site.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 2.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. 10.1056/NEJMcp1001110 [DOI] [PubMed] [Google Scholar]
- 3.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013;132:1272–6. 10.1002/ijc.27965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology 2002;122:1500–11. 10.1053/gast.2002.32978 [DOI] [PubMed] [Google Scholar]
- 5.Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther 2011;34:1255–68. 10.1111/j.1365-2036.2011.04887.x [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 2012;61:646–64. 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 7.Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808–25. 10.1111/j.1572-0241.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 8.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15:1–20. 10.1111/j.1523-5378.2009.00738.x [DOI] [PubMed] [Google Scholar]
- 9.Gatta L, Vakil N, Vaira D, et al. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 2013;347:f4587 10.1136/bmj.f4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008;5:321–31. 10.1038/ncpgasthep1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 2007;45:4006–10. 10.1128/JCM.00740-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi I, Murakami K, Kato M, et al. The current prevalence of drug-resistant Helicobacter pylori in Japan—a summary of 2006 surveillance of resistant bacteria and a 5-year summary. J Japan Soc Helicobacter Res 2009;10:98–103 (in Japanese). [Google Scholar]
- 13.Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy—a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group). J Gastroenterol Hepatol 2014;29(Suppl 4):29–32. 10.1111/jgh.12796 [DOI] [PubMed] [Google Scholar]
- 14.Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther 2011;339:412–20. 10.1124/jpet.111.185314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 2010;335:231–8. 10.1124/jpet.110.170274 [DOI] [PubMed] [Google Scholar]
- 16.Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011;81:1145–51. 10.1016/j.bcp.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011;337:797–804. 10.1124/jpet.111.179556 [DOI] [PubMed] [Google Scholar]
- 18.Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015;6:e94 10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–48. 10.1111/apt.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015;42:685–95. 10.1111/apt.13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asaka M, Sugiyama T, Kato M, et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001;6:254–61. 10.1046/j.1523-5378.2001.00037.x [DOI] [PubMed] [Google Scholar]
- 22.Sakurai Y, Hirayama M, Hashimoto M, et al. Population pharmacokinetics and proton pump inhibitory effects of intravenous lansoprazole in healthy Japanese males. Biol Pharm Bull 2007;30:2238–43. 10.1248/bpb.30.2238 [DOI] [PubMed] [Google Scholar]
- 23.Murakami K, Fujioka T, Okimoto T, et al. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents 2002;19:67–70. 10.1016/S0924-8579(01)00456-3 [DOI] [PubMed] [Google Scholar]
- 24.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 1990;9:1447–54. 10.1002/sim.4780091208 [DOI] [PubMed] [Google Scholar]
- 25.Friede T, Mitchell C, Müller-Velten G. Blinded sample size reestimation in non-inferiority trials with binary endpoints. Biom J 2007;49:903–16. 10.1002/bimj.200610373 [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. CLSI document M100-S17 [ISBN 1-56238-625-5].
- 27.Sugizaki K, Sakata Y, Arai T, et al. A multicenter prospective observational study of triple therapy with rabeprazole, amoxicillin and metronidazole for Helicobacter pylori in Japan. Intern Med 2012;51:3103–8. 10.2169/internalmedicine.51.8510 [DOI] [PubMed] [Google Scholar]
- 28.Erah PO, Goddard AF, Barrett DA, et al. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 1997;39:5–12. 10.1093/jac/39.1.5 [DOI] [PubMed] [Google Scholar]
- 29.Gustavson LE, Kaiser JF, Edmonds AL, et al. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother 1995;39:2078–83. 10.1128/AAC.39.9.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IARC Helicobacter pylori Working Group. Helicobacter pylori eradication as a strategy for preventing gastric cancer Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8), 2014. http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed 19 Oct 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis of eradication rates (%) by bacterial susceptibility to CLR (MIC) 4 weeks after completion of first-line eradication therapy
gutjnl-2015-311304supp_table.pdf (257KB, pdf)
Time course of arithmetic mean serum gastrin levels (Safety Analysis Set) during A) first-line triple therapy and B) second-line triple therapy
gutjnl-2015-311304supp_figure.pdf (106.3KB, pdf)