Abstract
Background
Little is known about the comparative effects of different glucocorticoids on the adrenal and growth hormone (GH) axes in children with congenital adrenal hyperplasia (CAH). We sought to compare the effects of hydrocortisone (HC), prednisone (PDN), and dexamethasone (DEX) in children with classic CAH and to investigate a potential role of pharmacogenetics.
Methods
Subjects were randomly assigned to three sequential 6-week courses of HC, PDN, and DEX, each followed by evaluation of adrenal hormones, IGF-1, GH, and body mass index (BMI). Single nucleotide polymorphism (SNP) analysis of genes in the glucocorticoid pathway was also performed.
Results
Nine prepubertal subjects aged 8.1 ± 2.3 years completed the study. Mean ACTH, androstenedione, and 17-hydroxyprogesterone (17-OHP) values were lower following the DEX arm of the study than after subjects received HC (p ≤ 0.016) or PDN (p ≤ 0.002). 17-OHP was also lower after HC than PDN (p < 0.001). There was no difference in IGF-1, GH, or change in BMI. SNP analysis revealed significant associations between hormone concentrations, pharmacokinetic parameters, and variants in several glucocorticoid pathway genes (ABCB1, NR3C1, IP013, GLCCI1).
Conclusions
DEX resulted in marked adrenal suppression suggesting that its potency relative to hydrocortisone and prednisone was underestimated. SNPs conferred significant differences in responses between subjects. Although preliminary, these pilot data suggest that incorporating pharmacogenetics has the potential to eventually lead to targeted therapy in children with CAH.
Keywords: Congenital adrenal hyperplasia, Hydrocortisone, Prednisone, Dexamethasone, Pharmacogenetics
Background
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency results from an enzymatic block in the biosynthesis of cortisol and aldosterone. Treatment of classic CAH in children involves a challenging balance between androgen excess leading to bone age advancement due to under treatment with glucocorticoids and cortisol excess leading to impaired linear growth from overtreatment [1]. Hydrocortisone (HC) is the preferred glucocorticoid in children with CAH due to potential concerns of linear growth suppression associated with longer-acting and more potent glucocorticoid formulations [1, 2]. However, while experience is limited, dexamethasone (DEX) and single or multiple doses of prednisone (PDN) have been used successfully in children with CAH [3, 4].
Little is known about the comparative effects of HC, PDN, and DEX on the hypothalamic-pituitary-adrenal (HPA) and growth hormone axes in CAH. The aim of this pilot study was to compare hormonal and pharmacokinetic profiles after short-term treatment with HC, PDN, and DEX in children with CAH. Additional studies were performed to determine if variants in genes in the glucocorticoid pathway were associated with individual differences in responses to treatment.
Methods
Subjects and study design
Prepubertal children between the ages of 4 and 12 years with classic CAH followed at Riley Hospital for Children were eligible for enrollment. Exclusion criteria included medical problems that affect growth, absorption, or clearance of glucocorticoids and medications known to affect the absorption or clearance of glucocorticoids. The study was approved by the Institutional Review Board at Indiana University, and written informed consent from parents and assent from subjects, when appropriate, was obtained. All visits were conducted at the Indiana University General Clinical Research Center (GCRC). At the time of enrollment, a physical exam was performed, including height, weight, vital signs, and Tanner staging. A bone age radiograph was obtained if one had not been done within the past 6 months. Baseline blood tests at enrollment included 17-hydroxyprogesterone (17-OHP), androstenedione, and electrolytes.
Subjects were assigned to three sequential 6-week treatment courses arranged in random order during which they received the following medications: hydrocortisone (5 mg tablets; Pfizer, New York, NY) 15 mg/m2/day divided three times a day administered at 08:00, 15:00, and 21:00; prednisone (1 mg tablets; Roxane Laboratories Inc., Columbus, OH) 3 mg/m2/day divided twice a day administered at 08:00 and 21:00; and dexamethasone (0.5 mg/5 mL elixir; Morton Grove Pharmaceuticals Inc., Morton Grove, IL) 0.3 mg/m2/day administered daily at 21:00. All medications were taken orally. The bioequivalence and potency conversion of HC to PDN to DEX was estimated to be 1 to 5 to 50, based on clinical practice guidelines from the Endocrine Society regarding glucocorticoid dosing in CAH [1]. A pill cutter and oral syringe were given to each subject. Subjects remained on their usual dose of mineralocorticoid replacement throughout the study. Subjects were instructed to administer stress dose steroids by tripling the oral dose of glucocorticoid in times of illness or fever. If stress dosing was administered within a week of a scheduled GCRC admission, it was postponed until the subject had not received stress dose steroids for at least 7 days.
At the end of each 6-week period, subjects were admitted to the GCRC for 25 h. A physical exam was performed, including height, weight, vital signs, and Tanner staging. A peripheral IV was placed from which all blood samples were obtained. At 08:00, sodium, potassium, IGF-1, 17-OHP, and androstenedione were measured. For those taking HC, cortisol was measured before the 08:00 dose and then at 20, 40, 60, 80, 120, and 240 min in order to evaluate cortisol metabolism and clearance. While on PDN, cortisol was measured as a surrogate for PDN metabolism and clearance. When patients were on DEX, a dexamethasone level was drawn at 20:00 before the 21:00 DEX dose was administered. Measurements of 17-OHP were obtained every 2 h (13 total per each overnight stay); growth hormone every hour from 20:00 to 08:00 (13 total per each overnight stay); adrenocorticotropic hormone (ACTH) at 14:00 and then every 2 h from 00:00 to 06:00 (5 total per each overnight stay); and androstenedione at 20:00 (2 total per each overnight stay). At the first GCRC admission, 5 mL of blood was drawn and frozen in the GCRC Pharmacogenetic Core Lab to be used for DNA extraction.
All subjects were contacted by telephone two weeks after starting a new glucocorticoid regimen to review administration and compliance. Pill counts and determination of elixir volumes were performed at GCRC admissions to assess compliance. At the end of the 18-week study, subjects returned to their pre-study glucocorticoid regimen.
Laboratory assays
All blood samples were analyzed at the GCRC, except for genomics and dexamethasone samples which were analyzed at Esoterix (Calabasas Hills, CA) by high performance liquid chromatography (HPLC) tandem mass spectrometry. Standard clinical laboratory methods were used to measure serum electrolytes. 17-OHP was measured by radioimmunoassay (RIA) (MP Biomedicals, Orangeburg, NY). The following hormones were measured by enzyme-linked immunosorbent assay (ELISA): cortisol, androstenedione, ACTH, IGF-1 (ALPCO Diagnostics, Salem, NH) and growth hormone (Alpha Diagnostic, Inc., San Antonio, TX).
DNA was extracted with QIAGEN QIAamp® DNA Blood Mini and Maxi kits (QIAGEN, Valencia, CA) and genotyped for single nucleotide polymorphisms (SNPs) in genes in the glucocorticoid pathway as previously described [5]. SNPs analyzed included those in the following genes: importin 13 or IPO13 (rs6671164, rs4448553, rs1990150, rs2240447, rs2486014, rs2301993, rs7412307, rs2428953), nuclear receptor subfamily 3, group C, member 1 or NR3C1 (rs41423247), glucocorticoid-induced transcript 1 or GLCCI1 (rs37973), ATP-binding cassette, subfamily B, member 1 or ABCB1 (rs1045642, rs2032582, rs1128503), cytochrome P450, subfamily IIIA, polypeptide 7 or CYP3A7 (rs2257401, rs2687133), stress-induced phosphoprotein 1 or STIP1 (rs2236647), low density lipoprotein, oxidized, receptor 1 or OLR1 (rs3736233), adenylate cyclase 2 or ADCY2 (rs2230739), corticotropin-releasing hormone receptor 1 or CRHR1 (rs242941, rs1876828), and Fc fragment of IgE, low affinity II, receptor for or FCER2 (rs28364072).
Statistical analysis
Statistical analyses were performed to determine if there was an association between the three glucocorticoids and biochemical and anthropometric outcomes. These outcomes were measured over time, and thus each participant had multiple measures for each outcome. Generalized linear models were used to analyze the data in order to account for repeated measures and to ensure that the proper covariance structure was used. Pairwise comparisons were also performed using a Bonferroni correction. Overall analyses were considered significant at p ≤ 0.05 and pairwise comparisons among the three drugs were considered significant at p ≤ 0.017. All analytic assumptions were verified and log transformed when the data did not follow the normal distribution.
For 17-OHP, additional analyses were performed to determine the proportion of time spent within the predetermined target range of 100–1000 ng/dL. Each time point was connected with a straight line to its next neighbor and integration methods were performed to derive the total area and specified target areas. ANOVA models were used to determine if there was a significant difference in the proportion of time within the target range between the study drugs.
BMI z-scores were generated using the Center for Disease Control’s growth chart training resource. ANCOVA models were used to determine if there was a significant difference in BMI z-scores following treatment with the three different study drugs. As the glucocorticoids were administered at different combinations, this analysis was adjusted for the previous drug that the subject was on.
All analytic assumptions were verified. All analyses were performed using SAS v9.3 (SAS Institute, Cary, NC).
The pharmacokinetic measurements were based on three summaries: average, area under the concentration curve (AUC), and decreasing rate or slope. The average was calculated based on the average measurement among all sampling time points. The AUC was calculated based on the trapezoid rule, and the decreasing rate was calculated based on the measurements from the last three sampling time points.
The associations between relevant phenotypes and SNPs were assessed through three different genetic models: dominant, recessive, and additive. The dominant model compares the genotype homozygous dominant (XX) with the genotypes containing one or both recessive, or variant alleles (Xx or xx). The recessive model compares the genotype homozygous recessive (xx) versus genotypes with one or both dominant alleles (XX or Xx). An additive model examines whether each copy of an allele contributes to an additional risk, so XX would be the highest, Xx would have less risk, and xx would have no risk [6]. Linear regression was applied to analyze these genetic associations. T-tests were used to determine directionality. As this was an exploratory analysis, any association with a p-value ≤ 0.05 was considered statistically significant. The Bonferroni justification was not applied in the genetic data analysis. The genetic data analyses were conducted in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/).
Results
Subjects
Nine subjects with classic CAH (8 with salt-wasting and 1 with simple virilizing) aged 8.1 ± 2.3 years (range: 4.8–11.6 years) completed the study. Four additional subjects were enrolled but withdrew due to difficulty maintaining peripheral IV access. One additional subject was excluded when his screening visit revealed he was pubertal. No other subjects met exclusion criteria. Patient characteristics including baseline hormonal values at study entry are summarized in Table 1. Electrolytes and vital signs were normal in all subjects.
Table 1.
Baseline characteristics at enrollment of subjects who completed all 3 arms of the study
| Number of subjects | 9 (5 female) |
|---|---|
| Glucocorticoid regimen prior to entering the study | Hydrocortisone (7), prednisone (2) |
| Dose of hydrocortisone (mg/m2/day) equivalent | 15.6 ± 4.0 |
| Age (years) | 8.1 ± 2.3 |
| Bone age (years) | 8.6 ± 2.9 |
| Height (cm); z-score | 127.6 ± 17.9; 0.0 ± 1.2 |
| Weight (kg); z-score | 39.1 ± 24.0; 1.0 ± 1.4 |
| BMI (kg/m2); z-score | 22.0 ± 8.1; 1.2 ± 1.1 |
| 17-hydroxyprogesterone (ng/dL) | 991.1 ± 978.5 89.4 (0.4–2500.0) |
| Androstenedione (ng/dL) | 35.2 ± 22.5 22.3 (2.0–392.0) |
Values are mean ± standard deviation, with 17-hydroxyprogesterone and androstenedione also including median (minimum – maximum) due to data skewness
Hormonal studies and BMI z-scores
Mean ACTH, androstenedione, and 17-OHP values were all significantly lower following treatment with DEX when compared to HC (p = 0.016, p = 0.016, p < 0.001) and PDN (p < 0.001, p = 0.002, p < 0.001). Interestingly, 17-OHP levels were significantly lower following treatment with HC than on PDN (p < 0.001). No other differences in adrenal hormones between treatment groups were seen. IGF-1 levels did not differ among the three treatment groups (p = 0.980), nor did GH levels (p = 0.127), or change in BMI z-scores (p = 0.648). Mean IGF-1 SDS (adjusted for age, gender, and Tanner stage) also did not differ for each glucocorticoid (p = 0.957): HC (1.57), PDN (1.61), and DEX (1.78). Representative GH profiles showing preserved GH pulsatility from three of the subjects are shown in Fig. 1. None of the subjects exhibited clinical signs of glucocorticoid excess during the study. The significant differences in hormone levels between treatment pairs are summarized in Table 2.
Fig. 1.
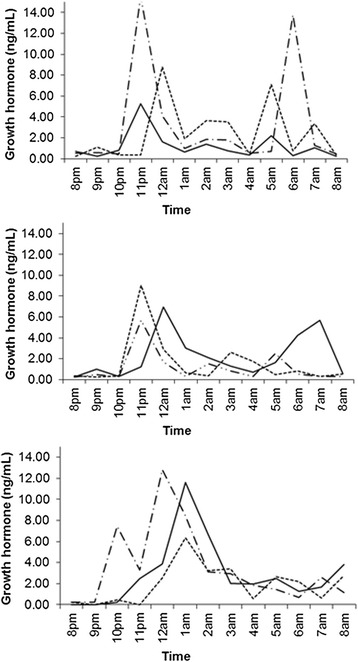
Representative GH profiles in three subjects. There is normal variability and pulsatility of GH secretion for each glucocorticoid (solid line for PDN, solid-dash line for HC, and dashed line for DEX)
Table 2.
Summary of differences of log transformed data between treatment pairs
| Drug pair | ACTH | Androstenedione | 17-OHP | |||
|---|---|---|---|---|---|---|
| Difference (95 % CI) | p-value | Difference (95 % CI) | p-value | Difference (95 % CI) | p-value | |
| DEX-HC | −0.55 (−0.99, −0.12) | 0.016** | −0.64 (−1.15, −0.14) | 0.016** | −1.59 (−1.94, −1.23) | < 0.001** |
| DEX-PDN | −0.90 (−1.33, −0.47) | < 0.001** | −0.90 (−1.40, −0.40) | 0.002** | −2.44 (−2.80, −2.09) | < 0.001** |
| HC- PDN | −0.35 (−0.78, 0.09) | 0.110 | −0.26 (−0.76, 0.25) | 0.293 | −0.86 (−1.21, −0.50) | < 0.001** |
Values are expressed as the difference between drug groups (first drug minus the second drug) with 95 % confidence interval (CI)
** Significant p-value is ≤ 0.017
Mean 17-OHP values were lowest on DEX and highest on PDN at each measured time point (Fig. 2). The majority of 17-OHP values during treatment with each glucocorticoid were outside of the target range (75 % for DEX, 70 % for HC, and 80 % for PDN) and did not differ between groups (p = 0.714).
Fig. 2.
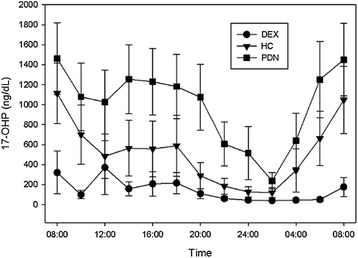
Mean 17-hydroxyprogesterone (17-OHP) concentration by time points. 17-OHP was measured every 2 h during each inpatient stay from 08:00 to 08:00 the next day. 17-OHP was lowest on DEX and highest on PDN at each time point (p < 0.001). Bars represent the 95 % confidence interval for the standard error
Dexamethasone levels
Eight subjects had trough DEX levels of < 30 ng/dL. Only one subject had a detectable DEX level of 56 ng/dL, which corresponded to low 17-OHP and androstenedione concentrations. However, 17-OHP measurements in three subjects with undetectable DEX levels were nearly all < 100 ng/dL and androstenedione levels were also low.
Pharmacokinetics and pharmacogenetics
As illustrated in Fig. 3, there was variability in glucocorticoid metabolism and pharmacokinetics between subjects. Due to the small sample size, data analysis is reported using dominant genetic models, which examine the associations between carrying the dominant allele for each SNP and downstream pharmacokinetic effects. Based on the dominant model analyses, gene variants were correlated with hormone concentrations. First, for ABCB1 (rs2032582 and rs1045642), subjects carrying at least one copy of the variant (GA or AA) had significantly lower concentrations of 17-OHP on the DEX arm as compared to subjects with the homozygous wild-type (GG) genotype (p = 0.025 for both). Showing a similar trend for ABCB1 (rs1128503) when on DEX, subjects carrying at least one variant allele (CA or AA) had significantly lower concentrations of 17-OHP compared to homozygous wild-type (CC) subjects (p = 0.025). For NR3C1 (rs41423247), subjects with at least one copy of the variant allele (GC or CC) had significantly higher concentrations of androstenedione when on PDN as compared to homozygous wild-type (GG) subjects (p = 0.004). There were no significant genetic associations identified when subjects were on HC. These results are shown in Table 3 and Fig. 4.
Fig. 3.
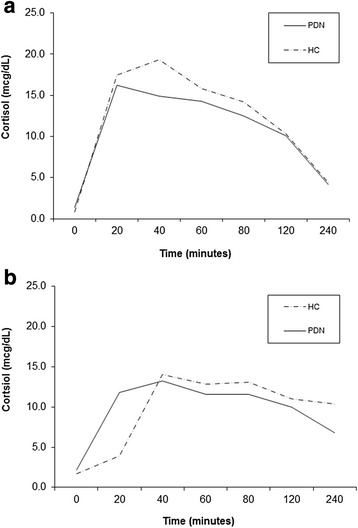
Variable cortisol pharmacokinetics were noted in subjects taking hydrocortisone (HC) and prednisone (PDN). For example, the subject in (a) metabolized and cleared glucocorticoids faster than the subject in (b)
Table 3.
Average association analysis using a dominant model of genetic variants and their effect on hormone levels during glucocorticoid treatments
| Hormone | Drug | Gene | CHR | SNP | Basepair | Minor allele | N | P value |
|---|---|---|---|---|---|---|---|---|
| Androstenedione | Prednisone | NR3C1 | 5 | rs41423247 | 142778575 | C | 8 | 0.004 |
| 17-OHP | Dexamethasone | ABCB1 | 7 | rs1045642 | 87138645 | A | 9 | 0.025 |
| 17-OHP | Dexamethasone | ABCB1 | 7 | rs2032582 | 87160618 | A | 9 | 0.025 |
| 17-OHP | Dexamethasone | ABCB1 | 7 | rs1128503 | 87179601 | A | 9 | 0.025 |
Abbreviations: CHR chromosome, SNP single nucleotide polymorphism, N number, 17-OHP 17-hydroxyprogesterone, NR3C1 nuclear receptor subfamily 3 group C member 1, ABCB1 ATP-binding cassette sub-family B
Fig. 4.
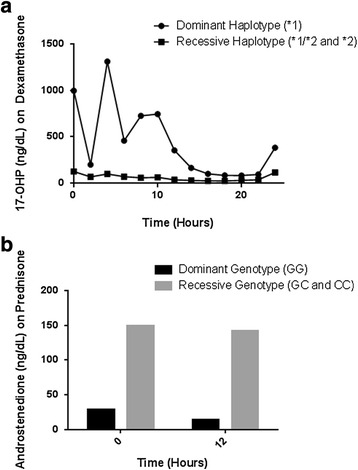
In a, when comparing the ABCB1 *1 dominant haplotype with the ABCB1 recessive haplotypes *1/*2 and *2, the subjects homozygous for the *1 haplotype had less suppression of 17-OHP on DEX compared to the others. In b, those expressing the dominant genotype had greater suppression of androstenedione on PDN than the recessive genotype
To investigate total drug exposure over time, area under the concentration curve (AUC) analysis was performed, with significant associations noted only during the PDN arm of the study (Table 4). For importin-13 (IPO13, rs6671164 and rs7412307), subjects homozygous for the variant allele had the least exposure to glucocorticoid followed by those who were heterozygous for the variant followed by those homozygous for the wild-type (p = 0.015 for all). Inversely, for IPO13 variants rs4448553, rs1990150, rs2240447, rs2301993, and rs2428953, subjects who were homozygous for the variant allele had the most exposure to glucocorticoid followed by those who were homozygous for the wild type followed by subjects who were heterozygous for the variant (p ≤ 0.023 for all). Lastly, subjects who were heterozygous for the variant allele for the glucocorticoid induced transcript 1 (GLCCI1, rs37973) had the least exposure to glucocorticoid followed by those homozygous for the wild-type followed by subjects who were homozygous for the variant allele (p = 0.037 for all). There were no significant correlations identified for these gene variants when subjects were on HC or DEX.
Table 4.
Area under the curve and slope analysis of cortisol for genetic variants
| Drug | Gene | CHR | SNP | Basepair | Minor allele | N | P value |
|---|---|---|---|---|---|---|---|
| Area under the curve analysis: | |||||||
| Prednisone | IPO13 | 1 | rs6671164 | 44403489 | G | 8 | 0.015 |
| Prednisone | IPO13 | 1 | rs4448553 | 44411589 | A | 9 | 0.007 |
| Prednisone | IPO13 | 1 | rs1990150 | 44414127 | G | 9 | 0.023 |
| Prednisone | IPO13 | 1 | rs2240447 | 44415415 | C | 9 | 0.007 |
| Prednisone | IPO13 | 1 | rs2301993 | 44426025 | C | 9 | 0.007 |
| Prednisone | IPO13 | 1 | rs7412307 | 44433864 | G | 8 | 0.015 |
| Prednisone | IPO13 | 1 | rs2428953 | 44443459 | A | 9 | 0.023 |
| Prednisone | GLCCI1 | 7 | rs37973 | 8007876 | G | 9 | 0.038 |
| Slope analysis: | |||||||
| Hydrocortisone | ABCB1 | 7 | rs1045642 | 87138645 | A | 9 | 0.026 |
| Hydrocortisone | ABCBI | 7 | rs2032582 | 87160618 | A | 9 | 0.026 |
| Hydrocortisone | ABCB1 | 7 | rs1128503 | 87179601 | A | 9 | 0.026 |
Abbreviations: CHR chromosome, SNP single nucleotide polymorphism, IPO13 importin-13, GLCCI1 glucocorticoid-induced transcript 1, ABCB1 ATP-binding cassette sub-family B member 1
Slope analysis was performed to determine cortisol clearance (Table 4). Using the dominance model, subjects homozygous for ABCB1 variants (rs1045642, rs2032582, and rs1128503) had faster clearance of cortisol while on HC as compared to homozygous wild-type subjects (p = 0.026, p = 0.026, and p = 0.026, respectively). There were no significant correlations between gene variants and cortisol clearance for subjects on PDN.
Discussion
This pilot study compared the hormonal effects and pharmacokinetic profiles of HC, PDN, and DEX in nine prepubertal children with classic CAH. We used a potency conversion between HC to PDN to DEX of 1 to 5 to 50. However, our data demonstrate that DEX was more potent than both HC and PDN as children on the DEX arm had significantly lower adrenal hormone levels compared to those on either HC or PDN. Although glucocorticoid equivalencies are debatable, our findings are in line with other studies suggesting that DEX is at least 70 times [3] and up to 80 to 100 times or more [7–9] potent than HC in regards to suppressing adrenal androgen production. Unlike for DEX, we did not find any difference in ACTH or androstenedione concentrations during treatment with HC and PDN. In contrast, 17-OHP values were significantly higher when children were receiving PDN than when they were treated with HC. This suggests that PDN may be ≤ 5 fold more potent than HC and conflicts with other reports suggesting that PDN is 10 to 15 times more potent than HC [9, 10]. It is important to note that these findings may not reflect what is seen with prednisolone, which is the active metabolite of PDN.
Laboratory monitoring during glucocorticoid therapy in children with CAH is difficult since hormone concentrations can vary widely depending on the time of day and most recent glucocorticoid dose [11]. By measuring frequent hormone levels during each inpatient stay, we were able to characterize the degree of hormone variability. In particular, there were wide fluctuations in 17-OHP levels within individual subjects demonstrating that a single 17-OHP measurement is problematic in terms of reflecting the degree of biochemical control. This marked variability in 17-OHP has been attributed to circadian patterns [12, 13] and exaggerated responses to stress [14, 15]. Androstenedione also has a circadian rhythm but the magnitude of variability is less than that seen with 17-OHP [16] and levels often correlate better with overall CAH control [17]. Although not routinely used in the management of CAH, ACTH levels have been shown to be a useful adjunct [18], and as seen in our study, often correlate with other hormone markers of biochemical control.
Nightly dosing of DEX can lead to overtreatment with greater suppression of early morning adrenal steroid concentrations [19, 20]. All but one of our subjects had an undetectable trough DEX level, but many of our subjects on DEX had low 17-OHP and androstenedione levels. This implies that trough DEX levels do not accurately reflect the biological effect at a tissue or cellular level. Despite disparities in adrenal steroids, there was no difference in GH, IGF-1, or BMI during treatment with the three glucocorticoids. However, given the short duration of our study, any presumption regarding the implication of this observation on long-term growth would be premature.
Very few studies, also with small sample sizes, have compared different glucocorticoids in either children or adults with CAH [7, 20–22], and ours is the only one to compare three different preparations in a prepubertal cohort. While overall results have been conflicting, other investigators have also noted a greater degree of suppression than expected with DEX [7], as was the case in our study. In our study, significant correlations were found between ABCB1, NR3C1, IPO13 and GLCCI1 genotypes and hormone concentrations and/or clearance. While such findings could be spurious, it is intuitive that differences in glucocorticoid-related genes could impact individual physiologic responses. The ABCB1 gene encodes for P-glycoprotein, which is a transmembrane transporter that acts as a cellular drug efflux pump for various substances, including glucocorticoids [23]. Clinical response to glucocorticoids has been shown to be influenced by individual SNPs as well as haplotypes in ABCB1 [24, 25]. For example, polymorphisms in ABCB1 have been associated with steroid resistance in children with nephrotic syndrome [26, 27] and steroid response in those with inflammatory bowel disease [28, 29]. In our study, subjects with specific ABCB1 genetic variants were found to metabolize and clear cortisol faster when on HC. Polymorphisms in ABCB1 and other relevant genes might explain why some patients with CAH require unexpectedly low or high doses of glucocorticoids to achieve optimal biochemical control, and preemptive genetic testing may facilitate selection of a more appropriate starting dose for individual patients. Furthermore, experimental regimens and novel medical therapies currently under investigation may eventually make a one glucocorticoid approach obsolete in the treatment of CAH.
The NR3C1 gene encodes for the glucocorticoid receptor (GR). Other research has shown that individuals with the BclI (rs41423247) polymorphism have increased sensitivity and responsiveness to glucocorticoids [30, 31]. This SNP has also been associated with increased BMI, hypertension, and cardiovascular disease in the general population [32]. Adults with CAH who possess the BclI polymorphism have also been found to have a higher BMI, waist circumference, and systolic blood pressure [33] but this has not been found in children [34]. We did not evaluate for correlations with BMI in our subjects with this SNP given the small size of our population. However, we did find significant associations between the rs41423247 variant and androstenedione concentrations when subjects were on PDN.
Subjects in our study with genetic variants in IPO13 and GLCCI1 had variable exposure to glucocorticoids when on PDN. Both IPO13 and GLCCI1 genotypes are associated with glucocorticoid exposure in children on PDN [35, 36]. IPO13 regulates the nuclear translocation of the GR. Polymorphisms of IPO13 have been associated with airway hyper-responsiveness and reactivity in children with asthma, suggesting that IPO13 variation might improve endogenous glucocorticoid bioavailability in the cell nucleus [35]. The function of GLCCI1 is not well understood. SNPs in GLCCI1 in patients with asthma have been associated with a decreased response to inhaled glucocorticoids [36]. However, differences in gene variants of GLCCI1 have not been shown to be significant in other diseases, such as steroid resistant nephrotic syndrome [37].
Limitations of our pilot study include the small sample size, lack of a washout period, previous treatment with different glucocorticoids, and short exposure to each glucocorticoid. Although significant pharmacogenetic correlations were detected, it is premature to draw firm conclusions about the possible functional role of these genetic variants [38]. Another limitation is that hormone levels were measured using RIA and ELISA instead of more precise techniques, such as liquid chromatography and tandem mass spectrometry; however, this is similar to the clinical setting. Finally, we acknowledge that the potency conversion used in our study is open to debate. Determining equivalent glucocorticoid dosing is complex, varies between individuals and can be based on anti-inflammatory effect, growth-retarding effect, and/or androgen-suppressive effect. For example, while DEX may be up to 80 times more potent than cortisol for growth-retarding effect, it appears to be only 30 times more potent than cortisol in regards to anti-inflammatory effect [39]. While extremely preliminary, our findings are intriguing and warrant replication in a larger population to see if there is a true differential versus a dose or equivalency-related effect.
Conclusions
It would be unrealistic to expect there to be one best glucocorticoid regimen for all children with classic CAH. In our subjects, DEX resulted in over dosing while PDN often lead to under dosing. During treatment with each glucocorticoid, there was a great deal of individual variability in hormone concentrations. The majority of 17-OHP values were outside of the target range, suggesting that 17-OHP measurements to guide glucocorticoid dosing are problematic at best. Given inherent differences in individual physiology, pharmacogenetics may help to predict steroid sensitivity and response in patients with CAH. Ultimately, such information could lead to more targeted dosing for individual patients resulting in improved clinical care.
Acknowledgements
The authors want to acknowledge and thank the patients that participated in the study.
Funding
This work was supported by an investigator-initiated research grant from Pfizer. The authors have nothing else to declare.
Availability of data and materials
The research data are presented in the text of the paper as well as in the figures and tables of the paper.
Authors’ contributions
TDN served as the study coordinator, helped to design and execute the study, enrollment of patients, data collection, data analysis, performed literature search, and drafted the manuscript. JLR was involved in the study design, pharmacogenetic analysis, data interpretation, intellectual content, and writing of the pharmacogenetics aspects of the paper. ZMN was involved in recruitment of patients, revision of the manuscript, interpretation of data, and statistical analysis. SER was involved in the pharmacogentic analysis and data interpretation. JES was involved in statistical analysis and data interpretation. LL was involved in pharmacogenetics analysis and data interpretation. ECW was involved in the original study conception and design, revision of the manuscript, and interpretation of data. EAE was involved in study conception and design, interpretation of the data, intellectual content, and critical revision of the manuscript. Of note, all authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All patient information is de-identified. All patients signed a consent (and assent when appropriate).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) at Indiana University. The IRB study number is 0203-26. Written informed consent from parents and assent from subjects, when appropriate, was obtained.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- BMI
Body mass index
- CAH
Congenital adrenal hyperplasia
- DEX
Dexamethasone
- GCRC
General clinical research center
- GH
Growth hormone
- HC
Hydrocortisone
- HPA
Hypothalamic-pituitary-adrenal
- PDN
Prednisone
- SNP
Single nucleotide polymorphism
Contributor Information
Todd D. Nebesio, Phone: 317-944-3889, Email: tdnebesi@iu.edu
Jamie L. Renbarger, Email: jarenbar@iu.edu
Zeina M. Nabhan, Email: znabhan@iu.edu
Sydney E. Ross, Email: sydeross@iupui.edu
James E. Slaven, Email: jslaven@iu.edu
Lang Li, Email: lali@iu.edu.
Emily C. Walvoord, Email: ewalvoor@iu.edu
Erica A. Eugster, Email: eeugster@iu.edu
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–60. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint LWPES/ESPE CAH Working Group Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab. 2002;87:4048–53. doi: 10.1210/jc.2002-020611. [DOI] [PubMed] [Google Scholar]
- 3.Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics. 2000;106:767–73. doi: 10.1542/peds.106.4.767. [DOI] [PubMed] [Google Scholar]
- 4.Huseman CA, Varma MM, Blizzard RM, Johnson A. Treatment of congenital adrenal virilizing adrenal hyperplasia with single and multiple daily doses of prednisone. J Pediatr. 1977;90:538–42. doi: 10.1016/S0022-3476(77)80362-4. [DOI] [PubMed] [Google Scholar]
- 5.Haas DM, Lehmann AS, Skaar T, Philips S, McCormick CL, Beagle K, et al. The impact of drug metabolizing enzyme polymorphisms on outcomes after antenatal corticosteroid use. Am J Obstet Gynecol. 2012;206:447. doi: 10.1016/j.ajog.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horita N, Kaneko T. Genetic model selection for a case-control study and a meta-analysis. Meta Gene. 2015;5:1–8. doi: 10.1016/j.mgene.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen JW, Loriaux DL. Variable efficacy of glucocorticoids in congenital adrenal hyperplasia. Pediatrics. 1976;57:942–7. [PubMed] [Google Scholar]
- 8.Rivkees SA, Stephenson K. Low-dose dexamethasone therapy from infancy of virilizing congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2009;2009:274682. doi: 10.1186/1687-9856-2009-274682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivkees SA. Dexamethasone therapy of congenital adrenal hyperplasia and the myth of the “growth toxic” glucocorticoid. Int J Pediatr Endocrinol. 2010;2010:569680. doi: 10.1155/2010/569680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr. 2003;143:402–5. doi: 10.1067/S0022-3476(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 11.Dauber A, Kellogg M, Majzoub JA. Monitoring of therapy in congenital adrenal hyperplasia. Clin Chem. 2010;56:1245–51. doi: 10.1373/clinchem.2010.146035. [DOI] [PubMed] [Google Scholar]
- 12.Atherden SA, Barnes ND, Grant DB. Circadian variation in plasma 17-hydroxyprogesterone in patients with congenital adrenal hyperplasia. Arch Dis Child. 1972;47:602–4. doi: 10.1136/adc.47.254.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisch H, Parth K, Schober E, Swoboda W. Circadian patterns of plasma cortisol, 17-hydroxyprogesterone, and testosterone in congenital adrenal hyperplasia. Arch Dis Child. 1981;56:208–13. doi: 10.1136/adc.56.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boninsegni R, Salerno R, Giannotti P, Andreuccetti T, Busoni P, Santoro S, Forti G. Effects of surgery and epidural or general anaesthesia on testosterone, 17-hydroxyprogesterone and cortisol plasma levels in prepubertal boys. J Steroid Biochem. 1983;19:1783–7. doi: 10.1016/0022-4731(83)90360-6. [DOI] [PubMed] [Google Scholar]
- 15.Ersch J, Beinder E, Stallmach T, Bucher HU, Torresani T. 17-Hydroxyprogesterone in premature infants as a marker of intrauterine stress. J Perinat Med. 2008;36:157–60. doi: 10.1515/JPM.2008.013. [DOI] [PubMed] [Google Scholar]
- 16.Young MC, Walker RF, Riad-Fahmy D, Hughes IA. Androstenedione rhythms in saliva in congenital adrenal hyperplasia. Arch Dis Child. 1988;63:624–8. doi: 10.1136/adc.63.6.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuqua JS, Rotenstein D, Lee PA. Duration of suppression of adrenal steroids after glucocorticoid administration. Int J Pediatr Endocrinol. 2010;2010:712549. doi: 10.1186/1687-9856-2010-712549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaFranchi S. Plasma adrenocorticotrophic hormone in congenital adrenal hyperplasia. Importance in long-term management. Am J Dis Child. 1980;134:1068–72. doi: 10.1001/archpedi.1980.02130230048015. [DOI] [PubMed] [Google Scholar]
- 19.Young MC, Hughes IA. Dexamethasone treatment for congenital adrenal hyperplasia. Arch Dis Child. 1990;65:312–4. doi: 10.1136/adc.65.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauber A, Feldman HA, Majzoub JA. Nocturnal dexamethasone versus hydrocortisone for the treatment of children with congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010. doi: 10.1155/2010/347636. [DOI] [PMC free article] [PubMed]
- 21.Horrocks PM, London DR. A comparison of three glucocorticoid suppressive regimes in adults with congenital adrenal hyperplasia. Clin Endocrinol. 1982;17:547–56. doi: 10.1111/j.1365-2265.1982.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith R, Donald RA, Espiner EA, Glatthaar C, Abbott G, Scandrett M. The effect of different treatment regimens on hormonal profiles in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1980;51:230–6. doi: 10.1210/jcem-51-2-230. [DOI] [PubMed] [Google Scholar]
- 23.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, et al. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–52. [PubMed] [Google Scholar]
- 24.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–76. doi: 10.2165/00003088-200443090-00001. [DOI] [PubMed] [Google Scholar]
- 26.Wasilewska A, Zalewski G, Chyczewski L, Zoch-Zwierz W. MDR-1 gene polymorphisms and clinical course of steroid-responsive nephrotic syndrome in children. Pediatr Nephrol. 2007;22:44–51. doi: 10.1007/s00467-006-0275-3. [DOI] [PubMed] [Google Scholar]
- 27.Choi HJ, Cho HY, Ro H, Lee SH, Han KH, Lee H, et al. Polymorphisms of the MDR1 and MIF genes in children with nephrotic syndrome. Pediatr Nephrol. 2011;26:1981–8. doi: 10.1007/s00467-011-1903-0. [DOI] [PubMed] [Google Scholar]
- 28.Krupoves A, Mack D, Seidman E, Deslandres C, Amre D. Associations between variants in the ABCB1 (MDR1) gene and corticosteroid dependence in children with Crohn’s disease. Inflamm Bowel Dis. 2011;17:2308–17. doi: 10.1002/ibd.21608. [DOI] [PubMed] [Google Scholar]
- 29.De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17:1095–108. doi: 10.3748/wjg.v17.i9.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol. 2003;59:585–92. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- 31.De Iudicibus S, Stocco G, Martelossi S, Drigo I, Norbedo S, Lionetti P, et al. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut. 2007;56:1319–20. doi: 10.1136/gut.2006.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manenschijn L, van den Akker EL, Lamberts SW, van Rossum EF. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann N Y Acad Sci. 2009;1179:179–98. doi: 10.1111/j.1749-6632.2009.05013.x. [DOI] [PubMed] [Google Scholar]
- 33.Moreira RP, Gomes LG, Mendonca BB, Bachega TA. Impact of glucocorticoid receptor gene polymorphisms on the metabolic profile of adult patients with the classical form of 21-hydroxylase deficiency. PLoS One. 2012;7:e44893. doi: 10.1371/journal.pone.0044893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira RP, Gomes LG, Madureira G, Mendonca BB, Bachega TA. Influence of the A3669G glucocorticoid receptor gene polymorphism on the metabolic profile of pediatric patients with congenital adrenal hyperplasia. Int J Endocrinol. 2014;2014:594710. doi: 10.1155/2014/594710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raby BA, Van Steen K, Lasky-Su J, Tantisira K, Kaplan F, Weiss ST. Importin-13 genetic variation is associated with improved airway responsiveness in childhood asthma. Respir Res. 2009;10:67. doi: 10.1186/1465-9921-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–83. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheong HI, Kang HG, Schlondorff J. GLCCI1 single nucleotide polymorphisms in pediatric nephrotic syndrome. Pediatr Nephrol. 2012;27:1595–9. doi: 10.1007/s00467-012-2197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–76. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hindmarsh PC. Management of the child with congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:193–208. doi: 10.1016/j.beem.2008.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data are presented in the text of the paper as well as in the figures and tables of the paper.


