Abstract
In enterococci and in Streptomyces coelicolor, a glycopeptide nonproducer, the glycopeptide resistance genes vanHAX are colocalized with vanRS. The two-component system (TCS) VanRS activates vanHAX transcription upon sensing the presence of glycopeptides. Amycolatopsis balhimycina, the producer of the vancomycin-like glycopeptide balhimycin, also possesses vanHAXAb genes. The genes for the VanRS-like TCS VnlRSAb, together with the carboxypeptidase gene vanYAb, are part of the balhimycin biosynthetic gene cluster, which is located 2 Mb separate from the vanHAXAb. The deletion of vnlRSAb did not affect glycopeptide resistance or balhimycin production. In the A. balhimycina vnlRAb deletion mutant, the vanHAXAb genes were expressed at the same level as in the wild type, and peptidoglycan (PG) analyses proved the synthesis of resistant PG precursors. Whereas vanHAXAb expression in A. balhimycina does not depend on VnlRAb, a VnlRAb-depending regulation of vanYAb was demonstrated by reverse transcriptase polymerase chain reaction (RT-PCR) and RNA-seq analyses. Although VnlRAb does not regulate the vanHAXAb genes in A. balhimycina, its heterologous expression in the glycopeptide-sensitive S. coelicolor ΔvanRSSc deletion mutant restored glycopeptide resistance. VnlRAb activates the vanHAXSc genes even in the absence of VanS. In addition, expression of vnlRAb increases actinorhodin production and influences morphological differentiation in S. coelicolor.
Introduction
Bacteria need to respond to changes in their environment. Therefore, they require adequate means to gain and process information on the immediate surroundings. Such means are represented by two-component systems (TCSs), which are ubiquitous in all prokaryotes. A typical TCS consists of a sensor histidine kinase (HK) and a response regulator (RR).1 The HK measures a specific external signal and autophosphorylates at a conserved histidine residue within the cytosol. This phosphoryl group is transferred to the associated RR. The activated RR initiates the cellular response.2 Most RRs are transcription factors that not only change the gene expression pattern of one or more genes of the cell, but also post-transcriptional and post-translational regulation of RNAs and proteins, respectively, by RRs has been reported.3 The ability of bacteria to sense the signal enables them to react with an adaptive response.
Of special interest is the glycopeptide-sensing TCS VanRS that controls the expression of glycopeptide resistance genes in gram-positive pathogens,4,5 some glycopeptide producers, and other actinomycetes.6 VanS is a membrane-standing HK. Its C-terminus extends into the cytoplasm and contains the kinase domain and the phosphorylation site.7 VanS senses the presence of glycopeptides and catalyses adenosine triphosphate-dependent autophosphorylation of a specific histidine residue. Subsequently, VanS transfers the phosphate group to an aspartate residue of VanR, which then activates the transcription of the resistance genes. However, under noninduction conditions, VanS acts as a phosphatase, removing the phosphate group from VanR.8
Glycopeptides such as vancomycin, teicoplanin, and telavancin are used for treating infections caused by gram-positive pathogens. They act by binding to the N-acyl-d-alanyl-d-alanine (d-Ala-d-Ala) termini of peptidoglycan (PG) and its precursor lipid II. This binding effectively sequesters the substrate for the transglycosylases and the d,d-transpeptidases, two key enzymes of cell wall synthesis, resulting in an inability to grow and subsequently to cell death.
Glycopeptide resistance is mediated by reprogramming cell wall biosynthesis. Ten types of resistances have been characterized so far (VanA-N).9,10 In each case, the terminal d-alanine (d-Ala) in the pentapeptide side chain of the PG of gram-positive bacteria is substituted either by a d-lactate (d-Lac) (VanA, B, D, F, and M) or a d-serine (VanC, E, G, L and VanN). These substitutions result in a 1000-fold11 or 6-fold12 decreased binding affinity of the glycopeptide to its target, respectively. Categorization into the different phenotypes is based on the inducibility, the breadth of resistance to individual compounds, and the level of resistance.9
Three of those phenotypes, VanC,13 VanD,14 and VanN,15,16 are constitutively expressed. All others are inducible to different degrees by different glycopeptides. It was shown that enterococcus and staphylococcus strains expressing glycopeptide resistance genes constitutively are impaired in growth in comparison with strains where the genes are inducible.17,18 Apparently, careful control of the expression of these genes is advantageous.
Streptomyces coelicolor A3(2) is neither a pathogen nor a glycopeptide producer, but it is likely to encounter glycopeptides in its natural habitat. Therefore, it benefits from carrying vanRSSc, vanHAXSc, vanKSc, and vanJSc (Fig. 1C). VanHSc is a d-stereospecific lactate dehydrogenase that converts pyruvate to d-Lac. VanASc is a d-Ala-d-Ala-ligase family protein that ligates d-Ala and d-Lac to d-Ala-d-Lac-depsipeptides. VanXSc is a highly selective carboxypeptidase that cleaves the remaining d-Ala-d-Ala-dipeptide. VanKSc belongs to the Fem family of enzymes, which add the cross-bridging amino acid(s) to the stem pentapeptide of PG precursors.19 VanY is a membrane protein conferring resistance to teicoplanin. To identify the precise nature of the ligand signal that activates glycopeptide resistance in S. coelicolor A3(2), the VanB-type HK VanSSc, sensing vancomycin, but not teicoplanin, was investigated.21,22 Investigation on VanSSc revealed opposed results. On the one hand, it was shown by cross-linking experiments that vancomycin is the direct ligand of the VanSSc.21 On the other hand, Kwun et al.22 demonstrated that VanSSc is activated by vancomycin in complex with the d-Ala-d-Ala termini of PG precursors.
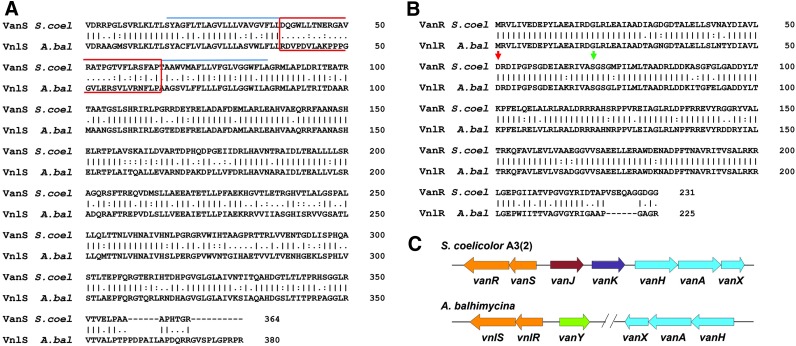
FIG. 1.
(A, B) EMBOSS stretcher pairwise sequence alignment of VnlRSAb and VanRSSc. (A) EMBOSS stretcher pairwise sequence alignment of VnlSAb and VanSSc. Transmembrane domains are indicated in blue. The extracytosolic domain is highlighted by a red box. (B) EMBOSS stretcher pairwise sequence alignment of VnlRAb and VanRSc. The site of aspartate phosphorylation is indicated by red and that of the proposed autophosphorylation by green arrow. (C) Organization of the resistance genes in Streptomyces coelicolor compared with that of Amycolatopsis balhimycina. “-” for a mismatch or a gap; “.” for any small positive score; “:” for a similarity, which scores more than 1.0; and “I” for an identity where both sequences have the same residue.
The actinomycete Amycolatopsis balhimycina produces the glycopeptide balhimycin, a vancomycin-type glycopeptide differing from vancomycin only in the glycosylation pattern.23,24 The balhimycin biosynthesis gene cluster contains all genes necessary for balhimycin production25 as well the vanRS-like regulatory genes, vnlRSAb and the accessory resistance gene vanYAb (Fig. 1C).26 VanYAb is a carboxypeptidase which cleaves the endstanding D-Ala-D-Ala-dipeptide from the PG precursors.27 However, the vanHAXAb genes are encoded more than 2 Mb apart from the balhimycin biosynthesis cluster. Although VnlRAb does not regulate glycopeptide resistance in A. balhimycina,27 its heterologous expression in the glycopeptide-sensitive S. coelicolor strain M600 J23016 (ΔvanRSSc) revealed unexpected effects. VnlRAb activates the vanHAXSc genes, increases actinorhodin production, and influences morphological differentiation in S. coelicolor.
Materials and Methods
Bacterial, strains, plasmids, and primers
The strains and plasmids used for this study are listed in Table 1, the primers used for this study are listed in Table 2.
Table 1.
Bacterial Strains Used in This Study
| Relevant feature(s) | References | |
|---|---|---|
| Strains | ||
| Streptomyces coelicolor A3(2) | ||
| M600 | SCP1- SCP2- | 30 |
| M600 ΔvanRSSc (J2301) | vanRSSc deletion mutant | 6 |
| M600 ΔvanRSSc [vnlRSAb] | ΔvanRSSc complemented with pRM4vnlRSAb | This study |
| M600 ΔvanRSSc [vnlRAb] | ΔvanRSSc complemented with pRM4vnlRAb | This study |
| M600 ΔvanRSSc [vnlSAb] | ΔvanRSSc complemented with pRM4vnlSAb | This study |
| M600 ΔvanRSSc [vnlRAbD51A] | ΔvanRSSc complemented with pRM4vnlRAbD51A | This study |
| Amycolatopsis balhimycina DSM 5908 | ||
| A. balhimycina WT DSM 5908 | Wildtype | 24 |
| A. balhimycina ΔvnlRAb | vnlRAb deletion mutant | 27 |
| A. balhimycina [vnlRAb] | Overexpression of vnlRAb in A. balhimycina, using pRM4vnlRAb | This study |
| A. balhimycina ΔvnlRAb [vnlRAb] | ΔvnlRAb complemented with pRM4vnlRAb | This study |
| A. balhimycina ΔvnlSAb | vnlSAb deletion mutant | This study |
| Escherichia coli | ||
| XL1-blue | recA1; endA1; gyrA96; tji-1; hsdR17; supE44; relA1; lac [F'proAB, lacqZΔM15Tn10(tetr)] | 28 |
| ET 12567 pUZ8002 | pUZ8002; kanr | 34 |
| ET 12567 | F-; dam13::Tn9; dcm-6; hsdM; hsdR; recF143; zjj201::Tn10; galK2; galT22; ara14; lacY1; xyl15; leuB6; thi1; tonA31; rpsL136; hisG4; tsx78; mtli; glnV44 | 29 |
| Plasmids | ||
| pRM4 | pSET152 ermEp*, RBS, Φ31 attP-int-derived integration vector | 31 |
| pRM4vnlRSAb | Expression plasmid for vnlRSAb | This study |
| pRM4vnlRAb | Expression plasmid for vnlRAb | This study |
| pRM4vnlSAb | Expression plasmid for vnlSAb | This study |
| pRM4vnlRAbD51A | Expression plasmid for vnlRAb, | This study |
| Exchange of Asp at position 51 with Ala | ||
| pSP1 | Inactivation vector in A. balhimycina | 32 |
| Erythromycin and ampicillin resistance | ||
| pSPΔvnlSAb | pSP1 carrying a 1579 bp upstream and a 1509 bp downstream fragment of vnlSAb | This study |
WT, wild type.
Table 2.
Primers Used in This Study
| Primer | Sequence | Relevant feature(s) | References | |
|---|---|---|---|---|
| 1 | vnlRS-compl.1 | CATCGGCATATGCGCGTGCTGATCGTCGAG | Cloning of vnlRSAb | This study |
| 2 | vnlRS-compl. 2 | GAATTCCTGCGCGACTCCAGCGTTTCTCAGCGGAAG | ||
| 3 | vnlR-over | GAATTCAGGCGTAGCTGAGG | Cloning of vnlRAb | This study |
| 4 | vnlS-cloning | ATCATATGAGCGTCCGCCTCAAAC | Cloning of vnlSAb | This study |
| 5 | vnlRD51A1 | GAATATCCCGGgCGAGGACGG | Recombinant primers for aa exchange | This study |
| 6 | vnlRD51A2 | CCGTCCTCGcCCGGGATATT | ||
| 7 | vnlSdelFrg1for | TTAGAATTCGATTGTCCGCGAGAAATG | Cloning of vnlSAb upstream region | This study |
| 8 | vnlSdelFrg1rev | TAATCTAGACCCGGCCGCTCTGTC | ||
| 9 | vnlSdelFrg2for | TTATCTAGACCGGCCGGGTCACCTC | Cloning of vnlSAb downstream region | This study |
| 10 | vnlSdelFrg2rev | ATTGCATGCGGGCGCAAGTGAGTTTCGGTCATCG | ||
| 11 | pSETerme rev | ATGCTAGTCGCGGTTGA | Integration of Plasmid and Insert | This study |
| 12 | attBli-fwd | TTCTGGAAATCCTCGAAGGC | Integration of plasmid through ΦC31 | |
| 13 | attPint-rev | TGTGCATGCGCCCACGAATG | ||
| 14 | ery for | AAGGGAGAAAGGCGGACAGG | Proof of erythromycin resistance cassette | This study |
| 15 | ery rev | GTCGCTTCTGCGCAAGTACC | ||
| 16 | vnlSproof for | TGCTCGAAGTCCTCGTTTCC | Integration and deletion verification | This study |
| 17 | vnlSproof rev | GCAAGTACGTGAGCGATCAG | ||
| 18 | vanSSc_RT_1 | CTCCAACTGACCACGAACCT | RT-PCR analysis in S. coelicolor M600 | This study |
| 19 | vanSSc_RT_2 | GGTCGGTGTGTATGCGTTC | ||
| 20 | vanRSc_RT_1 | TGCTGAGTGTCAACGCCTAC | ||
| 21 | vanRSc_RT_2 | CGAACTGCTTCCTGGTCAAC | ||
| 22 | vanASc_RT_1 | ACCGTGACAGGAGACGAGAC | ||
| 23 | vanASc_RT_2 | CTGGTGGATCCGGAAGAAT | ||
| 24 | hrdBSc_RT_1 | TGACCAGATTCCGGCCACTC | This study | |
| 25 | hrdBSc_RT_2 | CTTCGCTGCGACGCTCTTTC | ||
| 26 | sigB for | CGTAGGTCGAGAACTTGAAC | RT-PCR analysis in A balhimycina DSM5908 | 41 |
| 27 | sigB rev | GTGTCTACCTCAACGGTATC | ||
| 28 | vanH1 | GGGACAAGCCCATCAAGAAC | 27 | |
| 29 | vanA2 | GAGCGGACTTGACGGAGATG | ||
| 30 | vanY_RT_fwd | TCGGCACGAGGATTG | ||
| 31 | vanY_RT_rev | TTCACGCACAGTTCG |
RT-PCR, reverse transcriptase polymerase chain reaction.
Escherichia coli XL1-blue28 was used for cloning purposes, and the methylation-deficient strain E. coli ET1256729 was used to obtain unmethylated DNA for A. balhimycina transformations.
A. balhimycina24 is the balhimycin-producing wild type (WT) and was used to generate the vnlS deletion as well as the vnlR-overexpressing strains (this study). Furthermore, ΔvnlR deletion27 was used for complementation (this study).
S. coelicolor M60030 were used to generate S. coelicolor M600 ΔvanRS.6 This deletion strain was used to generate complementations with vnlRSAb, vnlRAb, vnlSAb, and vnlRAbD51A (this study).
The overexpression plasmids pRM4vnlRSAb, pRM4vnlRAb, pRM4vnlSAb, and pRM4vnlRAbD51A are derived from pRM431, a pSET152-derived nonreplicative, ΦC31 integration vector with an integrated constitutive ermEp* promoter, an artificial ribosomal binding site, and an apramycin resistance cassette.
The deletion vector pSPΔvnlSAb is derived from pSP132 in which flanking regions of vnlSAb were cloned.
Media and culture conditions
A. balhimycina grown in 100 ml TSB medium (Difco) for 48 hr and 2 ml of this preculture were used to inoculate the main cultures either in 100 ml R530 or in TSB medium. R5 medium was used to stimulate balhimycin production, while TSB medium was used when balhimycin production should be prevented. After 48 hr of cultivation, the mycelium was used to isolate PG precursors, to extract DNA, or to perform resistance assays against different glycopeptides. To isolate RNA, the cells were grown 15/39/63 hr. Balhimycin production assays were performed after 5 days of growth.
S. coelicolor M145 and M600 were grown on Cullum-agar plates for sporulation. Isolated spores were used to inoculate 10 ml R5 medium as preculture for cell wall precursor extraction or DNA extraction. For RNA isolation, 2 ml of a 48-hr-old TSB preculture was used to inoculate 100 ml of HA medium. The cells were harvested after 69 hr.
To compare the growth of the different S. coelicolor strains, 10 μl spores (∼1.5 × 107) of each strain were streaked on a YM plate. The plate was incubated for 7 days.
A. balhimycina and S. coelicolor were grown at 30°C, and liquid cultures were shaken at 180 rpm.
A. balhimycina and S. coelicolor strains were cultivated in 100 ml of R5 medium in an orbital shaker (220 rpm) in 500-ml baffled Erlenmeyer flasks at 27°C.
Liquid/solid media were supplemented with 100 μg/ml apramycin to select for strains carrying integrated antibiotic resistance genes.
E. coli was grown in Luria-Bertani broth (Roth) at 37°C using 100 μg/μl apramycin or 150 μg/μl ampicillin for selection of plasmid-containing colonies. Liquid cultures were shaken at 180 rpm.
Plasmid construction
For the heterologous expression in S. coelicolor ΔvanRSSc, the entire coding regions of the vnlRAb (Table 2 primer 1 + 3), vnlRSAb (Table 2 primer 1 + 2), and vnlSAb (primer 4 + 2) were amplified using Kapa-Hifi proofreading polymerase and the corresponding primers in brackets.
The vnlRAb (758 bp), vnlRSAb (1908 bp), and vnlSAb (1198 bp) polymerase chain reaction (PCR) products were integrated into pRM431 through the primer-attached restriction sites (Nde-EcoRI) downstream of the ermEp* promoter.
Site-directed mutagenesis by overlap extension33 was performed for the exchange of aspartate at position 51 to an alanine with the primers 5 + 6 (Table 2). The 758 bp PCR product vnlRAbD51A was integrated into pRM4 through the primer-attached restriction sites (Nde-EcoRI) downstream of the ermEp* promoter.
For the in-frame deletion of vnlSAb (1125 bp), a 1579 bp upstream fragment (Table 2 primer 7 + 8) and a 1509 bp downstream fragment (Table 2 primer 9 + 10) of vnlSAb were amplified from A. balhimycina genomic DNA using Kapa-Hifi proofreading polymerase and the corresponding primers in brackets. The plasmid pSPΔvnlS was constructed by integration of the fragments in pSP130 through the primer-attached restriction sites at the 5′ and 3′ ends (EcoRI/XbaI and XbaI/SphI) (pSPΔvnlS).
DNA transfer
Transformation of E. coli XL1-blue28 and ET12567 (pUZ8002)34 was performed as described previously.35,36
Plasmids pRM4vnlRSAb, pRM4vnlRAb, pRM4vnlSAb, and pRM4vnlRAbD51A were transferred into S. coelicolor through intergeneric conjugation.30 Plasmid integration was confirmed by colony PCR using the primer pair 12 + 13 (Table 2) or primer 11 (Table 2) in combination with a reverse primer of corresponding gene.
pRM4vnlRAb and pRM4vnlRAbD51A were transferred into A. balhimycina through the direct transformation method32,37 using unmethylated plasmid DNA isolated from E. coli ET12567. Integration of plasmid was verified by PCR using primer pair 11 + 3 (Table 2).
For deletion of vnlSAb, A. balhimycina WT was transformed with pSPΔvnlSAb by direct transformation. The integration of the plasmid into the chromosome through homologous recombination was confirmed by PCR screening for the erythromycin resistance cassette, using primers ery for and ery rev (Table 2). To obtain deletion mutants, a second homologous recombination event was provoked by stressing plasmid-carrying colonies as described by Puk et al.38 Colonies were examined for sensitivity to erythromycin, and the deletions were verified by PCR analysis, using primers 16 + 17 (Table 2).
Sequence alignment
The amino acid (AA) sequences of VnlRSAb, VanRSc, and VanSSc are available under accession number Y16952 (named VanRS), (SCO3590), and (SCO3589), respectively.
Alignment of the AA sequences was performed by EMBOSS stretcher39; (www.ebi.ac.uk/Tools/psa/emboss_stretcher/).
Resistance test, reverse transcriptase polymerase chain reaction analyses, PG precursor, and cell wall analysis
Resistance test, reverse transcriptase polymerase chain reaction (RT-PCR) analyses, extraction of PG precursors, PG isolation, and the high-performance liquid chromatography–mass spectrometry (HPLC-MS) analyses were performed as described.27,40
Balhimycin concentration
The balhimycin concentration in 1 ml culture was quantified using HPLC with a photodiode array detector (HPLC-DAD) as described.40 The balhimycin concentration was calculated to 100 μg/ml total DNA.
Inference of biomass concentration from DNA quantification
For the quantification of total DNA in 1 ml culture, an acid extraction of DNA coupled with a colorimetric method41 was performed by measuring the absorbance at 600 nm. To analyze the amount of DNA, a standard curve with salmon sperm DNA was generated.
Results
A. balhimycina includes a VanRS homologous TCS (VnlRSAb) encoded in the balhimycin biosynthetic gene cluster
In most of the antibiotic-producing bacteria, the antibiotic biosynthetic gene clusters include resistance genes. One exception is the balhimycin producer A. balhimycina. In this study, the glycopeptide resistance genes vanHAXAb are located 2 Mb apart from the balhimycin biosynthetic gene cluster. In addition, the resistance is characterized by another unusual feature: the counterpart of the well-known TCS VanRS, which is known to regulate vanHAX expression in pathogens and in S. coelicolor is encoded by genes (vnlRSAb), which are part of the biosynthetic gene cluster and are therefore not colocated with the vanHAXAb genes.
VanRSSc of S. coelicolor was reported to sense glycopeptides and to activate the expression of the vanHAXSc genes.19 To elucidate the differences of the two actinomycete TCSs, we compared the AA sequence of VnlRSAb with the sequence of VanRSSc. Sequence alignment using EMBOSS stretcher39 revealed 82% sequence similarity between VnlRAb and VanRSc (Sco3590) and 73% between VnlSAb and VanSSc (Sco3589) (Fig. 1A, B). Based on the high similarity, a corresponding function of both RRs could be proposed.
In S. coelicolor, VanSSc phosphorylates VanRSc at the aspartate at AA position 51. Replacement of this residue with an alanine completely destroyed the activity of VanRSc.6 It has been shown that in S. coelicolor, in the absence of vancomycin, acetylphosphate phosphorylates VanRSc, whereas VanSSc acts as a phosphatase to decrease the level of VanRSc∼P. On exposure to vancomycin, VanS activity switches from a phosphatase to a kinase and vancomycin resistance is induced.6 Furthermore, Novotna et al.42 specified a serine residue at AA position 69 important for autophosphorylation through acetyl phosphate.
Sequence comparison revealed that VnlRAb contains both, a conserved aspartate at AA position 51 (D51) and a serine at AA position 69, the position that probably becomes autophosphorylated through acetyl phosphate (Fig. 1B), indicating an analogous phosphorylation pattern of VnlRAb compared with VanRSc.
The RR VnlRAb does not control expression of the vanHAXAb genes in A. balhimycina
In A. balhimycina, vanHAXAb expression does not depend on VnlRSAb. The deletion of vnlRAb had no effect on glycopeptide resistance and did not result in any obvious phenotype.27 This raises the interesting question on the function of this TCS in A. balhimycina.
Since overexpression of RRs of two-component signal transduction systems often modulates multidrug resistance,43,44 we overexpressed vnlRAb in A. balhimycina to analyze the effects on resistance and antibiotic production. vnlRAb was cloned under the control of the constitutive promoter ermE*p into the integrative plasmid pRM4 (pRM4vnlRAb). pRM4vnlRAb was transferred into A. balhimycina WT and into the A. balhimycina ΔvnlRAb mutant,27 resulting in the recombinant strains A. balhimycina [vnlRAb] and A. balhimycina ΔvnlRAb [vnlRAb], respectively. The phenotypes of the recombinant strains overexpressing VnlRAb and (as a control) that of the deletion mutant A. balhimycina ΔvnlRAb were compared with the WT phenotype (Fig. 2). All strains produced balhimycin at the same level (Fig. 2A). No differences in resistance against balhimycin were observed. Using a method optimized for actinomycetes,27 muropeptides from all A. balhimycina strains cultivated under balhimycin production conditions were isolated. HPLC/MS chromatograms showed the similar muropeptide composition pattern for all strains (Fig. 2B).
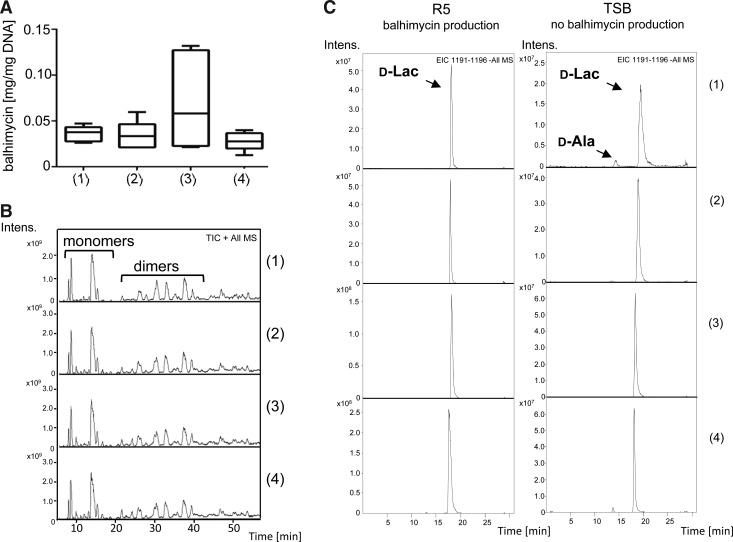
FIG. 2.
Analysis of balhimycin production, muropeptide composition, and PG precursors in A. balhimycina WT (1), A. balhimycina ΔvnlRAb (2), A. balhimycina [vnlRAb] (3), and A. balhimycina ΔvnlRAb [vnlRAb] (4). (A) Production of balhimycin measured by HPLC (n = 5). (B) HPLC/MS chromatogram of the muropeptides (positive mode). The first bracket embraces the peaks representing muropeptide monomers, the second the muropeptide dimers. (C) Extracted ion chromatograms of the negative mode from the PG precursors isolated from cells grown in R5 (balhimycin production) and in TSB (no balhimycin production). d-Lac, Pentapeptide precursors ending on d-Ala-d-Lac 1194 m/z at retention time ∼18 min. d-Ala, Pentapeptide precursors ending on d-Ala-d-Ala 1193 m/z at retention time ∼12 min. HPLC, high-performance liquid chromatography; MS, mass spectrometry; PG, peptidoglycan; WT, wild type.
In addition to muropeptides, the PG precursors were analyzed. For this purpose, we cultivated the strains under balhimycin production conditions and conditions under which balhimycin production is disabled. Under production as well as under nonproduction conditions, A. balhimycina WT, A. balhimycina [vnlRAb], A. balhimycina ΔvnlRAb, and A. balhimycina ΔvnlRAb [vnlRAb] produced resistant PG precursors ending with d-Ala-d-Lac (Fig. 2C). Only A. balhimycina WT and A. balhimycina ΔvnlRAb [vnlRAb] produced traces of precursors ending with d-Ala-d-Ala under nonproduction conditions (Fig. 2C). These results suggest that VnlRAb does not regulate the synthesis of resistance PG in A. balhimycina.
RT-PCR analyses revealed that a vanHAXAb transcript was detectable in A. balhimycina ΔvnlRAb, confirming that the expression of vanHAXAb is independent of vnlRAb (Fig. 3).
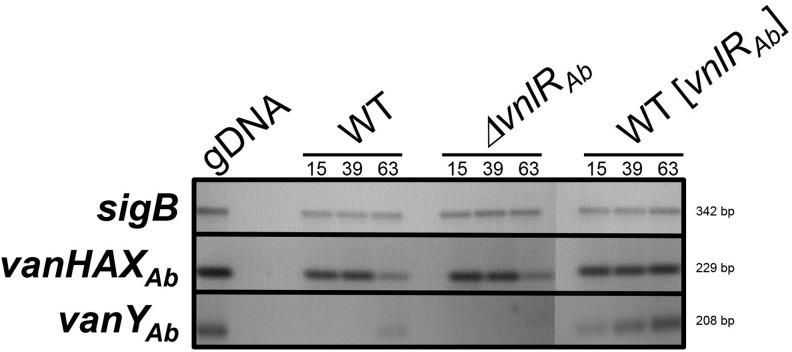
FIG. 3.
RT-PCR analyses of vanHAXAb and vanYAb in A. balhimycina WT, A. balhimycina ΔvnlRAb, and in A. balhimycina WT overexpressing vnlRAb (WT [vnlRAb]). RNA was isolated at different time points (15/39/63 hr) from the three strains cultivated in balhimycin production medium R5. sigB: transcription of the housekeeping gene sigB. vanHAXAb and vanYAb: transcription of vanHAXAb and vanYAb. For PCR, genomic DNA (gDNA) was used as positive control.
In A. balhimycina, sensing of glycopeptides through VnlSAb is not required for expressing the resistance genes
In enterococci and in S. coelicolor, the RR VanRSc becomes phosphorylated by the HK VanSSc. To analyze whether and how VnlRAb interacts with VnlSAb, we constructed an in-frame ΔvnlSAb mutant of A. balhimycina using the inactivation plasmid pSPΔvnlSAb. This plasmid containing a 1509 bp downstream fragment and a 1579 bp upstream fragment of vnlSAb was introduced into A. balhimycina through direct transformation. Successive homologous recombination resulted in the deletion of vnlSAb. A. balhimycina ΔvnlSAb showed neither a defect in balhimycin production nor resistance toward glycopeptides. In addition, no changes in the PG precursor and in the nascent PG composition in comparison with A. balhimycina WT were observed (data not shown). These results suggested that sensing the presence of glycopeptides does not correlate with balhimycin production and glycopeptide resistance. Apparently, the expression of the vanHAXAb genes occurs independently of VnlSAb.
VnlRAb is able to activate vanHAXSc transcription in S. coelicolor
In silico analyses revealed similar characteristics of VnlRSAb compared with VanRSSc. However, as shown above, the VnlRSAb system in A. balhimycina, in contrast to VanRSSc in S. coelicolor, does not regulate the vanHAXAb. To clarify the contradictory findings, the genes encoding the TCS VnlRSAb as well as VnlRAb and VnlSAb individually were transferred into the S. coelicolor mutant strain, in which the vanRSSc genes were deleted,6 to elucidate the ability of VnlRAb to activate the vanHAXSc genes in the S. coelicolor mutant. vnlRSAb, vnlRAb, and vnlSAb were introduced into S. coelicolor ΔvanRSSc under the control of the constitutive promoter ermEp* using the integrative plasmid pRM4vnlRAb. The growth of the recombinant strains was tested on glycopeptide-containing plates.
Introduction of vnlRSAb and of vnlRAb alone into S. coelicolor M600 ΔvanRSSc resulted in balhimycin-resistant strains (Fig. 4). In contrast, expression of vnlSAb alone did not change the glycopeptide-sensitive phenotype of the S. coelicolor ΔvanRSSc mutant. These results indicated that VnlRAb from A. balhimycina is able to activate the transcription of vanHAXSc in S. coelicolor M600 also in the absence of VnlSAb. Since in S. coelicolor M600 VanRSc∼P can be generated in a VanSSc-independent manner using acetylphosphate,6 we suggest a similar activation of VnlRAb in the absence of VnlSAb or VanSSc.
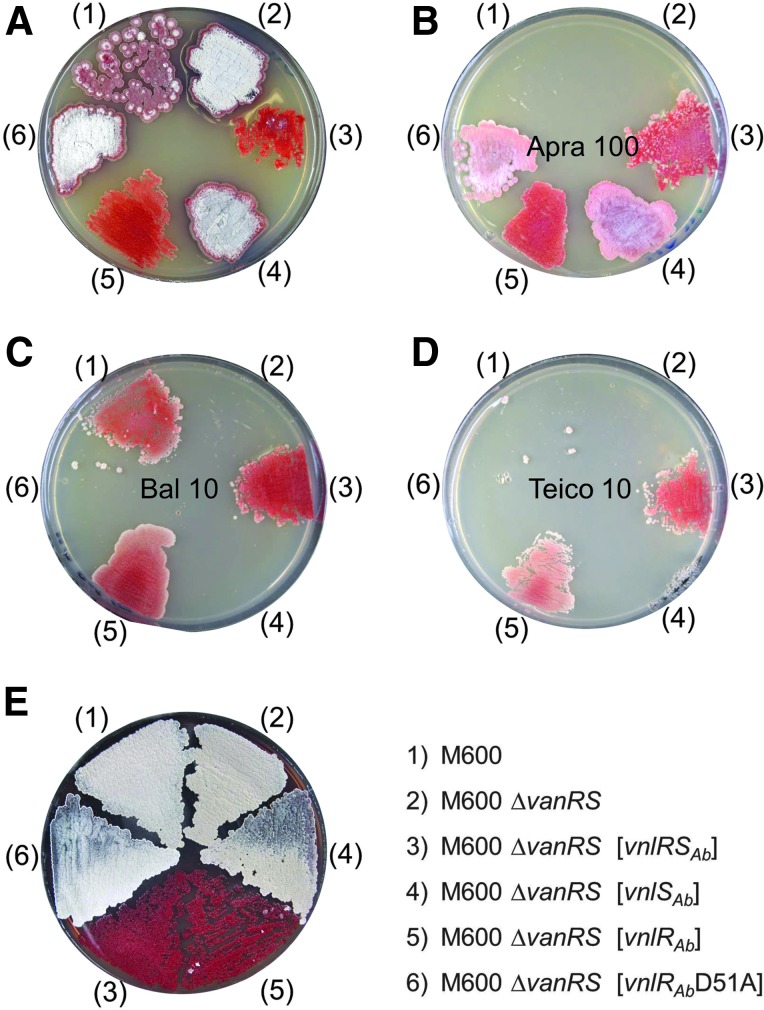
FIG. 4.
Growth and resistance of the S. coelicolor M600 ΔvanRSSc complemented with different combinations of vnlRSAb. (A) Growth on YM agar containing no antibiotic. (B) Growth on YM agar containing apramycin (100 mg/ml) (Apra 100) to prove plasmid integration. (C) Growth on YM agar containing balhimycin (10 mg/ml) (Bal 10). (D) Growth on YM agar containing teicoplanin (10 mg/ml) (Teico 10). (E) Growth on YM agar containing no antibiotic. M600, S. coelicolor M600.
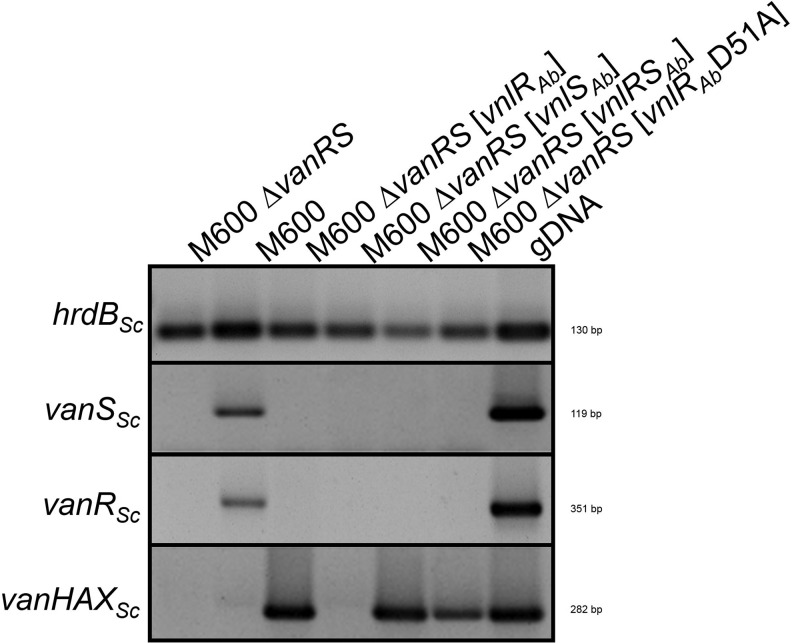
The activation of the vanHAXSc genes in the complemented S. coelicolor M600 ΔvanRSSc mutant with vnlRSAb and vnlRAb was further analyzed by RT-PCR. For this purpose, RNA was isolated from 25-hr-old liquid cultures grown without addition of any glycopeptide. A vanHAXSc transcript was detected when S. coelicolor M600 ΔvanRSSc was complemented with vnlRSAb or with vnlRAb alone. However, in S. coelicolor M600 and in the S. coelicolor M600 ΔvanRSSc mutant, transcription of the vanHAXSc failed (Fig. 5), confirming the functionality of VnlRAb as transcriptional activator in S. coelicolor.
FIG. 5.
RT-PCR analyses of S. coelicolor M600 and different S. coelicolor M600 mutants. RNA was isolated after 25 hr of cultivation in the absence of any glycopeptide. hrdB: transcription of the housekeeping gene hrdBSc. vanSSc, vanRSc, and vanHAXSc: transcription of vanSSc, vanRSc, and vanHAXSc. For PCR, genomic DNA (gDNA) was used as positive control.
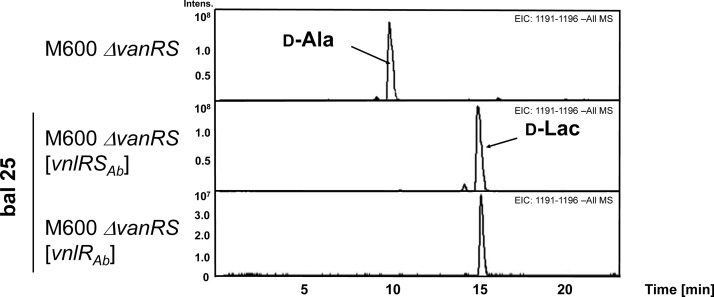
To investigate whether transcription of the vanHAXSc genes indeed resulted in the formation of glycopeptide-resistant PG precursors, which caused the resistant phenotype, we used HPLC/MS to analyze the PG precursor composition of S. coelicolor M600 ΔvanRSSc and of S. coelicolor M600 ΔvanRSSc complemented either with vnlRSAb or vnlRAb. Complementing S. coelicolor M600 ΔvanRSSc with vnlRSAb or with vnlRAb restored the synthesis of resistant PG precursors. In the presence of balhimycin exclusively, PG precursors ending with d-Ala-d-Lac were synthesized (Fig. 6C, D). The PG precursor composition of the glycopeptide-sensitive S. coelicolor M600 ΔvanRSSc mutant was analyzed after growing the strain in the absence of balhimycin. In this mutant, only sensitive cell wall precursors ending on d-Ala-d-Ala (1193 m/z) eluting at a retention time of 10–11 min were detected (Fig. 6A).
FIG. 6.
Extracted ion chromatograms (negative mode) of the PG precursors isolated from cells grown in R5 medium without antibiotic or with 25 mg/ml balhimycin (bal25). M600 ΔvanRSSc: S. coelicolor M600 ΔvanRSSc; d-Lac, pentapeptide precursors ending on d-Ala-d-Lac 1194 m/z at retention time ∼18 min; d-Ala, pentapeptide precursors ending on d-Ala-d-Ala 1193 m/z at retention time ∼14 min.
The phosphorylation site D51 is essential for the function of VnlRAb
To define the phosphorylation site of VnlRAb, D51, which was identified as a likely phosphorylation site by sequence composition (Fig. 1), was replaced by an alanine by exchanging nucleotide A to C at position 161 of vnlRAb using the recombinant PCR method. The exchange was verified by sequence analysis. The mutated gene was cloned into the integrative vector pRM4 under the control of the ermEp* promoter and introduced into S. coelicolor M600 ΔvanRSSc. The resulting recombinant strain was not able to grow in the presence of the tested glycopeptides (Fig. 4). Therefore, we propose D51 as the VnlRAb phosphorylation site.
VnlRAb expands the glycopeptide resistance in S. coelicolor
The glycopeptide resistance mechanism in S. coelicolor belongs to the VanB type of resistance, meaning that glycopeptide resistance can only be induced by vancomycin or vancomycin-type glycopeptides, whereas teicoplanin (a type IV glycopeptide) fails to activate resistance,42 resulting in a teicoplanin-sensitive phenotype of S. coelicolor. In contrast, A. balhimycina is resistant against vancomycin- as well as teicoplanin-type glycopeptides. To analyze whether the RR is responsible for determination of the glycopeptide resistance type, the recombinant strains S. coelicolor ΔvanRSSc [vnlRSAb], S. coelicolor ΔvanRSSc [vnlRAb], S. coelicolor ΔvanRSSc [vnlSAb], and S. coelicolor ΔvanRSSc [vnlRSAb D51A] were grown on teicoplanin-containing plates. Surprisingly, the recombinant strains (S. coelicolor ΔvanRSSc [vnlRSAb], S. coelicolor ΔvanRSSc [vnlRAb]) were able to grow also on teicoplanin-containing plates, whereas growth of S. coelicolor M600 WT was inhibited (Fig. 4). These results indicated that VnlRAb is able to induce teicoplanin resistance in S. coelicolor M600 by probably activating further genes required for teicoplanin resistance.
VnlRAb influences antibiotic production in S. coelicolor
To analyze if the heterologous expression of VnlRAb, in addition to the activation of the vanHAXSc genes, causes further (morphological) changes in S. coelicolor M600, the growth and production of actinorhodin were investigated without the addition of any antibiotic. Similar titers of spores (1.5 × 107) of S. coelicolor M600, S. coelicolor ΔvanRSSc, S. coelicolor ΔvanRSSc [vnlRSAb], S. coelicolor ΔvanRSSc [vnlRAb], S. coelicolor ΔvanRSSc [vnlSAb], and S. coelicolor ΔvanRSSc [vnlRSAb D51A] were plated on YM medium. Surprisingly, the heterologous expression of vnlRSAb or vnlRAb alone in S. coelicolor M600 ΔvanRSSc caused retardation in growth and increased actinorhodin production (Fig. 4E).
These results suggested that VnlRAb is not only able to activate the vanHAXSc genes in S. coelicolor M600 and to change its glycopeptide resistance type but it also has effects on other genes in S. coelicolor M600.
VnlRAb is responsible for the activation of vanYAb
Heterologous expression of VnlRAb in S. coelicolor confirmed that it can take over the VanRSc function to induce the expression of the vanHAXAb genes and, in addition, can apparently induce the expression of further genes. In contrast, it is not involved in regulation of the vanHAXAb genes in A. balhimycina. Since regulatory genes are often colocalized with its target genes, we speculated that VnlRSAb might control vanYAb, which is located directly adjacent to vnlRAb and which encodes a carboxypeptidase. Previous studies showed that VanYAb cleaves the d-Ala-d-Ala dipeptide from the PG precursors, but it is not able to cleave the d-Ala-d-Lac depsipeptide.27 To investigate, whether VnlRAb regulates the expression of vanYAb, transcriptional analyses were performed. RT-PCR analyses revealed that vanYAb was only transcribed when vnlRAb was expressed under the control of the strong promoter ermE*p (Fig. 2 (A. balhimycina [vnlRAb]). In A. balhimycina WT, transcription was detectable on a low level only after 63 hr of cultivation and in the A. balhimycina ΔvnlRAb mutant, vanYAb transcription was not induced at all (Fig. 2). This result was confirmed by RNA-seq analyses where we compared the transcription level of vanYAb in the A. balhimycina WT and A. balhimycina ΔvnlRAb (data not shown). The transcription of vanYAb was 25-fold decreased in A. balhimycina ΔvnlRAb compared with A. balhimycina WT. We therefore concluded that the RR VnlRAb in A. balhimycina is involved in controlling the expression of resistance mediated by VanYAb.
Discussion
Glycopeptide resistance in pathogens and in S. coelicolor is mediated by the action of VanHAX. The expression of the vanHAX genes is regulated by the TCS VanRS, the genes of which are colocalized with vanHAX. In the presence of glycopeptides, VanS becomes autophosphorylated and phosphorylates VanR, which subsequently activates transcription of vanHAX. VanH, VanA, and VanH reprogram the biosynthesis of the PG precursors, resulting in lipid II with an N-terminal d-Ala-d-Lac depsipeptide instead of the normally occurring d-Ala-d-Ala termini, the target of the glycopeptides. A. balhimycina produces the vancomycin-like glycopeptide balhimycin and has to protect itself from the action of the glycopeptide.45 The genome of A. balhimycina includes vanHAXAb genes and vanRS-like genes (vnlRSAb). However, in contrast to other glycopeptide-resistant bacteria, the vanHAXAb genes in A. balhimycina are located 2 Mb apart from the vnlRSAb genes, which are part of the balhimycin biosynthetic gene cluster.40
RT-PCR experiments revealed that VnlRAb is not involved in the activation of the vanHAXAb genes in A. balhimycina. Subsequent PG analyses confirmed that a vnlRAb deletion mutant cannot synthesize resistant muropeptides. Since vnlRAb is colocalized with the balhimycin biosynthetic genes, an alternative role of VnlRAb as regulator of balhimycin synthesis was assumed, but the deletion of the vnlRAb did not affect balhimycin production. Hence, VnlRAb is not the central regulator activating the vanHAXAb resistance genes or the balhimycin biosynthetic genes.
To further investigate the potential target gene(s) of VnlRAb, we analyzed the transcription of vanYAb, which encodes a carboxypeptidase and which is located adjacent to the vnlRSAb genes in the balhimycin biosynthetic gene cluster.25 RT-PCR and RNA-seq analyses revealed that vanYAb expression was 25-fold decreased in A. balhimycina ΔvnlRAb compared with A. balhimycina WT.
VanYAb is a d,d-carboxypeptidase, which cleaves the endstanding d-Ala from lipid II, resulting in the formation of tetrapeptides.27 In contrast to other described carboxypeptidases,46 VanYAb has no d,d-carboxyesterase activity. The tetrapeptides are the substrates for the l,d-transpeptidase (Ldt), which subsequently cross-links the tetrapeptide acyl donors at the third AA. This results in PG with 3–3 cross-linked tetra- and tripeptides, which are devoid of the d-Ala-d-Ala-ending peptides, and which can therefore not serve as target of glycopeptides anymore.47 Investigations of the PG of A. balhimycina revealed the presence of 3–3 cross-linked tetra- and tripeptides.40,45 Furthermore, we could identify at least three ldt genes in the genome of A. balhimycina.45 We therefore speculate that by activating the expression of vanYAb, VnlRAb is involved in regulating an alternative, VanHAXAb-independent glycopeptide resistance mechanism in A. balhimycina. This fact is further confirmed by RT-PCR analysis, where it was shown that VanYAb is expressed in A. balhimycina ΔvanHAXAb.40
This observation is in accordance with the findings in Nonomuraea ATCC 39727, the producer of the dalbavancin precursor A40926. Nonomuraea ATCC 39727 does not encode VanHAX homologs, but possesses a VanY homolog (VanYn) for the synthesis of a resistant PG precursor.48 As described for A. balhimycina, VanYn cleaves the C-terminal d-Ala from the pentapeptide as well as from the d-Ala-d-Ala dipeptide. The tetrapeptides are subsequently cross-linked by Ldt, resulting in glycopeptide-resistant cell wall.47 The surprising features of VnlRAb are that although it does not regulate the transcription of the vanHAXAb genes in A. balhimycina, it is able to activate vanHAXSc transcription in S. coelicolor, and that it activates teicoplanin resistance in S. coelicolor.
Activation of the vanHAXSc transcription can be explained by the binding of VnlRAb at the promoter region of vanHAXSc. Sequence comparison of the promoter regions of vanHAXSc and vanHAXAb not only revealed conserved motives but also some differences (data not shown). Although many attempts have been made to analyze putative promoter sequences in gel mobility assays, no shifts could be observed. This is probably due to the fact that after purification, the protein lost its functionality (data not shown). Therefore, determination of the exact binding motive of VnlRAb still requires alternative approaches.
VnlRAb was not only able to restore vancomycin resistance in an S. coelicolor ΔvanRSAb mutant after heterologous expression but it even conferred teicoplanin resistance to this mutant, although S. coelicolor WT is sensitive toward teicoplanin. Recent comparative study of the VanR-VanS systems from two Streptomyces strains, S. coelicolor and Streptomyces toyocaensis (the producer of the sugarless glycopeptide A47934), indicated that the glycopeptide antibiotic inducer specificity is determined solely by the differences between the AA sequences of the VanR-VanS TCS present in each strain rather than by any inherent differences in general cell properties, including cell wall structure and biosynthesis.42 On the one hand, the results obtained in this work support this finding; since vnlRAb is under the control of the ermEp* promoter, VnlRAb is constitutively expressed and activates the transcription of the vanHAXJKSc genes in S. coelicolor independent from the presence of any glycopeptide. The activation of vanHAXJKSc resulted in the synthesis of PG with pentapeptides ending on d-Ala-d-Lac depsipeptide, which are resistant against vancomycin and teicoplanin. On the other hand, the second explanation contradicts the work of Novotna et al.42; it is likely that VnlRAb activates the transcription of additional unknown genes, which mediate teicoplanin resistance.
The diverse functionality of VnlRAb in the glycopeptide producer A. balhimycina and in the nonproducer S. coelicolor provides the starting point of evolutionary analyses of glycopeptide resistance. In pathogenic bacteria and in S. coelicolor of glycopeptides, resistance is strictly regulated and is induced by the presence of glycopeptides. In contrast, glycopeptide producers overcome this regulation not only by the constitutive expression of the vanHAX genes but also by the development of a vanHAX-independent resistance mechanism. However, the ability of VnlRAb to activate transcription of vanHAX in S. coelicolor is an indication of a common origin of the three glycopeptide resistance mechanisms, the inducible one, the constitutively expressed, and the vanHAX-independent mechanism. Whether and how the complex resistance mechanism has evolved in the glycopeptide producers and whether and how it was transferred into resistance pathogens have to be subjects of future investigation.
Acknowledgments
The Deutsche Forschungsgemeinschaft (DFG) supported this work by the SFB 766 program TP-A03. The authors thank Matthew I. Hutchings, Hee-Jeon Hong, and Mark J. Buttner for providing the S. coelicolor J3201 mutant and Tobias Busche and Joern Kalinowski for the RNA-seq analyses. The authors further thank Vera Rosenberger and Tobias Martin for competent assistance during the experiments, Sigrid Stockert for technical support, and Günther Muth for sharing his excellent expertise in the research and writing process.
Disclosure Statement
The authors disclose that there are no commercial associations that might create a conflict of interest in connection with submitted manuscripts.
References
- 1.Laub M.T., and Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 2.Perry J., Koteva K., and Wright G. 2011. Receptor domains of two-component signal transduction systems. Mol. Biosyst. 7:1388–1398 [DOI] [PubMed] [Google Scholar]
- 3.Galperin M.Y. 2010. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 13:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur M., Molinas C., and Courvalin P. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 174:2582–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardete S., and Tomasz A. 2014. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Invest. 124:2836–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchings M.I., Hong H.J., and Buttner M.J. 2006. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 59:923–935 [DOI] [PubMed] [Google Scholar]
- 7.Cheung J., and Hendrickson W.A. 2010. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 13:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold H.S. 2001. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin. Infect. Dis. 33:210–219 [DOI] [PubMed] [Google Scholar]
- 9.Pootoolal J., Neu J., and Wright G.D. 2002. Glycopeptide antibiotic resistance. Annu. Rev. Pharmacol. Toxicol. 42:381–408 [DOI] [PubMed] [Google Scholar]
- 10.Xu X., Lin D., Yan G., Ye X., Wu S., Guo Y., Zhu D., Hu F., Zhang Y., Wang F., Jacoby G.A., and Wang M. 2010. vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob. Agents Chemother. 54:4643–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bugg T.D., Wright G.D., Dutka-Malen S., Arthur M., Courvalin P., and Walsh C.T. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 30:10408–10415 [DOI] [PubMed] [Google Scholar]
- 12.Billot-Klein D., Blanot D., Gutmann L., and van Heijenoort J. 1994. Association constants for the binding of vancomycin and teicoplanin to N-acetyl-D-alanyl-D-alanine and N-acetyl-D-alanyl-D-serine. Biochem. J. 304 (Pt 3):1021–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds P.E., Snaith H.A., Maguire A.J., Dutka-Malen S., and Courvalin P. 1994. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem. J. 301 (Pt 1):5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perichon B., Reynolds P., and Courvalin P. 1997. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob. Agents Chemother. 41:2016–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebreton F., Depardieu F., Bourdon N., Fines-Guyon M., Berger P., Camiade S., Leclercq R., Courvalin P., and Cattoir V. 2011. D-Ala-D-Ser VanN-type transferable vancomycin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 55:4606–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura T., Tanimoto K., Shibayama K., Arakawa Y., Fujimoto S., Ike Y., and Tomita H. 2012. Identification of VanN-type vancomycin resistance in an Enterococcus faecium isolate from chicken meat in Japan. Antimicrob. Agents Chemother. 56:6389–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foucault M.L., Courvalin P., and Grillot-Courvalin C. 2009. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2354–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foucault M.L., Depardieu F., Courvalin P., and Grillot-Courvalin C. 2010. Inducible expression eliminates the fitness cost of vancomycin resistance in enterococci. Proc. Natl. Acad. Sci. U S A 107:16964–16969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong H.J., Hutchings M.I., Neu J.M., Wright G.D., Paget M.S., and Buttner M.J. 2004. Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol. Microbiol. 52:1107–1121 [DOI] [PubMed] [Google Scholar]
- 20.Novotna G., Hill C., Vincent K., Liu C., Hong H.J. 2012. A novel membrane protein, VanJ, conferring resistance to teicoplanin. Antimicrob. Agents Chemother. 56:1784–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koteva K., Hong H.J., Wang X.D., Nazi I., Hughes D., Naldrett M.J, Buttner M.J., and Wright G.D. 2010. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 6:327–329 [DOI] [PubMed] [Google Scholar]
- 22.Kwun M.J., Novotna G., Hesketh A.R., Hill L., and Hong H.-J. 2013. In vivo studies suggest that induction of VanS-dependent vancomycin resistance requires binding of the drug to D-Ala-D-Ala termini in the peptidoglycan cell wall. Antimicrob. Agents. Chemother. 57:4470–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee S., Vijayakumar E.K.S., Nadkarni S.R., Patel M.V., Blumbach J., Ganguli B.N., Fehlhaber H.W., Kogler H., and Vertesy L. 1994. Balhimycin, a New Glycopeptide Antibiotic with an unusual hydrated 3-amino-4-oxoaldopyranose sugar moiety. J. Org. Chem. 59:3480–3484 [Google Scholar]
- 24.Nadkarni S.R., Patel M.V., Chatterjee S., Vijayakumar E.K., Desikan K.R., Blumbach J., Ganguli B.N., and Limbert M. 1994. Balhimycin, a new glycopeptide antibiotic produced by Amycolatopsis sp. Y-86,21022. Taxonomy, production, isolation and biological activity. J. Antibiot. (Tokyo). 47:334–341 [DOI] [PubMed] [Google Scholar]
- 25.Pelzer S., Sussmuth R., Heckmann D., Recktenwald J., Huber P., Jung G., and Wohlleben W. 1999. Identification and analysis of the balhimycin biosynthetic gene cluster and its use for manipulating glycopeptide biosynthesis in Amycolatopsis mediterranei DSM5908. Antimicrob. Agents Chemother. 43:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shawky R.M., Puk O., Wietuorrek A., Pelzer S., Takano E., Wohlleben W., Stegmann E. 2007. The border sequence of the balhimycin biosynthetic gene cluster from Amycolatopsis balhimicina contains bbr, encoding a StrR-like pathway-specific regulator. J. Mol. Microbiol. Biotechnol. 13:76–88 [DOI] [PubMed] [Google Scholar]
- 27.Schäberle T.F., Vollmer W., Frasch H.J., Huttel S., Kulik A., Rottgen M., von Thaler A.K., Wohlleben W., and Stegmann E. 2011. Self-resistance and cell wall composition in the glycopeptide producer Amycolatopsis balhimycina. Antimicrob. Agents Chemother. 55:4283–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullock W.O., Fernandez J.M., and Short J.M. 1987. Xl1-Blue—a high-efficiency plasmid transforming Reca Escherichia-Coli strain with beta-galactosidase selection. Biotechniques. 5:376–378 [Google Scholar]
- 29.MacNeil D.J., Gewain K.M., Ruby C.L., Dezeny G., Gibbons P.H., and MacNeil T. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 111:61–68 [DOI] [PubMed] [Google Scholar]
- 30.Kieser T., Bibb M.J., Buttner M.J., Chater K.F., and Hopwood D.A. 2000. Practical Streptomyces Genetics: John Innes Foundation, Norwich Research Park, Colney, Norwich, United Kingdom [Google Scholar]
- 31.Menges R., Muth G., Wohlleben W., and Stegmann E. 2007. The ABC transporter Tba of Amycolatopsis balhimycina is required for efficient export of the glycopeptide antibiotic balhimycin. Appl. Microbiol. Biotechnol. 77:125–134 [DOI] [PubMed] [Google Scholar]
- 32.Pelzer S., Reichert W., Huppert M., Heckmann D., and Wohlleben W. 1997. Cloning and analysis of a peptide synthetase gene of the balhimycin producer Amycolatopsis mediterranei DSM5908 and development of a gene disruption/replacement system. J. Biotechnol. 56:115–128 [DOI] [PubMed] [Google Scholar]
- 33.Ho S.N., Hunt H.D., Horton R.M., Pullen J.K., and Pease L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 77:51–59 [DOI] [PubMed] [Google Scholar]
- 34.Flett F., Mersinias V., and Smith C.P. 1997. High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol. Lett. 155:223–229 [DOI] [PubMed] [Google Scholar]
- 35.Cohen S.N., Chang A.C., and Hsu L. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA. 69:2110–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison D.A. 1979. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 68:326–331 [DOI] [PubMed] [Google Scholar]
- 37.Madon J., and Hutter R. 1991. Transformation system for Amycolatopsis (Nocardia) mediterranei: direct transformation of mycelium with plasmid DNA. J. Bacteriol. 173:6325–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puk O., Bischoff D., Kittel C., Pelzer S., Weist S., Stegmann E., Sussmuth R.D., and Wohlleben W. 2004. Biosynthesis of chloro-beta-hydroxytyrosine, a nonproteinogenic amino acid of the peptidic backbone of glycopeptide antibiotics. J. Bacteriol. 186:6093–6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice P., Longden I., and Bleasby A. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. TIG. 16:276–277 [DOI] [PubMed] [Google Scholar]
- 40.Frasch H.J., Kalan L., Kilian R., Martin T., Wright G., and Stegmann E. 2015. Alternative pathway to a glycopeptide-resistant cell wall in the balhimycin producer Amycolatopsis balhimycina. ACS Infect. Dis. 1:243–252 [DOI] [PubMed] [Google Scholar]
- 41.Burton K. 1956. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novotna G.B., Kwun M.J., and Hong H.J. 2015. In vivo characterization of the activation and interaction of the VanR-VanS two-component regulatory system controlling glycopeptide antibiotic resistance in two related Streptomyces species. Antimicrob. Agents Chemother. 60:1627–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino K., and Yamaguchi A. 2001. Overexpression of the response regulator EvgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 183:1455–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin M.F., Lin Y.Y., Yeh H.W., and Lan C.Y. 2014. Role of the BaeSR two-component system in the regulation of Acinetobacter baumannii adeAB genes and its correlation with tigecycline susceptibility. BMC Microbiol. 14:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegmann E., Frasch H.J., Kilian R., and Pozzi R. 2015. Self-resistance mechanisms of actinomycetes producing lipid II-targeting antibiotics. Int. J. Med. Microbiol. 305:190–195 [DOI] [PubMed] [Google Scholar]
- 46.Wright G.D., Molinas C., Arthur M., Courvalin P., and Walsh C.T. 1992. Characterization of vanY, a DD-carboxypeptidase from vancomycin-resistant Enterococcus faecium BM4147. Antimicrob. Agents Chemother. 36:1514–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hugonnet J.E., Haddache N., Veckerle C., Dubost L., Marie A., Shikura N., Mainardi J.L., Rice L.B., and Arthur M. 2014. Peptidoglycan cross-linking in glycopeptide-resistant Actinomycetales. Antimicrob. Agents Chemother. 58:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcone G.L., Beltrametti F., Binda E., Carrano L., Foulston L., Hesketh A., Bibb M., and Marinelli F. 2010. Novel mechanism of glycopeptide resistance in the A40926 producer Nonomuraea sp. ATCC 39727. Antimicrob. Agents Chemother. 54:2465–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]