Abstract
Pelvic injuries are not uncommon. The complex anatomy of the pelvic bones, the complex pattern of injuries, associated important structures such as neurovascular bundles, and difficult access make the reduction and fixation of these fractures difficult. Often the surgical outcomes are not satisfactory. Three-dimensional (3D) imaging using computed tomography (CT) scan (3DCT) has been the mainstay of preoperative evaluation since the 1980s, however, even with these images it may be difficult to understand complex injury patterns. Preoperative printing of a 3D model using the same CT scan data allows surgeons to hold the pelvis in their hands and then plan appropriate treatment. We report one such case of complex pelvic injury and its management using the novel method of preoperative 3D model printing.
Key words: 3D printing, fracture, preoperative planning, pelvis
Introduction
Pelvic injuries are not uncommon, especially in hospitals catering to high-impact trauma.[1] The primary aim in treating pelvic injuries is to restore the integrity of the pelvic ring and achieve symmetry. Treating these injuries poses many difficulties and challenges because of the complex anatomy, the complex patterns of these injuries, associated important structures such as the neurovascular bundles, and the difficulty in accessing these sites for surgical fixation. Hence, the final outcome may not be satisfactory.
Routine imaging with computed tomography (CT) scan gives only a two-dimensional idea of the anatomy of the pelvis and the injuries. Three-dimensional (3D) reconstruction using CT scans (3DCT) provides more detail and orientation, and has been the mainstay of evaluation for the last 25 years.[2] However, for the best possible operative outcome, it is of utmost importance to understand the anatomy clearly from all dimensions.
3D printing of bone pathology allows surgeons to evaluate fracture patterns and pathology in vitro in detail. By holding the models in their hands, surgeons are able to get a clear perspective of the pathology, without having to imagine the abnormality in their minds, using computer-based 3D data. These models also help better communicate the pathology with patients and relatives and eventually lead to better surgical outcomes, reduced operation times, and reduced postoperative complications.[3]
Case History
A 45-year-old man presented with a history of road traffic accident and pelvic injury. A plain radiograph of the pelvis was obtained that showed a fracture of the right acetabulum [Figure 1]. A CT scan of the pelvis was then performed and confirmed a comminuted fracture of the right acetabulum, associated with protrusion acetabuli [Figure 2] with a transverse and posterior column fracture pattern. There was no fracture involving the proximal femur. Hence, surgery was planned.
Figure 1.
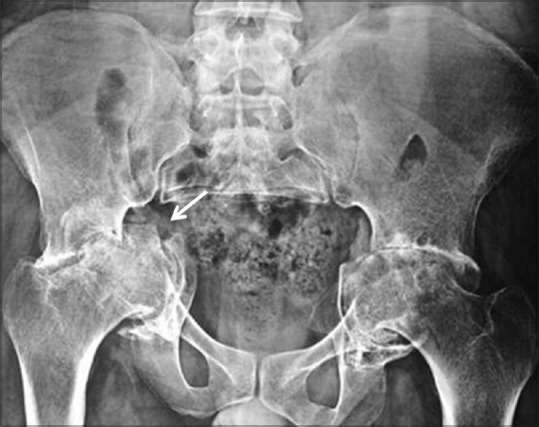
Frontal radiograph of the pelvis with both hips shows a displaced tranverse fracture (arrow) involving the right acetabulum with protrusion acetabuli
Figure 2 (A-C).
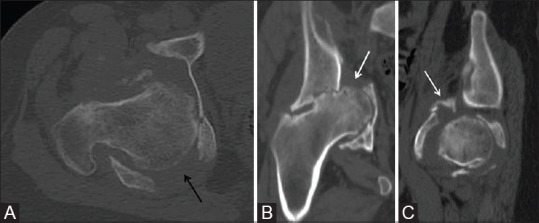
Computed tomography scan of the right hemipelvis. Axial (A), coronal (B), and sagittal (C) images demonstrate a comminuted fracture with a transverse (white arrow) and posterior column (black arrow) pattern of injury involving the right acetabulum
However, the CT scan images including the 3DCT reconstructions [Figure 3] did not provide sufficient information to understand the anatomy of the comminuted fracture. Hence, a 3D model of the pelvis with both hips was prepared to better understand the anatomy and pathology [Figures 4 and 5].
Figure 3 (A-D).
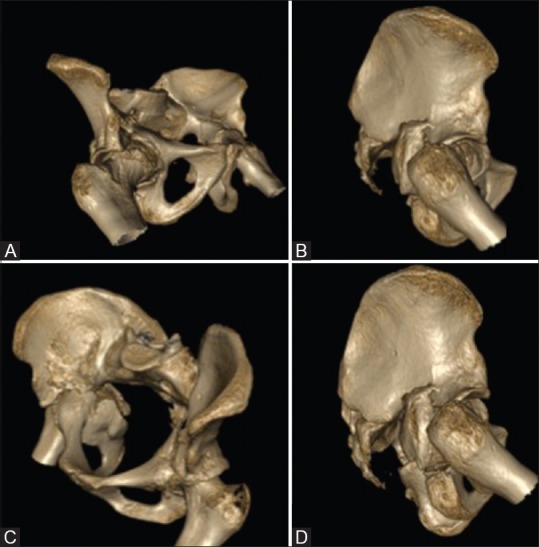
(A-D) Three-dimensional computed tomography scan reconstructions of the pelvis with both hips in various projections showing the acetabular fracture patterns well
Figure 4 (A-D).
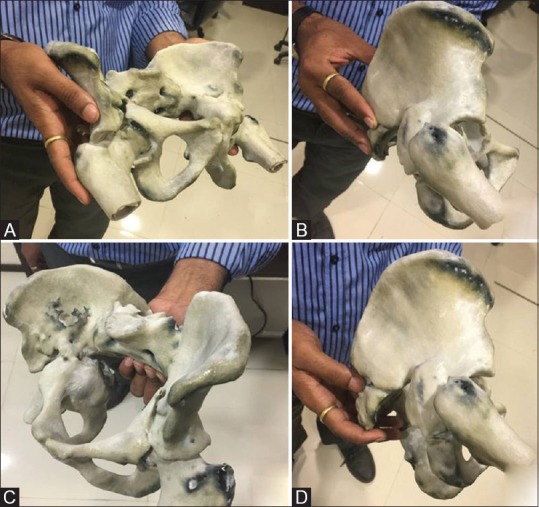
(A-D) Images of the three-dimensional printed model of pelvis with both hips, held in the hand of the surgeon and rotated in real-time to understand the acetabular fracture pattern and plan the treatment on the model prior to the actual surgery
Figure 5 (A and B).
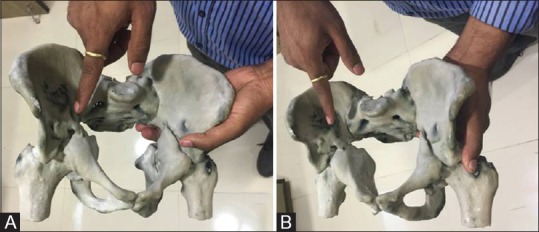
(A and B) Images of the three-dimensional printed model of pelvis with both hips, held in the hand with the surgeon pointing at the acetabular fracture seen very well
For the purpose of 3D model printing, the axial CT scan data was reconstructed into 0.75 mm thin slices at 0.5 mm intervals. The DICOM images were then converted into the standard triangular language (STL) format that is used for rapid prototyping. The STL file was then loaded on a commercial 3D printer (Projet 660, 3D Systems, USA) workstation, and the model was created in approximately 4 h and 30 min. The material used was a proprietary powder that is bound by a chemical solution to create a bone-like model.
The model was then delivered to the surgeon. Holding the model in his hand and by viewing the fracture pattern from all possible angles, the surgeon was able to successfully plan the exact surgery for this particular injury [Figures 4–6], as against merely viewing the 3D images on a workstation or on film.
Figure 6.
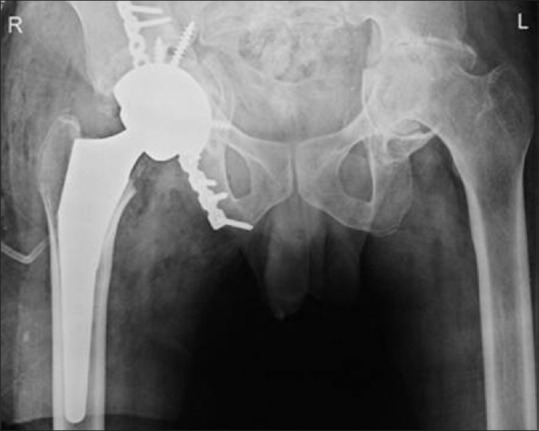
Postoperative frontal radiograph of the pelvis with both hips shows screw and plate fixation of the right acetabular fracture with total hip replacement
The surgeon was able to accurately classify the fracture only after carefully observing the injury on the model and rotating the model in all directions. The complex pattern of injury involving both the transverse and posterior column pattern of fracture was confirmed with the careful assessment of the fracture on the model. The various fracture fragments and their relationship with each other was easily and accurately understood because of the 3D model.
Long-term function depends on achieving good reduction, which in turn depends on accurately classifying the acetabular fracture pattern. Thus, it was possible to achieve good fracture reduction because the fracture pattern was accurately classified. It has been proven that a 3D model is more accurate in the assessment and classification of the acetabular fracture than radiographs and CT scans alone.[4]
Following are the advantages the surgeon got in this case by using the 3D model than the routine 3D reconstructions of CT scan images:
By holding the model in his hand, the surgeon was more confident of the pattern of acetabular fracture and the relationships of the fracture fragments to each other
This led to the accurate classification of this acetabular fracture as both transverse and posterior column fracture, which was confusing on routine 3D CT images
As there were comminuted fracture fragment and the 3D model clearly showed extension of the fracture medially in the acetabulum, the surgical plan was changed. Eventually total hip replacement (THR) was planned because there was significant risk of medial migration of the femoral head after surgery, even if good reduction was achieved. Without the 3D model, only reduction of the fracture would have been performed which would have worsened the situation over time, ultimately requiring THR
Because the surgeon had already taken preoperative measurements on the model and decided on the exact size of the instruments used in the surgery, this led to significant reduction in the intraoperative time by approximately 25 min
The surgeon could also easily explain the pattern of injury and the surgical plan to the patients and relatives and conveniently with the 3D model held in his hand.
Hence, overall, in this case, the surgeon could manage the acetabular fracture effectively and easily with the use of 3D model than he would have with routine 3D CT images alone.
Discussion
Pelvic injuries are usually complex, with comminution, displacement, and rotation abnormalities. These lead to various deformities including shortening and poor morbidity if not properly treated.[5] The primary aim in treating pelvic injuries is to restore the integrity of the pelvic ring and to achieve symmetry. For this, good reduction and fixation of the fractures are essential, but often difficult to perform. The trauma may also lead to various complications including deformities and injuries to the adjacent nerves and blood vessels.[6]
Hence, preoperative planning is crucial to achieve the best possible results. So far, routine 3D CT imaging has been the gold standard for preoperative planning.[2] However, with the advent of 3D printing, new doors have opened for better preoperative assessment and surgical outcomes. A better understanding of the pathology leads to reduced operative time, improved surgical efficiency, and reduced iatrogenic complications.[3]
There are various methods of rapid prototyping for 3D printing. These include STL, selective laser sintering, fused deposition modelling, and multijet modelling.[7] We used the STL method for printing 3D models with the ability to add colour using the .zpr format. Various materials including bone-like powder, plastic, ceramic, and metal are available, all of which are machine specific.[7]
Thin <0.75 mm CT scan slices are required to produce high-resolution 3D models with sharp details. A 3D model is created in the traditional way on the workstation and then converted to a.stl or .zpr file. Printing time varies from 3–6 h depending on the vertical height of the model. The models thus printed are air-dried and fixed using different fixatives such as varnish or proprietary solutions.
Because these models are life-size, distance between various anatomic landmarks can be measured and compared with the CT data and the intraoperative measurements to assess the accuracy of the 3D model. The models are usually kept in the operating theatre to allow a real-time understanding of the pathology.
There are many advantages of 3D printing.
The surgeon can hold the model in his/her hand
This helps the surgeon to understand the complex anatomy and obtaina 3D orientation of the pathology
The surgeon can also practice the planned surgery on the model and assess the result. The surgeon can perform reduction followed by fixation to see the final outcome and can then modify the plan for better results. Osteotomy, wedge resection, screw, wires, and plate fixation can all be performed on the model and the implant or prosthesis can also be designed accordingly, if required
This also allows reduction in surgical time by up to 17%[8]
These models can also be used as educational aids for surgeons and students.
3D printing has a role to play in many different fields apart from orthopedics, such as maxillofacial surgery,[8] otorhinolaryngology,[9,10] neurosurgery,[11,12] and cardiovascular interventions[13] where preoperative printing of models is of immense help to surgeons for better surgical planning. 3D printing can be used not only in cases of trauma, as presented in this case report, but also in various complex congenital anomalies as well as tumors for preoperative planning.
There are a few limitations and drawbacks associated with the use of 3D models in preoperative planning. Different materials are needed for different organ systems. The same bone-like material may not be ideal when it comes to 3D printing of the heart or vessels, where soft plastic-based materials may be better. There is also limited data on the use of 3D models in medical practice.
The models created with proprietary powder are also fragile and can break while taking the model out from the tray, during fixation process, transportation, and also while handling. Hence, careful handling and effectively protecting the model while transportation is a must to prevent the model from breaking.
The other limitations are the resolution and accuracy of the printed 3D model.[14] The resolution of the 3D model depends upon the resolution of the scan, printer, and the material used for printing. Thus, to achieve better resolution the scan must be of high resolution with thin slices, as specified earlier, and the printer as well as the powder used for printing must be capable of generating high resolution models. The accuracy of the printed models depends on the printer, material, and the method of printing. With proper calibration and maintenance of the printer, higher accuracy can be achieved.
Cost is also a major limiting factor at present. The cost of the 3D model depends on the size of the part to be printed, the material used for printing, and the cost of operating the printer.[14] Printing life-size model increases the cost, hence when not required, small-sized models can be printed to reduce the cost, without significantly affecting the resolution or the accuracy.
There are some concerns regarding the safety as well. A recent study suggests emission of ultrafine particles from the 3D printers.[15] These small sized particles can pass into the smaller airways and get deposited in the lungs as well as in the brain and can lead to pulmonary and neurological complaints. Most printers have filters to reduce these particles and fumes. In addition, proper ventilation and personal safety measures such as wearing mask and gloves while operating the printer are a must to reduce these complications. The high voltage used for printing may also result in hot surfaces.
Thus, overall, fragility, resolution, accuracy, cost and health hazards are potential drawbacks of using 3D printing. However, with the advancement in technology and availability of better printing techniques, high resolution printers, and new materials for printing, all these issues can be effectively addressed in future.
3D printing, though very effective for preoperative planning, has not yet been adopted widely in medical practice, especially in a country like India. Multiple issues are involved such as lack of awareness, reservation of surgeons, lack of availability, and cost. However, with better understanding of the usefulness of 3D printing and increased awareness it is likely that the utility of this technique will increase.
Conclusion
3D printing of bone models is a feasible and effective method for preoperative planning of complex pelvic injuries. A 3D printed model can be readily printed within 24 h and be part of a routine 3DCT protocol. A 3D printed model helps understand the complex anatomy and pathology of bone lesions better, leading to overall improved surgical outcome and better quality of life for the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Demetriades D, Karaiskakis M, Toutouzas K, Alo K, Velmahos G, Chan L. Pelvic fractures: Epidemiology and predictors of associated abdominal injuries and outcomes. J Am CollSurg. 2002;195:1–10. doi: 10.1016/s1072-7515(02)01197-3. [DOI] [PubMed] [Google Scholar]
- 2.Jankharia B, Shroff M, Shah S. Three-dimensional computed tomography in acetabular trauma. Ind J RadiolImag. 1992;2:97–106. [Google Scholar]
- 3.Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatricorthopedic disorders. PedRadiol. 2014;44:216–21. doi: 10.1007/s00247-013-2788-9. [DOI] [PubMed] [Google Scholar]
- 4.Hurson C, Tansey A, O'Donnchadha B, Nicholson P, Rice J, McElwain J. Rapid prototyping in the assessment, classification and preoperative planning of acetabular fractures. Injury. 2007;38:1158–62. doi: 10.1016/j.injury.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 5.McLaren AC, Rorabeck CH, Halpenny J. Long-term pain and disability in relation to residual deformity after displaced pelvic ring fractures. Canadian J Surg. 1990;33:492–4. [PubMed] [Google Scholar]
- 6.Poole GV, Ward EF, Griswold JA, Muakkassa FF, Hsu HS. Complications of pelvic fractures from blunt trauma. Am Surg. 1992;58:225–31. [PubMed] [Google Scholar]
- 7.Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196:W683–8. doi: 10.2214/AJR.10.5681. [DOI] [PubMed] [Google Scholar]
- 8.D'Urso PS, Barker TM, Earwaker WJ, Bruce LJ, Atkinson RL, Lanigan MW, et al. Stereolithographicbiomodelling in cranio-maxillofacial surgery: A prospective trial. J CranioMaxFacialSurg. 1999;27:30–7. doi: 10.1016/s1010-5182(99)80007-9. [DOI] [PubMed] [Google Scholar]
- 9.Karayazgan-Saracoglu B, Gunay Y, Atay A. Fabrication of an auricular prosthesis using computed tomography and rapid prototyping technique. J CraniofacSurg. 2009;20:1169–72. doi: 10.1097/SCS.0b013e3181acdb95. [DOI] [PubMed] [Google Scholar]
- 10.Rose AS, Kimbell JS, Webster CE, Harrysson OL, Formeister EJ, Buchman CA. Multi-material 3D Models for temporal bone surgical simulation. Ann OtolLaryngolRhinol. 2015;124:528–36. doi: 10.1177/0003489415570937. [DOI] [PubMed] [Google Scholar]
- 11.Maravelakis E, David K, Antoniadis A, Manios A, Bilalis N, Papaharilaou Y. Reverse engineering techniques for cranioplasty: A case study. J Med EngTechnol. 2008;32:115–21. doi: 10.1080/03091900600700749. [DOI] [PubMed] [Google Scholar]
- 12.Muller A, Krishnan KG, Uhl E, Mast G. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. J CraniofacSurg. 2003;14:899–914. doi: 10.1097/00001665-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Schievano S, Migliavacca F, Coats L, Khambadkone S, Carminati M, Wilson N, et al. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology. 2007;242:490–7. doi: 10.1148/radiol.2422051994. [DOI] [PubMed] [Google Scholar]
- 14.Fredieu J, Kerbo J, Herron M, Klatte R, Cooke M. Anatomical Models: A Digital Revolution. Medical science educator. 2015;25:183–94. [Google Scholar]
- 15.Stephens B, Azimi P, El Orch Z, Ramos T. Ultrafine particle emissions from desktop 3D printers. Atmos Environ. 2013;79:334–9. [Google Scholar]


