Abstract
Alkyne and azide analogs of natural compounds that can be coupled to sensitive tags by click chemistry are powerful tools to study biological processes. Arachidonic acid (AA) is a FA precursor to biologically active compounds. 19-Alkyne-AA (AA-alk) is a sensitive clickable AA analog; however, its use as a surrogate to study AA metabolism requires further evaluation. In this study, AA-alk metabolism was compared with that of AA in human cells. Jurkat cell uptake of AA was 2-fold greater than that of AA-alk, but significantly more AA-Alk was elongated to 22:4. AA and AA-alk incorporation into and remodeling between phospholipid (PL) classes was identical indicating equivalent CoA-independent AA-PL remodeling. Platelets stimulated in the presence of AA-alk synthesized significantly less 12-lipoxygenase (12-LOX) and cyclooxygenase products than in the presence of AA. Ionophore-stimulated neutrophils produced significantly more 5-LOX products in the presence of AA-alk than AA. Neutrophils stimulated with only exogenous AA-alk produced significantly less 5-LOX products compared with AA, and leukotriene B4 (LTB4)-alk was 12-fold less potent at stimulating neutrophil migration than LTB4, collectively indicative of weaker leukotriene B4 receptor 1 agonist activity of LTB4-alk. Overall, these results suggest that the use of AA-alk as a surrogate for the study of AA metabolism should be carried out with caution.
Keywords: cyclooxygenase, lipoxygenase, eicosanoid, phospholipid remodeling, leukotriene
The click chemistry reactions that easily conjugate molecules together are now widely used to discover new drugs and inhibitor targets (1–4). Furthermore, alkyne and azide analogs of naturally occurring compounds that have minimal structure modifications and that can be coupled to sensitive tags by click chemistry are powerful emerging tools to study biological processes (5, 6). A large number of alkyne and azide analogs and tags have been described, and these are very practical tools that can replace radioactive tracers in many applications. Alkyne-lipid analogs have been shown to be particularly useful for the isolation and identification of individual species of lipids from complex mixtures and for profiling protein lipidation because of the ability to specifically extract labeled compounds (7–12).
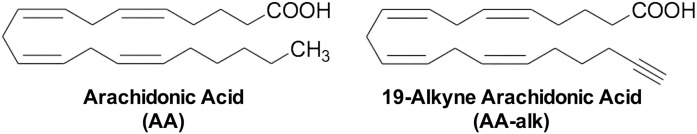
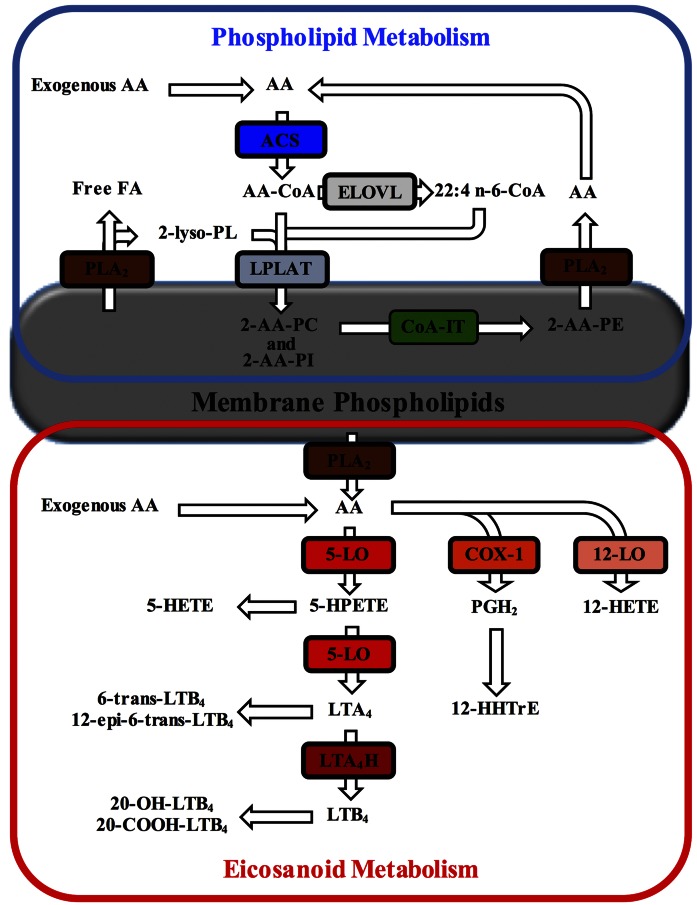
Clickable lipid analogs, including alkyne FAs, have proved to be very useful as surrogates to study FA metabolism and FA protein interactions in complex mixtures (12–17). Among these, 19-alkyne arachidonic acid (AA-alk) (Fig. 1) has been suggested to be a sensitive probe for the study of cellular arachidonic acid (AA) metabolism (17, 18). AA is the PUFA precursor to a number of potent biologically active molecules such as prostaglandins and leukotrienes; thus this probe may serve as a suitable tracker for cellular AA metabolism that is under tight regulation (Fig. 2). However, prior to utilizing any new analog as a surrogate in metabolic studies, it is important to make sure that its metabolism and regulation resembles that of AA itself. Mammalian cells cannot synthesize AA de novo and must obtain this essential FA from exogenous sources as intact AA or as one of its precursors. Cells mainly store AA in the sn-2 position of membrane PLs, although AA can also be elongated to 22:4 n-6 prior to its incorporation into PLs. AA undergoes a specific pattern of incorporation into PLs where initial acylation is primarily in PC and PI resulting from reactions catalyzed by ACSs and lyso-PLATs. Once AA is incorporated into PC species, it is then transferred primarily into 1-radyl PE species by a CoA-IT-catalyzed reaction (19–24).
Fig. 1.
The structures of AA and its clickable analog AA-alk.
Fig. 2.
Schematic representation of cellular AA incorporation and metabolism. Once incorporated into cells, free AA is activated by acyl-CoA synthetases (ACSs) to produce the AA-CoA required for its incorporation into phospholipids (PLs) by the action of lysophospholipid acyl-CoA acyltransferases (lyso-PLATs). The action of phospholipase A2 (PLA2) is required to generate the 2-lyso-PL substrate. AA-CoA can also be elongated to 22:4 n-6 following the action of elongases of very long chain FAs (ELOVL). Once incorporated into PLs, AA can also be directly transferred between PL species by CoA-independent transacylase (CoA-IT)-catalyzed reactions. Upon cell stimulation, PLA2 catalyzes the hydrolysis of AA from PLs, which can be converted into various lipid mediators (eicosanoids) by the action of cyclooxygenases (COXs) and lipoxygenases (LOXs). 12-HHTrE, 12-hydroxyheptadecatrienoic acid; LTA4H, leukotriene A4 hydrolase; LTB4, leukotriene B4; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PGH2, prostaglandin H2; PI, phosphatidylinositol.
Upon appropriate cell stimulation, AA can be hydrolyzed from PL by PLA2 and can be converted by LOXs and COXs into many different bioactive lipid mediators, called eicosanoids, which include HETEs, leukotrienes, prostaglandins, and lipoxins (24–27). These enzymes can also catalyze the conversion of exogenous AA. The enzymes expressed in any particular cell type dictate the bioactive lipid product profile. Significantly, these compounds are important lipid mediators of inflammation and have been shown to participate in the maintenance of homeostasis, including host immunity, as well as in numerous pathologies (27–29).
The aim of this study is to evaluate whether AA-alk is a suitable traceable analog of AA for studying cellular arachidonate-PL and eicosanoid metabolism. Overall, the results suggest that AA-alk may be a good surrogate for studying aspects of cellular AA-PL metabolism but may not be suitable for studies in bioactive lipid mediator production and activity.
MATERIALS AND METHODS
Reagents
Boron trifluoride (14% in methanol) was obtained from Sigma-Aldrich (Oakville, ON, Canada). The [3H]AA was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO). The 1,2-diheptadecanoyl-PC was from Biolynx (Brockville, ON, Canada). FA methyl esters (FAMEs) and FFAs were obtained from Nu-check Prep (Elysian, MN). AA-alk was purchased from NuChem Thérapeutiques Inc. (Montreal, QC, Canada).
Cell culture and pulse labeling (remodeling)
Jurkat cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 10 mM HEPES, d-glucose (to 25 mM) and 1 mM sodium pyruvate at 37°C in a 5% CO2 atmosphere. For FA incorporation studies, Jurkat cells were incubated in the presence of 20 μM AA or 20 μM AA-alk for 2 h at 37°C. Cells were then washed twice with culture medium, and cellular lipids were extracted in chloroform using heptadecanoyl-PC as an internal standard (30). For pulse-labeling experiments, Jurkat cells (6 × 107) were pulse labeled in 3 ml of culture medium (2% FBS) containing 20 μM [3H]AA or 20 μM AA-alk for 2 h at 37°C. Cells were then washed twice with culture medium and incubated for another 0, 4, or 24 h before cellular lipid extraction. Cellular lipids were extracted in chloroform (30), PL classes were separated by reverse phase-HPLC (RP-HPLC) (31), and fractions containing neutral lipids, PE, PI, phosphatidylserine (PS), and PC were collected using elution times determined with PL standards. The internal standard heptadecanoyl-PC was added to each fraction prior to further analyses.
FA analysis
Cellular lipid extracts or HPLC fractions were dried and saponified with 400 µl of 0.5 M KOH in methanol at 100°C for 15 min, and FAMEs were then prepared by adding 1 ml of 14% BF3 in methanol and heating at 100°C for 10 min. FAMEs were extracted in hexane and quantified by gas chromatography with flame ionization detection (GC/FID) using a 30 m trace-FAME column on a Thermo Trace gas chromatograph (Thermo Electron Corporation, Mississauga, ON, Canada) (32). Authentic FAME standards were used for the identification of FA peak retention times and for standard curve quantification. For pulse-label studies, the radioactivity was also measured in each fraction by liquid scintillation counting (Beckman Instruments LS 5000 CE).
To confirm the identity of the 22:4-alkyne, FAMEs were analyzed and measured by positive ion chemical ionization GC/MS using a Polaris Q mass spectrometer (Thermo). The positive chemical ionization ion trap scan was 300–350 u, the reagent gas was methane (0.6 ml/min, 180°C), and helium was the damping gas (0.3 ml/min).
Preparation and stimulation of human platelets
Platelets were isolated from heparinized blood obtained from healthy donors as previously described (33). Briefly, blood was centrifuged at 200 g for 10 min at room temperature. The platelet-rich plasma fraction (upper phase) was collected and centrifuged at 400 g for 2 min to remove remaining erythrocytes. Platelets were then pelleted following centrifugation at 1,300 g for 10 min, and platelets (300 × 106 cells/ml) were resuspended in Tyrode buffer (134 mM NaCl, 2.9 mM KCl, 20 mM HEPES, 5 mM CaCl2, 1 mM MgCl2, 5 mM glucose, 0.34 mM Na2HPO4, 12 mM NaHCO3, and 0.5 mg/ml BSA, pH 7.4). Stimulation of platelets was initiated with the addition of 10 μM calcium ionophore A23187 (Sigma-Aldrich) in the presence of 10 μM AA or 10 μM AA-alk for 15 min at 37°C. Stimulations were stopped with the addition of 2 vol of cold methanol containing 50 ng of 19-OH-prostaglandin B2 (PGB2; internal standard), and samples were stored at −20°C prior to analysis by RP-HPLC.
Preparation and stimulation of human neutrophils
Neutrophils were isolated from heparinized blood obtained from healthy donors as previously described (34). Briefly, blood was centrifuged at 200 g for 10 min at room temperature, the platelet-rich plasma was discarded, and erythrocytes were removed following dextran sedimentation. Following centrifugation on a lymphocyte separation medium cushion (density, 1.077 g/ml) (Wisent, St. Bruno, QC, Canada) at 900 g for 20 min at room temperature, mononuclear cells were eliminated, and neutrophils (>96%) were obtained from the pellet after hypotonic lysis in purified water to eliminate contaminating erythrocytes. Neutrophils suspended in HBSS (1 × 107 cells/ml) containing 1.6 mM CaCl2 and 0.4 U/ml of adenosine deaminase were stimulated with 10 μM calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk for 5 min at 37°C. For autocrine loop experiments, neutrophils were stimulated with varying concentrations of AA or AA-alk for 5 min at 37°C (35). All neutrophil stimulations were stopped with the addition of 0.5 vol of cold methanol containing 25 ng of 19-OH-PGB2, and samples were stored at −20°C prior to analysis.
Preparation and stimulation of HEK293 cells
HEK293 cells that were stably transfected to express human 5-LOX and human 5-LOX activating protein (FLAP) (36, 37) were cultured in DMEM medium supplemented with 10% FBS at 37°C in a humidified 5% CO2 environment. Cells were washed, suspended in HBSS (1 × 107 cells/ml) containing 1.6 mM CaCl2, and stimulated with 10 μM calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk for 15 min at 37°C. Stimulations were stopped with the addition of 0.5 vol of cold methanol containing 25 ng of 19-OH-PGB2, and samples were stored at −20°C prior to analysis by RP-HPLC.
Eicosanoid analysis by RP-HPLC
Samples were centrifuged at 300 g to remove precipitated proteins, and the supernatants were diluted with water to obtain a final methanol content of 20% (v/v). Samples were then subjected to in-line solid phase extraction and RP-HPLC analysis with UV detection optimized to separate LOX products as previously described (38) with some variations. Briefly, samples were injected onto an Agilent 1100 HPLC equipped with an Oasis HLB online cartridge column (3.9 × 20 mm, 15 μm particle size; Waters, Milford, MA) for in-line extraction using 0.1% acetic acid as mobile phase at a flow rate of 3 ml/min for 3 min. The solvent was then changed over 0.1 min to solvent A (54% water:23% methanol:23% acetonitrile:0.0025% H3PO4), and a Rheodyne® valve was switched to direct the flow to a Chromolith® HighResolution RP-18 end-capped column (100 × 4.6 mm) (EMD Millipore, Etobicoke, ON, Canada) at a flow rate of 2.2 ml/min. After 5.11 min, the mobile phase was then changed to 85% solvent A and 15% solvent B (5% water:32% methanol:63% acetonitrile:0.01% H3PO4) for 1 min, followed by a linear gradient to 55% solvent A and 45% solvent B over the next 0.3 min, and held at that proportion for an additional 1.3 min. The gradient was then changed in a linear fashion to 30% solvent A and 70% solvent B over a 1.3 min period and held for an additional 1.3 min at which time the mobile phase was changed to 100% solvent B over 0.2 min and held for 3.5 min. Peaks were quantified by absorbance at 236 nm and 270 nm using a diode array detector.
LC/MS/MS analyses
Selected peaks eluting from the above-mentioned HPLC analyses were collected for further characterization by MS. LC/MS/MS analysis was performed using a Dionex Ultimate 3000 liquid chromatograph coupled to a Thermo-Fisher Scientific Linear Ion Trap (LTQ-XL) using a Hypersil Gold C18 column (150 mm × 2.1 mm inner diameter) with a solvent gradient of 50% to 100% methanol (solvent B) over 40 min at a flow rate of 100 μl/min. Solvent A consisted of water, and both solvents were of HPLC grade with no buffer additives. The sample injection volume was constant for all samples at 5 μl. The mass spectrometer was operated in negative ion mode with LC/MS spectra collected in full scan mode over an m/z range of 200–800 and LC/MS/MS spectra collected from 90 to 400. The MS/MS collision energy was set to 35% with an isolation mass width of 3. Interface parameters for the mass spectrometer were as follows: sheath gas (15, arbitrary units), auxiliary gas (1, arbitrary units), capillary temperature (250°C), capillary voltage (−45 volts), and tube lens voltage (−150 volts). Full scan MS and MS/MS spectra were collected for separate LC injections of 5 μl.
Neutrophil migration assay
In order to produce LTB4 and LTB4-alk for functional studies, HEK293 cells that were stably transfected to express 5-LOX and FLAP (36, 37) were stimulated with A23187 in the presence of 40 μM of AA or AA-alk, respectively. 5-LOX products were separated by RP-HPLC as described above, and the LTB4 and LTB4-alk peaks were collected, dried, and resuspended in ethanol. Control experiments were performed with nontransfected HEK293 cells that do not express 5-LOX and FLAP and do not produce 5-LOX products (36).
To measure the chemoattractant activity of LTB4 and LTB4-alk, 200 µl of neutrophil suspension (2.5 × 106 cells/ml HBSS containing 1.6 mM CaCl2 and 5% FBS) preincubated with 0.3 U/ml adenosine deaminase were transferred to cell culture inserts (3.0 µm pore size; Falcon). Neutrophils were allowed to migrate (2 h, 37°C, 5% CO2) to the lower chamber containing 700 µl HBSS/1.6 mM CaCl2 along with 10 nM of LTB4-alk, LTB4, or diluent as negative control. Inserts were then discarded, and cells that had migrated to the lower chamber were counted using the MOXI Z Mini automated cell counter (Orflo Technologies, Ketchum, ID). Calculations were performed as previously described (39).
Statistical analyses
Statistical analyses were performed using Prism software (GraphPad Software Inc., La Jolla, CA) as described in the figure legends. Data show the means ± SEM for n = 3 to n = 6 independent experiments.
Ethics
This study was approved by the Université de Moncton institutional Review Committee for Research involving human subjects. All subjects provided informed consent prior to their participation in the study.
RESULTS
Incorporation of AA-alk and AA into cells
FAMEs were prepared from pure AA and AA-alk and were separated by GC. It was determined that AA-alk-methyl ester eluted at a retention time of ∼17.5 min, thus about 6 min later than that of AA-methyl ester (not shown).
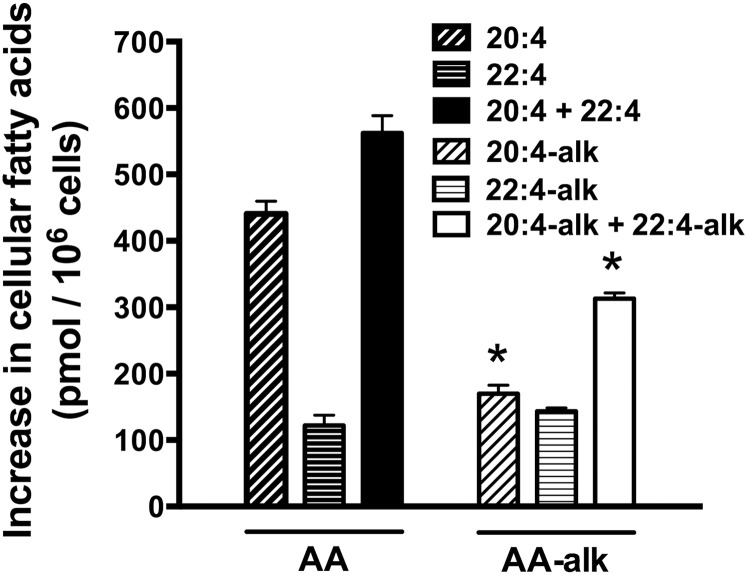
To compare the ability of cells to take up exogenous AA-alk and AA, Jurkat cells were incubated with 20 μM of each FA for 2 h, cells were washed, lipids were extracted, FAMEs were prepared, and cellular FAs were measured. In cells incubated with AA-alk, a peak corresponding to AA-alk was observed on chromatograms, as well as second peak eluting at 21 min (supplemental Figure S1). Based on the relative elution profiles of AA-alk, AA, and 22:4n-6, this second peak eluted at a retention time where the elongation product 22:4-alk would be expected. The identity of the putative 22:4-alk was confirmed by MS where the molecular mass of the FAME was determined to be m/z 343.3, which is the expected mass of the protonated methyl-22:4-alk. The quantities of AA-alk and 22:4-alk were approximately equivalent suggesting a very efficient elongation of AA-alk during this 2 h incubation period (Fig. 3). In cells incubated with exogenous AA, both the cellular AA and 22:4 content increased following the 2 h incubation period, though the increase in cellular AA was ∼4-fold greater than that of 22:4 (Fig. 3). When the total uptake of exogenous AA and AA-alk were measured, including their elongation products, cells incorporated nearly two times more AA than AA-alk during this 2 h incubation period (Fig. 3). Overall this indicates that while the capacity to incorporate AA into cells was greater than that of AA-alk, a greater proportion of the AA-alk was elongated compared with AA.
Fig. 3.
The incorporation and elongation of exogenous AA and AA-alk into Jurkat cells. Jurkat cells were incubated with 20 μM AA, 20 μM AA-alk, or their diluent controls for 2 h. Cells were then washed, cellular lipids were extracted, FAs were hydrolyzed and transmethylated, and FAMEs were measured by GC/FID. The results show the increase in the cellular content of AA, 22:4, AA-alk, and 22:4-alk compared with controls following the 2 h incubation period. Data are the means ± SEM of three independent experiments (n = 3). * P < 0.05, different from cells incubated with AA as determined by two-sided Student’s t-tests.
Arachidonate-PL remodeling
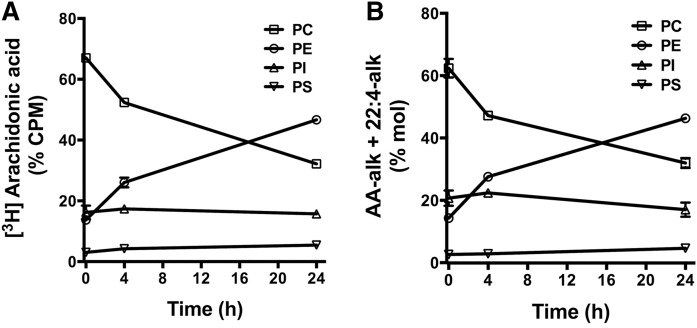
The uptake of exogenous AA into glycerophospholipids is known to follow a distinct pattern where the initial incorporation of AA is primarily into PC species, followed by a CoA-IT-driven remodeling into PE species (Fig. 2) (20–24). To compare the incorporation and remodeling of AA and AA-alk, their distribution in cellular glycerophospholipid classes was measured over a 24 h period following a 2 hour pulse with 20 μM of [3H]AA or with 20 μM of AA-alk. The initial incorporation of [3H]AA was primarily into PC with a subsequent redistribution toward PE species over time (Fig. 4A). Approximately 20% of the [3H]AA incorporated into glycerophospholipids was in PI, and the proportion of radiolabel associated with PI did not change significantly during the subsequent 24 h incubation period. This pattern of [3H]AA labeling and remodeling in glycerophospholipid classes is consistent with previous reports (21–23, 40). AA-alk incorporation and remodeling in glycerophospholipids followed a nearly identical pattern to that observed with [3H]AA, with the characteristic remodeling between PC and PE classes and a stable incorporation into PI (Fig. 4B). Because labeling with [3H]AA tracks both [3H]AA and its [3H]22:4 elongation product, the remodeling pattern of AA-alk was calculated using the sum of AA-alk and 22:4-alk.
Fig. 4.
Arachidonate-PL remodeling in Jurkat cells. Jurkat cells were pulse labeled with 20 μM [3H]AA (A) or 20 μM AA-alk (B) for 2 h, then washed and incubated for the indicated times prior to lipid extraction. PL classes were separated by HPLC, and PC, PE, PI, and PS were collected separately. The FAs from each fraction were hydrolyzed and transmethylated, and AA-alk and 22:4-alk FAMEs were measured by GC/FID. The radioactivity associated with each fraction was measured by liquid scintillation counting. Values represent the mean ± SEM of three independent experiments (n = 3).
Eicosanoid biosynthesis in human platelets
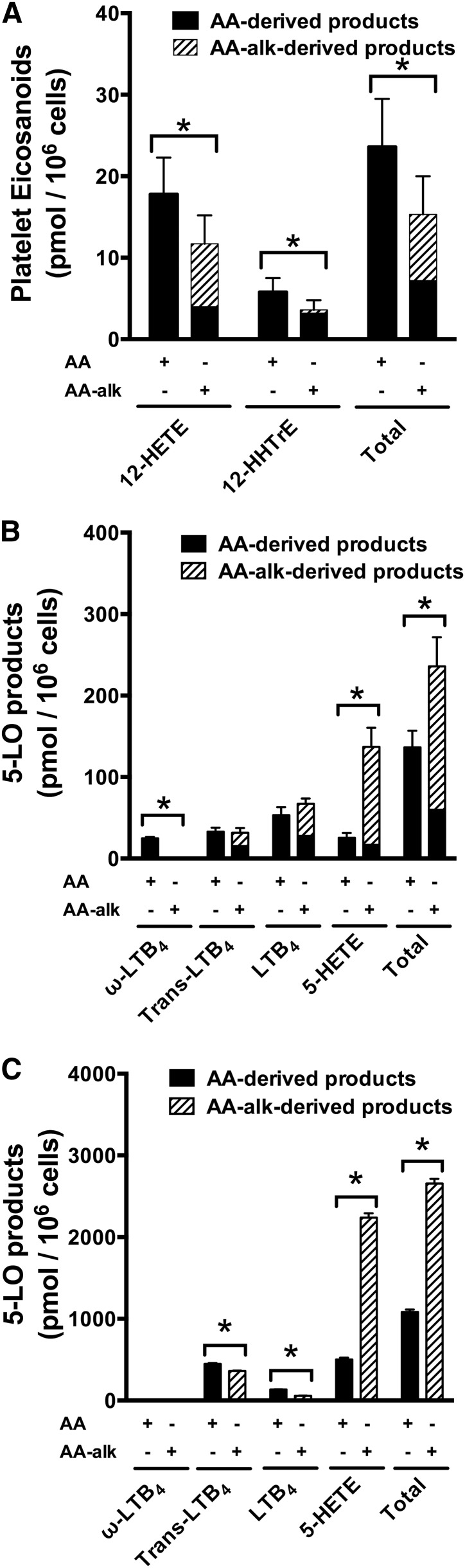
Stimulated human platelets primarily convert exogenous AA into bioactive eicosanoids through the 12-LOX and the COX-1 pathways. 12-LOX catalyzes the oxygenation of AA into 12-hydroperoxyeicosatetraenoic acid that is then rapidly converted to 12-HETE. COX-1 catalyzes the conversion of AA into PGH2, which, in platelets, is then rapidly and concurrently converted to thromboxane A2 and 12-HHTrE by thromboxane synthase (26, 41). To compare the ability of human platelets to convert AA and AA-alk to eicosanoids in each pathway, platelets were stimulated with calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk. The lipid mediators 12-HETE and HHtrE (or their alkyne analogs) were then measured following separation by RP-HPLC with UV detection. In platelets stimulated in the presence of exogenous AA, the typical product profile measured by RP-HPLC was obtained with detectable quantities of 12-HETE and HHTrE (supplemental Figure S2A). In platelets incubated in the presence of AA-alk, a peak with the typical spectrum and λ-max (237 nm) of HETEs was observed on HPLC chromatograms that elutes ∼4 min earlier than 12-HETE (supplemental Figure S2B) and was termed 12-HETE-alk. Similarly, a peak with the typical spectrum and λ-max (232 nm) of HHTrE was observed on HPLC chromatograms that elutes ∼4 min earlier than HHTrE and was termed 12-HHTrE-alk (supplemental Figure S2B). 12-HETE-alk was the major AA-alk product (7.6 ± 2.2 pmol/106 cells, mean ± SEM) as very little 12-HHTrE (0.4 ± 0.09 pmol/106 cells, mean ± SEM) was synthesized by stimulated platelets (Fig. 5A).
Fig. 5.
Eicosanoid biosynthesis by human platelets, neutrophils, and 5-LOX-transfected HEK293 cells stimulated in the presence of exogenous AA or AA-alk. A: Freshly isolated human platelets were stimulated with 10 μM calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk for 15 min at 37°C, and stimulations were stopped with organic solvents. B: Freshly isolated human neutrophils were stimulated with 10 μM calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk for 5 min at 37°C, and stimulations were stopped with organic solvents. C: HEK293 cells that were stably transfected with 5-LOX and FLAP were stimulated with 10 μM calcium ionophore A23187 in the presence of 10 μM AA or 10 μM AA-alk for 15 min at 37°C, and stimulations were stopped with organic solvents. AA-derived and AA-alk-derived products were separated by HPLC and measured by UV absorption at 270 nm and 236 nm. * P < 0.05, significantly different as determined by two-sided Student’s t-tests. Data are the means ± SEM of six independent experiments (n = 6) for the platelets, three independent experiments (n = 3) for the neutrophils, and four independent experiments for the HEK293 cells (n = 4). Trans-LTB4, sum of 6-trans-LTB4 and 12-epi-6-trans-LTB4; ω-LTB4, sum of 20-hydroxy-LTB4 and 20-carboxy-LTB4.
Because mouse COX-2 was shown to produce 11-HETE-alk (18), the identity of the peak identified as 12-HETE-alk was verified by LC/MS/MS. A parent ion with the expected m/z of 315 for a HETE-alk product was observed (supplemental Figure S3A). Additionally, its expected dehydration/decarboxylation products with m/z of 297 and 253, respectively, were present, as were the expected fragmentation ions of a 12-hydroxylated product with m/z of 179 and 208 (supplemental Figure S3B) (18, 42). Importantly, the expected fragmentation ion of 11-HETE-alk with an m/z of 167 (18, 42) was absent. Platelets stimulated in the presence of exogenous AA-alk also produced 12-HETE and HHTrE generated from endogenous AA. Overall, the biosynthesis of 12-LOX and COX-1 products was significantly lower in platelets stimulated in the presence of AA-alk than in platelets stimulated in the presence of exogenous AA (Fig. 5A).
Eicosanoid biosynthesis in human neutrophils and HEK293 cells
The main AA-derived metabolites synthesized by stimulated human neutrophils are products of the 5-LOX pathway. Freshly isolated human neutrophils do not synthesize COX products or express COX enzymes (43, 44). When human neutrophils were stimulated with calcium ionophore A23187 in the presence of 10 μM exogenous AA; the 5-LOX products 5-HETE, LTB4, 6-trans-LTB4, and 12-epi-6-trans-LTB4; and the omega-oxidation products 20-hydroxy-LTB4 and 20-carboxy-LTB4 were measured following separation by HPLC with UV detection (supplemental Figure S4). When neutrophils were stimulated in the presence of 10 μM exogenous AA-alk, several peaks were detected by HPLC that corresponded to expected 5-LOX products with the appropriate absorbance spectra, but whose elution times on HPLC were earlier than those measured for AA metabolites (supplemental Figure S4). One main difference with the AA-alk metabolite profile was the absence of omega-oxidation products. Another difference was that 5-HETE-alk was the predominant 5-LOX product derived from AA-alk, whereas 5-HETE was a much less prominent 5-LOX product synthesized by the cells stimulated in the presence of exogenous AA (Fig. 5B).
The peak identified as LTB4-alk was characterized by LC/MS/MS, and its profile was compared with that of commercial LTB4. Parent ions were observed with the expected m/z of 335 for LTB4 (LIPID MAPS® LM_ID: LMFA03020001) (45, 46) and m/z of 331 for LTB4-alk (supplemental Figures S5 and S6). Fragmentation ions with m/z of 195, 151, and 129 were measured for both LTB4-alk and LTB4, as would be expected based on the structures associated with the respective ions that do not include the omega end of the molecule (46). Several ions from collision-induced dissociation of LTB4 retain the omega end of the molecule such as ions resulting from one and two dehydrations (m/z 317 and 299) and their additional decarboxylations (m/z 273 and 255), as well as fragmentation ion m/z 203 (46). Importantly, corresponding ions with m/z 313, 295, 269, 251, and 199 were observed in the MS/MS product ion spectra of LTB4-alk that are analogous to fragments generated from LTB4 but with m/z values shifted by 4 units, consistent with ions containing an omega-terminal alkyne (supplemental Figure S6B). Additionally, the peak identified as 5-HETE-alk showed the expected parent ion and fragmentation pattern that was previously reported for 5-LOX-catalyzed 5-HETE-alk synthesis (supplemental Figure S7) (18).
The profile of 5-LOX products was also measured in HEK293 cells that were stably transfected with human 5-LOX and FLAP (37). This is an attractive model to measure 5-LOX product biosynthesis from exogenous substrates because they do not release endogenous AA when stimulated and the control cells transfected with a control vector do not produce 5-LOX products. When control cells were stimulated in the presence of AA or AA-alk, no measurable 5-LOX products were produced (not shown). When the 5-LOX/FLAP-transfected cells were stimulated in the presence of exogenous substrate, 5-LOX products were synthesized in the same proportions as those measured in neutrophil, with more AA-alk products produced than those of AA, and with 5-HETE-alk as the predominant AA-alk product (Fig. 5C). Overall, the biosynthesis of total 5-LOX products (AA-derived and AA-alk-derived) was significantly greater in neutrophils or HEK293 cells stimulated in the presence of AA-alk than in cells stimulated in the presence of exogenous AA (Fig. 5B, C).
Functional studies in AA-alk and LTB4-alkyne
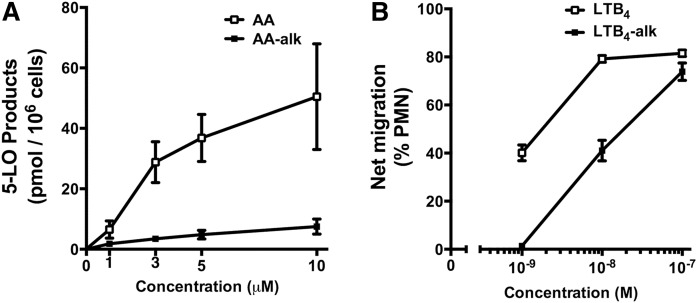
AA can stimulate human neutrophils though an autocrine stimulatory loop where exogenous AA is converted to LTB4, which then activates the leukotriene B4 receptor 1 (BLT1) allowing a greater calcium-dependent conversion of exogenous AA via the 5-LOX pathway (35). When neutrophils were incubated with exogenous AA or AA-alk to induce the autocrine stimulatory loop, exogenous AA induced the expected robust stimulation of 5-LOX product biosynthesis, whereas AA-alk induced a markedly smaller cellular response (Fig. 6A) suggesting that the autocrine stimulatory loop was not induced by AA-alk.
Fig. 6.
Autocrine stimulation of neutrophils with exogenous AA and AA-alk and neutrophil chemoattractant activity of LTB4 and LTB4 alk. A: Freshly isolated human neutrophils were stimulated with various concentrations of AA or AA-alk for 5 min at 37°C, and stimulations were stopped with organic solvents. AA-derived and AA-alk-derived products were separated by HPLC and measured by UV absorption at 270 nm and 236 nm. 5-LOX products are the sum of 5-HETE, LTB4, 6-trans-LTB4, 12-epi-6-trans-LTB4, 20-hydroxy-LTB4, and 20-carboxy-LTB4. B: Freshly isolated human neutrophils were suspended in cell culture inserts (3.0 µm pore size) and were allowed to migrate for 2 h at 37°C to the lower chamber containing the indicated concentrations LTB4-alk, LTB4, or their diluent. Cells that had migrated to the lower chamber were counted, and results show the percent of cells that had migrated from the upper to the lower chamber. Data are the means ± SEM of three independent experiments (n = 3).
One of the main biological functions of LTB4 is its potent chemotactic activity toward human neutrophils. To compare the biological activities of LTB4 and LTB4-alk, their ability to induce BLT1-dependent neutrophil chemotaxis was measured. Using a transwell chemotaxis assay, LTB4 was ∼12 times more potent at stimulating neutrophil chemotaxis [apparent EC50 1.7 nM; 95% confidence interval (CI), 0.8–3.5 nM] than LTB4-alkyne (apparent EC50 20.9 nM; 95% CI, 13.0–33.7 nM) (Fig. 6B).
DISCUSSION
The use of alkyne or azide analogs of biological compounds that are amenable to click chemistry reactions has attracted substantial recent interest. This is due to the ability to tag the compounds with fluorescent or affinity probes, thus providing sensitive methods of identifying their potential metabolites or ascertaining macromolecules with which the compound may interact within complex biological mixtures. The use of these compounds can also greatly simplify the ability to purify the compounds or their partners of interest from complex milieus. However, to be truly useful as physiologically relevant probes it is critical to understand the extent to which a clickable compound may behave like its natural counterpart.
FAs are attractive candidates for click chemistry probes because the methyl (or omega) end of the aliphatic chain, which is generally not considered to be a biologically reactive region of the molecule, can be modified into a terminal alkyne. Such FA alkynes have proved to be useful in studying the subcellular localization of palmitoylated proteins such as hedgehog, tubulin, and Ras (8, 12, 15) that are subject to posttranscriptional acylation. Among the FAs that have been developed as click chemistry probes is the 19-alkyne derivative of AA, AA-alk (Fig. 1) (17, 18). AA is the precursor of numerous biologically active lipid mediators and as such undergoes unique mechanisms of regulation compared with other FAs, likely because of its role in regulating several biological processes including inflammation. The current study compared the cellular metabolism of AA and AA-alk in the lymphocytic leukemia Jurkat cell line that rapidly remodels AA between PL classes, as well as in human platelets that express 12-LOX and COX-1 and in human neutrophils that express 5-LOX, all enzymes that catalyze the conversion of AA into biologically active eicosanoids. Overall, AA-alk behaved similarly to AA with regard to cellular uptake and distribution into cellular PLs; however, its metabolism as a precursor for biologically active eicosanoids differed considerably from its native counterpart AA.
Rapidly proliferating cells typically have an enhanced requirement for unsaturated FAs to support membrane biogenesis required for sustained cell proliferation (23, 47). When incubated with exogenous AA or AA-alk, rapidly dividing Jurkat cells effectively incorporated exogenous AA. However, the capacity to incorporate exogenous AA-alk was significantly inferior with ∼50% of the capacity measured for AA. Long chain FAs like AA are incorporated into cells by incompletely defined mechanisms that can include simple diffusion and saturable transport processes. Proteins involved in the uptake of FAs include FA binding protein, caveolin-1, FAT/CD36, and the FA transport protein family of proteins (19, 48, 49). With respect to long chain PUFAs in particular, ACS activity has been shown to be important for their incorporation into cells and five isoforms of human long chain ACS have been identified (19, 50–52) with the ACS4 isoform showing specificity for AA. While cellular AA incorporation can involve the participation of FA transfer proteins and ACSs, the exact contribution of different proteins isoforms associated with AA uptake is not fully elucidated, and likely several mechanisms can be at play in any given cell type. Although the mechanism of cellular uptake in lymphocytic leukemia cells has not been elucidated, the results of the current study suggest that the AA-specific transport mechanisms are not as effective with AA-alk as a substrate indicating that the use of AA-alk as a tool or substrate to study AA transport proteins or mechanisms should be performed with caution.
Once incorporated into cells, AA is usually converted by ACSs to AA-CoA thioesters. AA can then be incorporated into glycerophospholipids by reactions catalyzed by lyso-PLATs or undergo elongation to 22:4 by a reaction catalyzed by ELOVLs prior to incorporation into glycerophospholipids. Although AA-alk was not as effectively incorporated into cells as AA, approximately half of the AA-alk that was incorporated underwent elongation to its 22:4 elongation product compared with only 20% of the newly incorporated AA. This suggests that compared with AA, AA-alk is more effectively utilized as a substrate for the ELOVL enzymes expressed in Jurkat cells. This observation could reflect differences in substrate preference of the ELOVL enzymes, or these enzymes may elongate a smaller proportion of exogenous AA because cells already contain its elongation product 22:4 and thus substrate/product equilibrium is reached more rapidly than with AA-alk. Regardless of the mechanism explaining this difference in elongation potential, these observations once again suggest that results obtained with AA-alk as a surrogate for the study of AA elongation must be carefully interpreted.
AA undergoes a pattern of incorporation into and remodeling between glycerophospholipid classes that appears to be specific for AA compared with other FAs (20, 23, 53, 54). This unique remodeling pattern is believed to have evolved partly because AA is the precursor of very potent bioactive metabolites, and cells have developed mechanisms to tightly control its cellular distribution and bioavailability. Unlike cellular uptake and elongation reactions, the measured patterns of incorporation of AA-alk and AA into the main glycerophospholipid classes were nearly identical with between 60% and 70% of the initial incorporation occurring in PC species, 15% to 20% into PE and PI species, and less than 5% into PS species. This suggests that the lyso-PLATs that catalyze the incorporation into glycerophospholipid classes appear to utilize AA-CoA and AA-alk-CoA in a near-identical fashion. Furthermore, the typical CoA-IT-driven CoA-IT remodeling of newly incorporated AA from PC species to PE species was identical for AA and AA-alk. Therefore, AA-alk appears to be a very good surrogate of AA when studying AA incorporation into and remodeling between glycerophospholipid classes.
Some cell types like leukocytes and platelets can readily transform exogenous AA into bioactive eicosanoids by reactions catalyzed by LOXs and COXs. When these cells are activated by stimuli like calcium ionophores, exogenous AA, as well as endogenous AA hydrolyzed from PLs by PLA2, is rapidly transformed by these enzymes into a diversity of products. Stimulated platelets utilized exogenous AA-alk as a substrate for both 12-LOX and COX-1 product biosynthesis. However, smaller quantities of 12-HETE-alk and the HHTrE-alk were produced by these cells compared with AA metabolites suggesting that AA-alk is not as efficiently transformed by platelets as AA. These results are consistent with a previous report comparing the kinetics of AA and AA-alk transformation by crude human 12-LOX preparations where the transformation of AA was slightly more efficient than that of AA-alk (18). Similarly, purified murine COXs were reported to efficiently oxidize AA-alk but poorly catalyzed the cyclization of dioxalanyl intermediates to endoperoxides (18), which is consistent with the very limited biosynthesis of HHTrE-alk by stimulated platelets. However, the murine COX-catalyzed production of 11-HETE-alk that resulted from the poor cyclization was not detected in stimulated human platelets.
Stimulated human neutrophils metabolize AA through the 5-LOX pathway. When stimulated in the presence of AA-alk, alkyne derivatives of the expected 5-LOX products were synthesized with the exception of the LTB4-alk degradation products 20-OH-LTB4-alk and 20-COOH-LTB4-alk. This is likely because CYP4F3A, the cytochrome P450 protein responsible for the oxidation of the omega methyl end of AA (55), cannot catalyze the oxidation of the 19-alkyne moiety at the omega end of the AA-alk molecule. Alkynes are also known to inhibit cytochrome P450 enzymes (56). Despite the inability to degrade LTB4, cells stimulated in the presence of AA-alk did not show an accumulation of LTB4-alk compared with LTB4 measured in cells stimulated in the presence of AA. In contrast to platelet eicosanoids, neutrophils produced more 5-LOX metabolites when stimulated in the presence of AA-alk than in the presence of AA, and this was primarily due to the significantly greater production of 5-HETE-alk compared with 5-HETE. In fact, there was a significant shift in product profiles where 5-HETE-alk was the predominant AA-alk product, while LTB4 and its derivatives were the predominant AA-derived products. Near identical 5-LOX product profiles were also measured in 5-LOX/FLAP-transfected HEK293 cells. This suggests that the lipoxygenation of AA-alk at carbon-5 generating 5-hydroperoxyeicosatetraenoic acid-alk (5-HpETE-alk) is efficient, but that the second pseudolipoxygenation that involves abstraction of a hydrogen at carbon-10 from 5-HpETE-alk to generate the LTA4-alk epoxide is not as efficient as with the AA-derived substrate. Thus, the presence of the 19-alkyne structure appears to impact the substrate specificity of 5-LOX differently for each of the two 5-LOX-catalyzed reactions. The ultimate outcome is an altered product profile where (LTB4 + derivatives)/5-HETE ratios are significantly different depending on the substrate. These observations suggest that the use of AA-alk as a surrogate for the study of AA-derived lipid mediator metabolism must be performed with caution.
In the absence of other stimuli, exogenous AA can stimulate human neutrophils though an autocrine stimulatory loop. In this stimulation model, basal 5-LOX activity converts exogenous AA into LTB4 in a calcium-independent manner, which then activates the BLT1 receptor allowing a more robust cellular stimulation with a calcium-dependent activation of 5-LOX and more extensive conversion of exogenous AA via the 5-LOX pathway (35). However, AA-alk produced a much weaker stimulation of human neutrophils through this autocrine stimulatory loop than that of exogenous AA. This may be because AA-alk is preferentially metabolized to 5-HETE rather than LTB4, thus lessening the strength of the autocrine stimulatory loop that relies on a basal production of LTB4. Alternatively, it may indicate that LTB4-alk is a weaker BLT1 agonist than LTB4 and at low concentrations may not be potent enough to drive the calcium-dependent autocrine stimulation of neutrophils. Regardless of the mechanism responsible for this diminished stimulation by exogenous AA-alk, this indicates that cellular responses to exogenous AA-alk are not as robust as those measured in response to exogenous AA.
In light of the more muted stimulation of neutrophils in response to AA-alk compared with AA, the biological activity of LTB4-alk was compared with that of LTB4. LTB4 is best known as a very potent chemotactic agent toward human leukocytes (57–59). When freshly isolated human neutrophil migration was measured in a transwell migration assay, LTB4 exhibited chemotactic activity in the low nanomolar range as expected (60). While LTB4-alk also exhibited chemotactic activity toward neutrophils, it was less potent than that of LTB4 by a full order of magnitude. Because LTB4-induced chemotaxis is dependent on stimulation of the BLT1 receptor (58–60), this indicates that LTB4-alk is a weaker BLT1 agonist than LTB4 and suggests that the biological activity of other AA-alk-derived eicosanoids may warrant investigation. Once again, these observations regarding the weaker ability of AA-alk and LTB4-alk to stimulate cells suggests that experiments performed using AA-alk-derived eicosanoids should be carefully considered.
Reagents that are amenable to click chemistry are proving to be extremely useful analytical and discovery tools due to structural and biological similarities to their natural analogs, and the ease with which they can be detected, labeled and purified in complex biological matrices. Omega-terminal alkyne derivatives of FAs are among these reagents and are certainly powerful tools that have already yielded new important information on FA-protein interactions. While the current study does not invalidate the use of AA-alk as a potentially powerful tool to investigate AA metabolism and behavior in biological systems, some important differences in its metabolism and biological activity have been delineated that should be useful to investigators who will use these tools to investigate AA in biological systems.
Supplementary Material
Acknowledgments
The authors thank Natalie Levesque for technical support with FA analysis by GC/MS.
Footnotes
Abbreviations:
- 12-HHTrE
- 12-hydroxyheptadecatrienoic acid
- AA
- arachidonic acid
- AA-alk
- 19-alkyne arachidonic acid
- ACS
- acyl-CoA synthetase
- alk
- alkyne
- BLT
- leukotriene B4 receptor 1
- CoA-IT
- CoA-independent transacylase
- COX
- cyclooxygenase
- ELOVL
- elongases of very long chain FAs
- FAME
- FA methyl ester
- GC/FID
- gas chromatography with flame ionization detection
- FLAP
- 5-LOX activating protein
- LOX
- lipoxygenase
- LTB4
- leukotriene B4
- lyso-PLAT
- lysophospholipid acyl-CoA acyltransferase
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PGB2
- prostaglandin B2
- PI
- phosphatidylinositol
- PL
- phospholipid
- PLA2
- phospholipase A2
- PS
- phosphatidylserine
- RP-HPLC
- reverse phase-HPLC
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the New Brunswick Health Research Foundation (NBHRF) awarded to M. E. Surette. P. P. Robichaud was supported by Doctoral Scholarships from the CIHR, the NBHRF, and the Fonds de recherche sur l’arthrite et les maladies rhumatismales de l’université Laval. S. J. Poirier was supported by Doctoral Scholarships from the CIHR and the NBHRF. L. H. Boudreau was supported by the New Brunswick Innovation Foundation. E. Boilard is recipient of a New Investigator Award from the CIHR. M. E. Surette is the recipient of a New Brunswick Innovation Research Chair.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Kolb H. C., and Sharpless K. B.. 2003. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 8: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 2.Cravatt B. F., Wright A. T., and Kozarich J. W.. 2008. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 77: 383–414. [DOI] [PubMed] [Google Scholar]
- 3.Niphakis M. J., and Cravatt B. F.. 2014. Enzyme inhibitor discovery by activity-based protein profiling. Annu. Rev. Biochem. 83: 341–377. [DOI] [PubMed] [Google Scholar]
- 4.Nwe K., and Brechbiel M. W.. 2009. Growing applications of “click chemistry” for bioconjugation in contemporary biomedical research. Cancer Biother. Radiopharm. 24: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay C. S., and Finn M. G.. 2014. Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem. Biol. 21: 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best M. D. 2009. Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry. 48: 6571–6584. [DOI] [PubMed] [Google Scholar]
- 7.Milne S. B., Tallman K. A., Serwa R., Rouzer C. A., Armstrong M. D., Marnett L. J., Lukehart C. M., Porter N. A., and Brown H. A.. 2010. Capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. Nat. Chem. Biol. 6: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konitsiotis A. D., Jovanovic B., Ciepla P., Spitaler M., Lanyon-Hogg T., Tate E. W., and Magee A. I.. 2015. Topological analysis of Hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J. Biol. Chem. 290: 3293–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jao C. Y., Roth M., Welti R., and Salic A.. 2009. Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. USA. 106: 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martín-Couce L., Martín-Fontecha M., Capolicchio S., López-Rodríguez M. L., and Ortega-Gutiérrez S.. 2011. Development of endocannabinoid-based chemical probes for the study of cannabinoid receptors. J. Med. Chem. 54: 5265–5269. [DOI] [PubMed] [Google Scholar]
- 11.Tate E. W., Kalesh K. A., Lanyon-Hogg T., Storck E. M., and Thinon E.. 2015. Global profiling of protein lipidation using chemical proteomic technologies. Curr. Opin. Chem. Biol. 24: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X., and Hannoush R. N.. 2014. Method for cellular imaging of palmitoylated proteins with clickable probes and proximity ligation applied to Hedgehog, tubulin, and Ras. J. Am. Chem. Soc. 136: 4544–4550. [DOI] [PubMed] [Google Scholar]
- 13.Gaebler A., Milan R., Straub L., Hoelper D., Kuerschner L., and Thiele C.. 2013. Alkyne lipids as substrates for click chemistry-based in vitro enzymatic assays. J. Lipid Res. 54: 2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tully S. E., and Cravatt B. F.. 2010. Activity-based probes that target functional subclasses of phospholipases in proteomes. J. Am. Chem. Soc. 132: 3264–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X., and Hannoush R. N.. 2014. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat. Chem. Biol. 10: 61–68. [DOI] [PubMed] [Google Scholar]
- 16.Thiele C., Papan C., Hoelper D., Kusserow K., Gaebler A., Schoene M., Piotrowitz K., Lohmann D., Spandl J., Stevanovic A., et al. . 2012. Tracing fatty acid metabolism by click chemistry. ACS Chem. Biol. 7: 2004–2011. [DOI] [PubMed] [Google Scholar]
- 17.Windsor K., Genaro-Mattos T. C., Kim H. Y., Liu W., Tallman K. A., Miyamoto S., Korade Z., and Porter N. A.. 2013. Probing lipid-protein adduction with alkynyl surrogates: application to Smith-Lemli-Opitz syndrome. J. Lipid Res. 54: 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beavers W. N., Serwa R., Shimozu Y., Tallman K. A., Vaught M., Dalvie E. D., Marnett L. J., and Porter N. A.. 2014. omega-Alkynyl lipid surrogates for polyunsaturated fatty acids: free radical and enzymatic oxidations. J. Am. Chem. Soc. 136: 11529–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita A., Hayashi Y., Nemoto-Sasaki Y., Ito M., Oka S., Tanikawa T., Waku K., and Sugiura T.. 2014. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 53: 18–81. [DOI] [PubMed] [Google Scholar]
- 20.Kramer R. M., and Deykin D.. 1983. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J. Biol. Chem. 258: 13806–13811. [PubMed] [Google Scholar]
- 21.Fonteh A. N., and Chilton F. H.. 1992. Rapid remodeling of arachidonate from phosphatidylcholine to phosphatidylethanolamine pools during mast cell activation. J. Immunol. 148: 1784–1791. [PubMed] [Google Scholar]
- 22.Boilard E., and Surette M. E.. 2001. Anti-CD3 and concanavalin A-induced human T cell proliferation is associated with an increased rate of arachidonate-phospholipid remodeling. Lack of involvement of group IV and group VI phospholipase A2 in remodeling and increased susceptibility of proliferating T cells to CoA-independent transacyclase inhibitor-induced apoptosis. J. Biol. Chem. 276: 17568–17575. [DOI] [PubMed] [Google Scholar]
- 23.Robichaud P. P., Boulay K., Munganyiki J. E., and Surette M. E.. 2013. Fatty acid remodeling in cellular glycerophospholipids following the activation of human T cells. J. Lipid Res. 54: 2665–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichaud P. P., and Surette M. E.. 2015. Polyunsaturated fatty acid-phospholipid remodeling and inflammation. Curr. Opin. Endocrinol. Diabetes Obes. 22: 112–118. [DOI] [PubMed] [Google Scholar]
- 25.Rådmark O., and Samuelsson B.. 2010. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem. Biophys. Res. Commun. 396: 105–110. [DOI] [PubMed] [Google Scholar]
- 26.Smith W. L., Urade Y., and Jakobsson P. J.. 2011. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 111: 5821–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis E. A., and Norris P. C.. 2015. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rådmark O., Werz O., Steinhilber D., and Samuelsson B.. 2015. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta. 1851: 331–339. [DOI] [PubMed] [Google Scholar]
- 29.Boyce J. A. 2008. Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 8: 335–349. [DOI] [PubMed] [Google Scholar]
- 30.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 31.Chilton F. H. 1990. Separation and characterization of arachidonate-containing phosphoglycerides. Methods Enzymol. 187: 157–167. [DOI] [PubMed] [Google Scholar]
- 32.Surette M. E., Koumenis I. L., Edens M. B., Tramposch K. M., and Chilton F. H.. 2003. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 25: 948–971. [DOI] [PubMed] [Google Scholar]
- 33.Cloutier N., Tan S., Boudreau L. H., Cramb C., Subbaiah R., Lahey L., Albert A., Shnayder R., Gobezie R., Nigrovic P. A., et al. . 2013. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Böyum A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97: 77–89. [PubMed] [Google Scholar]
- 35.Surette M. E., Krump E., Picard S., and Borgeat P.. 1999. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A(2a) receptor agonists and crucial role of autocrine activation by leukotriene B(4). Mol. Pharmacol. 56: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau L. H., Bertin J., Robichaud P. P., Laflamme M., Ouellette R. J., Flamand N., and Surette M. E.. 2011. Novel 5-lipoxygenase isoforms affect the biosynthesis of 5-lipoxygenase products. FASEB J. 25: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 37.Allain E. P., Boudreau L. H., Flamand N., and Surette M. E.. 2015. The intracellular localisation and phosphorylation profile of the human 5-lipoxygenase delta13 isoform differs from that of its full length counterpart. PLoS One. 10: e0132607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borgeat P., Picard S., Vallerand P., Bourgoin S., Odeimat A., Sirois P., and Poubelle P. E.. 1990. Automated on-line extraction and profiling of lipoxygenase products of arachidonic acid by high-performance liquid chromatography. Methods Enzymol. 187: 98–116. [DOI] [PubMed] [Google Scholar]
- 39.Provost V., Larose M. C., Langlois A., Rola-Pleszczynski M., Flamand N., and Laviolette M.. 2013. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J. Leukoc. Biol. 94: 213–222. [DOI] [PubMed] [Google Scholar]
- 40.Balsinde J. 2002. Roles of various phospholipases A2 in providing lysophospholipid acceptors for fatty acid phospholipid incorporation and remodelling. Biochem. J. 364: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall E. R., and Tai H. H.. 1981. Purification of thromboxane synthetase and evidence of two distinct mechanisms for the formation of 12-L-hydroxy-5,8,10-heptadecatrienoic acid by porcine lung microsomes. Biochim. Biophys. Acta. 665: 498–503. [DOI] [PubMed] [Google Scholar]
- 42.Wheelan P., Zirrolli J. A., and Murphy R. C.. 1993. Low-energy fast atom bombardment tandem mass spectrometry of monohydroxy substituted unsaturated fatty acids. Biol. Mass Spectrom. 22: 465–473. [DOI] [PubMed] [Google Scholar]
- 43.Fasano M. B., Wells J. D., and McCall C. E.. 1998. Human neutrophils express the prostaglandin G/H synthase 2 gene when stimulated with bacterial lipopolysaccharide. Clin. Immunol. Immunopathol. 87: 304–308. [DOI] [PubMed] [Google Scholar]
- 44.Walsh C. E., Waite B. M., Thomas M. J., and DeChatelet L. R.. 1981. Release and metabolism of arachidonic acid in human neutrophils. J. Biol. Chem. 256: 7228–7234. [PubMed] [Google Scholar]
- 45.Sud M., Fahy E., Cotter D., Brown A., Dennis E. A., Glass C. K., Merrill A. H. Jr., Murphy R. C., Raetz C. R., Russell D. W., et al. . 2007. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35: D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheelan P., Zirrolli J. A., and Murphy R. C.. 1996. Negative ion electrospray tandem mass spectrometric structural characterization of leukotriene B4 (LTB 4) and LTB 4-derived metabolites. J. Am. Soc. Mass Spectrom. 7: 129–139. [DOI] [PubMed] [Google Scholar]
- 47.Belkaid A., Duguay S. R., Ouellette R. J., and Surette M. E.. 2015. 17beta-Estradiol induces stearoyl-CoA desaturase-1 expression in estrogen receptor-positive breast cancer cells. BMC Cancer. 15: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soupene E., and Kuypers F. A.. 2008. Mammalian long-chain acyl-CoA synthetases. Exp. Biol. Med. (Maywood). 233: 507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abumrad N., Coburn C., and Ibrahimi A.. 1999. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim. Biophys. Acta. 1441: 4–13. [DOI] [PubMed] [Google Scholar]
- 50.Digel M., Ehehalt R., Stremmel W., and Fullekrug J.. 2009. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol. Cell. Biochem. 326: 23–28. [DOI] [PubMed] [Google Scholar]
- 51.Ellis J. M., Frahm J. L., Li L. O., and Coleman R. A.. 2010. Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 21: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durgan D. J., Smith J. K., Hotze M. A., Egbejimi O., Cuthbert K. D., Zaha V. G., Dyck J. R., Abel E. D., and Young M. E.. 2006. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am. J. Physiol. Heart Circ. Physiol. 290: H2480–H2497. [DOI] [PubMed] [Google Scholar]
- 53.Sugiura T., Katayama O., Fukui J., Nakagawa Y., and Waku K.. 1984. Mobilization of arachidonic acid between diacyl and ether phospholipids in rabbit alveolar macrophages. FEBS Lett. 165: 273–276. [DOI] [PubMed] [Google Scholar]
- 54.Chilton F. H., Fonteh A. N., Surette M. E., Triggiani M., and Winkler J. D.. 1996. Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta. 1299: 1–15. [DOI] [PubMed] [Google Scholar]
- 55.Kikuta Y., Kusunose E., Endo K., Yamamoto S., Sogawa K., Fujii-Kuriyama Y., and Kusunose M.. 1993. A novel form of cytochrome P-450 family 4 in human polymorphonuclear leukocytes. cDNA cloning and expression of leukotriene B4 omega-hydroxylase. J. Biol. Chem. 268: 9376–9380. [PubMed] [Google Scholar]
- 56.Blobaum A. L. 2006. Mechanism-based inactivation and reversibility: is there a new trend in the inactivation of cytochrome p450 enzymes? Drug Metab. Dispos. 34: 1–7. [DOI] [PubMed] [Google Scholar]
- 57.Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., and Smith M. J.. 1980. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 286: 264–265. [DOI] [PubMed] [Google Scholar]
- 58.Jackson W. T., Boyd R. J., Froelich L. L., Mallett B. E., and Gapinski D. M.. 1992. Specific inhibition of leukotriene B4-induced neutrophil activation by LY223982. J. Pharmacol. Exp. Ther. 263: 1009–1014. [PubMed] [Google Scholar]
- 59.Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., and Parent C. A.. 2012. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell. 22: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chouinard F., Lefebvre J. S., Navarro P., Bouchard L., Ferland C., Lalancette-Hebert M., Marsolais D., Laviolette M., and Flamand N.. 2011. The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J. Immunol. 186: 3188–3196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.