Abstract
Clinical chorioamnionitis at term (TCC) is the most common obstetrical infliction diagnosed in labor and delivery units worldwide and is associated with a substantial increase in maternal and neonatal morbidity and mortality. This obstetrical complication is a heterogeneous condition, as only half of patients have detectable microorganisms in the amniotic cavity. Because bioactive lipids play a key role in the initiation and resolution of an inflammatory response, we aimed to characterize the amniotic fluid lipidome in patients with TCC. We studied the amniotic fluid of patients in the following groups: 1) spontaneous labor at term without clinical chorioamnionitis (TLB) and 2) spontaneous labor at term with clinical chorioamnionitis (TCC). The TCC group was subdivided into a) those with microbial invasion of the amniotic cavity (TCC-MIAC) and b) those without microbial invasion of the amniotic cavity (TCC-noMIAC). The amniotic fluid concentration of proinflammatory lipid mediators did not differ between patients in TLB with TCC. In contrast, concentration of lipids with anti-inflammatory/proresolution properties was significantly lower in all patients with TCC than in those with TLB. These results suggest that while proinflammatory lipid mediators are involved in infection-driven intra-amniotic inflammation, a relative deficiency of anti-inflammatory/proresolution lipid mediator biosynthesis is a characteristic of TCC.
Keywords: lipoxygenase, eicosanoids, inflammation, omega-3 fatty acids, lipidomics, intra-amniotic inflammation, infection, parturition, epoxy fatty acids, epoxygenase
Intra-amniotic inflammation in both preterm labor patients with intact membranes and in preterm prelabor rupture of membranes is often subclinical in nature (1–3). However, this process can lead to a systemic maternal inflammatory response that is known as clinical chorioamnionitis (4). Clinical chorioamnionitis is the most common infection-related condition diagnosed in labor and delivery units around the world. It is most frequent in young primiparous women and is estimated to have affected nearly 38,000 live births in the United States alone in 2014 (5, 6). In addition to intense maternal inflammatory response, intra-amniotic infection elicits acute histologic chorioamnionitis (7) and, frequently, a fetal inflammatory response, which is manifested as funisitis or chorionic vasculitis (8–11). Clinical chorioamnionitis and puerperal endomyometritis are the leading causes of infection-related complications in pregnant women. In addition to the maternal morbidity associated with clinical chorioamnionitis and puerperal infections, neonates born to mothers with clinical chorioamnionitis at term (TCC) are at increased risk for long-term complications, notably cerebral palsy (11–13).
The features of clinical chorioamnionitis include maternal fever, maternal or fetal tachycardia, leukocytosis, uterine tenderness, and foul-smelling amniotic fluid (14–16). We recently reported that TCC is a heterogeneous condition, as only 54% of patients have intra-amniotic infection (also called microbial-associated intra-amniotic inflammation), 24% have intra-amniotic inflammation without detectable microorganisms (sterile intra-amniotic inflammation), and 22% have no intra-amniotic inflammation (6). While the precise causes of sterile intra-amniotic inflammation are unknown, studies have shown that damage-associated molecular patterns, such as high mobility gene box-1, can elicit a sterile inflammatory response (17) and are elevated in the amniotic fluid of patients with TCC (18), and that this alarmin can induce labor (19). There is an urgent need for tests allowing the differential diagnosis of the three subgroups of patients with clinical chorioamnionitis, as these patients require different treatment. For example, patients with microbial-associated intra-amniotic inflammation should be managed with antibiotics, whereas the administration of these agents is unnecessary for those without intra-amniotic inflammation/infection. Identification of biomarkers to delineate clinical chorioamnionitis in the presence and absence of intra-amniotic infection would significantly advance our understanding of the disease as well as meet our diagnostic needs.
Bioactive lipids derived from the metabolism of PUFAs such as arachidonic acid act as mediators in the inflammatory response to injury caused by both microorganisms (bacterial or viral) and cellular damage (sterile) (20–23). These lipids also ensure resolution of inflammation (21, 22). Prostaglandins (PGs) play an important role in parturition at term (24–26), and it is possible that an increase in amniotic fluid inflammatory lipids such as PGE2, leukotriene B4 (LTB4), and so forth, in clinical chorioamnionitis can be observed. In fact, amniotic fluid concentrations of LTB4 (as well as other 5-lipoxygenase-derived metabolites of arachidonic acid) are significantly higher in patients with clinical chorioamnionitis and microbial-associated intra-amniotic inflammation than in those with sterile intra-amniotic inflammation (27). Recent evidence suggests that, in the normal course of events, a typical inflammatory response is followed by the synthesis of proresolution mediators such as lipoxins, resolvins, and protectins from PUFAs to inhibit further leukocyte infiltration (20, 21). Biosynthesis of these resolution mediators is facilitated by the same polymorphonuclear leukocytes that generate inflammatory lipid mediators, following an acute inflammatory response (28–30). Such lipid mediator class switching is crucial to limit inflammation to a homeostatic function and prevent a chronic inflammatory response (28). As an acute inflammatory condition, TCC is expected to elicit an amniotic fluid lipid mediator profile distinct from that of spontaneous labor at term (TLB). Therefore, the bioactive lipid profiles of amniotic fluid in TCC may reflect an imbalance between pro- and anti-inflammatory lipid mediators in favor of the proinflammatory state when compared with spontaneous labor.
To test this hypothesis, the amniotic fluid fatty acyl lipidome of patients at term in spontaneous labor with or without clinical chorioamnionitis was analyzed, utilizing an unbiased LC/MS approach. Results of this study demonstrate that the concentrations of inflammatory lipid mediators such as PGE2, known to participate in the initiation of labor, are similar, while a significantly lower concentration of anti-inflammatory lipid mediators was observed in patients with clinical chorioamnionitis, especially those without detectable microbial invasion in the amniotic cavity.
MATERIALS AND METHODS
Study design and population
A retrospective cross-sectional study was conducted by searching the clinical database and Bank of Biological Samples of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch [Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NICHD/NIH)]. Women with singleton pregnancies who had amniotic fluid samples obtained by transabdominal amniocentesis were included. The details of patient groups are described elsewhere (27).
All patients provided written, informed consent before the collection of amniotic fluid samples. The collection and utilization of the samples were approved by the Human Investigation Committee of the Sótero del Río Hospital, a major affiliate of the Catholic University of Santiago, Chile, and the Institutional Review Board of the NICHD. Many of these samples have been used in previous studies in the biology of cytokines and inflammatory mediators (4, 6, 16, 31–33).
Clinical definitions
Spontaneous term labor was defined as the presence of regular uterine contractions with a frequency of at least one every 10 min and cervical changes after 37 weeks of gestation. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by two or more of the following criteria: 1) maternal tachycardia (heart rate >100 beats/min); 2) uterine tenderness; 3) foul-smelling odor of the amniotic fluid; 4) fetal tachycardia (heart rate >160 beats/min); and 5) maternal leukocytosis (leukocyte count >15,000 cells/mm3) (16).
Intra-amniotic inflammation was diagnosed when the amniotic fluid interleukin (IL)-6 concentrations were ≥2.6 ng/ml as determined by ELISA (34). Microbial invasion of the amniotic cavity (MIAC) was defined according to the results of amniotic fluid cultures and/or PCR/ESI/MS (Ibis® Technology - Athogen, Carlsbad, CA). Based on the results of amniotic fluid cultures and/or PCR/ESI/MS, patients were classified as 1) having MIAC or 2) those without MIAC.
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for evaluation of the microbial and inflammatory status of the amniotic cavity in patients presenting with TCC, whereas patients approaching term underwent an amniocentesis for assessment of fetal lung maturity. This information was used by obstetricians and neonatologists in the management of mothers and neonates in terms of treatment with antibiotics. Women at term in labor consisted of those who were admitted for suspected preterm labor because of uncertain dates and those who had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering whether these patients were at term in labor were derived retrospectively, and were as follows: 1) spontaneous labor; 2) delivery within 24 h of amniocentesis; 3) analysis of amniotic fluid consistent with fetal lung maturity; 4) birth weight >2,500 g; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by a pediatrician that was consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell count (35), glucose concentration (36), and Gram stain (37) were also performed shortly after collection, as previously described. The results of these tests were used for clinical management. Results of IL-6 concentrations in amniotic fluid were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged at 1,300 g for 10 min at 4°C, and the supernatant was stored at −70°C.
Detection of microorganisms with molecular methods
In addition to standard cultivation techniques, the amniotic fluid of patients with clinical chorioamnionitis was analyzed using broad-range real-time PCR/ESI/MS (Ibis®Technology - Athogen), as previously described (34). Briefly, DNA was extracted from 300 µl of amniotic fluid using a method that combines bead-beating cell lysis with a magnetic-bead-based extraction method (38, 39). The extracted DNA was amplified by the previously described broad bacteria and candida detection assay, according to the manufacturer’s instructions. PCR/ESI/MS can identify 3,400 bacteria and 40 Candida spp., which are represented in the platform’s signature database (40).
After PCR amplification, 30 µl aliquots of each PCR product were desalted and analyzed via ESI/MS. The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared with a database. The sensitivity (lower limit of detection) of the assay for the detection of bacteria in blood is, on average, 100 colony-forming units (CFUs)/ml (95% confidence interval, 6–600 CFU/ml). A comparison of detection limits between blood and amniotic fluid shows that the assays have comparable detection limits (100 CFU/ml).
Determination of IL-6 concentrations in amniotic fluid
Concentrations of IL-6 in amniotic fluid were determined by sensitive and specific enzyme immunoassays obtained from R&D Systems (Minneapolis, MN). The initial assay validation was performed in our laboratory prior to the conduction of this study. The concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The sensitivity of the assay for IL-6 was 0.09 pg/ml.
Sample preparation and LC/MS analysis
Fatty acyl lipids from amniotic fluid samples were extracted and analyzed by LC/MS, as described earlier (26). However, the LC/MS analysis included multiple reaction monitoring (MRM) of metabolites of linoleic, eicosapentaenoic, and docosahexaenoic acids in addition to arachidonic acid. A complete list of the analyzed PUFA metabolites, the internal standards used, mass spectrometric conditions, and the detection limits of individual lipid mediators are presented in supplemental Table S1. Under standardized conditions, the detection limits of most eicosanoids are ∼2 pg on the column, and the limit of quantitation was 5 pg at a signal/noise ratio of 3. Because the sample volume used was 200 µl, this translates to an assay sensitivity of 0.03 nM for an average molecular mass of 330 of the detected eicosanoids.
Statistical analysis
For any detectable lipid analyte in a subject group, a zero value observed in any sample was replaced with half the average detection limit of the LC/MS method used for the eicosanoids (i.e., 0.015 nM). This ensures that information from all samples was used in the statistical analysis and that the fold change between groups was finite for each analyte. A Wilcoxon test, which does not rely on any distributional assumptions about the data, was used for all pair-wise group comparisons. The significance of P value from the Wilcoxon test is independent from the choice of the threshold concentration used to replace the values below the quantitation limits of the assay.
A parametric alternative to the Wilcoxon test was also applied by using a t-test for analytes with concentrations above the quantitation limits of the assay in all samples or using censored regression otherwise. In the presence of a perfect separation between groups, the maximum likelihood estimation involved in censored regression could not be applied, and hence a t-test was used instead.
To account for multiple testing, the P value obtained for all analytes in a given group comparison was adjusted to control the false discovery rate (FDR) (41). A threshold of 10% on the FDR was used to infer significance.
All analyses were performed under the R statistical language and environment, version 3.0 (www.r-project.org), using the censReg R package for the censored regression analysis (42).
RESULTS
Clinical characteristics of the study population
The demographic and clinical characteristics of patients are described in Table 1. The patient groups include women at term gestation with spontaneous labor (TLB, n = 35) and those with TCC (n = 24). Patients with TCC were further subdivided into 1) those with MIAC (TCC-MIAC, n = 12) and 2) those without MIAC (TCC-noMIAC, n = 12). IL-6 concentrations in amniotic fluid were greater in the TCC-MIAC group than in the TCC-noMIAC group; however, the statistical significance was marginal (P = 0.07). The TLB group had yet lower IL-6 concentrations than the TCC-noMIAC group.
TABLE 1.
Clinical and obstetrical characteristics of women in TLB and with TCC
| Characteristic | TLB (n = 35) | TCC-noMIAC (n = 12) | TCC-MIAC (n = 12) | P (TCC-noMIAC vs. TCC-MIAC |
| Maternal age (years) | 23 (20–29) | 21.5 (19.5–23.5) | 19 (17.8–25.3) | 0.4 |
| GA at amniocentesis and delivery (weeks) | 39 (38–40.2) | 39.6 (38.9–40.0) | 40.5 (39.9–40.8) | 0.04 |
| IL-6 (ng/ml) | 1.08* (0.76–1.71) | 3.2 (2.0–12.0) | 13.9 (5.0–22.0) | 0.07 |
| Birth weight (g) | 3,250 (3,100–3,730) | 3,500 (2,978–3,725) | 3,710 (3,565–3,795) | 0.09 |
GA, gestational age; IL, interleukin; TCC-MIAC, clinical chorioamnionitis at term with microbial invasion of the amniotic cavity; TCC-noMIAC, clinical chorioamnionitis at term without microbial invasion of the amniotic cavity; TLB, spontaneous labor at term. Values are expressed as median (interquartile range). *, Detectable only in 11 of 35 patients. P: Wilcoxon rank sum test.
LC/MS analysis of amniotic fluid fatty acyl lipidome
The LC/MS method included MRM transitions to encompass stable metabolites of linoleic acid, arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid produced by cyclooxygenase (COX), lipoxygenase, and the epoxygenase pathways of PUFA metabolism (details of the metabolites analyzed and the standard abbreviations are provided in supplemental Table S1). Each detected fatty acyl lipid was positively identified by a combination of matching HPLC retention time with the authentic standard and the unique MRM transition. Coefficient of variation between different LC/MS runs of samples was <5%. Of the total 144 individual PUFA metabolites monitored by the LC/MS method, 51 metabolites were detectable in more than 50% of the samples in any group (supplemental Table S2). Only the lipid mediators that showed significant differences between TLB and TCC-noMIAC with P < 0.005 were included in the figures.
PGs
Concentrations of PGs and their metabolites such as PGE2, PGF2α, bicyclo PGE2, and so forth, derived primarily from ω-6 PUFAs in the COX pathway were comparable in the amniotic fluid of patients with TLB and those with TCC with MIAC (TCC-MIAC) and without MIAC (TCC-noMIAC) (Table 2). These similarities were also evident when the median concentrations of primary PGs (PGE2 and PGF2α) and their corresponding downstream metabolites (15-keto PGE2, PGA2, 13,14-dihydro-15-keto PGE2 (measured as bicyclo PGE2) from PGE2, and 15-keto PGF2α, 13,14-dihydro-15-keto PGF2α from PGF2α) were summed to assess their aggregate biosynthesis (supplemental Table S3). Despite higher concentrations in TCC-MIAC and lower concentrations in TCC-noMIAC groups compared with the TLB group, there was no statistically significant difference in the median concentrations of the COX-derived eicosanoids between any of the patient groups. The exception was 13,14-dihydro-15-keto PGF2α, which was higher in the TCC-MIAC than in the TLB group (Table 2).
TABLE 2.
COX pathway-derived lipid mediators from PUFAs in human amniotic fluid in TLB with or without clinical chorioamnionitis
| Lipid | TLB | TCC-no MIAC | TCC-MIAC | P (TLB vs. TCC-noMIAC) | P (TLB vs. TCC-MIAC) | P (TCC-noMIAC vs. TCC-MIAC) |
| PGE2 | 65.2 (28.5–157.4) [34] | 37.5 (20.9–105.5) [11] | 94.4 (64.3–128.7) [12] | 0.19 | 0.67 | 0.11 |
| Bicyclo PGE2 | 173.0 (101.2–264.0) [35] | 123.6 (39.4–206.6) [11] | 185.1 (109.1–334.3) [12] | 0.21 | 0.34 | 0.13 |
| PGA2 | 56.3 (39.7–85.6) [34] | 44.6 (22.1–73.0) [11] | 52.5 (32.4–101.1) [12] | 0.33 | 0.59 | 0.24 |
| 19-Hydroxy PGE2 | 147.7 (88.4–243.3) [35] | 132.7 (95.6–157.7) [12] | 171.5 (82.0–299.4) [12] | 0.48 | 0.78 | 0.44 |
| PGE3 | 18.0 (12.8–27.1) [34] | 13.0 (8.12–20.2) [11] | 20.7 (18.0–31.2) [11] | 0.24 | 0.37 | 0.13 |
| Bicyclo PGE1 | 12.4 (5.54–16.3) [27] | 3.29 (0.02–12.4) [6] | 12.7 (5.26–19.1) [9] | 0.07 | 0.83 | 0.15 |
| 19-Hydroxy PGE1 | 33.7 (21.7–60.3) [32] | 30.9 (17.7–49.1) [12] | 37.5 (21.0–66.2) [12] | 0.53 | 0.85 | 0.29 |
| PGJ2 | 25.9 (12.1–33.2) [29] | 24.9 (5.20–27.7) [9] | 26.8 (14.3–45.6) [10] | 0.46 | 0.58 | 0.37 |
| PGF2α | 10.2 (0.02–24.6) [23] | 3.86 (0.02–14.8) [6] | 22.5 (0.02–43.6) [8] | 0.41 | 0.30 | 0.22 |
| 15-Keto PGF2α | 0.02 (0.02–13.2) [13] | 0.02 (0.02–0.02) [2] | 0.02 (0.02–16.1) [5] | 0.21 | 0.69 | 0.21 |
| 13,14-Dihydro-15-keto-PGF2α | 10.8 (0.02–20.3) [24] | 15.2 (9.15–22.0) [10] | 18.5 (17.2–28.3) [12] | 0.26 | 0.01 | 0.17 |
All concentrations are in nM. Values in parentheses are interquartile ranges of analyte concentrations in nM, and those in brackets are number of samples with detectable levels of the corresponding lipid mediators. All P values are derived from Wilcoxon tests. P values <0.05 are italicized.
Lipoxygenase pathway metabolites
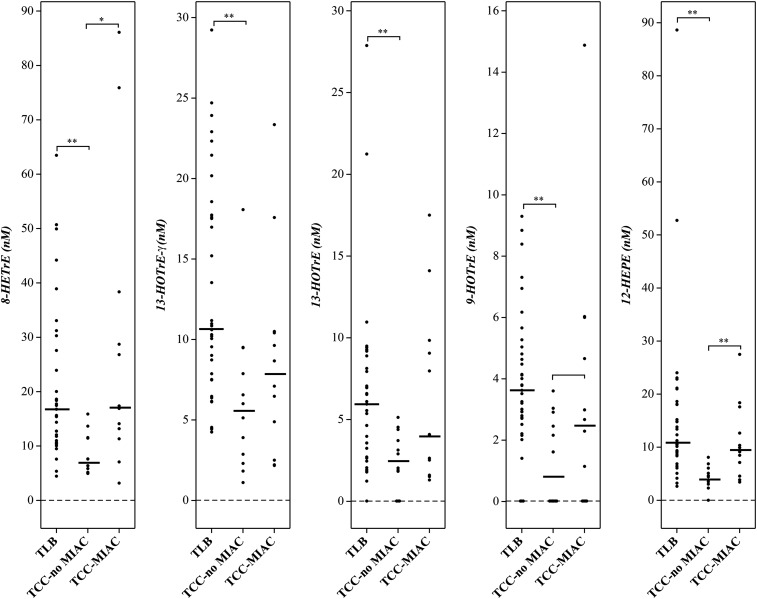
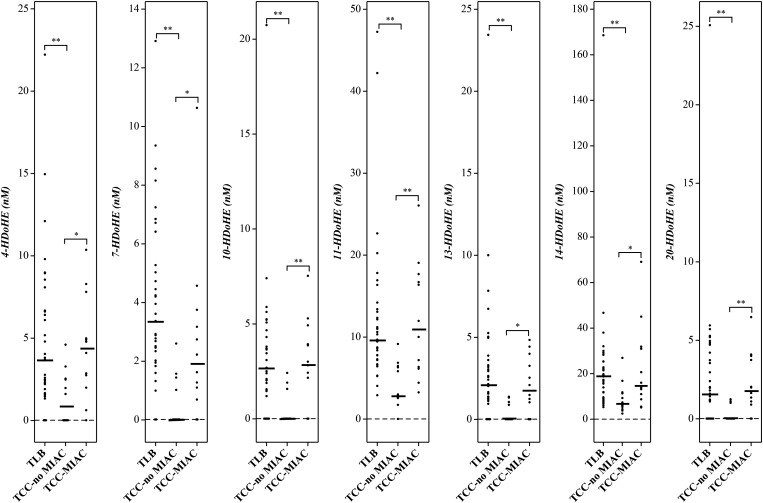
Hydroxy fatty acids derived from both ω-6 and ω-3 PUFAs in the lipoxygenase pathway were detectable in all three patient groups (Table 3). There were no significant differences between TLB and TCC-MIAC with respect to the median concentrations of the hydroxy fatty acids from either ω-6 or ω-3 PUFAs, except for 12-HETE, 15-HETE, and 11-HEPE, which are higher in TCC-MIAC than in the TLB group (Table 3). On the other hand, virtually all hydroxy fatty acids derived from ω-3 PUFAs were significantly lower in patients with clinical chorioamnionitis without detectable microorganisms in the amniotic cavity (TCC-noMIAC) than in those with TLB (Table 3; Figs. 1, 2). While 5 of 9 hydroxy fatty acids from ω-6 PUFAs (Table 3) were also lower in patients with TCC-noMIAC than in those with TLB, the difference in concentration was more pronounced in ω-3 PUFA-derived hydroxy fatty acids (HOTrEs, HEPEs, and HDoHEs, 5-fold lower in TCC-noMIAC than TLB) than in ω-6 PUFA-derived hydroxy fatty acids (HOTrE-γ, HEDE, and HETEs, 2-fold lower in TCC-noMIAC than TLB) (supplemental Table S2).
TABLE 3.
Lipoxygenase pathway-derived lipid mediators from ω-3 and ω-6 PUFAs in human amniotic fluid in TLB with or without clinical chorioamnionitis
| Lipid | TLB | TCC-noMIAC | TCC-MIAC | P (TLB vs. TCC-noMIAC) | P (TLB vs. TCC-MIAC) | P (TCC-noMIAC vs. TCC-MIAC) |
| 9(S)-HOTrE (ω-3) | 3.63 (2.72–4.92) [32] | 0.82 (0.02–2.57) [6] | 2.48 (0.02–4.99) [8] | 5.32 × 10−4 | 0.18 | 0.22 |
| 13(S)-HOTrE (ω-3) | 5.95 (2.98–8.51) [34] | 2.47 (1.38–3.88) [9] | 4.07 (2.29–9.25) [12] | 2.28 × 10−3 | 0.75 | 0.13 |
| 5-HEPE (ω-3) | 0.02 (0.02–1.89) [16] | 0.02 (0.02–0.02) [1] | 0.02 (0.02–1.44) [5] | 0.02 | 0.56 | 0.06 |
| 11-HEPE (ω-3) | 2.01 (1.66–2.24) [30] | 2.67 (1.99–3.45) [12] | 2.50 (2.17–3.02) [12] | 0.02 | 0.01 | 0.67 |
| 12-HEPE (ω-3) | 10.9 (8.74–18.1) [35] | 4.11 (3.37–5.34) [11] | 9.53 (6.48–13.9) [12] | 1.99 × 10−6 | 0.25 | 2.32 × 10−3 |
| 15-HEPE (ω-3) | 1.50 (0.02–2.58) [23] | 0.02 (0.02–0.17) [3] | 2.18 (0.02–2.95) [8] | 0.01 | 0.73 | 0.02 |
| 4-HDoHE (ω-3) | 3.66 (1.79–6.65) [32] | 0.81 (0.02–2.51) [6] | 4.44 (2.56–5.70) [11] | 4.69 × 10−3 | 0.81 | 0.01 |
| 7-HDoHE (ω-3) | 3.37 (2.40–5.85) [33] | 0.02 (0.02–1.13) [4] | 1.93 (1.00–3.32) [10] | 1.35 × 10−5 | 0.05 | 0.01 |
| 10-HDoHE (ω-3) | 2.69 (1.51–4.06) [29] | 0.02 (0.02–1.58) [4] | 2.92 (2.37–4.18) [10] | 8.07 × 10−4 | 0.49 | 1.09 × 10−3 |
| 11-HDoHE (ω-3) | 9.70 (7.26–13.3) [35] | 2.98 (2.64–6.36) [11] | 11.0 (6.31–17.0) [12] | 3.18 × 10−5 | 0.97 | 1.11 × 10−3 |
| 13-HDoHE (ω-3) | 2.15 (1.24–3.46) [30] | 0.02 (0.02–0.93) [4] | 1.79 (0.79–3.45) [9] | 1.14 × 10−4 | 0.46 | 0.01 |
| 14-HDoHE (ω-3) | 19.2 (11.8–24.0) [35] | 6.81 (5.17–11.4) [12] | 14.9 (10.4–31.2) [12] | 4.93 × 10−4 | 0.80 | 0.02 |
| 17-HDoHE (ω-3) | 3.29 (1.67–5.79) [30] | 1.27 (0.02–2.51) [7] | 5.06 (2.72–8.04) [11] | 0.01 | 0.47 | 0.01 |
| 20-HDoHE (ω-3) | 1.61 (1.14–4.47) [27] | 0.02 (0.02–0.27) [3] | 1.82 (1.06–3.82) [10] | 3.35 × 10−4 | 0.92 | 1.77 × 10−3 |
| 13(S)-HOTrE (γ) (ω-6) | 10.6 (7.50–17.7) [35] | 5.57 (2.73–8.28) [12] | 7.88 (4.29–10.4) [12] | 1.03 × 10−3 | 0.07 | 0.27 |
| 8-HETrE (ω-6) | 16.8 (11.4–28.9) [35] | 7.00 (5.67–11.5) [12] | 17.1 (12.7–31.1) [12] | 1.55 × 10−4 | 0.76 | 0.01 |
| 8-HETE (ω-6) | 7.43 (4.66–11.9) [34] | 3.25 (2.62–4.46) [11] | 11.6 (6.27–16.6) [12] | 5.3 × 10−4 | 0.09 | 7.17 × 10−5 |
| 11-HETE (ω-6) | 19.2 (11.7–29.5) [35] | 10.9 (4.64–16.5) [12] | 28.9 (15.1–39.9) [12] | 0.009 | 0.25 | 0.005 |
| 12-HETE (ω-6) | 144.6 (108.7–207.1) [35] | 100.7 (69.2–137.8) [12] | 252.8 (163.7–373.4) [12] | 0.02 | 0.005 | 2.74 × 10−4 |
| 15-HETE (ω-6) | 21.8 (15.5–30.4) [35] | 14.7 (6.51–22.7) [12] | 54.6 (24.7–83.8) [12] | 0.09 | 0.03 | 0.003 |
HDoHE, hydroxy docosahexaenoic acid; HEDE, hydroxy eicosadienoic acid; HEPE, hydroxy eicosapentaenoic acid; HETrE, hydroxy eicosatrienoic acid; HOTrE, hydroxy octadecatrienoic acid. All concentrations are in nM. Values in parentheses are interquartile ranges of analyte concentrations in nM, and those in brackets are number of samples with detectable levels of the corresponding lipid mediators. All P values are derived from Wilcoxon tests. P values <0.05 are italicized.
Fig. 1.
Lipoxygenase pathway metabolites of ω-6 PUFAs [dihomo-γ-linolenic (8-HETrE) and γ-linolenic (13-HOTrE-γ) acids] and ω-3 PUFAs [α-linolenic (9-HOTrE and 13-HOTrE) and eicosapentaenoic (12-HEPE) acids] detected in amniotic fluid from women in TLB, compared with those with TCC without (TCC-noMIAC) or with MIAC (TCC-MIAC). Cross bars: median concentrations. * P < 0.05; ** P < 0.005.
Fig. 2.
Lipoxygenase pathway metabolites of docosahexaenoic acid (ω-3 PUFAs) that exhibited significant difference between patient groups. Patient group abbreviations and other details are the same as in Fig. 1.
Epoxygenase pathway metabolites
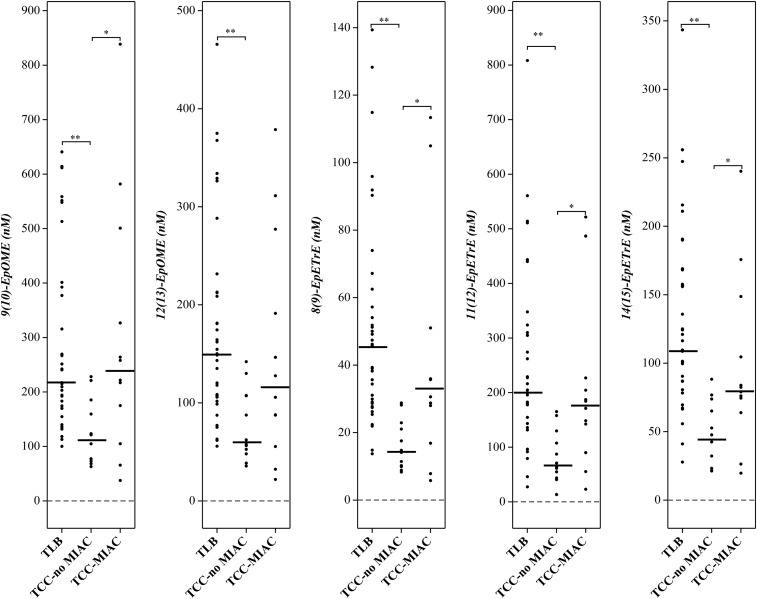
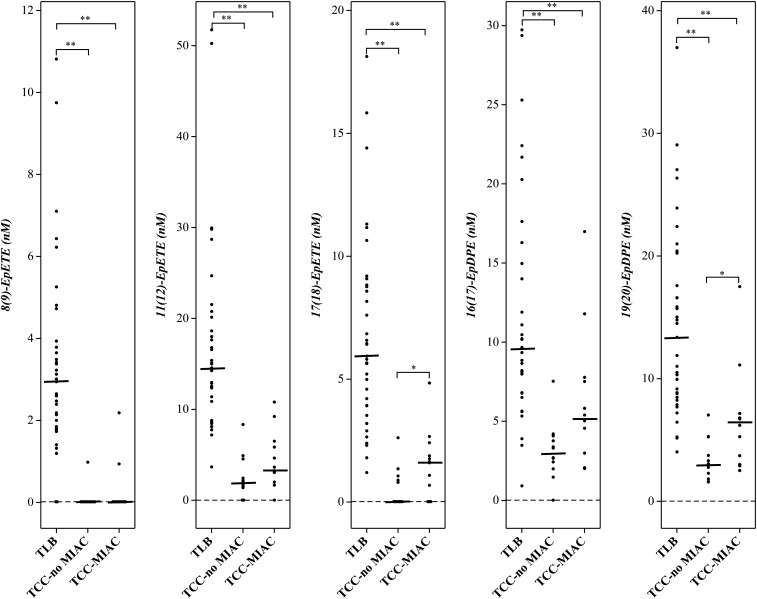
The concentration of epoxy fatty acids derived from the metabolism of PUFAs in the epoxygenase pathway, especially from ω-3 PUFAs (EpETEs and EpDPEs), were significantly lower in patients with TCC, regardless of the status of MIAC (TCC-noMIAC and TCC-MIAC) than in TLB (P < 10−7 to 10−3) (Table 4; Figs. 3, 4). Alternatively, the epoxy fatty acids derived from the epoxygenase metabolism of ω-6 PUFAs (EpOMEs and EpETrEs) were only lower in patients with clinical chorioamnionitis without MIAC (TCC-noMIAC, P < 10−7 to 10−4), but not in those with TCC-MIAC, (P = 0.1 to 0.97) than in those with TLB (Table 4; Fig. 3). Vicinyl dihydroxy PUFAs resulting from the hydrolysis of these epoxy fatty acids were also included in the analysis (supplemental Table S1) but were not detectable in any of these samples (data not shown).
TABLE 4.
Epoxygenase pathway-derived lipid mediators from ω-6 and ω-3 PUFAs in human amniotic fluid in TLB with or without clinical chorioamnionitis
| Lipid | TLB | TCC-noMIAC | TCC-MIAC | P (TLB vs. TCC-noMIAC) | P (TLB vs. TCC-MIAC) | P (TCC-noMIAC vs. TCC-MIAC) |
| 9(10)-EpOME | 217.2 (172.2–384.9) [35] | 113.0 (76.2–166.1) [12] | 239.8 (157.4–370.2) [12] | 1.20 × 10−4 | 0.97 | 0.03 |
| 12(13)-EpOME | 150.2 (106.4–212.7) [35] | 59.9 (51.5–107.3) [12] | 116.7 (79.5–212.7) [12] | 1.06 × 10−4 | 0.28 | 0.16 |
| 8(9)-EpETrE | 45.7 (28.9–59.9) [35] | 14.4 (10.2–21.5) [12] | 33.2 (25.2–39.7) [12] | 4.97 × 10−7 | 0.21 | 0.02 |
| 11(12)-EpETrE | 200.2 (166.1–307.6) [35] | 67.4 (52.1–113.1) [12] | 177.8 (129.3–210.0) [12] | 6.77 × 10−6 | 0.24 | 0.01 |
| 14(15)-EpETrE | 109.6 (83.7–162.9) [35] | 45.1 (23.2–67.3) [12] | 80.7 (71.8–115.6) [12] | 2.86 × 10−6 | 0.10 | 0.02 |
| 8(9)-EpETE (ω-3) | 2.94 (1.83–3.86) [33] | 0.02 (0.02–0.02) [1] | 0.02 (0.02–0.02) [2] | 1.26 × 10−6 | 5.33 × 10−6 | NA |
| 11(12)-EpETE (ω-3) | 14.6 (11.1–19.4) [35] | 1.94 (1.42–2.96) [10] | 3.45 (2.79–6.00) [11] | 6.87 × 10−7 | 7.70 × 10−8 | 0.07 |
| 17(18)-EpETE (ω-3) | 5.94 (3.92–8.80) [35] | 0.02 (0.02–0.93) [5] | 1.62 (0.51–2.02) [9] | 5.81 × 10−7 | 7.55 × 10−6 | 0.04 |
| 16(17)-EpDPE (ω-3) | 9.62 (7.39–14.5) [35] | 3.00 (2.32–3.84) [11] | 5.21 (2.76–7.58) [12] | 6.01 × 10−6 | 3.55 × 10−3 | 0.05 |
| 19(20)-EpDPE (ω-3) | 13.4 (8.63–18.9) [35] | 3.02 (2.17–4.12) [12] | 6.46 (3.54–6.89) [12] | 1.06 × 10−6 | 1.55 × 10−4 | 0.02 |
EpDPE, epoxy docosapentaenoic acid; EpETE, epoxy eicosatetraenoic acid; EpETrE, epoxy eicosatrienoic acid; EpOME, epoxy octadecenoic acid. All concentrations are in nM. Values in parentheses are interquartile ranges of analyte concentrations in nM, and those in brackets are number of samples with detectable levels of the corresponding lipid mediators. All P values are derived from Wilcoxon tests. P values <0.05 were italicized.
Fig. 3.
Epoxygenase pathway metabolites of linoleic and arachidonic acids (ω-6 PUFAs) that exhibited significant difference between patient groups. Patient group abbreviations and other details are the same as in Fig. 1.
Fig. 4.
Epoxygenase pathway metabolites of eicosapentaenoic and docosahexaenoic acids (ω-3 PUFAs) that exhibited significant difference between patient groups. Patient group abbreviations and other details are the same as in Fig. 1.
DISCUSSION
Principal findings of the study include the following: 1) the amniotic fluid concentrations of anti-inflammatory/proresolution lipid mediators are significantly lower in women with TCC without detectable microorganisms in the amniotic cavity than in those with TLB; and 2) there were no significant differences in the amniotic fluid concentrations of PGs and other known inflammatory lipid mediators between spontaneous labor and women in labor at term with clinical chorioamnionitis.
Amniotic fluid PG concentrations are similar between patients with TLB regardless of the presence or absence of intra-amniotic inflammation
Spontaneous parturition at term is akin to inflammation by virtue of leukocyte infiltration in gestational tissues (43, 44), as well as elevated concentrations of inflammatory cytokines such as IL-1α, IL-1β (45), IL-6 (44, 46), IL-8 (47), TNF-α (48), and monocyte chemoattractant protein-1 (49). Induction of COX-2 expression in placental membranes in response to elevated cytokines also results in enhanced biosynthesis of PGs such as PGE2 and PGF2α in amniotic fluid, even in the absence of intra-amniotic infection and precedes the onset of labor at term (24, 25, 50–52). Our recent MS-based lipidomic analysis of human amniotic fluid at term confirmed significantly higher concentrations of PGs in TLB than in term without labor (26). In addition, we have identified several other arachidonic acid derived lipid mediators of epoxygenase and lipoxygenase pathways.
The median IL-6 concentrations in amniotic fluid of patients with TCC are significantly higher than in those with TLB (Table 1). However, the difference in the concentrations of IL-6 are not significantly different between the two subgroups of clinical chorioamnionitis (P = 0.07). Regardless of the presence of microorganisms in the amniotic cavity in clinical chorioamnionitis (TCC-noMIAC and TCC-MIAC), there was no statistically significant increase in the inflammatory eicosanoids such as PGE2 or other arachidonic acid-derived lipid mediators over TLB (Table 2). In fact, the concentrations of PGs (both PGE2 and PGF2α, as well as their downstream metabolites; supplemental Table S3) were lower in clinical chorioamnionitis compared with TLB, albeit not statistically significant. LTB4, the chemotactic leukotriene, and other 5-lipoxygenase pathway-derived metabolites are virtually undetectable in patients with clinical chorioamnionitis without MIAC and intra-amniotic inflammation (sterile intra-amniotic inflammation) or in those with TLB, except in patients with clinical chorioamnionitis with intra-amniotic inflammation and infection (27). Therefore, sterile intra-amniotic inflammation associated with clinical chorioamnionitis is not due to a higher concentration of PGs and other inflammatory lipid mediators that are normally increased in amniotic fluid during spontaneous labor (and play an important role in the physiological process of parturition). Thus, despite higher amniotic fluid concentrations of the inflammatory cytokine IL-6, in clinical chorioamnionitis compared with TLB, there is no corresponding increase in PGs. These data strongly suggest a dissociation in the normally coordinated expression of inflammatory cytokines and COX-2 mediated PG biosynthesis, at least in the context of clinical chorioamnionitis with or without intra-amniotic infection.
Anti-inflammatory and precursors of specialized proresolution lipid mediators are lower in the amniotic fluid of patients with TCC
Our recent eicosanomic analysis of human amniotic fluid in labor at term demonstrated the presence of substantial concentrations of epoxygenase pathway metabolites of arachidonic acid (median concentration of EpETrEs: 39–191 nM) (26). An expanded fatty acyl lipidomic analysis to encompass metabolites of other essential PUFAs such as linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid, along with arachidonic acid, revealed the presence of epoxides of these PUFAs as well in human amniotic fluid in TLB (Table 4; supplemental Table S2). However, the concentration of epoxygenase pathway metabolites of PUFAs, especially those derived from ω-3 PUFAs, were significantly lower in the amniotic fluid of patients with clinical chorioamnionitis, regardless of the presence/absence of bacteria in the amniotic fluid, than in those with TLB (Table 4; Figs. 3, 4). As can be expected, not all epoxy PUFA concentrations were lower (in relation to PGs) to the same extent (Table 4; supplemental Table S3). While 8(9)-EpETE is undetectable in both clinical chorioamnionitis groups and 17(18)-EpETE was not detectable in TCC-noMIAC, the rest of the epoxy PUFAs were lower to varying degrees (Table 4). The differences in the concentrations (as well as the differences in reduced concentrations in clinical chorioamnionitis) of the epoxy PUFAs may represent different, yet unknown, physiological effects in clinical chorioamnionitis. This lower concentration of the epoxy PUFAs is not due to their metabolism by epoxide hydrolase, because little, if any, of the corresponding dihydroxy PUFAs were detectable in amniotic fluid of any of these patients. Although an effective efflux of the epoxide hydrolysis products from the amniotic cavity is a possibility, their complete absence in amniotic fluid, despite high concentrations of the PUFA epoxides, suggests minimal, if any, epoxide hydrolase activity in the amniotic cavity. Further studies on the expression and enzymatic activity of epoxide hydrolases in placental membranes are needed to confirm these results.
Concentrations of lipoxygenase pathway metabolites of ω-3 PUFAs (α-linolenic, eicosapentaenoic, and docosahexaenoic acids) (Figs. 1, 2) were also significantly lower in patients with TCC without bacteria in the amniotic cavity (TCC-noMIAC) than in patients with TLB or those with TCC with intra-amniotic infection (TCC-MIAC) (Table 3). Any differences in concentration of hydroxy PUFAs between TLB and TCC-MIAC were not significant. Thus, while the COX pathway metabolism essentially remains unchanged in labor at term regardless of clinical chorioamnionitis, epoxygenase and lipoxygenase pathway metabolism, especially that of ω-3 PUFAs, is significantly lower only in TCC-noMIAC.
Metabolites of ω-3 PUFAs are generally considered anti-inflammatory by virtue of their partial agonist/antagonist properties against the eicosanoid receptors (53–55). Metabolism of ω-6 PUFAs, especially arachidonic acid, is also inhibited by their ω-3 analogs (56, 57). In addition to the reduced agonist activity of ω-3 PUFA-derived PGs, resolution of inflammation by lipoxygenase metabolites of ω-3 PUFAs as an active process is now firmly established as a new paradigm by the identification and biological activity of lipoxins, resolvins, and protectins [collectively termed as specialized proresolution mediators (SPMs)] (21, 58, 59). While SPMs such as resolvins D1 and E1, protectin D1, and lipoxins A4, B4, and A5 were included in the analysis, they were not detected in these samples of human amniotic fluid. However, the detected hydroxy fatty acids of ω-3 PUFAs such as HEPEs and HDoHEs are known precursors of SPMs and several additional proresolution mediators that were reported in recent years (60, 61). Significantly lower concentrations or absence of these SPM precursors likely signify a failure of resolution of inflammation that characterizes clinical chorioamnionitis in the absence of microbial invasion of the amniotic cavity (TCC-noMIAC).
Although little is known about the role of epoxy PUFAs in reproductive biology, studies in cardiovascular and renal physiology showed that epoxy PUFAs are anti-inflammatory by inhibition of cytokine-mediated activation of nuclear factor κB as well as their analgesic, antihypertensive, and antifibrotic activities (62–64). It is likely that the anti-inflammatory activities of epoxy PUFAs provide a balance to the proinflammatory properties of PGs (e.g., PGE2) in human amniotic fluid during TLB as a normal physiological response. Thus, the ratio of PGs to the epoxy PUFAs in TLB likely signifies a homeostatic response in a normal physiological event. Any significant change in this ratio in favor of PGs, either by an increase in PGs or by a decrease in epoxy PUFAs, could result in an inflammatory condition. Indeed, the ratios of both PGE2 and PGF2α (as well as the sums of the metabolites of each of these PGs) tilted 50% to 100% in favor of PGs, primarily due to decreased concentrations of epoxy PUFAs (supplemental Table S3). This suggests an intriguing possibility that a loss of balance between pro- and anti-inflammatory lipid mediators in TCC, represented by COX and epoxygenase metabolites, respectively, manifests in sterile intra-amniotic inflammation. This inflammatory response is further exacerbated by a failure of proresolution mediator biosynthesis as suggested by lower concentrations of hydroxy ω-3 PUFAs, the precursors of SPMs (vide supra). Furthermore, it can be surmised that physiological responses such as fever and tachycardia that are mediated by PG, are held in abeyance during TLB by the antipyretic, analgesic, and anti-inflammatory actions of the epoxygenase- and lipoxygenase-derived lipid mediators. This represents a novel concept that clinical chorioamnionitis, in the absence of MIAC, is a result of failed endogenous anti-inflammatory lipid mediator biosynthesis in an ordered and latent “inflammatory” physiological process (i.e., parturition).
In conclusion, the amniotic fluid fatty acyl lipidomic profile of TCC is distinctly different from that of spontaneous labor. Clinical chorioamnionitis, either driven by intra-amniotic infection or idiopathic, is characterized by significantly lower concentrations of anti-inflammatory and proresolution lipid mediators in human amniotic fluid. Spontaneous parturition at term is characterized by increased expression of PUFAs metabolizing enzymes such as COX-2 (65, 66), elevated cytokine expression (44, 45, 48, 49, 67), and leukocyte chemotaxis (44) and is generally considered a physiological inflammatory response. Recent unbiased, myometrial transcriptomic analysis further confirmed the overexpression of inflammatory genes in TLB (68, 69). Despite the heightened inflammatory molecular signatures during spontaneous parturition at term, return to homeostasis is normally uneventful, except in clinical chorioamnionitis. While intra-amniotic infection (microbial-associated intra-amniotic inflammation) can be understood as a pathological event, data from the lipidomic analysis presented in this report strongly suggest that TCC in the absence of MIAC (as well as that resulting from intra-amniotic infection, at least in part) is a result of “unchecked inflammation” of a physiological process due to insufficient anti-inflammatory and proresolution lipid mediator response to return the system to homeostasis. Further studies to elucidate the mechanisms of this anti-inflammatory and proresolution insufficiency could identify valuable biomarkers of impending clinical chorioamnionitis and/or pharmacological targets to alleviate both short- and long-term complications associated with clinical chorioamnionitis for the neonate.
Supplementary Material
Footnotes
Abbreviations:
- CFU
- colony-forming unit
- COX
- cyclooxygenase
- EpDPE
- epoxy docosapentaenoic acid
- EpETE
- epoxy eicosatetraenoic acid
- EpETrE
- epoxy eicosatrienoic acid
- HDoHE
- hydroxy docosahexaenoic acid
- HEDE
- hydroxy eicosadienoic acid
- HEPE
- hydroxy eicosapentaenoic acid
- HOTrE
- hydroxy octadecatrienoic acid
- IL
- interleukin
- LTB4
- leukotriene B4
- MIAC
- microbial invasion of the amniotic cavity
- MRM
- multiple reaction monitoring
- PG
- prostaglandin
- SPM
- specialized proresolution mediator
- TCC
- clinical chorioamnionitis at term
- TCC-MIAC
- clinical chorioamnionitis at term with microbial invasion of the amniotic cavity
- TCC-noMIAC
- clinical chorioamnionitis at term without microbial invasion of the amniotic cavity
- TLB
- spontaneous labor at term
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, Department of Health and Human Services; in part with federal funds from NICHD (Foundation for the National Institutes of Health, contract no. HHSN275201300006C); and in part by the National Center for Research Resources (Foundation for the National Institutes of Health, grant S10RR027926) and a Perinatal Virtual Discovery Grant from Wayne State University (K.R.M.). This article is a continuation of our biochemical characterization of clinical chorioamnionitis at term and represents the eighth in the series. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gibbs R. S., Romero R., Hillier S. L., Eschenbach D. A., and Sweet R. L.. 1992. A review of premature birth and subclinical infection. Am. J. Obstet. Gynecol. 166: 1515–1528. [DOI] [PubMed] [Google Scholar]
- 2.Romero R., Dey S. K., and Fisher S. J.. 2014. Preterm labor: one syndrome, many causes. Science. 345: 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravett M. G., Hummel D., Eschenbach D. A., and Holmes K. K.. 1986. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet. Gynecol. 67: 229–237. [DOI] [PubMed] [Google Scholar]
- 4.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Dong Z., Chaiyasit N., et al. . 2016. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J. Perinat. Med. 44: 77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malloy M. H. 2014. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J. Perinatol. 34: 611–615. [DOI] [PubMed] [Google Scholar]
- 6.Romero R., Miranda J., Kusanovic J. P., Chaiworapongsa T., Chaemsaithong P., Martinez A., Gotsch F., Dong Z., Ahmed A. I., Shaman M., et al. . 2015. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J. Perinat. Med. 43: 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C. J., Romero R., Chaemsaithong P., Chaiyasit N., Yoon B. H., and Kim Y. M.. 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213: S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero R., Espinoza J., Goncalves L. F., Kusanovic J. P., Friel L., and Hassan S.. 2007. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 25: 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R., Gotsch F., Pineles B., and Kusanovic J. P.. 2007. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 65: S194–S202. [DOI] [PubMed] [Google Scholar]
- 10.Christiaens I., Zaragoza D. B., Guilbert L., Robertson S. A., Mitchell B. F., and Olson D. M.. 2008. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 79: 50–57. [DOI] [PubMed] [Google Scholar]
- 11.Becroft D. M. O., Thompson J. M. D., and Mitchell E. A.. 2010. Placental chorioamnionitis at term: epidemiology and follow-up in childhood. Pediatr. Dev. Pathol. 13: 282–290. [DOI] [PubMed] [Google Scholar]
- 12.Elovitz M. A., Brown A. G., Breen K., Anton L., Maubert M., and Burd I.. 2011. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 29: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas W., and Speer C. P.. 2011. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology. 99: 177–187. [DOI] [PubMed] [Google Scholar]
- 14.Tita A. T. N., and Andrews W. W.. 2010. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 37: 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton E. R. 1993. Chorioamnionitis and intraamniotic infection. Clin. Obstet. Gynecol. 36: 795–808. [DOI] [PubMed] [Google Scholar]
- 16.Romero R., Chaemsaithong P., Korzeniewski S. J., Kusanovic J. P., Docheva N., Martinez-Varea A., Ahmed A. I., Yoon B. H., Hassan S. S., Chaiworapongsa T., et al. . 2016. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J. Perinat. Med. 44: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G. Y., and Nunez G.. 2010. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R., Chaiworapongsa T., Savasan Z. A., Hussein Y., Dong Z., Kusanovic J. P., Kim C. J., and Hassan S. S.. 2012. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J. Matern. Fetal Neonatal Med. 25: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Lopez N., Romero R., Plazyo O., Panaitescu B., Furcron A. E., Miller D., Roumayah T., Flom E., and Hassan S. S.. 2016. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am. J. Reprod. Immunol. 75: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan C. N., Chiang N., and Van Dyke T. E.. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan C. N., and Savill J.. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 22.Ricciotti E., and Fitzgerald G. A.. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris S. G., Padilla J., Koumas L., Ray D., and Phipps R. P.. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23: 144–150. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell M. D. 1981. Prostaglandins during pregnancy and the perinatal period. J. Reprod. Fertil. 62: 305–315. [DOI] [PubMed] [Google Scholar]
- 25.Romero R., Gonzalez R., Baumann P., Behnke E., Rittenhouse L., Barberio D., Cotton D. B., and Mitchell M. D.. 1994. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot. Essent. Fatty Acids. 50: 97–104. [DOI] [PubMed] [Google Scholar]
- 26.Maddipati K. R., Romero R., Chaiworapongsa T., Zhou S. L., Xu Z., Tarca A. L., Kusanovic J. P., Munoz H., and Honn K. V.. 2014. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 28: 4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maddipati K. R., Romero R., Chaiworapongsa T., Chaemsaithong P., Zhou S-L., Xu Z., Tarca A. L., Kusanovic J. P., Gomez R., Chaiyasit N., et al. . 2016. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. FASEB J. In press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy B. D., Clish C. B., Schmidt B., Gronert K., and Serhan C. N.. 2001. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2: 612–619. [DOI] [PubMed] [Google Scholar]
- 29.Serhan C. N. 2005. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. 73: 141–162. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann C., Homann J., Ball A-K., Blöcher R., Kleinschmidt T. K., Basavarajappa D., Angioni C., Ferreirós N., Häfner A-K., Rådmark O., et al. . 2015. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 29: 5029–5043. [DOI] [PubMed] [Google Scholar]
- 31.Romero R., Chaemsaithong P., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Dong Z., Docheva N., Martinez-Varea A., et al. . 2016. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J. Perinat. Med. 44: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Chaiyasit N., Dong Z., et al. . 2016. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J. Perinat. Med. 44: 53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Kusanovic J. P., Yoon B. H., Kim J. S., Chaiyasit N., Ahmed A. I., Qureshi F., et al. . 2016. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J. Perinat. Med. 44: 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R., Miranda J., Chaiworapongsa T., Chaemsaithong P., Gotsch F., Dong Z., Ahmed A. I., Yoon B. H., Hassan S. S., Kim C. J., et al. . 2014. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 71: 330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R., Quintero R., Nores J., Avila C., Mazor M., Hanaoka S., Hagay Z., Merchant L., and Hobbins J. C.. 1991. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am. J. Obstet. Gynecol. 165: 821–830. [DOI] [PubMed] [Google Scholar]
- 36.Romero R., Jimenez C., Lohda A. K., Nores J., Hanaoka S., Avila C., Callahan R., Mazor M., Hobbins J. C., and Diamond M. P.. 1990. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am. J. Obstet. Gynecol. 163: 968–974. [DOI] [PubMed] [Google Scholar]
- 37.Romero R., Emamian M., Quintero R., Wan M., Hobbins J. C., Mazor M., and Edberg S.. 1988. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am. J. Obstet. Gynecol. 159: 114–119. [DOI] [PubMed] [Google Scholar]
- 38.Eshoo M. W., Crowder C. C., Rebman A. W., Rounds M. A., Matthews H. E., Picuri J. M., Soloski M. J., Ecker D. J., Schutzer S. E., and Aucott J. N.. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 7: e36825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J. H., Ranken R., Sefers S. E., Lovari R., Quinn C. D., Meng S., Carolan H. E., Toleno D., Li H., Lee J. N., et al. . 2013. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J. Clin. Microbiol. 51: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzgar D., Frinder M., Lovari R., Toleno D., Massire C., Blyn L. B., Ranken R., Carolan H. E., Hall T. A., Moore D., et al. . 2013. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J. Clin. Microbiol. 51: 2670–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y., and Hochberg Y.. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 57: 289–300. [Google Scholar]
- 42.Henningsen A. 2011. censReg: censored regression (Tobit) models. In R Package, Version 0.5. (Accessed September 6, 2011, at http://cran.r-project.org/package=censReg. )
- 43.Liggins G. 1981. Cervical ripening as an inflammatory reaction. In The Cervix in Pregnancy and Labour: Clinical and Biochemical Investigations. D. A. Ellwood and A. B. M. Anderson, editors. Churchill Livingstone, Edinburgh. 1–9. [Google Scholar]
- 44.Osman I., Young A., Ledingham M. A., Thomson A. J., Jordan F., Greer I. A., and Norman J. E.. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 45.Romero R., Mazor M., Brandt F., Sepulveda W., Avila C., Cotton D. B., and Dinarello C. A.. 1992. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am. J. Reprod. Immunol. 27: 117–123. [DOI] [PubMed] [Google Scholar]
- 46.Romero R., Sepulveda W., Kenney J. S., Archer L. E., Allison A. C., and Sehgal P. B.. 1992. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found. Symp. 167: 205–220; discussion 220–223. [DOI] [PubMed] [Google Scholar]
- 47.Romero R., Ceska M., Avila C., Mazor M., Behnke E., and Lindley I.. 1991. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am. J. Obstet. Gynecol. 165: 813–820. [DOI] [PubMed] [Google Scholar]
- 48.Maymon E., Ghezzi F., Edwin S. S., Mazor M., Yoon B. H., Gomez R., and Romero R.. 1999. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am. J. Obstet. Gynecol. 181: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 49.Esplin M. S., Romero R., Chaiworapongsa T., Kim Y. M., Edwin S., Gomez R., Gonzalez R., and Adashi E. Y.. 2003. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J. Matern. Fetal Neonatal Med. 14: 51–56. [DOI] [PubMed] [Google Scholar]
- 50.Lee S. E., Romero R., Park I. S., Seong H. S., Park C. W., and Yoon B. H.. 2008. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J. Matern. Fetal Neonatal Med. 21: 89–94. [DOI] [PubMed] [Google Scholar]
- 51.Romero R., Munoz H., Gomez R., Parra M., Polanco M., Valverde V., Hasbun J., Garrido J., Ghezzi F., Mazor M., et al. . 1996. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot. Essent. Fatty Acids. 54: 187–191. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell M. D., Romero R. J., Edwin S. S., and Trautman M. S.. 1995. Prostaglandins and parturition. Reprod. Fertil. Dev. 7: 623–632. [DOI] [PubMed] [Google Scholar]
- 53.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M.. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narumiya S., Sugimoto Y., and Ushikubi F.. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu T. 2009. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49: 123–150. [DOI] [PubMed] [Google Scholar]
- 56.Wada M., DeLong C. J., Hong Y. H., Rieke C. J., Song I., Sidhu R. S., Yuan C., Warnock M., Schmaier A. H., Yokoyama C., et al. . 2007. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 282: 22254–22266. [DOI] [PubMed] [Google Scholar]
- 57.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. . 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J. Biol. Chem. 285: 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., and Serhan C. N.. 2005. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174: 4345–4355. [DOI] [PubMed] [Google Scholar]
- 59.Serhan C. N., and Petasis N. A.. 2011. Resolvins and protectins in inflammation resolution. Chem. Rev. 111: 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colas R. A., Shinohara M., Dalli J., Chiang N., and Serhan C. N.. 2014. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307: C39–C54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan C. N. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., and Liao J. K.. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Oltman C. L., Lu T., Lee H. C., Dellsperger K. C., and VanRollins M.. 2001. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 280: H2430–H2440. [DOI] [PubMed] [Google Scholar]
- 64.Morisseau C., Inceoglu B., Schmelzer K., Tsai H-J., Jinks S. L., Hegedus C. M., and Hammock B. D.. 2010. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 51: 3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi S. J., Oh S., Kim J. H., and Roh C. R.. 2007. Changes of nuclear factor kappa B (NF-kappaB), cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9) in human myometrium before and during term labor. Eur. J. Obstet. Gynecol. Reprod. Biol. 132: 182–188. [DOI] [PubMed] [Google Scholar]
- 66.Fuentes A., Spaziani E. P., and O’Brien W. F.. 1996. The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins. 52: 261–267. [DOI] [PubMed] [Google Scholar]
- 67.Keelan J. A., Blumenstein M., Helliwell R. J., Sato T. A., Marvin K. W., and Mitchell M. D.. 2003. Cytokines, prostaglandins and parturition—a review. Placenta. 24: S33–S46. [DOI] [PubMed] [Google Scholar]
- 68.Mittal P., Romero R., Tarca Adi L., Gonzalez J., Draghici S., Xu Y., Dong Z., Nhan-Chang C-L., Chaiworapongsa T., Lye S., et al. . 2010. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J. Perinat. Med. 38: 617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bollapragada S., Youssef R., Jordan F., Greer I., Norman J., and Nelson S.. 2009. Term labor is associated with a core inflammatory response in human myometrium and cervix. Am. J. Obstet. Gynecol. 200: 104.e1–104.e11. [Erratum. 2009. Am. J. Obstet. Gynecol. 201: 214.] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.