Abstract
The accumulation of lipids is a histologic and biochemical hallmark of obesity-associated nonalcoholic fatty liver disease (NAFLD). A subset of NALFD patients develops progressive liver disease, termed nonalcoholic steatohepatitis, which is characterized by hepatocellular apoptosis and innate immune system-mediated inflammation. These responses are orchestrated by signaling pathways that can be activated by lipids, directly or indirectly. In this review, we discuss palmitate- and lysophosphatidylcholine (LPC)-induced upregulation of p53-upregulated modulator of apoptosis and cell-surface expression of the death receptor TNF-related apoptosis-inducing ligand receptor 2. Next, we review the activation of stress-induced kinases, mixed lineage kinase 3, and c-Jun N-terminal kinase, and the activation of endoplasmic reticulum stress response and its downstream proapoptotic effector, CAAT/enhancer binding homologous protein, by palmitate and LPC. Moreover, the activation of these stress signaling pathways is linked to the release of proinflammatory, proangiogenic, and profibrotic extracellular vesicles by stressed hepatocytes. This review discusses the signaling pathways induced by lethal and sublethal lipid overload that contribute to the pathogenesis of NAFLD.
Keywords: nonalcoholic fatty liver disease, steatohepatitis, lipotoxicity, apoptosis, cell death, cell signaling, extracellular vesicles, exosomes, fatty acids, lysophosphatidylcholine, nonalcoholic steatohepatitis
Obesity-associated liver steatosis, referred to as nonalcoholic fatty liver disease (NAFLD), is characterized by the accumulation of neutral triglyceride droplets within hepatocytes (1, 2). Liver lipidomic analyses in NAFLD have demonstrated increased diacylglycerol, free cholesterol, and lysophosphatidylcholine (LPC), in addition to the morphologically obvious triglyceride (Table 1) (3–7). A host of metabolic perturbations, dietary factors, genetic influences, and environmental determinants predispose to steatosis, and these have been comprehensively reviewed elsewhere (8–11). A subset of NAFLD patients develops progressive liver injury, termed nonalcoholic steatohepatitis (NASH), characterized histologically by hepatic inflammation, fibrosis, and cirrhosis (12, 13), and molecularly by hepatocyte apoptosis (12). This group is clinically relevant due to the risks of liver failure, hepatocellular carcinoma, and the need for life-saving liver transplantation (2). Mechanistic studies of lipotoxicity have thus focused on NASH, due to its progressive nature and associated clinical risks.
TABLE 1.
Total and individual lipid classes significantly increased in liver tissue of NASH patients
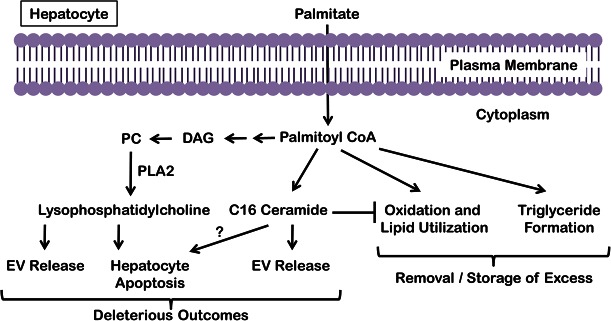
The lipids that can activate these processes are defined as toxic lipids in NASH pathogenesis. These toxic lipids can activate proapoptotic signaling and myriad stress responses in hepatocytes (14–20). In general, the saturated free fatty acids, palmitate and stearate, are directly cytotoxic, whereas the monounsaturated free fatty acids, such as oleate and palmitoleate, may protect from saturated fatty acid-induced toxicity, though the latter may impart sensitization to death receptor-mediated apoptosis (14, 15, 21). Furthermore, palmitate can induce the formation of both ceramide and LPC (6, 16, 22). Ceramide can be directly synthesized from palmitate via the de novo pathway at the endoplasmic reticulum (ER) (22, 23). LPC is formed via phospholipase A2 (PLA2) action on phosphatidylcholine, which in turn is derived from diacylglycerol; palmitate-induced diacylglycerol accumulation thus indirectly leads to LPC formation (Fig. 1) (6). LPC can activate proapoptotic signaling akin to palmitate (6, 16), and lead to extracellular vesicle (EV) release (24). Palmitate-derived C16:0 ceramide is associated with insulin resistance and steatohepatitis (25, 26), and at a cellular level can induce EV release (27). In this review, we discuss the signaling through which these lipids lead to cell death and inflammation (15, 28). Short of causing cell death, lipid overload leads to ballooned hepatocytes (29), which do not progress to cell death (30). We will also discuss ways in which these metabolically stressed cells induce distinct signaling pathways, such as sonic hedgehog (30, 31) and release of proinflammatory EVs (27, 32, 33), that contribute to lipotoxic damage.
Fig. 1.
Fate of palmitate in hepatocytes. Excess palmitate delivered to hepatocytes is converted to palmitoyl-CoA and can induce the formation of ceramide via the de novo pathway at the ER and LPC via PLA2 action on phosphatidylcholine (PC), which in turn is derived from diacylglycerol (DAG). Though palmitate can activate the proapoptotic machinery, palmitate itself and palmitate-derived LPC and ceramide all lead to the release of EVs from hepatocytes. Palmitate is buffered in hepatocytes by either oxidation or conversion to triglyceride, both of which mitigate palmitate-induced lipotoxicity.
LIPID-ACTIVATED PROAPOPTOTIC SIGNALING
Several studies have elucidated the apoptotic pathways activated in hepatocytes by saturated free fatty acids, palmitate in particular, and the phospholipid, LPC (6, 14–20, 34). Though there are other modes of cell death, such as necrosis and necroptosis (35), their contribution to NASH pathogenesis is less well-studied. Therefore, we have focused on proapoptotic signaling. Lipid-induced activation of mixed lineage kinase 3 (MLK3) and c-Jun N-terminal kinase (JNK) (24, 32), and the ER stress response (36, 37) are key cellular pathways that mediate proapoptotic signaling initiated by toxic saturated free fatty acids and in the steatotic liver (38). Sphingolipids, too, are elevated in NASH (25), and C16:0 ceramide has recently been linked to the pathophysiology of NASH (39); therefore, we discuss these pathways and concepts below.
Free fatty acid-induced proapoptotic signaling by death receptors
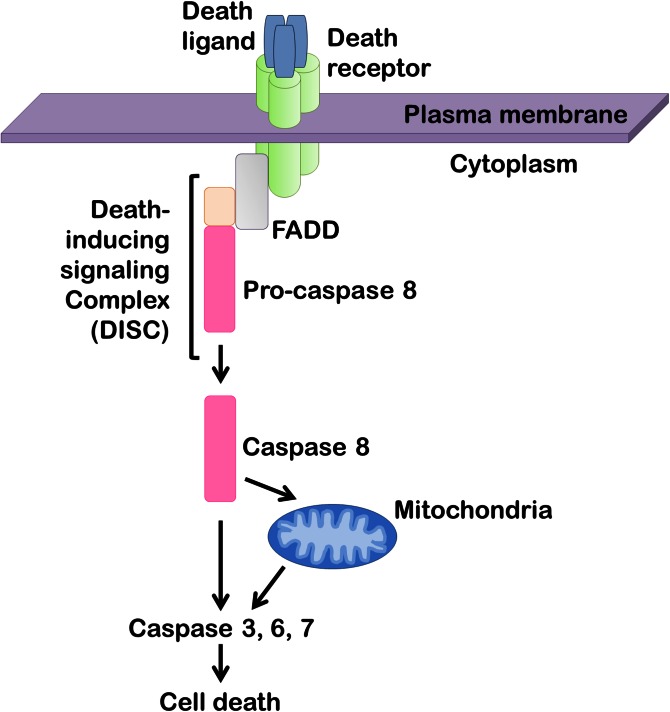
The plasma membrane receptors termed death receptors belong to TNF receptor superfamily and their activation on the cell surface initiates the extrinsic pathway of apoptosis (40–42). The principal death receptors expressed in the liver are Fas, TNF receptor 1 (TNFR1), and TNF-related apoptosis-inducing ligand (TRAIL) receptor (TRAIL-R)1 and TRAIL-R2 (42). Stimulation of these receptors leads to receptor oligomerization followed by the formation of a death-inducing signaling complex, which consists of caspase 8 and several adaptor proteins (Fig. 2) (41, 42). Caspase 8 then directly activates effector caspases 3, 6, and 7, which execute cellular demise. In some cell types, including hepatocytes, death receptor-activated proapoptotic signals converge on mitochondria-mediated cell death pathways (42). In this paradigm, activated caspase 8 cleaves Bid generating truncated Bid, which then induces egress of proapoptotic factors from the intermitochondrial space to the cytosol. This includes cytochrome c, which leads to activation of effector caspases 3, 6, and 7 (Figs. 2, 3).
Fig. 2.
Death receptor-mediated apoptosis. Upon binding of a ligand to the extracellular portion of the death receptor, the receptor intracellular domain recruits adaptor proteins, such as Fas-associated protein with death domain (FADD) and pro-caspase 8, to form a signaling platform termed the death-inducing signaling complex. Caspase 8 then undergoes proteolytic autoactivation, resulting in direct or indirect (via mitochondria) activation of caspases 3, 6, and 7 that execute the final steps of cellular demolition.
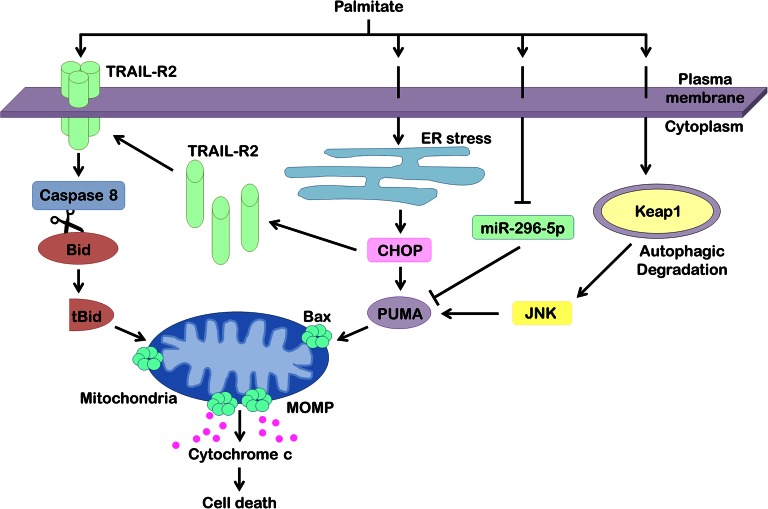
Fig. 3.
Apoptotic signaling networks in hepatocyte lipotoxicity. Hepatocyte treatment with palmitate results in ligand-independent clustering and activation of the TRAIL-R2, causing caspase-dependent cell death. Active caspase 8 cleaves Bid into its truncated form (tBid), which translocates to mitochondria to promote release of proapoptotic factors, such as cytochrome c. Palmitate also induces ER stress, which upregulates proapoptotic PUMA and TRAIL-R2 via transcription factor CHOP. Increased levels of PUMA facilitate hepatocyte apoptosis via the mitochondrial pathway. Palmitate has also been found to cause cellular depletion of miR-296-5p, a microRNA that, via complementary binding to the 3′-UTR of PUMA mRNA, causes its degradation. Loss of miR-296-5p during lipotoxicity removes this break on PUMA posttranscriptional regulation, thereby increasing cellular levels of PUMA. Finally, palmitate induces autophagic degradation of Keap1, resulting in JNK activation and PUMA upregulation. Along with other proapoptotic inputs, PUMA sensitizes the hepatocyte to Bax activation and MOMP culminating in cell death.
Death receptors expressed in the liver have been considered as potential mediators of hepatic lipotoxicity (43). Liver expression of death receptor Fas is significantly increased in patients with NAFLD compared with healthy controls (44, 45). In animal models of NAFLD, lipid-loaded hepatocytes were greatly susceptible to Fas-mediated apoptosis upon administration of anti-Fas antibody (46, 47). Nevertheless, there are no in vivo studies implicating Fas in the development of NASH. Also, hepatocytes isolated from Fas-deficient lpr mice readily underwent palmitate-induced lipoapoptosis comparable to wild-type cells, suggesting that apoptosis induced by toxic lipids is not dependent on Fas (18). Similar to Fas, TNFR1 is also upregulated in liver of NASH patients (48, 49). TNFR1 and its cognate ligand, TNFα, play an important role in proinflammatory signaling, development of insulin resistance, and, perhaps, steatosis; however, they are not directly implicated in mediating lipoapoptosis in the liver (43).
Among the death receptors, TRAIL-R2 (also known as DR5) appears to be a major mediator of lipotoxicity in hepatocytes (18, 50). Again, liver expression of TRAIL-R2 is significantly increased in patients with NASH, as well as in a murine nutrient-excess model of NASH (51, 52). Palmitate-induced apoptosis is significantly decreased in primary hepatocytes and the hepatocellular carcinoma cell line lacking TRAIL-R2, but not TRAIL-R1 (18). In wild-type hepatocytes, palmitate treatment increases TRAIL-R2 expression and changes organization of plasma membrane domains causing clustering and ligand-independent activation of TRAIL-R2, leading to caspase 8-dependent cell death (Fig. 3) (18). Increased expression of TRAIL-R2, but not other death receptors, and ligand-independent activation of the TRAIL-R2 causing caspase 8-dependent apoptosis have recently also been described in cells with persistent ER stress (53, 54). Because saturated fatty acids also cause ER stress (see below), this TRAIL-R2 proapoptotic signaling may also contribute to free fatty acid-induced lipoapoptosis. It is important to note that palmitate also upregulates proapoptotic proteins, such as p53-upregulated modulator of apoptosis (PUMA) and Bim, which contribute to lipoapoptosis in the context of TRAIL-R2 activation (see below). Upregulation of these proapoptotic proteins may be critical for lipoapoptosis, as TRAIL receptor ligand-dependent activation is harmless to healthy hepatocytes, but is cytotoxic to steatotic hepatocytes, both in vitro and in vivo (14, 51, 55). Thus, upregulation of proapoptotic proteins by palmitate or LPC in hepatocytes is key to their sensitization to apoptosis. Finally, in a murine model of Western diet-induced NASH (56), TRAIL receptor deficiency remarkably protects against hepatocyte apoptosis and all other associated pathogenic features of NASH, such as liver injury, inflammation, and fibrosis (50). Altogether, these studies suggest that TRAIL receptor signaling plays a key role in hepatocyte lipotoxicity.
Engagement of Bcl-2 proteins by free fatty acids
Many proapoptotic signaling cascades, including those initiated by death receptors, converge on the mitochondria triggering mitochondrial outer membrane permeabilization (MOMP) (57). The MOMP results in egress of proapoptotic mediators, such as cytochrome c and second mitochondrial activator of apoptosis, into the cytosol where they promote activation of effector caspases culminating in cellular disassembly. MOMP is regulated by proteins of the Bcl-2 family (57). Anti-apoptotic members include Bcl-2, Bcl-xL, and Mcl-1; these proteins act to prevent MOMP. The proapoptotic molecules are Bax and Bak, which oligomerize to induce the MOMP. The family of BH3-only proteins act as biosensors of proapoptotic stimuli, and induce MOMP either by deactivating (i.e., Bmf, Bad, Hrk, Bik) the anti-apoptotic proteins or directly activating Bax or Bak (i.e., Bim, Bid, Noxa, PUMA) (57, 58).
Lipotoxic stimuli have been shown to modulate expression and function of Bcl-2 family proteins in hepatocytes (16–20, 59). In particular, PUMA is induced by palmitate and LPC in cultured hepatocytes and hepatocyte-derived cell lines, and hepatocytes genetically deficient in PUMA are resistant to lipoapoptosis (20). Thus, PUMA is likely a key sensitizer of hepatocytes to lipoapoptosis. PUMA appears to be upregulated by multiple mechanisms during palmitate-induced hepatocyte lipoapoptosis. For example, an ER stress pathway involving CAAT/enhancer binding homologous protein (CHOP) and activator protein (AP)-1, the latter activated by JNK signaling pathways, potently induce PUMA expression by enhancing its transcription (17, 20). AP-1 and CHOP form a transcription complex inducing PUMA expression following incubation of hepatocytes with palmitate. Loss of miR-296-5p during palmitate-induced hepatocyte apoptosis may also contribute to upregulation of PUMA expression by enhancing its translation (19). Interestingly, miR-296-5p is reduced in human liver specimens from patients with NASH, and varies inversely with PUMA mRNA levels in these specimens (19). These observations imply that this mechanism of enhanced PUMA protein expression during lipotoxicity is germane to human NASH. Cellular levels of Kelch-like ECH-associated protein 1 (Keap1) also regulate hepatocyte expression of PUMA (59). Keap1 is an adaptor protein for E3 ligases and initiates the proteasomal degradation of several proteins [e.g., nuclear factor (erythroid-derived 2)-like 2, (Nrf2)]. Hepatocyte-specific genetic deletion of Keap1 is associated with increased liver steatosis and greater sensitivity of hepatocytes to palmitate-mediated lipoapoptosis due to increased expression of both Bim and PUMA (59). Loss of Keap1 during palmitate treatment of hepatocytes appears to be via autophagic degradation of Keap1. Thus, multiple signaling networks initiated by lipotoxic stress in hepatocytes converge to enhance PUMA expression, which sensitizes the cells to apoptosis (Fig. 3).
Bim may also be upregulated by lipotoxic stimuli through activation of the transcription factor, FoxO3a (34). Lipotoxic stimuli, such as saturated fatty acids, activate phosphatases, which dephosphorylate FoxO3a promoting its translocation into the nucleus and induction of Bim transcription (60). These BH3-only proteins, Bim and PUMA, likely activate Bax during lipotoxic stress, as active Bax is present on mitochondria (15) and lysosomes (61) in palmitate-treated hepatocytes and Bax inhibitors attenuate free fatty acid-induced hepatocyte apoptosis (61). Although high concentrations of saturated free fatty acids will also result in loss of the protective protein, Mcl-1, by enhancing its proteasomal degradation (34), hepatocyte-specific Mcl-1 knockout mice are only minimally more susceptible to diet-induced fatty liver disease than are wild-type mice (unpublished observations). Because hepatocytes seem to express only Mcl-1 and Bcl-xL from the anti-apoptotic Bcl-2 family of proteins, these findings suggest that Bcl-xL may contribute to hepatocyte cytoprotection during lipotoxic insults in vivo.
It has long been recognized that unsaturated free fatty acids antagonize the proapoptotic effects of saturated fatty acids in a variety of cell types (21, 62, 63). Indeed, the monounsaturated free fatty acid, palmitoleate, is quite cytoprotective during palmitate treatment of hepatocytes (21). Palmitoleate reduces expression of both Bim and PUMA, likely by diminishing palmitate-induced ER stress; this observation further supports a pivotal role for these two BH3-only proteins in hepatocyte injury by lipotoxic mediators. The cytoprotective effect of palmitoleate may be due to more efficient sequestration of palmitate as triglyceride in hepatocytes treated with a combination of palmitate and palmitoleate (21), similar to the effect of oleate on palmitate-treated CHO cells (64). In both these studies, the combination of a monounsaturated fatty acid with palmitate mitigated palmitate-induced cell death, while enhancing triglyceride deposition.
The role of Bcl-2 proteins in liver lipotoxicity warrants further study. Important insights will be gained from their study in animal models of NASH. As Bax or Bak pharmacologic inhibitors become available, they will also help define the role of mitochondrial apoptosis in models of NASH. Along these lines, we remain intrigued that delivering miR-296-5p to the liver using nanoparticles could also be salutary in human NASH by preventing PUMA upregulation.
Free fatty acid-induced MLK3 and JNK signaling
MLK3, a ubiquitously expressed member of a family of serine/threonine protein kinases that function in a phospho-relay module to control the activity of downstream MAPKs (65, 66), is implicated in NASH pathogenesis (24) and palmitate-induced JNK activation (67, 68). We reported that MLK3 genetic deficiency in a murine model of diet-induced obesity, insulin resistance, and NASH is protective against disease progression by decreasing liver steatosis, injury, inflammation, and fibrosis (24). These effects were accompanied by a reduction in the activating phosphorylation of JNK in the liver (24).
JNK is a member of the MAPK family (69). Of the three JNK genes, JNK1 and JNK2 are expressed in the liver (69). JNK signaling is activated in dietary and genetic animal models of NASH (70–72) and in human NASH (20, 73). NASH-associated JNK activation was reported in both hepatocytes and macrophages, causing apoptosis and liver inflammation, respectively (74, 75). Interestingly, JNK in macrophages is required for tissue infiltration and proinflammatory polarization (28). Both JNK1 and JNK2 have been implicated in insulin resistance and steatohepatitis (69). In a mouse model of obesity, JNK phosphorylates insulin receptor substrate-1, suppressing insulin receptor signaling and inducing insulin resistance (76). JNK is directly implicated in inhibition of fatty acid oxidation and susceptibility to steatosis by its hepatic regulation of PPARα (77). JNK-induced repression of PPARα suppresses the production of fibroblast growth factor 21, consequently inhibiting mitochondrial and peroxisomal fat oxidation and ketogenesis (77). The specific roles of JNK1 and JNK2 in NASH are divergent, depending on the dietary model used (71, 72). JNK1 mediates mouse steatohepatitis due to a diet deficient in methionine and choline (71); whereas, when mice are fed a high fat diet, JNK1 predominantly mediates steatosis and liver injury, though JNK2 contributes to proapoptotic protein expression (72). Because JNK1 knockout mice are resistant to obesity while on a high fat diet, the improved metabolic and hepatic profiles cannot be differentiated from the effects of minimal weight gain (72). Notably, the anti-obesity effect of genetic JNK1 deletion may be mediated via its signaling in the pituitary gland (78).
At a cellular level, similar to the in vivo data, JNK1 appears to be the predominant form mediating palmitate-induced c-Jun phosphorylation and downstream transcriptional responses (20). In palmitate-induced lipotoxicity, JNK1 induces the expression of PUMA, as discussed above (Fig. 3) (20, 79). Additionally, other proapoptotic proteins are also JNK targets in lipotoxicity. JNK can phosphorylate and activate the proapoptotic BH3-only proteins, Bim, Bad, and Bax, which triggers the mitochondrial pathway of apoptosis (15, 79, 80). In addition, JNK induces TRAIL-R2 expression, which sensitizes steatotic hepatocytes to TRAIL-mediated hepatotoxicity (14, 81). We speculate that inhibition of this kinase cascade via proximal inhibition of MLK3, or more distally of JNK, is a potential therapeutic strategy for the treatment of human NASH.
LPC-induced proapoptotic signaling
LPC is a phospholipid generated from phosphatidylcholine by partial hydrolysis, which removes one of the fatty acid groups. This reaction is usually catalyzed by PLA2 (6, 28). LPC is also formed in the plasma by lecithin-cholesterol acyltransferase activity (82). Thus, hepatocytes can be exposed to LPC derived from at least two sources. These are extracellular plasma LPC and LPC generated intracellularly within hepatocytes via PLA2 activity. The relative proportion of these two pathways to LPC accumulation in hepatocytes in vivo remains to be clarified.
LPC has been implicated in mediating free fatty acid-induced lipotoxicity in hepatocytes (6, 16). Han et al. (6) observed that conversion products of palmitate, rather than palmitate itself, induced hepatocyte lipoapoptosis. Interestingly, in this study, the metabolism of palmitate to lipid intermediates in the sphingolipid-ceramide pathway was not responsible for hepatocyte lipoapoptosis. However, hepatocytes were protected against palmitate-induced apoptosis when the conversion of palmitate was blocked by triacsin C, an acyl-CoA synthetase inhibitor. In addition, palmitate-induced cell death was also significantly attenuated by inhibition of PLA2, suggesting that formation of LPC plays a principal role in palmitate lipotoxicity. In line with these observations, LPC intracellular levels in cultured hepatocytes increase proportionally to the concentration of palmitate treatment (16). Conversely, inhibition of PLA2 in hepatocytes leads to decreased fatty acid uptake and decreased concentration of intracellular LPC (83). Hepatocyte treatment with exogenous LPC readily induces lipoapoptosis in primary hepatocytes and human hepatocellular carcinoma cell lines (16, 33). Whether exogenous LPC is taken up by cells or acts at the plasma membrane level to induce signaling is not well-understood. In the study by Han et al. (6), LPC-induced lipoapoptosis appeared to be dependent on an unidentified G protein-coupled receptor. Kakisaka et al. (16) found that hepatocyte treatment with exogenous LPC induced JNK activation, similarly to palmitate. In a JNK-dependent manner, LPC also increased the expression of CHOP, an effector protein of ER stress response (16). CHOP-mediated upregulation of PUMA, a potent proapoptotic BH3-only protein of the Bcl-2 family, then triggered caspase-dependent cell death. Being an intracellular metabolite, exogenous LPC treatment might be less physiologic than treatment with palmitate itself. On the other hand, LPC-induced lipoapoptosis is dependent on mechanisms largely indistinguishable from palmitate-induced lipoapoptosis, consistent with the notion that LPC mediates palmitate-induced lipotoxicity in hepatocytes.
Finally, the potential role of LPC in human disease has also been highlighted by the accumulation of this lipid in the liver of NASH patients (3, 6). The total phospholipid content in the NASH liver appears to be decreased and, therefore, the reduced phospholipid/LPC ratio may indicate an increase in PLA2 activity (3, 83). Consistent with this notion, PLA2 deletion in ob/ob mice, a genetic model of NAFLD, significantly decreased the development of spontaneous liver steatosis in (84). Future studies will need to confirm whether PLA2 activity is increased during NASH.
Ceramides in NASH
Ceramides are sphingolipids that function as lipid second messengers. Ceramide is generated at the ER via condensation of palmitoyl-CoA and serine, in a reaction catalyzed by serine palmitoyltransferase, to form 3-ketosphinganine, which is converted to ceramide by a series of enzymatic steps in the de novo ceramide synthesis pathway (22). A link between ceramide and lipoapoptosis was suggested by the work of Unger and colleagues in pancreatic β cells and diabetic mice (85). Palmitate loading of β cells led to increased serine palmitoyltransferase activity and ceramide formation. In the same study, inhibition of de novo ceramide synthesis in vivo in prediabetic rats mitigated β cell lipoapoptosis. Subsequently, ceramide accumulation in fatty livers has been reported in both human NAFLD and mouse models of NAFLD and NASH (5, 25, 39). In fact, some species of ceramides could differentiate steatosis from NASH in one study (5), suggesting a role in disease progression. Ceramide can also be generated by hydrolysis of sphingolipids by sphingomyelinases in the plasma membrane or lysosomes, via salvage pathways, without direct synthesis from palmitate (23). Though sphingomyelinases have been studied in alcohol-induced liver disease (86), their contribution to obesity-associated NASH is an area that warrants additional studies.
Two recent studies have underscored the importance of C16:0 ceramide in the pathogenesis of dietary steatohepatitis in mice and suggested a role for this in humans. Raichur et al. (39) generated mice haploinsufficient for ceramide synthase (CerS)2. These mice displayed enhanced susceptibility to high fat diet-induced liver injury with associated hepatic accumulation of C16:0 ceramide. Hepatocytes isolated from these mice demonstrated impaired mitochondrial fatty acid oxidation, due to impaired electron transport chain. Turpin et al. (25) demonstrated increased expression of CerS6 in white adipose tissue of a large cohort of obese subjects. They subsequently deleted CerS6 in mice. These mice were protected from diet-induced obesity, insulin resistance, and hepatosteatosis. Interestingly, CerS6-deleted hepatocytes exhibited increased palmitate oxidation, and mice lacking CerS6 in brown adipose tissue also showed increased lipid oxidation, suggesting that C16:0 ceramide generated by CerS6 inhibits lipid utilization in the liver and brown adipose tissue in obesity. Conversely, inhibition of ceramide synthesis by myriocin or degradation of ceramides by transgenic overexpression of acid ceramidases improved hepatic steatosis (87). The expression of a fatty acid transporter, CD36, was reduced in these livers, suggesting that ceramide promotes hepatic steatosis by increasing hepatocyte uptake of fatty acids.
In cultured hepatocytes, palmitate-induced apoptosis is associated with increased cellular ceramide concentrations (88). Studies that have examined the effects of inhibition of ceramide synthesis on palmitate-induced lipoapoptosis have given mixed results (6, 88). Sphingomyelinases and the salvage pathway of ceramide synthesis are linked to death receptor-induced apoptosis in hepatocytes (89–91). Fas ligand-activated Fas signaling led to acid sphingomyelinase-dependent ceramide synthesis with subsequent formation of reactive oxygen species in rat hepatocytes (89). In mouse and rat hepatocytes undergoing TNFα-induced apoptosis, endogenous C16:0 ceramide levels were found to be elevated (90). Furthermore, an increase in lysosomal ceramide and sphingosine was reported in liver cell lines undergoing apoptosis via TNFα/cyclohexamide-induced lysosomal permeabilization (91). This was mediated by both acid sphingomyelinase and neutral sphingomyelinase. Whether ceramides directly alter the apoptotic pathway or machinery in palmitate-loaded hepatocytes remains to be elucidated.
Lipid-activated ER stress response
The unfolded protein response, mediated by three transmembrane ER proteins, inositol requiring enzyme-1α, protein kinase-like ER kinase (PERK), and activating trancription factor 6α, is increasingly linked to both lipid metabolism and lipotoxic signaling (92). Given that this topic is covered in another review in this series, we will limit our discussion to the context of palmitate or LPC-mediated hepatocyte apoptosis. Palmitate-induced hepatocyte apoptosis is mediated, in part, by ER stress-induced activation of CHOP (18). Moreover, CHOP upregulates the proapoptotic protein, PUMA, and the cell surface death receptor, TRAIL-R2 (17, 18), though additional pathways may play a role (93). Surprisingly, CHOP knockout mice were sensitized to the development of high fat diet-induced steatohepatitis and methionine-deficient choline-deficient diet-induced steatohepatitis (94). This was due to a dominant in vivo effect of CHOP deletion in macrophages, which allowed the persistence and accumulation of macrophages in the liver, thus increasing the proinflammatory milieu (94).
SUBLETHAL INFLAMMATION AND INJURY
Palmitate can activate signaling events in macrophages that either occur independently of cell death or precede cell death. In macrophages, palmitate and other saturated free fatty acids can directly activate toll-like receptors (TLRs), with subsequent activation of the proinflammatory transcription factor, nuclear factor-κB (NF-κB). This nonlethal proinflammatory signaling is discussed below. Palmitate can activate several kinase pathways in myriad cell types (95–100). Palmitate-induced activation of protein kinase C (PKC), protein kinase B (PKB), and double-stranded RNA-dependent protein kinase (PKR) are well-studied in cell types other than hepatocytes and, therefore, are only briefly mentioned. Furthermore, in recent years, nonlethal proapoptotic signaling events generated in lipotoxic hepatocytes have gained importance due to improvements in our ability to dissect the signaling events emanating from these stressed steatotic hepatocytes. In this context, we discuss the importance of ballooned hepatocytes and lipotoxic hepatocyte-derived EVs.
Free fatty acid-induced TLR and NF-κB activation
TLRs are a family of pattern recognition receptors, mediators of innate immune responses that are tightly associated with the detection of pathogen-associated molecular patterns and endogenous damage-associated molecular patterns (DAMPs), including the widely expressed high-mobility group box 1 (101, 102). Obesity-associated chronic low-grade inflammation in insulin target tissues is a major contributor to insulin resistance (103). This inflammation is mediated, at least in part, by saturated fatty acids that stimulate proinflammatory pathways in a TLR4-dependent manner in adipocytes and macrophages (104). TLR4 signaling leads to the activation of the transcription factors, NF-κB and AP-1, resulting in the production of inflammatory cytokines (104). Furthermore, saturated fatty acids can activate myeloid proinflammatory cells via TLR2/4 and JNK signaling pathways, thereby promoting inflammation and subsequent peripheral insulin resistance (105). Though endotoxin contamination of BSA has been a concern in palmitate-induced TLR4 activation (106), macrophages do activate TLR4 signaling when treated with palmitate without BSA (107). In an elegant study, Pal et al. (108) identified fetuin-A, a liver-derived circulating glycoprotein, as an endogenous ligand that directly links saturated fatty acids to TLR4 activation in adipocytes, leading to NF-κB activation and upregulation of the inflammatory cytokines, interleukin-6 and TNF-α, resulting in insulin resistance. Moreover, β cells respond to the palmitate via the TLR4 signaling pathway and produce chemokines that recruit proinflammatory monocytes/macrophages to the islets (109). Depletion of proinflammatory cells protected mice from palmitate-induced β cell dysfunction. In hepatocytes, palmitate-induced high-mobility group box 1 release mediates the activation of TLR4 signaling in an autocrine manner, leading to hepatocyte NF-κB activation and cytokine expression (110). Taken together, these studies provide evidence that palmitate-induced TLR activation in hepatocytes and macrophages enhances the release of inflammatory cytokines and contributes to the development of insulin resistance and the progression of NASH, though it is not linked to palmitate-induced hepatocyte apoptosis.
Other potentially important fatty acid-induced signaling pathways
A number of other signaling pathways have been shown, in other tissues, to be engaged during lipotoxic stress, but thus far, there is limited evidence that these pathways play a central role in the response to lipid metabolic stress in the liver. Palmitate can activate PKC isoforms in several cell types, including pancreatic β cells, adipocytes, skeletal myocytes, and hypothalamic neurons (87, 88, 91, 92, 94–97). In pancreatic β cell lines, palmitate-induced activation of PKC induces NADPH oxidase activity and leads to production of reactive oxygen species (111). In 3T3-L1 adipocytes, in addition to promoting insulin resistance, palmitate activation of PKC-θ signals downstream through JNK and NF-κB (100). In vascular smooth muscle cells, activation of several PKC isoforms was associated with palmitate-induced apoptosis (112). While oxidative stress and signaling through JNK and NF-κB have been implicated in NASH, the potential contributions of these PKC pathways to NAFLD progression are not well-understood.
Conversely, palmitate can inhibit PKB, a key mediator of cellular metabolic responses (113, 114). In hepatocytes, palmitate-induced apoptosis was associated with inhibition of PKB activation (115, 116) and decreased insulin-induced PKB phosphorylation (99, 117). In additional cell types, such as myocytes, insulin resistance due to palmitate is also manifest as PKB inhibition (118). Whether palmitate inhibition of PKB signaling contributes to progression of NAFLD is not known.
PKR signaling is activated by palmitate in cultured cells, including hepatocytes, adipocytes, β cells, and myocytes. Though PKR is convincingly activated by palmitate, its in vivo role is less clear. While early studies suggested that lipid-induced PKR activation in the liver contributed to insulin resistance (109–111), a recent study demonstrated that deletion of PKR in mice did not ameliorate high fat diet-induced hepatic steatosis or glucose metabolism (119). Furthermore, macrophages from PKR knockout mice had no defects in palmitate-induced proinflammatory signaling. In none of these studies, however, was there clear evidence that induction of the PKR signaling pathway in hepatocytes or Kupffer cells contributed to the development of NASH.
Ballooned hepatocytes
Hepatocytes with ballooning degeneration are a prominent histopathological feature of lipotoxic liver injury. Indeed, the magnitude of hepatocellular ballooning correlates with NASH severity and is used to calculate the disease activity score (120, 121). Ballooned hepatocytes are characterized by cellular swelling and enlargement, a central nucleus and reticulated cytoplasm, loss of keratin 8 and 18, and accumulation of ubiquitinated proteins (122). Another distinctive feature of ballooned hepatocytes is their production of sonic hedgehog, a ligand of the developmental hedgehog signaling pathway (31). In other cell types, sonic hedgehog generation has been described in response to stress in Drosophila melanogaster cells in which the cell death program has been initiated but cannot be executed (123). These cells exist in “undead” state and secrete various factors, including sonic hedgehog, to promote tissue remodeling. On this basis, Kakisaka et al. (30) suggested that ballooned hepatocytes have a cellular phenotype similar to undead cells and modeled undead ballooned hepatocytes in vitro by treating caspase 9-deficient liver-derived cell lines with palmitate. Caspase 9-deficient cells were not only protected against palmitate- and LPC-induced lipoapoptosis, but also displayed increased expression of sonic hedgehog upon palmitate and LPC treatment in a JNK-dependent manner. In this experimental paradigm, sonic hedgehog also served as an autocrine survival factor for the lipotoxic hepatocytes.
Tunicamycin-induced ER stress also enhances expression and secretion of sonic hedgehog by hepatocytes (31). In these studies, sonic hedgehog generated by hepatocytes has been suggested as a paracrine profibrotic factor for stromal cells, which are thought to be the major hedgehog-responsive cells. Hepatocytes also express components of the hedgehog signaling cascade, e.g., plasma membrane receptor smoothened, and may represent an important target of hedgehog signaling during lipotoxicity (51). Activation of Gli1, a transcription factor mediating canonical hedgehog signaling, increased production of osteopontin by hepatocytes, which in turn promoted macrophage-associated proinflammatory response in a paracrine fashion (124). Finally, inhibition of hedgehog signaling by pharmacological inhibitors of smoothened (vismodegib and erismodegib) or genetic deletion of smoothened in the liver parenchyma prevents liver injury, inflammation, and fibrosis in mouse models of NAFLD and NASH (51, 124).
EVs and inflammation
Cells release diverse types of membrane-bound nanoparticles into the extracellular milieu. These can be of endosomal or plasma membrane origin, termed exosomes or microvesicles, respectively, and EVs collectively. EVs act as messengers of information that mediate intercellular communication and regulate functions of target cells (125). EVs are constitutively released under physiologic conditions into various body fluids (125). Pathogenic stimuli may further increase the number of released vesicles and modify their cargo, as demonstrated by an elevated number of circulating EVs in mouse models of liver ischemia reperfusion injury (126) and NASH (33, 127).
EVs have been implicated in lipotoxic signaling in several recent studies (Fig. 4). One of the first reports of their significance in NASH was the observation that repeated injections of EVs isolated from the serum of high fat diet-fed mice to chow diet-fed mice resulted in hepatic inflammation and promoted the development of fatty liver disease (128). Later, several studies have clearly demonstrated that EV release from hepatocytes is significantly increased upon treatment with lipids such as palmitate, LPC, and even ceramide loading of cells (27, 33, 127, 129). Consistent with the emerging literature, lipotoxic EVs are heterogeneous. This applies to the mechanisms of biogenesis and release, cargo contained therein, and effects on target cells. For example, LPC-mediated EV release from hepatocytes is MLK3-dependent, and MLK3 regulates the chemotactic cargo of the EVs (129). Specifically, the abundance of the potent chemokine C-X-C motif ligand 10 (CXCL10) is significantly reduced in LPC-stimulated MLK3-deficient hepatocytes or by pharmacologic inhibition of MLK3. Likewise, MLK3 knockout mice fed a high fat, fructose, and cholesterol diet (56) have reduced CXCL10 levels in plasma EVs (129). This, in turn, is associated with hepatoprotection against diet-induced liver injury and inflammation.
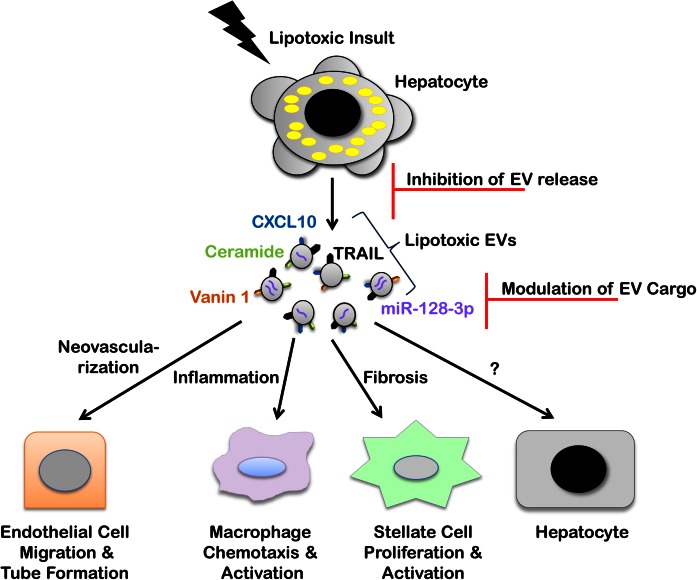
Fig. 4.
EVs in lipotoxic signaling and therapeutic opportunities. Hepatocyte lipotoxicity promotes EV release. Recent in vitro and in vivo studies have defined multiple roles of lipotoxic EVs in NASH pathogenesis through cell-to-cell communication via various cargoes. CXCL10 and ceramide-enriched EVs mediate monocyte/macrophage chemotaxis to the liver, while TRAIL-enriched EVs contribute to macrophage activation resulting in the sterile inflammatory response observed in a nutrient excess mouse model of NASH. Vanin 1-enriched EVs mediate endothelial cell migration and tube formation in vitro and neovascularization in a NASH mouse model, while miR-128-3p-laden EVs enhance HSC proliferation and activation in vitro. Inhibition of EV release and modulation of EV cargo to interrupt deleterious target cell responses are opportunities for EV-based therapies.
LPC-stimulated hepatocyte EVs appear to originate mainly from the plasma membrane, as their release is sensitive to inhibition by fasudil, an inhibitor of Rho-associated coiled-coil containing protein kinase 1 (33). This phenomenon precedes cell death; however, it requires activation of the TRAIL receptor proapoptotic pathway, consisting of the TRAIL receptor → caspase 8 → caspase 3 signaling cascade. These EVs are biologically potent, as they induce macrophage activation, partly by a TRAIL-dependent mechanism. Consistent with these in vitro data, the Rho-associated coiled-coil containing protein kinase 1 inhibitor, fasudil, decreased NASH-induced circulating EVs, which was associated with a reduction in liver injury, inflammation, and fibrosis (33).
EVs derived from lipotoxic hepatocytes also mediate angiogenesis (127). Neovascularization is an important pathological feature of NASH and correlates with fibrosis severity (127). Lipotoxic EVs were laden with Vanin 1, an ectoenzyme with known cell migration and adherence properties. Vanin 1-containing EVs induced endothelial cell migration and tube formation in vitro, and angiogenesis in a NASH mouse model, in a Vanin 1-dependent mechanism. EVs derived from Vanin 1-deficient HepG2 cells failed to induce significant endothelial cell migration and tube formation. Likewise, administration of siRNA against Vanin 1 to mice on a methionine and choline-deficient diet protected these mice against steatohepatitis-induced pathological angiogenesis in the liver (127).
Lipid cargo within EVs is also important in NASH pathogenesis. It was recently demonstrated that palmitate-induced ER stress leads to an enhanced release of EVs (27). Furthermore, palmitate-induced EV release is mediated by the unfolded protein response sensor, inositol requiring enzyme-1α, and its downstream target, X-box binding protein 1 (XBP-1). Palmitate-induced EVs are enriched in C16:0 ceramide and mediate macrophage chemotaxis via ceramide-derived sphinogosine-1-phosphate signaling (27).
On the other hand, emerging data suggest that microRNA carried within EVs may be important in NASH-induced fibrosis. For example, miR-128-3p is enriched in EVs released by lipotoxic hepatocytes and efficiently internalized by hepatic stellate cells (HSCs) (130). The miR-128-mRNA regulatory network identified by the Ingenuity Pathway Analysis showed that miR-128-3p regulates several proteins involved in liver fibrosis and HSC activation. Among several hepatic pathological conditions, liver fibrosis was the third most regulated by miR-128-3p. Hepatic miR-128-3p levels were markedly increased in two diet-induced NAFLD/NASH mouse models (130). The exposure of HSC to miR-128-3p-depleted EVs resulted in downregulation of profibrogenic markers (e.g., α-smooth muscle actin, collagen1α1) and upregulation of the HSC quiescence regulator, PPAR-γ, compared with miR-128-3p-containing EVs. Likewise, miR-128-3p-depleted EVs attenuated HSC proliferation and migration.
There are likely multiple mediators of macrophage chemotaxis and activation within the lipotoxic hepatocyte EVs. In fact, our EV protein mass spectrometry analysis demonstrated that these EVs contain many DAMPs (32). These DAMPs are known to activate inflammatory responses in mammals (131). The role of DAMP-enriched EVs as potential macrophage activators and their regulatory relationship merits further studies.
REFLECTION AND VISION
What have we learned about hepatic lipotoxicity?
Considerable information has been gleaned from in vitro and in vivo model systems regarding hepatic lipotoxicity. We have reviewed above what we believe are the significant observations from both a scientific and clinical perspective. The best information in humans suggests that triglycerides in hepatic steatosis are derived 60% from circulating free fatty acids, 26% from hepatic de novo lipogenesis, and 14% from the diet (132). Moreover, despite the clinical fascination with fat in the liver, we now understand the formation of neutral triglycerides is not injurious to the liver and likely represents both an evolutionarily conserved pathway for fatty acid storage and also a detoxification pathway for saturated free fatty acids (64, 133–136). Indeed, inhibiting triglyceride synthesis improves hepatic steatosis, but exacerbates liver damage, in obese mice with NASH (136). This information supports the use of free fatty acids to study lipotoxicity in vitro. We have learned that NASH is a very proapoptotic disease (44), and inhibition of apoptosis may well be therapeutic in this disease (137). Consistent with these observations, palmitate and stearate induce robust apoptosis in hepatocytes (75); saturated fatty acids are more toxic than unsaturated fatty acids, but even unsaturated fatty acids, such as oleate, sensitize hepatocytes to death receptor-mediated apoptosis (14, 46). The role of ER stress in mediating lipotoxic events has also been clarified in a multitude of observations (38, 138). Thus, the role of free fatty acids in mediating primary hepatocyte apoptosis is clear and the relevance of this model to human NASH has been established. Nonetheless, primary hepatocyte lipotoxicity is likely insufficient to cause hepatic damage and cirrhosis, and likely progressive hepatic injury also requires activation of the innate immune system, especially macrophages (139). For example, inhibiting macrophage recruitment into the liver is salutary in a murine model of NASH (32). This information has led to the following working framework: the insulin resistance of obesity results in a surfeit of circulating free fatty acids which inundate the liver, resulting in free fatty acid-mediated ER stress, apoptosis, and inflammation culminating in human NASH.
What are the current unanswered questions?
There are myriad unanswered questions in NASH pathogenesis and therapy, of which we will discuss two major avenues of future pursuit. First, considerable data suggest that hepatic farnesoid X receptor agonists are beneficial in human NASH (140). We need to understand how these agonists disrupt hepatocyte apoptosis or render free fatty acids nontoxic. Such an understanding may provide insight into better therapeutic strategies for human NASH. Second, we need to understand how lipotoxicity promotes macrophage-associated inflammation in the liver. In keeping with this, we need to test to determine whether the lipotoxic liver secretes molecules that are directly proinflammatory. Early data from our laboratory suggest that lipotoxic hepatocytes release EVs laden with chemokines, DAMPs, and proinflammatory lipids (27, 33, 129). EVs are also known to contain microRNAs which can modulate target cell function. These observations raise several interesting scientific questions such as (Fig. 4): i) Do free fatty acids drive vesicle biogenesis and/or packaging of molecules into the vesicles? ii) What are the effects of these vesicles on innate immune cells, surrounding hepatocytes, endothelial cells, and stellate cells in the liver? iii) Can we modulate the generation of these proinflammatory vesicles as a therapeutic strategy for NASH? Finally, we need to better understand how the innate immune response modulates liver metabolism of free fatty acids. The next generation of questions in hepatic lipotoxicity promises to be very exciting and hopefully will advance therapeutic opportunities for NASH.
Acknowledgments
The authors are grateful to Ms. Courtney Hoover for superb administrative assistance.
Footnotes
Abbreviations:
- AP
- activator protein
- CerS
- ceramide synthase
- CHOP
- CAAT/enhancer binding homologous protein
- CXCL10
- C-X-C motif ligand 10
- DAMP
- damage-associated molecular pattern
- ER
- endoplasmic reticulum
- EV
- extracellular vesicle
- HSC
- hepatic stellate cell
- JNK
- c-Jun N-terminal kinase
- Keap1
- Kelch-like ECH-associated protein
- LPC
- lysophosphatidylcholine
- MLK3
- mixed lineage kinase 3
- MOMP
- mitochondrial outer membrane permeabilization
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NF-κB
- nuclear factor-κB
- PKB
- protein kinase B
- PKC
- protein kinase C
- PKR
- double-stranded RNA-dependent protein kinase
- PLA2
- phospholipase A2
- PUMA
- p53-upregulated modulator of apoptosis
- TLR
- toll-like receptor
- TNFR1
- TNF receptor 1
- TRAIL
- TNF-related apoptosis-inducing ligand
- TRAIL-R
- TNF-related apoptosis-inducing ligand receptor
This work was supported in part by Office of Extramural Research, National Institutes of Health Grants DK97178 (H.M.), DK41876 (G.J.G.), and KL2TR000136-09 (S.H.I.). Support was also provided to P.H. by the American Liver Foundation.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Unger R. H., Clark G. O., Scherer P. E., and Orci L.. 2010. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 1801: 209–214. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M. E. 2015. Nonalcoholic fatty liver disease: a systematic review. JAMA. 313: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 3.Puri P., Baillie R. A., Wiest M. M., Mirshahi F., Choudhury J., Cheung O., Sargeant C., Contos M. J., and Sanyal A. J.. 2007. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 4.Gorden D. L., Ivanova P. T., Myers D. S., McIntyre J. O., VanSaun M. N., Wright J. K., Matrisian L. M., and Brown H. A.. 2011. Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS One. 6: e22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorden D. L., Myers D. S., Ivanova P. T., Fahy E., Maurya M. R., Gupta S., Min J., Spann N. J., McDonald J. G., Kelly S. L., et al. . 2015. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 56: 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han M. S., Park S. Y., Shinzawa K., Kim S., Chung K. W., Lee J. H., Kwon C. H., Lee K. W., Park C. K., Chung W. J., et al. . 2008. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 49: 84–97. [DOI] [PubMed] [Google Scholar]
- 7.Araya J., Rodrigo R., Videla L. A., Thielemann L., Orellana M., Pettinelli P., and Poniachik J.. 2004. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. (Lond.). 106: 635–643. [DOI] [PubMed] [Google Scholar]
- 8.Satapathy S. K., and Sanyal A. J.. 2015. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin. Liver Dis. 35: 221–235. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. C., Horton J. D., and Hobbs H. H.. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinella M. E., and Sanyal A. J.. 2015. NAFLD in 2014: genetics, diagnostics and therapeutic advances in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 12: 65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L. A., Boerwinkle E., Cohen J. C., and Hobbs H. H.. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig J., Viggiano T. R., McGill D. B., and Oh B. J.. 1980. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55: 434–438. [PubMed] [Google Scholar]
- 13.Argo C. K., Northup P. G., Al-Osaimi A. M., and Caldwell S. H.. 2009. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 51: 371–379. [DOI] [PubMed] [Google Scholar]
- 14.Malhi H., Barreyro F. J., Isomoto H., Bronk S. F., and Gores G. J.. 2007. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 56: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhi H., Bronk S. F., Werneburg N. W., and Gores G. J.. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281: 12093–12101. [DOI] [PubMed] [Google Scholar]
- 16.Kakisaka K., Cazanave S. C., Fingas C. D., Guicciardi M. E., Bronk S. F., Werneburg N. W., Mott J. L., and Gores G. J.. 2012. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G77–G84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cazanave S. C., Elmi N. A., Akazawa Y., Bronk S. F., Mott J. L., and Gores G. J.. 2010. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G236–G243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cazanave S. C., Mott J. L., Bronk S. F., Werneburg N. W., Fingas C. D., Meng X. W., Finnberg N., El-Deiry W. S., Kaufmann S. H., and Gores G. J.. 2011. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J. Biol. Chem. 286: 39336–39348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazanave S. C., Mott J. L., Elmi N. A., Bronk S. F., Masuoka H. C., Charlton M. R., and Gores G. J.. 2011. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J. Lipid Res. 52: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cazanave S. C., Mott J. L., Elmi N. A., Bronk S. F., Werneburg N. W., Akazawa Y., Kahraman A., Garrison S. P., Zambetti G. P., Charlton M. R., et al. . 2009. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J. Biol. Chem. 284: 26591–26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akazawa Y., Cazanave S., Mott J. L., Elmi N., Bronk S. F., Kohno S., Charlton M. R., and Gores G. J.. 2010. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J. Hepatol. 52: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanada K. 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 1632: 16–30. [DOI] [PubMed] [Google Scholar]
- 23.Chaurasia B., and Summers S. A.. 2015. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26: 538–550. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim S. H., Gores G. J., Hirsova P., Kirby M., Miles L., Jaeschke A., and Kohli R.. 2014. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 34: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turpin S. M., Nicholls H. T., Willmes D. M., Mourier A., Brodesser S., Wunderlich C. M., Mauer J., Xu E., Hammerschmidt P., Bronneke H. S., et al. . 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20: 678–686. [DOI] [PubMed] [Google Scholar]
- 26.Huang H., Kasumov T., Gatmaitan P., Heneghan H. M., Kashyap S. R., Schauer P. R., Brethauer S. A., and Kirwan J. P.. 2011. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity (Silver Spring). 19: 2235–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakazu E., Mauer A. S., Yin M., and Malhi H.. 2016. Hepatocytes release ceramide-enriched proinflammatory extracellular vesicles in an IRE1α-dependent manner. J. Lipid Res. 57: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han M. S., Jung D. Y., Morel C., Lakhani S. A., Kim J. K., Flavell R. A., and Davis R. J.. 2013. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 339: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldwell S., Ikura Y., Dias D., Isomoto K., Yabu A., Moskaluk C., Pramoonjago P., Simmons W., Scruggs H., Rosenbaum N., et al. . 2010. Hepatocellular ballooning in NASH. J. Hepatol. 53: 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakisaka K., Cazanave S. C., Werneburg N. W., Razumilava N., Mertens J. C., Bronk S. F., and Gores G. J.. 2012. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. J. Hepatol. 57: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangwala F., Guy C. D., Lu J., Suzuki A., Burchette J. L., Abdelmalek M. F., Chen W., and Diehl A. M.. 2011. Increased production of sonic hedgehog by ballooned hepatocytes. J. Pathol. 224: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim S. H., Hirsova P., Tomita K., Bronk S. F., Werneburg N. W., Harrison S. A., Goodfellow V. S., Malhi H., and Gores G. J.. 2016. Mixed lineage kinase 3 mediates release of C–X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 63: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsova P., Ibrahim S. H., Krishnan A., Verma V. K., Bronk S. F., Werneburg N. W., Charlton M. R., Shah V. H., Malhi H., and Gores G. J.. 2016. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 150: 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuoka H. C., Mott J., Bronk S. F., Werneburg N. W., Akazawa Y., Kaufmann S. H., and Gores G. J.. 2009. Mcl-1 degradation during hepatocyte lipoapoptosis. J. Biol. Chem. 284: 30039–30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galluzzi L., Vitale I., Abrams J. M., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., Dawson T. M., Dawson V. L., El-Deiry W. S., Fulda S., et al. . 2012. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karaskov E., Scott C., Zhang L., Teodoro T., Ravazzola M., and Volchuk A.. 2006. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 147: 3398–3407. [DOI] [PubMed] [Google Scholar]
- 37.Wei Y., Wang D., Topczewski F., and Pagliassotti M. J.. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291: E275–E281. [DOI] [PubMed] [Google Scholar]
- 38.Malhi H., and Kaufman R. J.. 2011. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raichur S., Wang S. T., Chan P. W., Li Y., Ching J., Chaurasia B., Dogra S., Ohman M. K., Takeda K., Sugii S., et al. . 2014. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20: 687–695. [Erratum. 2014. Cell Metab 20: 919.] [DOI] [PubMed] [Google Scholar]
- 40.Wiens G. D., and Glenney G. W.. 2011. Origin and evolution of TNF and TNF receptor superfamilies. Dev. Comp. Immunol. 35: 1324–1335. [DOI] [PubMed] [Google Scholar]
- 41.Leist M., and Jaattela M.. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2: 589–598. [DOI] [PubMed] [Google Scholar]
- 42.Guicciardi M. E., and Gores G. J.. 2009. Life and death by death receptors. FASEB J. 23: 1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsova P., and Gores G. J.. 2015. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 1: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldstein A. E., Canbay A., Angulo P., Taniai M., Burgart L. J., Lindor K. D., and Gores G. J.. 2003. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 125: 437–443. [DOI] [PubMed] [Google Scholar]
- 45.Zou C., Ma J., Wang X., Guo L., Zhu Z., Stoops J., Eaker A. E., Johnson C. J., Strom S., Michalopoulos G. K., et al. . 2007. Lack of Fas antagonism by Met in human fatty liver disease. Nat. Med. 13: 1078–1085. [DOI] [PubMed] [Google Scholar]
- 46.Feldstein A. E., Canbay A., Guicciardi M. E., Higuchi H., Bronk S. F., and Gores G. J.. 2003. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 39: 978–983. [DOI] [PubMed] [Google Scholar]
- 47.Siebler J., Schuchmann M., Strand S., Lehr H. A., Neurath M. F., and Galle P. R.. 2007. Enhanced sensitivity to CD95-induced apoptosis in ob/ob mice. Dig. Dis. Sci. 52: 2396–2402. [DOI] [PubMed] [Google Scholar]
- 48.Crespo J., Cayon A., Fernandez-Gil P., Hernandez-Guerra M., Mayorga M., Dominguez-Diez A., Fernandez-Escalante J. C., and Pons-Romero F.. 2001. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 34: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro P. S., Cortez-Pinto H., Sola S., Castro R. E., Ramalho R. M., Baptista A., Moura M. C., Camilo M. E., and Rodrigues C. M.. 2004. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 99: 1708–1717. [DOI] [PubMed] [Google Scholar]
- 50.Idrissova L., Malhi H., Werneburg N. W., LeBrasseur N. K., Bronk S. F., Fingas C., Tchkonia T., Pirtskhalava T., White T. A., Stout M. B., et al. . 2015. TRAIL receptor deletion in mice suppresses the inflammation of nutrient excess. J. Hepatol. 62: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirsova P., Ibrahim S. H., Bronk S. F., Yagita H., and Gores G. J.. 2013. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 8: e70599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkmann X., Fischer U., Bahr M. J., Ott M., Lehner F., Macfarlane M., Cohen G. M., Manns M. P., Schulze-Osthoff K., and Bantel H.. 2007. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 46: 1498–1508. [DOI] [PubMed] [Google Scholar]
- 53.Lu M., Lawrence D. A., Marsters S., Acosta-Alvear D., Kimmig P., Mendez A. S., Paton A. W., Paton J. C., Walter P., and Ashkenazi A.. 2014. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 345: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi H., and Wang H. G.. 2004. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279: 45495–45502. [DOI] [PubMed] [Google Scholar]
- 55.Ashkenazi A., Pai R. C., Fong S., Leung S., Lawrence D. A., Marsters S. A., Blackie C., Chang L., McMurtrey A. E., Hebert A., et al. . 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charlton M., Krishnan A., Viker K., Sanderson S., Cazanave S., McConico A., Masuoko H., and Gores G.. 2011. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301: G825–G834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czabotar P. E., Lessene G., Strasser A., and Adams J. M.. 2014. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15: 49–63. [DOI] [PubMed] [Google Scholar]
- 58.Chen H. C., Kanai M., Inoue-Yamauchi A., Tu H. C., Huang Y., Ren D., Kim H., Takeda S., Reyna D. E., Chan P. M., et al. . 2015. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat. Cell Biol. 17: 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cazanave S. C., Wang X., Zhou H., Rahmani M., Grant S., Durrant D. E., Klaassen C. D., Yamamoto M., and Sanyal A. J.. 2014. Degradation of Keap1 activates BH3-only proteins Bim and PUMA during hepatocyte lipoapoptosis. Cell Death Differ. 21: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barreyro F. J., Kobayashi S., Bronk S. F., Werneburg N. W., Malhi H., and Gores G. J.. 2007. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J. Biol. Chem. 282: 27141–27154. [DOI] [PubMed] [Google Scholar]
- 61.Feldstein A. E., Werneburg N. W., Li Z., Bronk S. F., and Gores G. J.. 2006. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G1339–G1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maedler K., Spinas G. A., Dyntar D., Moritz W., Kaiser N., and Donath M. Y.. 2001. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 50: 69–76. [DOI] [PubMed] [Google Scholar]
- 63.Staiger K., Staiger H., Weigert C., Haas C., Haring H. U., and Kellerer M.. 2006. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 55: 3121–3126. [DOI] [PubMed] [Google Scholar]
- 64.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V. Jr., Ory D. S., and Schaffer J. E.. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallo K. A., and Johnson G. L.. 2002. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3: 663–672. [DOI] [PubMed] [Google Scholar]
- 66.Kim K. Y., Kim B. C., Xu Z., and Kim S. J.. 2004. Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-beta-induced apoptosis in hepatoma cells. J. Biol. Chem. 279: 29478–29484. [DOI] [PubMed] [Google Scholar]
- 67.Jaeschke A., and Davis R. J.. 2007. Metabolic stress signaling mediated by mixed-lineage kinases. Mol. Cell. 27: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma M., Urano F., and Jaeschke A.. 2012. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J. Hepatol. 56: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seki E., Brenner D. A., and Karin M.. 2012. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 143: 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirosumi J., Tuncman G., Chang L., Gorgun C. Z., Uysal K. T., Maeda K., Karin M., and Hotamisligil G. S.. 2002. A central role for JNK in obesity and insulin resistance. Nature. 420: 333–336. [DOI] [PubMed] [Google Scholar]
- 71.Schattenberg J. M., Singh R., Wang Y., Lefkowitch J. H., Rigoli R. M., Scherer P. E., and Czaja M. J.. 2006. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 43: 163–172. [DOI] [PubMed] [Google Scholar]
- 72.Singh R., Wang Y., Xiang Y., Tanaka K. E., Gaarde W. A., and Czaja M. J.. 2009. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 49: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puri P., Mirshahi F., Cheung O., Natarajan R., Maher J. W., Kellum J. M., and Sanyal A. J.. 2008. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 134: 568–576. [DOI] [PubMed] [Google Scholar]
- 74.Kodama Y., Kisseleva T., Iwaisako K., Miura K., Taura K., De Minicis S., Osterreicher C. H., Schnabl B., Seki E., and Brenner D. A.. 2009. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 137: 1467–1477.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deleted in proof. [Google Scholar]
- 76.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., and Hotamisligil G. S.. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 77.Vernia S., Cavanagh-Kyros J., Garcia-Haro L., Sabio G., Barrett T., Jung D. Y., Kim J. K., Xu J., Shulha H. P., Garber M., et al. . 2014. The PPARalpha-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 20: 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vernia S., Cavanagh-Kyros J., Barrett T., Jung D. Y., Kim J. K., and Davis R. J.. 2013. Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes Dev. 27: 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim B. J., Ryu S. W., and Song B. J.. 2006. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 281: 21256–21265. [DOI] [PubMed] [Google Scholar]
- 80.Dhanasekaran D. N., and Reddy E. P.. 2008. JNK signaling in apoptosis. Oncogene. 27: 6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malhi H., and Gores G. J.. 2008. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 28: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glomset J. A. 1968. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 83.Stremmel W., Staffer S., Wannhoff A., Pathil A., and Chamulitrat W.. 2014. Plasma membrane phospholipase A2 controls hepatocellular fatty acid uptake and is responsive to pharmacological modulation: implications for nonalcoholic steatohepatitis. FASEB J. 28: 3159–3170. [DOI] [PubMed] [Google Scholar]
- 84.Deng X., Wang J., Jiao L., Utaipan T., Tuma-Kellner S., Schmitz G., Liebisch G., Stremmel W., and Chamulitrat W.. 2016. iPLA2β deficiency attenuates obesity and hepatic steatosis in ob/ob mice through hepatic fatty-acyl phospholipid remodeling. Biochim. Biophys. Acta. 1861: 449–461. [DOI] [PubMed] [Google Scholar]
- 85.Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., and Unger R. H.. 1998. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273: 32487–32490. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez A., Matias N., Fucho R., Ribas V., Von Montfort C., Nuno N., Baulies A., Martinez L., Tarrats N., Mari M., et al. . 2013. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J. Hepatol. 59: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia J. Y., Holland W. L., Kusminski C. M., Sun K., Sharma A. X., Pearson M. J., Sifuentes A. J., McDonald J. G., Gordillo R., and Scherer P. E.. 2015. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martínez L., Torres S., Baulies A., Alarcón-Vila C., Elena M., Fabriàs G., Casas J., Caballeria J., Fernandez-Checa J. C., and García-Ruiz C.. 2015. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaining de novo ceramide synthesis. Oncotarget. 6: 41479–41496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reinehr R., Sommerfeld A., Keitel V., Grether-Beck S., and Haussinger D.. 2008. Amplification of CD95 activation by caspase 8-induced endosomal acidification in rat hepatocytes. J. Biol. Chem. 283: 2211–2222. [DOI] [PubMed] [Google Scholar]
- 90.Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., and Brenner D. A.. 2005. Roles for C16:0-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J. Biol. Chem. 280: 27879–27887. [DOI] [PubMed] [Google Scholar]
- 91.Ullio C., Casas J., Brunk U. T., Sala G., Fabrias G., Ghidoni R., Bonelli G., Baccino F. M., and Autelli R.. 2012. Sphingosine mediates TNFalpha-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J. Lipid Res. 53: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volmer R., van der Ploeg K., and Ron D.. 2013. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA. 110: 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfaffenbach K. T., Gentile C. L., Nivala A. M., Wang D., Wei Y., and Pagliassotti M. J.. 2010. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am. J. Physiol. Endocrinol. Metab. 298: E1027–E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malhi H., Kropp E. M., Clavo V. F., Kobrossi C. R., Han J., Mauer A. S., Yong J., and Kaufman R. J.. 2013. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J. Biol. Chem. 288: 18624–18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bell K. S., Schmitz-Peiffer C., Lim-Fraser M., Biden T. J., Cooney G. J., and Kraegen E. W.. 2000. Acute reversal of lipid-induced muscle insulin resistance is associated with rapid alteration in PKC-theta localization. Am. J. Physiol. Endocrinol. Metab. 279: E1196–E1201. [DOI] [PubMed] [Google Scholar]
- 96.Benoit S. C., Kemp C. J., Elias C. F., Abplanalp W., Herman J. P., Migrenne S., Lefevre A. L., Cruciani-Guglielmacci C., Magnan C., Yu F., et al. . 2009. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J. Clin. Invest. 119: 2577–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carvalho-Filho M. A., Carvalho B. M., Oliveira A. G., Guadagnini D., Ueno M., Dias M. M., Tsukumo D. M., Hirabara S. M., Reis L. F., Curi R., et al. . 2012. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology. 153: 5261–5274. [DOI] [PubMed] [Google Scholar]
- 98.Kim J. K., Fillmore J. J., Sunshine M. J., Albrecht B., Higashimori T., Kim D. W., Liu Z. X., Soos T. J., Cline G. W., O’Brien W. R., et al. . 2004. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Invest. 114: 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia Y., Wan X., Duan Q., He S., and Wang X.. 2007. Inhibition of protein kinase B by palmitate in the insulin signaling of HepG2 cells and the preventive effect of arachidonic acid on insulin resistance. Front. Med. China. 1: 200–206. [DOI] [PubMed] [Google Scholar]
- 100.Yang L., Qian Z., Ji H., Yang R., Wang Y., Xi L., Sheng L., Zhao B., and Zhang X.. 2010. Inhibitory effect on protein kinase Ctheta by Crocetin attenuates palmitate-induced insulin insensitivity in 3T3-L1 adipocytes. Eur. J. Pharmacol. 642: 47–55. [DOI] [PubMed] [Google Scholar]
- 101.Akira S., and Takeda K.. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4: 499–511. [DOI] [PubMed] [Google Scholar]
- 102.Park J. S., Svetkauskaite D., He Q., Kim J. Y., Strassheim D., Ishizaka A., and Abraham E.. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279: 7370–7377. [DOI] [PubMed] [Google Scholar]
- 103.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 104.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., and Flier J. S.. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen M. T., Favelyukis S., Nguyen A. K., Reichart D., Scott P. A., Jenn A., Liu-Bryan R., Glass C. K., Neels J. G., and Olefsky J. M.. 2007. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282: 35279–35292. [DOI] [PubMed] [Google Scholar]
- 106.Erridge C., and Samani N. J.. 2009. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29: 1944–1949. [DOI] [PubMed] [Google Scholar]
- 107.Huang S., Rutkowsky J. M., Snodgrass R. G., Ono-Moore K. D., Schneider D. A., Newman J. W., Adams S. H., and Hwang D. H.. 2012. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pal D., Dasgupta S., Kundu R., Maitra S., Das G., Mukhopadhyay S., Ray S., Majumdar S. S., and Bhattacharya S.. 2012. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 109.Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F., Yagi N., Ohto U., Kimoto M., Miyake K., et al. . 2012. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 15: 518–533. [DOI] [PubMed] [Google Scholar]
- 110.Li L., Chen L., Hu L., Liu Y., Sun H. Y., Tang J., Hou Y. J., Chang Y. X., Tu Q. Q., Feng G. S., et al. . 2011. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 54: 1620–1630. [DOI] [PubMed] [Google Scholar]
- 111.Morgan D., Oliveira-Emilio H. R., Keane D., Hirata A. E., Santos da Rocha M., Bordin S., Curi R., Newsholme P., and Carpinelli A. R.. 2007. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 50: 359–369. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y. M., Wang X., Nawaz A., Kong Z. H., Hong Y., Wang C. H., and Zhang J. J.. 2011. Wogonin ameliorates lipotoxicity-induced apoptosis of cultured vascular smooth muscle cells via interfering with DAG-PKC pathway. Acta Pharmacol. Sin. 32: 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cazzolli R., Carpenter L., Biden T. J., and Schmitz-Peiffer C.. 2001. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 50: 2210–2218. [DOI] [PubMed] [Google Scholar]
- 114.Fayard E., Tintignac L. A., Baudry A., and Hemmings B. A.. 2005. Protein kinase B/Akt at a glance. J. Cell Sci. 118: 5675–5678. [DOI] [PubMed] [Google Scholar]
- 115.Song Z., Song M., Lee D. Y., Liu Y., Deaciuc I. V., and McClain C. J.. 2007. Silymarin prevents palmitate-induced lipotoxicity in HepG2 cells: involvement of maintenance of Akt kinase activation. Basic Clin. Pharmacol. Toxicol. 101: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pardo V., Gonzalez-Rodriguez A., Muntane J., Kozma S. C., and Valverde A. M.. 2015. Role of hepatocyte S6K1 in palmitic acid-induced endoplasmic reticulum stress, lipotoxicity, insulin resistance and in oleic acid-induced protection. Food Chem. Toxicol. 80: 298–309. [DOI] [PubMed] [Google Scholar]
- 117.Mordier S., and Iynedjian P. B.. 2007. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem. Biophys. Res. Commun. 362: 206–211. [DOI] [PubMed] [Google Scholar]
- 118.Sabin M. A., Stewart C. E., Crowne E. C., Turner S. J., Hunt L. P., Welsh G. I., Grohmann M. J., Holly J. M., and Shield J. P.. 2007. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J. Cell. Physiol. 211: 244–252. [DOI] [PubMed] [Google Scholar]
- 119.Lancaster G. I., Kammoun H. L., Kraakman M. J., Kowalski G. M., Bruce C. R., and Febbraio M. A.. 2016. PKR is not obligatory for high-fat diet-induced obesity and its associated metabolic and inflammatory complications. Nat. Commun. 7: 10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guy C. D., Suzuki A., Zdanowicz M., Abdelmalek M. F., Burchette J., Unalp A., and Diehl A. M.. 2012. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 55: 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., et al. . 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 122.Lackner C., Gogg-Kamerer M., Zatloukal K., Stumptner C., Brunt E. M., and Denk H.. 2008. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J. Hepatol. 48: 821–828. [DOI] [PubMed] [Google Scholar]
- 123.Fuchs Y., and Steller H.. 2011. Programmed cell death in animal development and disease. Cell. 147: 742–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kwon H., Song K., Han C., Chen W., Wang Y., Dash S., Lim K., and Wu T.. 2016. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease (NAFLD). Hepatology. 63: 1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raposo G., and Stoorvogel W.. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teoh N. C., Ajamieh H., Wong H. J., Croft K., Mori T., Allison A. C., and Farrell G. C.. 2014. Microparticles mediate hepatic ischemia-reperfusion injury and are the targets of Diannexin (ASP8597). PLoS One. 9: e104376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Povero D., Eguchi A., Niesman I. R., Andronikou N., du Jeu X. D., Mulya A., Berk M., Lazic M., Thapaliya S., Parola M., et al. . 2013. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci. Signal. 6: ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng Z. B., Liu Y. L., Liu C. R., Xiang X. Y., Wang J. H., Cheng Z. Q., Shah S. V., Zhang S. Y., Zhang L. M., Zhuang X. Y., et al. . 2009. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 50: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ibrahim S. H., Hirsova P., Tomita K., Bronk S. F., Werneburg N. W., Harrison S. A., Goodfellow V. S., Malhi H., and Gores G. J.. 2016. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 63: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Povero D., Panera N., Eguchi A., Johnson C. D., Papouchado B. G., de Araujo Horcel L., Pinatel E. M., Alisi A., Nobili V., and Feldstein A. E.. 2015. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting peroxisome proliferator-activated receptor-γ. Cell. Mol. Gastroenterol. Hepatol. 1: 646–663.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schaefer L. 2014. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 289: 35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., and Parks E. J.. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garbarino J., Padamsee M., Wilcox L., Oelkers P. M., D’Ambrosio D., Ruggles K. V., Ramsey N., Jabado O., Turkish A., and Sturley S. L.. 2009. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death. J. Biol. Chem. 284: 30994–31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z. Z., Berk M., McIntyre T. M., and Feldstein A. E.. 2009. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J. Biol. Chem. 284: 5637–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McClain C. J., Barve S., and Deaciuc I.. 2007. Good fat/bad fat. Hepatology. 45: 1343–1346. [DOI] [PubMed] [Google Scholar]
- 136.Yamaguchi K., Yang L., McCall S., Huang J., Yu X. X., Pandey S. K., Bhanot S., Monia B. P., Li Y. X., and Diehl A. M.. 2007. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 45: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 137.Barreyro F. J., Holod S., Finocchietto P. V., Camino A. M., Aquino J. B., Avagnina A., Carreras M. C., Poderoso J. J., and Gores G. J.. 2015. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 35: 953–966. [DOI] [PubMed] [Google Scholar]
- 138.Malhi H. 2014. MicroRNAs in ER stress: divergent roles in cell fate decisions. Curr. Pathobiol. Rep. 2: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lackey D. E., and Olefsky J. M.. 2016. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12: 15–28. [DOI] [PubMed] [Google Scholar]
- 140.Neuschwander-Tetri B. A., Loomba R., Sanyal A. J., Lavine J. E., Van Natta M. L., Abdelmalek M. F., Chalasani N., Dasarathy S., Diehl A. M., Hameed B., et al. . 2015. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 385: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]