Abstract
Background
Fine particulate matter with aerodynamic diameters smaller than 2.5 μm (PM2.5) has been reported to cause adverse effects on human health. Evidence has shown the association between PM2.5 exposure and adverse perinatal outcomes, and the most common method is epidemiological investigation. We wished to investigate the impact of PM2.5 on placenta and prenatal outcomes and its related mechanisms in a rat model.
Material/Methods
Pregnant rats were exposed to a low PM2.5 dose (15 mg/kg) with intratracheal instillation at pregnant day 10 and day 18, while the controls received an equivalent volume normal saline. All rats received cesarean section 24 h after the last intratracheal instillation and were sacrificed with anesthesia. Blood routine tests (BRT) and interleukin-6 (IL-6) were detected for analyzing inflammation and blood coagulation. Placenta tissue sections underwent pathologic examination, and the levels of homogenate glutathione peroxidase (GSH-Px) and methane dicarboxylic aldehyde (MDA) were determined for oxidative stress estimation.
Results
Increased absorbed blastocysts, and lower maternal weight gain and fetal weight were found in the PM2.5 exposure group compared to controls (p<0.05). Exposure to PM2.5 caused a significant increase of blood mononuclear cells (PBMC), platelets, and IL-6 levels (P<0.01). There were no differences in GSH-Px and MDA of placenta homogenate between the 2 groups (P>0.05). Placenta pathological examination demonstrated thrombus and chorioamnionitis in the PM2.5 exposure group.
Conclusions
PM2.5 exposure can result in placental pathological changes and adverse perinatal outcomes. The placental inflammation and hypercoagulability with vascular thrombosis may play important roles in placental impairment, but oxidative stress appears to be less important.
MeSH Keywords: Particulate Matter, Pregnancy, Systemic Inflammatory Response Syndrome
Background
Fine particulate matter, especially that composed of particles with an aerodynamic diameter of less than 2.5 μm (PM2.5), has been reported to pose great public health hazards. It is an environmental factor in developing diseases such as type 2 diabetes [1], cardiovascular disease [2], and asthma [3]. Even a short-term increased PM2.5 concentration in the air results in elevated mortality and morbidity [4]. Recently, epidemiological evidence has shown the association between PM2.5 exposure and adverse perinatal outcomes, such as restricted fetal growth expressed as low birth weight (<2500 g or small for gestational age). An epidemiological study showed that PM2.5 exposure during the third trimester was significantly associated with a reduction of neonatal birth weight [5]. Neonatal head circumference and triceps and subscapular skinfold thicknesses were decreased significantly with in utero exposure to PM2.5 [6].
Adverse perinatal outcome is usually regarded as an indicator of placental dysfunction [7]. Fetal growth is dependent on nutrient availability, which in turn is related to the placenta. This oval organ is where maternal and fetal blood are brought into very close contact, so that the mother can supply oxygen and nutrients to the fetus and remove waste products from the fetal blood at the same time. This transport process depends on the morphological characteristics of placenta, such as the placental size and blood flow. In clinical and experimental studies, fetal growth retardation has been commonly regarded as a consequence of placental insufficiency. Moreover, placental insufficiency results in a variety of pregnancy complications throughout the 3 trimesters, including abortion, fetal death, and preterm labor [8,9].
Based on results of previous studies, we hypothesized that PM2.5 can hurt placental development, subsequently promoting placental morphological changes and insufficiency, finally resulting in pregnancy complications. To date, few studies have investigated the placental changes and perinatal consequences of air pollution, and even less attention has been paid to PM2.5.These limited papers simply focussed on reduction of placenta weight [10] or local placental inflammation [11] in response to air pollution.
The mechanisms by which PM2.5 causes illnesses are still not well understood. Inflammation, coagulation hyperfunction, and oxidative response are the most commonly proposed mechanisms. Systemic inflammation is generally believed to be the major mediator of chronic exposure to ambient PM2.5 [12]. The ultrafine particles in urban air may promote inflammation of lung epithelium, which would increase the concentrations of acute cytokines [13] like interleukin, and in turn cause systemic inflammation. Increased plasma viscosity [14] and elevated blood platelet level [15] are also associated with exposure to ambient particles. Enhanced thrombosis susceptibility is shown with diesel exhaust particles exposure [16], which indicates that ambient particles may promote the process of thrombosis in capillaries and then affect function of organs. Oxidative stress is also an important etiological mechanism underlying PM2.5-induced injury [17]. PM2.5 inhalation can exert toxic effects on blood cells and the antioxidant system, stimulating the production of free radicals or reactive oxygen. In lungs, the antioxidative enzyme activities and lipid peroxidation levels changed markedly with obvious dose-dependent PM2.5 exposure [18].
Inhaled PM2.5 can easily be transported from lung alveoli to capillaries, after which it is dissolved and circulated into the bloodstream via the pulmonary artery. During blood circulation, components of PM2.5 may enter the uteroplacental vascular system, resulting in placenta pathologic changes and decreased transplacental function, with consequent pregnancy complications such as restricted fetal growth through 1 or more action mechanisms. We designed this animal experiment to assess the effect of PM2.5 on perinatal outcomes, especially on placenta, and to explore the possible mechanism by which PM2.5 affects the placenta in utero.
Material and Methods
Animals
Eleven-week-old female and 12-week-old male Sprague–Dawley (SD) rats were acclimatized 2 weeks in separate cages prior to mating. The animals were maintained in a climate-controlled room under a 12-h alternating light/dark cycle, 22±2°C temperature, and 50±10% relative humidity. Pregnancy was confirmed by the presence of a vaginal mucus plug, designated as Day 1 of pregnancy. Pregnant rats were placed in individual cages under the conditions similar to the acclimatization period. All animal studies were approved by the Animal Experimental Ethics Committee of the Laboratory Animal Centre, Wenzhou Medical University, China.
PM2.5 sampling and processing
The samples of PM2.5 were collected by a particulate sampler (2031 Qingdao Laoying Instruments Co. Ltd., China) through a fiberglass filter between Jan and Jun 2014 in Wenzhou, China. Filters were weighed and cut into 1–2-cm2 squares. The filter squares were agitated in ultrapure water with an ultrasonic shaker for 30 min ×2 times. The solution was filtered through 8 layers of gauze and centrifuged at 12 000 rpm for 20 min. The sediment was collected by a vacuum freeze drier (Christ/ALPHA2-4 LD, Germany). The dry PM2.5 powder was diluted in sterile saline in a concentration of 30 mg/mL and kept at −20°C before experiments [19].
Animal groups and treatment
Twenty pregnant rats were randomly assigned to 2 groups: 10 in the PM2.5 exposure group and 10 in the control group. Rats in the PM2.5 exposure group received a low PM2.5 dose (15 mg/kg) with intratracheal instillation at day 10 and day 18. The cumulative dose of PM2.5 for the PM2.5 exposure group was 30 mg/kg. Rats in the control group received an equivalent volume of normal saline with intratracheal instillation on the same days. All the pregnant rats received cesarean section at 23 h after the last intratracheal instillation and were sacrificed with anesthesia. All fetuses were weighed on an electronic balance scale (Mettler Toledo, AL204) and morphologically evaluated.
Blood biomarkers
Blood was sampled from the arteria cruralis. Tubes were centrifuged at 1500 rpm for 10 min at 4°C. Blood routine tests (BRT) were performed within 2 h after sacrifice via a hematology analyzer (SYSMEX-XS-800i). The interleukin-6 (IL-6) levels were measured by an enzyme-linked immunosorbent assay (ELISA) kit (ab100772, Abcam, UK) following the manufacturer’s instructions.
Placenta biomarkers detections
At the endpoint of the exposure, placenta tissues were collected after cesarean section for further analysis. Placentas were cut in half longitudinally, one piece for pathologic examinations and the other for biomarker detection. The tissues for biomarker detection were homogenized and stored at −80°C. The activities of glutathione peroxidase (GSH-Px) and methane dicarboxylic aldehyde (MDA) were detected for oxidative stress estimation with a colorimetric method assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Placenta pathology
Placenta tissue sections were stained using hematoxylin and eosin (HE). For histological and morphometric analysis, 10–20 fields per section were observed with digital light microscopy (Qimaging, Q36955, Canada). Placenta histological findings were reported, including thrombotic vasculopathy, chorioamnionitis, and abnormal syncytiotrophoblast cells. Syncytiotrophoblastic nodules are characterized by 3 syncytiotrophoblastic cells fused in the villus or intervillous space. All of these pathologic processes have been linked with poor placental functions and adverse pregnancy outcomes [20–22]. Three independent, experienced observers performed semiquantitative evaluation of the slides.
Statistics
Statistical analysis was performed using SPSS20.0 IBM statistical software to assess the differences between the PM2.5 exposed rats and the non-exposed rats. Chi-squared tests were used for categorical data and paired t-test (two-way ANOVA) for continuous data. A p value of less than 0.05 was considered statistically significant.
Results
During the whole exposure process, 2 rats in the PM2.5 group and 1 in the control group aborted several hours after endotracheal intubation. At the end of our experiment there were 8 rats in the PM2.5 group and 9 in the control group.
Perinatal Outcomes
Blastocysts transferred into vacuoles in a single uterine segment are called absorbed blastocysts. As shown in Table 1, 17% of blastocysts were absorbed with PM2.5 exposure, but only 5% were absorbed in the control group (P<0.05). In contrast to results of previous research [10], there was no difference in placenta weights between the 2 groups (P=0.78). The mean maternal weight gain and fetal weight in the PM2.5 group were lower than in the control group (P<0.05). To determine whether any differences existed in the external morphology, all the fetuses were checked carefully, including counting forelimbs and hind legs; there were no obvious external malformations in fetuses delivered from either group.
Table 1.
Maternal-fetal-placenta weight (in grams, mean ±SEM) and the number of absorbed blastocyst in test group and control.
| Group | n | Maternal weight | Fetal weight | Fetus | Absorbed blastocyst | Placenta weight | |
|---|---|---|---|---|---|---|---|
| Day 1 | Day19 | ||||||
| Control | 9 | 288.22±20.27 | 368.89±34.01 | 4.08±1.10 | 109 | 6 | 0.66±0.10 |
| PM2.5 | 8 | 274.90±10.18 | 313.63±15.23* | 3.11±1.54* | 80 | 17* | 0.65±0.08 |
P<0.05 in PM2.5 exposure group compared with controls.
Blood cell counting and IL-6 level
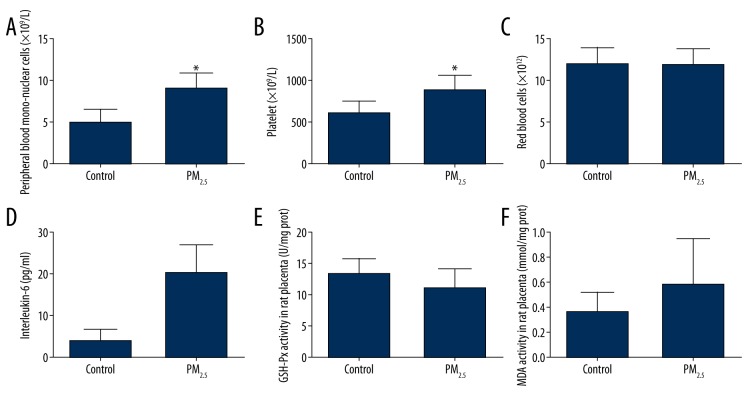
The various blood biochemical parameters are presented in Figure 1. Peripheral blood mononuclear cells (PBMC), platelets, and IL-6 were increased in the PM2.5 group compared to the control group (P<0.01). There was no significant difference in red blood cells (RBC) count between the 2 groups.
Figure 1.
Systemic inflammation, blood platelet count, and placenta oxidative stress with exposure to PM2.5. The peripheral blood mononuclear cells (PBMC) (A), platelet (B), and interleukin-6 (IL-6) (C) were higher in the test group compared to the control group (* p<0.01), but red blood cell count (D) was not. The glutathione peroxidase (GSH-Px) (E) and malondialdehyde (MDA) (F) of placenta homogenate were not significantly different between the 2 groups.
Placenta oxidative parameters expression
To determine if there was severe oxidative injury in placentas rats in the PM2.5 group, GSH-Px and MDA of placenta homogenate were detected and are illustrated in Figure 1. The placenta tissues with PM2.5 exposure tended to have a greater MDA (p=0.13) and a lower GSH-Px (p=0.11), but no significant difference was found between the 2 groups.
Placenta pathology
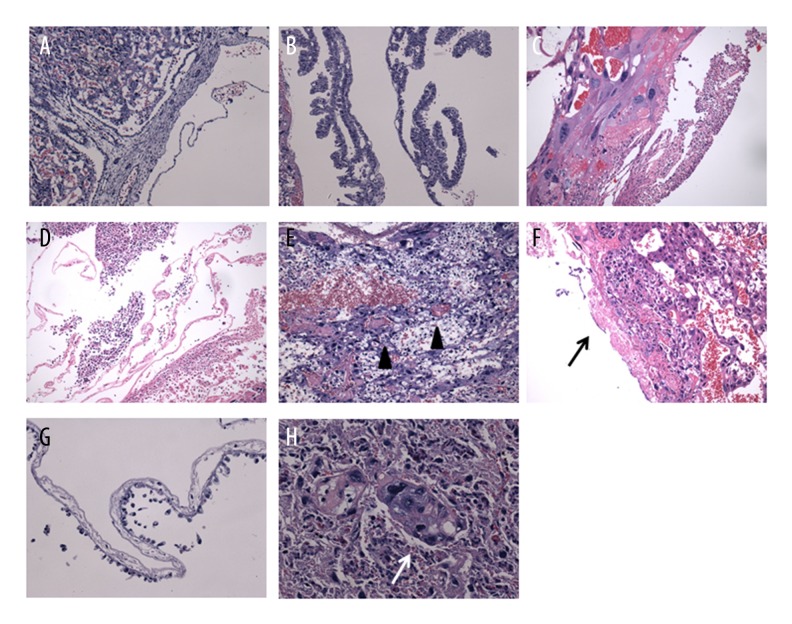
All the exposed placentas demonstrated at least 1 abnormal pathological finding (Figure 2C–2H). Extensive neutrophilic granulocyte infiltration (87.50%) was the most common microscopic finding (Figure 2C, 2D), followed by placental thrombus (62.50%, Figure 2E), fibrin deposition (37.50%, Figure 2F) in fetal surface, and serious amnionitis (25.00%) with papillae loss and epithelial cell necrocytosis and shedding (Figure 2G). Syncytiotrophoblast cells hyperplasia was common and some specimens showed syncytiotrophoblast nodules (25.00%, Figure 2H).
Figure 2.
Hematoxylin and eosin (HE) staining of placental tissue. (A, B) Non-exposed placental tissue did not shown abnormal pathological changes; (C–H) Placental tissues from PM2.5 exposure rats were described by: (C, D) placental infiltration of neutrophilic granulocytes involving amniotic membrane (E) thrombus (black triangle), (F) focal fibrinoid (black arrow), and (G) amnion with flat papillae and desquamated epithelial cells [=200×]. (H) Syncytiotrophoblast nodule (white arrow) observed from exposed placenta [=400×].
As expected, no pathological change was observed in any of the controls (Figure 2A, 2B). The placental villi were tortuous structures (Figure 2B) without neutrophilic granulocyte infiltration (Figure 2A).
Discussion
PM2.5, a complex mixture of particles with an aerodynamic diameter of less than 2.5 μm, consists of smoke, haze, and gas emissions. These are the smallest particles in the air with the highest likelihood of being inhaled, but they cannot be breathed out. They subsequently enter the systemic circulation, and ultimately cause negative health effects. Exposure to PM2.5 is known to induce failure of several organs and cause cardiovascular [4] and chronic pulmonary diseases [23].
A growing body of epidemiological evidence suggests that ambient air pollution has adverse effect on maternal and fetal development. Candace et al. found that maternal exposure to traffic-related air pollution increased the risk of gestational diabetes mellitus [24]. A population-based cohort study investigated the stillbirth rate and local PM2.5 concentration, and reported that exposure to high levels of PM2.5 in the third trimester of pregnancy was associated with 42% increased risk of stillbirth [25]. A study with a population consisting of 48 172 full-term live births in the state of Georgia, USA, used county-level PM2.5 data from the U.S. Environmental Protection Agency and reported that infants with maternal exposure to PM2.5 were at increased risk of low birth weight [26].
The tidal volume of rats is about 0.16 L and the respiratory rate is 70–110 breaths per minute [27]. The average PM2.5 concentration in Beijing in 2014 was as high as 85.9 μg/m3 [28]. Therefore, the equivalent daily PM2.5 exposure dose for rats would be about 1.4–2.2 mg/d, and the normal total dose in 19 pregnant days could be about 27–42 mg. In our study we used one-fifth of this normal total concentration (30 mg/kg). Under this PM2.5 exposure dose, the pregnant rats in our study still experienced more blastocysts lose, and retarded maternal weight gain and fetal weight compared to non-exposed rats. Our findings confirmed the effect of ambient particles on pregnant rats.
Combined with placenta pathological findings, these poor perinatal outcomes may be associated with placental abnormalities. The placenta acts as a bridge between mother and fetus. Fetal nutrient and oxygen supply, waste removal, and protection from xenobiotics rely on normal maternal-fetal circulation. The chorioamnionitis, amnionitis, and vessel thrombus observed in rats exposed to PM2.5 are regarded as an intrauterine inflammatory condition [29]. They may result in failure of the placenta to nourish the fetus and can result in pregnancy complications such as preterm birth [30], neonatal cerebral palsy [31], fetal growth restriction [32].
There are 3 potential mechanisms of PM2.5 exposure proposed by previous studies: high oxidative stress [33], hypercoagulability [34], and inflammation [12]. For placenta, PM2.5-mediated inflammation may play a major role during the exposure. Due to its small size, PM2.5 can reach the alveoli. Although these small airborne particles can be eliminated by bronchial epithelial cells, there are still some that remain and subsequently pass into the blood stream. The particles are recognized as foreign matter, the local immune response is activated, and proinflammatory cytokines are released [35]. Continued airborne particulate exposure leads to locally elevated concentrations of proinflammatory cytokines in the lung. This local immune response is thought to “spill over” into the circulation and trigger cellular inflammatory responses in various tissues. Finally, the exposure accelerates the transit of neutrophils, expands the leukocyte pool size, and increases levels of circulating cytokines such as IL-1 and IL-6, which are used to estimate the inflammatory reaction [13]. Brook et al. found increased PBMC and tumor necrosis factor-alpha neutrophil levels at 24 h after particulate matter exposure [36]. In a double-blind study, healthy men were exposed to diesel exhaust particulates for 1 h and the plasma IL-6 was obviously increased at 24 h after exposure [37]. In our study, elevated IL-6 and PBMC levels were also observed after PM2.5 exposure, indicating a more severe systemic inflammation reaction that may affect the placenta. Placenta chorioamnionitis with extensive neutrophilic exudate confirmed this inflammatory effect from another point of view. Moreover, PM2.5-exposed placenta shown amnionitis with obvious loss of papillae, which decreases surface area. The placental transport capacity may influence, and, in utero, cause oxygen and nutrient supply reduction. This could explain at least in part the high loss of blastocysts and low fetal weight with PM2.5 exposure.
Hypercoagulability may play an important role in PM2.5 exposure. Numerous studies have shown the relationship between air particulate pollutants and blood clotting. A study in China recruited a group of 76 young, healthy university students, determined their levels of plasminogen activator fibrinogen inhibitor-1 and tissue-type plasminogen activator. PM2.5 concentration was measured at 1 particulate matter supersite monitoring station 1 km from their campus. It revealed that urban air pollution was associated with blood coagulation in healthy young humans [38]. Rückerl found the plasma sCD40L, which reflects platelet activation, increased under ambient air pollution conditions in Germany [38,39]. Recently, Kloog et al. investigated effects of daily exposure to PM2.5 in each ZIP code of 453 413 deep vein thrombosis (DVT) patients and 151 829 pulmonary embolism (PE) patients. They found that a 10-μg m (−3) increase in short-term PM2.5 exposure was associated with a 0.63% increase in DVT admissions and a 6.98% increase in long-term exposure admissions. For PE, the associated risks were 0.38% and 2.67% [40]. Similarly, hypercoagulable state was found in our study. Thrombi were seen microscopically in most of the placentas of the PM2.5 exposure group and the average blood platelet count clearly increased after PM2.5 exposure. High platelet count may affect blood coagulation, perhaps due to direct interactions of circulating PM2.5 constituents with platelets, or due to an acute-phase response to the circulation inflammation, or other reasons. PM2.5 exposure resulted in thrombosis of placental capillaries. The vascular block reduces surface area for the transfer of substrates from mother to fetus and may contribute to placental dysfunction. Such placenta morphological and functional changes may consequently lead to decreased fetal growth.
Oxidative response may not be an important mechanism in placenta impairment. A state of high oxidative stress is a condition in which levels of free radicals or reactive oxygen are higher than normal. GSH-Px is an enzyme whose main biological role is to protect the body from oxidative damage. The biochemical function of GSH-Px is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water. MDA is the ultimate degraded products of oxygen free radical peroxidation. The levels of these markers are altered in many organs after ambient particles exposure, such as lung [41] and liver [42]. But in our study, these 2 biomarkers had not significantly different in placenta tissues of the 2 groups. Low-dose exposure during pregnancy may be one reason, and the differential expression level of the 2 makers due to the organ difference may be another reason. However, our findings indicate that oxidative stress does not play a major role in the placenta abnormality during PM2.5 exposure.
Conclusions
Our study provides direct evidence that PM2.5 exposure can cause adverse perinatal outcomes. Placenta of PM2.5-exposed rats revealed a number of pathologic changes in comparison to the controls. Placental inflammation and hypercoagulability with vascular thrombosis may play important roles in placental impairment, while oxidative stress may not. Pregnant women should avoid PM2.5 exposure.
Footnotes
Source of support: This study was supported by the Opening Project of Zhejiang Provincial Top Key Discipline of Clinical Medicine (LKFJ041)
References
- 1.Malmqvist E, Larsson HE, Jonsson I, et al. Maternal exposure to air pollution and type 1 diabetes – accounting for genetic factors. Environ Res. 2015;140:268–74. doi: 10.1016/j.envres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Zanobetti A, Schwartz J, Samoli E, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111:1188–93. doi: 10.1289/ehp.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dales R, Chen L, Frescura AM, et al. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: A panel study of schoolchildren with asthma. Eur Respir J. 2009;34:316–23. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- 4.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet. 2013;382:1039–48. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darrow LA, Klein M, Strickland MJ, et al. Ambient air pollution and birth weight in full-term infants in atlanta, 1994–2004. Environ Health Perspect. 2011;119:731–37. doi: 10.1289/ehp.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schembari A, de Hoogh K, Pedersen M, et al. Ambient air pollution and newborn size and adiposity at birth: Differences by maternal ethnicity (the born in bradford study cohort) Environ Health Perspect. 2015;123:1208–15. doi: 10.1289/ehp.1408675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlop K, Cedrone M, Staples JF, Regnault TR. Altered fetal skeletal muscle nutrient metabolism following an adverse in utero environment and the modulation of later life insulin sensitivity. Nutrients. 2015;7:1202–16. doi: 10.3390/nu7021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toal M, Chan C, Fallah S, et al. Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol. 2007;196:363.e1–7. doi: 10.1016/j.ajog.2006.10.897. [DOI] [PubMed] [Google Scholar]
- 9.Abramowicz JS, Sheiner E. In utero imaging of the placenta: Importance for diseases of pregnancy. Placenta. 2007;28(Suppl A):S14–22. doi: 10.1016/j.placenta.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Rocha ESIR, Lichtenfels AJ, Amador Pereira LA, Saldiva PH. Effects of ambient levels of air pollution generated by traffic on birth and placental weights in mice. Fertil Steril. 2008;90:1921–24. doi: 10.1016/j.fertnstert.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.de Melo JO, Soto SF, Katayama IA, et al. Inhalation of fine particulate matter during pregnancy increased il-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett. 2015;232:475–80. doi: 10.1016/j.toxlet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Brook RD, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 13.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: Relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 14.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: A link to mortality? Lancet. 1997;349:1582–87. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 15.Viehmann A, Hertel S, Fuks K, et al. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med. 2015;72:656–63. doi: 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- 16.Nemmar A, Hoet PH, Vermylen J, et al. Pharmacological stabilization of mast cells abrogates late thrombotic events induced by diesel exhaust particles in hamsters. Circulation. 2004;110:1670–77. doi: 10.1161/01.CIR.0000142053.13921.21. [DOI] [PubMed] [Google Scholar]
- 17.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–62. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 18.Meng Z, Qin G, Zhang B, et al. Oxidative damage of sulfur dioxide inhalation on lungs and hearts of mice. Environ Res. 2003;93:285–92. doi: 10.1016/s0013-9351(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 19.Choi JH, Kim JS, Kim YC, et al. Comparative study of pm2.5 – and pm10 – induced oxidative stress in rat lung epithelial cells. J Vet Sci. 2004;5:11–18. [PubMed] [Google Scholar]
- 20.Feeley L, Mooney EE. Villitis of unknown aetiology: Correlation of recurrence with clinical outcome. J Obstet Gynaecol. 2010;30:476–79. doi: 10.3109/01443611003802339. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JM, Gilstrap LC, Cox SM, et al. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol. 1998;91:725–29. doi: 10.1016/s0029-7844(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 22.Neres R, Marinho CR, Goncalves LA, et al. Pregnancy outcome and placenta pathology in plasmodium berghei anka infected mice reproduce the pathogenesis of severe malaria in pregnant women. PloS One. 2008;3:e1608. doi: 10.1371/journal.pone.0001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robledo CA, Mendola P, Yeung E, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–22. doi: 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFranco E, Hall E, Hossain M, et al. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PloS One. 2015;10:e0120594. doi: 10.1371/journal.pone.0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twum C, Zhu J, Wei Y. Maternal exposure to ambient pm and term low birthweight in the state of georgia. Int J Environ Health Res. 2016;(1):92–100. doi: 10.1080/09603123.2015.1061110. [DOI] [PubMed] [Google Scholar]
- 27.Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.jing Ny. Beijing: The annual concentration of pm2.5 fell 4.0% in 2014. 2015. Jan 04, [eb/ol]. Http://news.Xinhuanet.Com/local/2015-01/04/c_1113867637.Htm.
- 29.Conti N, Torricelli M, Voltolini C, et al. Term histologic chorioamnionitis: A heterogeneous condition. Eur J Obstet Gynecol Reprod Biol. 2015;188:34–38. doi: 10.1016/j.ejogrb.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Pappas A, Kendrick DE, Shankaran S, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168:137–47. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 32.Williams MC, O’Brien WF, Nelson RN, Spellacy WN. Histologic chorioamnionitis is associated with fetal growth restriction in term and preterm infants. Am J Obstet Gynecol. 2000;183:1094–99. doi: 10.1067/mob.2000.108866. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger B, Pryor WA, Cueto R, et al. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14:1371–77. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 34.Pekkanen J, Brunner EJ, Anderson HR, et al. Daily concentrations of air pollution and plasma fibrinogen in london. Occup Environ Med. 2000;57:818–22. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DM, Wilson MR, MacNee W, et al. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–99. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 36.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–67. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tornqvist H, Mills NL, Gonzalez M, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 38.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–76. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 39.Ruckerl R, Phipps RP, Schneider A, et al. Ultrafine particles and platelet activation in patients with coronary heart disease – results from a prospective panel study. Part Fibre Toxicol. 2007;4:1. doi: 10.1186/1743-8977-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kloog I, Zanobetti A, Nordio F, et al. Effects of airborne fine particles (pm2.5) on deep vein thrombosis admissions in the northeastern united states. J Thromb Haemost. 2015;13:768–74. doi: 10.1111/jth.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo B, Shi H, Wang L, et al. Rat lung response to pm2.5 exposure under different cold stresses. Int J Environ Res Public Health. 2014;11:12915–26. doi: 10.3390/ijerph111212915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araujo JA, Barajas B, Kleinman M, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–96. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]