ABSTRACT
In this study, we determined the number of peripheral blood circulating tumor cells (CTCs) pre- and post-cryosurgery in patients with colorectal cancer liver metastasis as a reference for understanding the relevance of any changes to the efficacy of cryosurgery. CTC numbers and CTC-related gene expression were measured in the peripheral blood of 55 patients with colorectal liver metastasis at 1 day before and 7 and 30 d after cryoablation using magnetic activated cell sorting (MACS) and fluorescence activated cell sorting (FACS) combined with real-time quantitative PCR (RT-qPCR). The number of CTCs decreased significantly with postoperative time (P < 0.01). Delta cycle threshold values for the CTC-related genes CEA, Ep-CAM, CK18 and CK19 increased significantly after cryoablation. Furthermore, the expression of CEA, Ep-CAM, CK18 and CK19 decreased significantly with time after cryoablation (P < 0.01). RT-qPCR and FACS combined with MACS has significant diagnostic and prognostic value for evaluating the efficacy of cryosurgery in patients with advanced colorectal cancer.
KEYWORDS: Circulating tumor cells, cryosurgery, colorectal cancer, flow cytometry, liver metastasis, reverse transcriptase polymerase chain reaction
Introduction
More than 90% of cancer-related deaths are due to metastatic disease rather than the primary tumor from which it arises 1. Among patients with colorectal cancer, 25% have detectable liver metastasis (CRLM) when first diagnosed and a further 20–30% have CRLM detected after primary resection.2 Thus, the identification of optimal diagnostic, predictive, surgical and perioperative methods to prevent death from CRLM is of paramount importance. Colorectal cancer is the third most common cancer in men and the second most common in women worldwide.3 About 50% of colorectal cancer patients develop metastases and a large proportion of these have CRLM; only about 20% of unresectable cancer who are able to achieve complete resection. Overall 5-year survival is 50–60%.4
Liver resection is the only treatment associated with long-term survival in patients with CRLM.5 For these patients, treatment has improved significantly over the past 2 decades, due principally to major advances in surgical techniques and the availability of more effective systemic therapies.6 However, only 10–20% of patients with CRLM are considered suitable candidates for hepatic metastasectomy because of the presence of extrahepatic disease or the anatomical distribution of their disease.7,8 To improve the eradication of metastases, local therapy by ablation is an attractive option when resection alone is inadequate due to insufficient remaining liver volume and to avoid overextended resection in an already complex procedure.9 It is difficult to treat these patients without surgery. Long term follow-up data suggest that cryoablation and local surgery achieve similar survival rates in patients with CRLM; moreover, cryoablation may reduce mortality rates in cancer patients.10 Liquid nitrogen has been used to freeze and destroy cancerous tissue at the cellular level, improving the efficiency of excision of CRLM.11 Recently, hepatic cryotherapy has emerged as a new treatment option in colorectal cancer patients with unresectable CRLM.12 Argon–helium surgery (Cryocare System) uses ultralow temperature cryoprobes to destroy tumor cells.13 In China, cryoablation is widely used to ablate lung, liver and kidney cancer. The Cryocare System offers distinct advantages over other cryomedical devices. It is the only device that can be used for percutaneous applications. Cryoablation as a local ablation method may be superior to surgery because it is suitable for multiple liver lesions and does not require massive resection of liver tissue.14,15 However, 60–70% of patients eventually relapse after treatment 16; therefore, the development of sensitive and robust circulating biomarkers is critical.
Circulating tumor cells (CTCs) are cancer cells shed from either the primary tumor or its metastases that circulate in the peripheral blood. CTCs may act as seeds for metastases and may indicate the spread of the disease.17,18 Increasingly, CTCs are being evaluated in liquid biopsies and their analysis hold great promise for the identification of patients at high risk for relapse, for the stratification of patients to specific adjuvant therapies and for monitoring response to treatment.19-21 The number of CTCs has been shown to be an independent prognostic biomarker in small cell and non-small cell lung cancer patients 22,23 and has been used in other epithelial cell tumors such as breast cancer,24,25 colorectal cancer 26 and prostate cancer.27 Because CTCs are often present in the blood of patients with tumor metastasis, detection of CTCs in peripheral blood is strongly correlated with early metastasis.28 The number of CTCs can also provide information on tumor biologic activity and enable real-time prediction of the prognosis of patients with distant metastases.26,27,29 In this study we used flow cytometry with immune magnetic beads and real-time quantitative PCR (qPCR) to measure CTCs in the peripheral blood of patients with CRML before and after cryosurgery, to explore the value of CTCs for evaluating the prognosis of patients with CRML who undergo cryosurgery.
Materials and methods
Patients
Patients who underwent cryoablation therapy for CRML in Fuda Cancer Hospital of Jinan University Affiliated Hospital were recruited from June 2014 to September 2015. The inclusion criteria were as follows: patient shown to have malignant CRML on imaging and pathology; patient agreed to undergo cryosurgery for CRML; patient able to understand the procedure and voluntarily signed informed consent; estimated survival of > 3 months after treatment; age > 18 y and < 85 years; Karnofsky performance status score > 60 points; and no obvious anomalies on routine blood tests or in liver and kidney function. The exclusion criteria were as follows: local/systemic chemotherapy ongoing or finished no more than 15 d previously; blood coagulation disorders or severe anemia; combined with other primary tumors; and concurrent sexually transmitted disease, leprosy, AIDS, HIV infection, hepatitis, tuberculosis or parasitic blood infection. Information for the 55 patients with CRML who met the inclusion criteria is shown in Table 1. Written informed consent was obtained from all patients before entry into the study. The protocol was approved by an institutional review board. This trial is registered at ClinicalTrials.gov as number NCT02450422.
Table 1.
Patient information and CTC numbers at baseline.
| Group | n | CTC number 1 day prior to treatment |
|---|---|---|
| Sex | ||
| Male | 28 | 22.21 ± 9.769 |
| Female | 27 | 25.22 ± 9.593 |
| Age (years) | ||
| ≤ 61 | 23 | 22.39 ± 7.981 |
| > 61 | 32 | 24.62 ± 10.628 |
| Differentiation of primary lesion | ||
| High differentiation | 16 | 24.19 ± 9.159 |
| Medium and low differentiation | 39 | 23.49 ± 9.875 |
| Number of lesions treated | ||
| 1 | 19 | 22.42 ± 8.009 |
| 2 | 19 | 23.69 ± 11.951 |
| 3 or more | 17 | 23.71 ± 8.601 |
| Treatment for liver metastasis before cryoablation | ||
| Surgery | 46 | 23.83 ± 9.907 |
| Chemotherapy | 7 | 21.71 ± 4.680 |
| Radiotherapy | 2 | 23.69 ± 19.092 |
| Tumor diameter (cm) | ||
| ≤ 1 | 24 | 24.63 ± 11.251 |
| > 1 | 31 | 22.97 ± 8.208 |
| Local Control | ||
| Local recurrence | 6 | 25.17 ± 13.091 |
| No recurrence | 49 | 23.51 ± 9.240 |
Percutaneous cryosurgery
Comprehensive cryosurgery was performed on all 55 patients. Obvious intrahepatic masses were cryoablated as previously reported.30,31 Percutaneous cryoablation was performed under double-row helical computed tomography (Somatom Emotion Duo; Siemens, Munich, Germany) or color ultrasound (ALOKA SSD-5500SA; Aloka, Tokyo, Japan) guidance. All cryosurgery was performed by Dr. Lizhi Niu and assistants (Haibo Li and Feng Mu). Each procedure comprised one to 3 freeze/thaw cycles accomplished using an argon gas-based cryosurgical unit (Endocare, Irvine, CA, USA).30,31 Depending on the location of the metastasis, probes were inserted percutaneously under ultrasound or CT guidance; 2 or 5 mm probes or, rarely, 10 mm probes (Cryo-42; Endocare, Irvine, CA, USA) were used according to the size of the tumor. Two or more probes were used simultaneously for large lesions. Individual tumors were frozen sequentially on a tumor-by-tumor basis. The duration of freezing depended on the formation of an “ice-ball” visible on ultrasonography as a hypoechogenic area > 1 cm larger than the diameter of the lesion. Thawing was achieved by input of helium for a period of time equal to the freezing time before the next freezing process was begun.
Cell line
Colorectal carcinoma cell line CX-1 (Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum at 37°C in a humidified atmosphere with 5% CO2.
Preparation of blood samples
Peripheral blood samples were collected at 3 time points: 1 day before cryoablation and 7 and 30 d postoperatively. On each occasion, approximately 20 mL blood was drawn by vein puncture from the 55 patients with liver cancer treated in our hospital and from 8 healthy volunteers. Blood from the healthy volunteers was used to plot a standard curve for flow cytometry experiments. To avoid contamination with skin cells, 5 mL blood was discarded before experimental samples were taken, as previously described. The samples were stored at room temperature and processed within 6 h after collection. Briefly, mononuclear cells were separated from other blood components using human peripheral blood lymphocyte separation liquid (Tianjin Haoyang Biological Manufacture Co., Ltd, Tianjin, China) and centrifuged at 1800g for 20 min at 4°C. Interface cells were removed and washed, and red blood cells were removed using BD Pharm Lyse™ (Becton Dickinson, San Jose, CA, USA). Following further washes, mononuclear cells were counted and samples were divided into 2 for RT-qPCR and multiparameter flow cytometry experiments (each sample contained at least 2–3 × 106 cells). Cell pellets were resuspended in phosphate-buffered saline (PBS) (Life Technologies, Shanghai, China) for multiparameter flow cytometry and then in TRIzol reagent following counting using a TC10™ automatic cell count meter (Bio-Rad, Hercules, CA, USA). Live cells were stained using Trypan Blue solution (Life Technologies, Carlsbad, CA, USA) and stored at −70°C until needed for RNA extraction.
Flow cytometry
After separation of blood using human peripheral blood lymphocyte separation liquid, mononuclear cells were washed twice with sterile Hank's balanced salt solution (Life Technologies, Shanghai, China). Isolated cells were enriched by binding to magnetic CD326 (Ep-CAM) MicroBeads (Miltenyi Biotech Ltd, Bergisch Gladbach, Germany) using magnetic activated cell sorting (MACS). Enriched isolated cells were then labeled with monoclonal antibodies targeting the epithelial cell antigens CD45, CD326 and cytokeratin 8, 18 and 19 (Miltenyi Biotech Ltd) and incubated in the dark at room temperature for 12 min. Antibodies specific for leukocytes (CD45) labeled with phycoerythrin (10 μL), specific for epithelial cells (cytokeratin 8, 18 and 19) labeled with fluorescein isothiocyanate (10 μL) and specific for epithelial cells (CD326/Ep-CAM) labeled with allophycocyan (10 μL) were added per 7.5 mL whole blood. Cell pellets were resuspended in 500 μL PBS and counted by flow cytometry using a BD FACSCanto™ II apparatus (Becton Dickinson, San Jose, CA, USA). Cells that were CD45 negative, CK positive and CD326 positive were defined as CTCs.
RT-qPCR
Primer sequences for GAPDH (reference)32 and the tumor markers CEA33 Ep-CAM,32 CK18 34 and CK1935 were obtained from the literature and are shown in Table 2. Primers were synthesized by Shanghai Yingweijieji Corp., Shanghai, China.
Table 2.
Pancreatic cancer CTC marker gene primers.
| Name of primer | Primer sequence (5'—3') | Tm (1 M Na+) | Product length(bp) |
|---|---|---|---|
| GADPH-F | TGCACCACCAACTGCTTAGG | 70.3 | 20 |
| GADPH-R | GGAGGCAGGGATGATGTTCT | 70.3 | 20 |
| CEA-F | AACTTCTCCTGGTCTCTCAGCT | 71.3 | 22 |
| CEA-R | GCAAATGCTTTAAGGAAGAAG | 65.0 | 21 |
| Ep-CAM-F | GGACCTGACAGTAAATGGGGAAC | 73.5 | 23 |
| Ep-CAM-R | CTCTTCTTTCTGGAAATAACCAGCAC | 72.9 | 26 |
| CK18-F | TGGTCACCACACAGTCTGCT | 70.3 | 20 |
| CK18-R | CCAAGGCATCACCAAGATTA | 66.2 | 20 |
| CK19-F | ATGAAAGCTGCCTTGGAAGA | 66.2 | 20 |
| CK19-R | TGATTCTGCCGCTCACTATCAG | 71.3 | 22 |
RNA was extracted from 1 mL TRIzol (Life Technologies, Carlsbad, CA, USA) that had been kept at −70°C. After thawing, 0.2 mL chloroform (Guangzhou Chemical Reagent Factory, Guangzhou, China) was added to the tube after centrifugation at 13,500g for 15 min at 4°C. The supernatant, containing intact RNA, was moved to a new tube then RNA precipitated with 500 μL isopropyl alcohol (Tianjin Fuyu Fine Chemical Co., Ltd, Tianjin, China) and washed with 75% ethanol (Tianjin Fuyu Fine Chemical Co., Ltd). The RNA was dissolved in 50 μL RNase-free water to the required concentration and its concentration and purity detected by Thermo Scientific Multiskan GO microplate spectrophotometer (Thermo Fisher, Shanghai, China). qPCR with SYBR® Green (Takara, Dalian, China) was used to detect the amplification products. For RNA synthesis by reverse transcription, cDNA template was used in the same reaction tube as used for the PCR amplification reaction. The total reaction system volume was 20 μL, including 10 μL of 2 × One Step SYBR® RT-PCR Buffer 4, 0.8 μL of PrimeScript Enzyme Mix 2 (Takara, Dalian, China), 0.8 μL of 10 μmol/L upstream and 0.8 μL downstream primers, 0.4 μL 50 × ROX Reference Dye, which was used to determine when the fluorescence signal had reached the cycle threshold (Ct) (Life Technologies, Carlsbad, CA, USA), 2 μL of total RNA and 5.2 μL of dH2O. The reverse transcription reaction took place at 42°C for 5 min and at 95°C for 10 s. PCR took place at 95°C for 5 s and 60°C for 34 s for a total of 40 cycles. A melting curve was drawn at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. The PCR system was found to be stable and to have good repeatability and did not lead to any nonspecific amplification.
Statistical analysis
Data were analyzed using SPSS version 20.0 (IBM, Armonk, NY, USA) and expressed as the mean ± standard deviation. Random analysis of variance was performed and P < 0.05 was considered statistically significant; P < 0.01 was considered statistically significant for expression differences. GraphPad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to plot all graphs.
Results
Changes in numbers of CTCs after cryotherapy
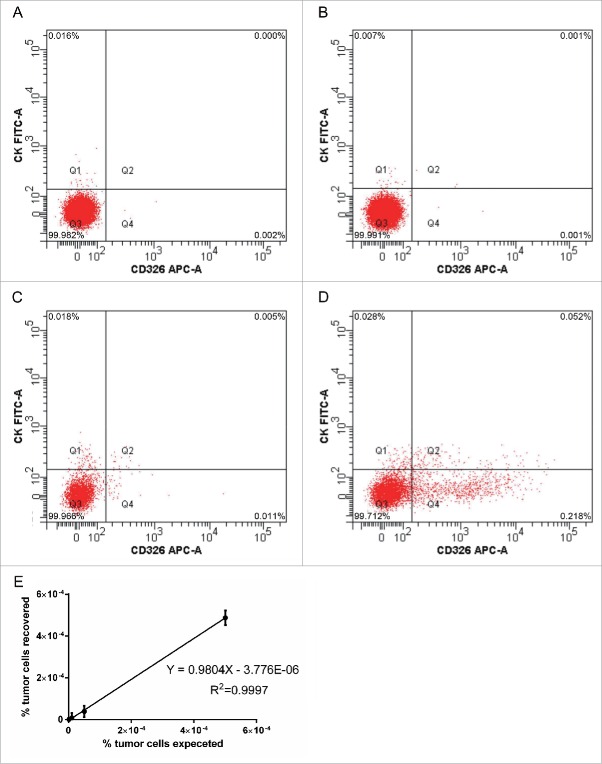
The numbers of CTCs in the peripheral blood of patients before and after cryotherapy for CRML were determined by flow cytometry. A standard curve for the determination of CTCs was generated by adding CX-1 cells to blood obtained from healthy volunteers. Analysis of serial dilutions (0.0001%, 0.001%, 0.005% and 0.05%) of human CX-1 tumor cells in normal human blood demonstrated that the lower detection limit for sensitivity of the method was 0.001%, which is one detected cell per 100,000 white blood cells (Fig. 1A-D). Below this level, background events were unpredictable. Recovery and linearity were highly reproducible according to correlation and regression analysis (Fig. 1E) and the number of tumor cell events recovered correlated positively with the expected number of tumor cell events based on the serial dilutions (R2 = 0.9997).
Figure 1.
Flow cytometry of CTCs in the peripheral blood of patients before and after cryotherapy for CRML. (A-D) Analysis of serial dilutions (0.0001%, 0.001%, 0.005% and 0.05%) of human CX-1 tumor cells in normal human blood. (E) Recovery and linear relationship across 3 separate experiments, every separate experiment conducts 10 times (n = 10).
Peripheral blood CTCs were analyzed in all 55 patients before and after cryosurgical treatment for CRML (Fig. 2). At 1 day before cryoablation (baseline), the mean number of CTCs was 23.69 ± 9.593; the mean numbers at 7 d and 30 d after the procedure were 19.29 ± 9.506 and 12.62 ± 6.178, respectively. Random analysis of variance (Table 3) showed that CTC numbers were decreased significantly at 7 and 30 d after cryoablation (P < 0.01 vs baseline).
Figure 2.

Flow cytometric enumeration of CTCs at 1 day before and 7 and 30 d after cryoablation therapy (P < 0.01, n = 55).
Table 3.
Pre- and post-treatment CTC numbers in peripheral blood.
| Patient ID | CTC number 1 day before treatment | CTC number 7 d after treatment | CTC number 30 d after treatment |
|---|---|---|---|
| P1 | 21 | 13 | 7 |
| P2 | 33 | 22 | 12 |
| P3 | 22 | 18 | 11 |
| P4 | 32 | 19 | 14 |
| P5 | 29 | 33 | 16 |
| P6 | 21 | 15 | 12 |
| P7 | 19 | 22 | 10 |
| P8 | 22 | 14 | 5 |
| P9 | 18 | 12 | 10 |
| P10 | 25 | 24 | 14 |
| P11 | 32 | 17 | 11 |
| P12 | 25 | 14 | 8 |
| P13 | 17 | 20 | 14 |
| P14 | 8 | 6 | 13 |
| P15 | 48 | 31 | 11 |
| P16 | 26 | 28 | 22 |
| P17 | 17 | 14 | 12 |
| P18 | 24 | 21 | 16 |
| P19 | 31 | 34 | 25 |
| P20 | 23 | 20 | 11 |
| P21 | 24 | 16 | 10 |
| P22 | 36 | 23 | 24 |
| P23 | 26 | 19 | 15 |
| P24 | 35 | 28 | 30 |
| P25 | 0 | 1 | 0 |
| P26 | 31 | 16 | 12 |
| P27 | 37 | 17 | 13 |
| P28 | 26 | 13 | 14 |
| P29 | 23 | 16 | 17 |
| P30 | 14 | 12 | 8 |
| P31 | 28 | 20 | 16 |
| P32 | 36 | 21 | 20 |
| P33 | 34 | 23 | 13 |
| P34 | 27 | 31 | 11 |
| P35 | 35 | 41 | 20 |
| P36 | 24 | 15 | 12 |
| P37 | 23 | 24 | 9 |
| P38 | 26 | 15 | 15 |
| P39 | 29 | 16 | 11 |
| P40 | 21 | 12 | 5 |
| P41 | 28 | 36 | 17 |
| P42 | 41 | 47 | 22 |
| P43 | 29 | 31 | 17 |
| P44 | 13 | 7 | 3 |
| P45 | 0 | 0 | 0 |
| P46 | 21 | 28 | 5 |
| P47 | 27 | 31 | 10 |
| P48 | 15 | 9 | 5 |
| P49 | 0 | 0 | 0 |
| P50 | 18 | 21 | 9 |
| P51 | 20 | 16 | 12 |
| P52 | 16 | 17 | 19 |
| P53 | 12 | 9 | 11 |
| P54 | 14 | 16 | 13 |
| P55 | 21 | 17 | 22 |
RT-qPCR
Changes in expression ΔCt values for CTC markers after cryotherapy
Measurement of ΔCt values for the CTC markers in the 55 patients with CRML before and after cryotherapy showed that CTC tumor markers decreased after cryotherapy. This indicated that cryotherapy reduced the number of CTCs in the patients' peripheral blood, which demonstrates the efficacy of cryoablation treatment.
Variable patterns of ΔCt value increases were observed. ΔCt for CEA increased from 2.27 ± 5.203 at baseline to 5.9204 ± 4.995 and 9.77 ± 5.551 at 7 and 30 d postoperatively, respectively; ΔCt for Ep-CAM increased from 2.60 ± 5.853 to 6.81 ± 5.816 and 10.56 ± 6.046; ΔCt for CK18 increased from 4.01 ± 6.049 to 8.23 ± 5.53 and 11.57 ± 5.530; and ΔCt for CK19 increased from 2.68 ± 5.506 to 6.09 ± 4.851 and 9.06 ± 6.005. Random analysis of variance (Fig. 3) showed that the △Ct values for the CTC markers were increased significantly at 7 and 30 d after cryoablation (P < 0.01 vs baseline).
Figure 3.
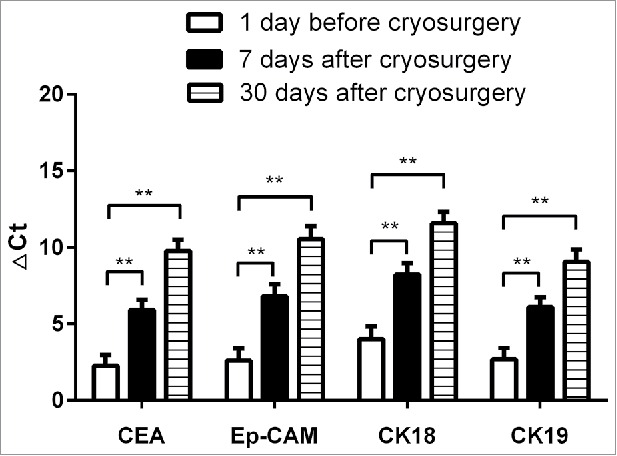
△Ct values for CTC markers at 1 day before and 7 and 30 d after cryoablation therapy (**P < 0.01, n = 55).
Changes in tumor marker gene expression after cryotherapy
Tumor marker-related gene expression before and after cryotherapy was analyzed using the 2−△△Ct method. Compared with baseline, CEA expression showed fold changes of 0.50 ± 1.496 and 0.05 ± 0.094 at 7 and 30 d after cryotherapy; Ep-CAM showed fold changes of 0.27 ± 0.523 and 0.08 ± 0.203; CK18 showed fold changes of 0.20 ± 0.324 and 0.05 ± 0.113; and CK19 showed fold changes of 0.53 ± 1.849 and 0.20 ± 0.730. All of the measured 2−△△Ct values for the target genes were less than 1, demonstrating that the expression of CTC markers decreased with time after treatment (Fig. 4).
Figure 4.
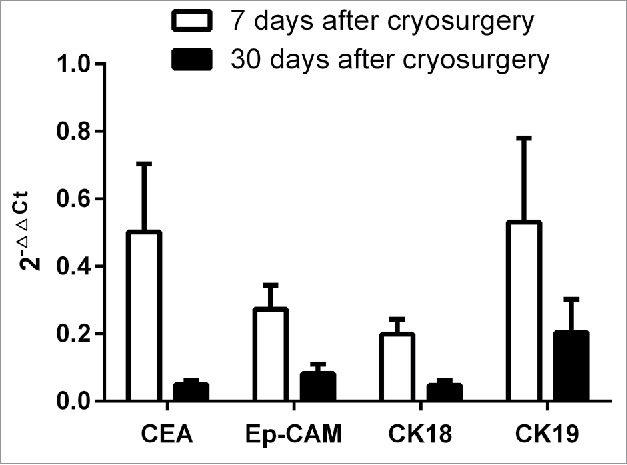
Changes in CTC marker expression at 1 day before and 7 and 30 d after cryoablation therapy (2−△△Ct method).
Discussion
Identification of an effective method for the diagnosis and treatment of locally advanced colorectal cancer is important. CTCs are increasingly being evaluated in liquid biopsies and CTC analysis holds great promise for the identification of patients at high risk for relapse, for the stratification of patients to specific adjuvant therapies and for monitoring response to treatment.19-21 Dynamic detection of peripheral blood CTCs is a promising prognostic indicator for cancer patients. This technique allows early identification of groups at high risk for recurrence, thus reducing the risk of tumor recurrence and significantly improving survival rates. The technology for CTC isolation and identification has developed rapidly in recent years, with FACS combined with MACS being the most widely used approach. This method can be used for sorting and quantitative analysis of single cells or other biological particles at the functional level. Detection of CTCs in human peripheral blood using flow cytometry approaches relies on the expression of epithelial cell-specific markers such as cytokeratins, which are expressed on epithelial cells but not on leukocytes.36 Cytokeratins are proteins comprising keratin-containing intermediate filaments in the intracytoplasmic cytoskeleton; their expression primarily depends on the type of epithelium, the time point in the course of terminal differentiation and the stage of development.37 CD326 (Ep-CAM) is another common surface marker for positive selection of cell populations,38 whereas a specific leukocyte surface marker (CD45) is used for negative selection 39 of white blood cells. Thus, in the present study CTCs were defined as CD45−CK+CD326+ cells on the basis of flow cytometry.
Cryoablation as a local ablation treatment method has the advantage of treating multiple liver lesions and does not require massive resection of liver tissue comparing with surgery. Unfortunately, there are still a majority of patients eventually relapse after treatment. CTCs as a robust method of circulating biomarker maybe can monitoring the process of cryoablation treatment. However, in recent years, there are seldom research in this region. We used the above-mentioned method to measure CTCs in the peripheral blood of 55 patients with CRML before and after cryoablation therapy. We found that the number of peripheral blood CTCs had decreased significantly at 7 and 30 d after cryoablation (P < 0.01). It is suggested that enumeration of peripheral blood CTCs can be used to evaluate the efficacy of cryosurgery. Previous meta-analyses40 have shown that CTCs are correlated with tumor size, with small tumors being associated with failure to detect CTCs in peripheral blood. The decline of CTC numbers after cryosurgery may be related to shrinkage of the tumor mass. Tumor tissue shrinks after cryoablation, with most tumor cells damaged and dying, which reduces the number of CTCs released from the lesion into the blood. The decrease of CTCs observed in the present study may therefore reflect the efficacy of the cryosurgery. Although argon–helium knife cryoablation is commonly used to treat CRML in China, there is still no effective method for evaluation of the curative effect of this treatment. The results of our study may provide a new and robust reference for the assessment of cryosurgery for CRML.
At 7 d after surgery, the number of CTCs in peripheral blood was increased compared with the preoperative number in 17/55 patients (30.91%), but all patients exhibited a marked decrease at 30 d after surgery (Fig. 5). This phenomenon may be explained by the immunologic response to cryoablation. Cryoablation of tumor tissue causes cells to undergo coagulative necrosis. On thawing, necrotic tumor cells within the ice-ball release intact tumor antigens, proinflammatory cytokines, nuclear proteins and high-mobility group box protein 1, which stimulate the innate immune response and attract granulocytes, macrophages and natural killer cells. These cells release cytokines and chemokines after activation. Dendritic cells, the professional antigen-presenting cells, then reach the damaged tissue and take up tumor antigens in a background of inflammation and abundant cytokines.41 By contrast, cells at the periphery of the ice-ball die through apoptosis. Recognition and phagocytosis of apoptotic cells prevents them releasing their intracellular contents, inhibiting the release of proinflammatory cytokines. Antigens are presented in the absence of an immune stimulus, leading to immune tolerance. Thus, the ratio of necrosis to apoptosis might play a critical role in determining the stimulatory or suppressive nature of the immune response to cryoablation.42 The speed of freezing could also play a role in determining the immunologic effects of cryoablation.42 High speed freezing causes necrosis and activates the immune response; low speed freezing leads to a reduced necrosis:apoptosis ratio resulting in immune suppression. Additionally, when a large amount of tumor is frozen, the large quantities of immune complexes generated may cause “high zone tolerance,” or antigen overloading that may lead to immunosuppression.43 As a result, in patients undergoing cryotherapy, an immunosuppressive effect early after the procedure may reduce immune system function so that the number of CTCs detected in peripheral blood is reduced, whereas later (30 d after cryosurgery) immune stimulation increases immune system function so that the number of CTCs detected is increased. However, although the timing of these immune status changes is clear according to our findings, the specific mechanism of the immune stimulatory and immunosuppressive effects at different time points after cryosurgery is unclear and will be explored in our future research.
Figure 5.
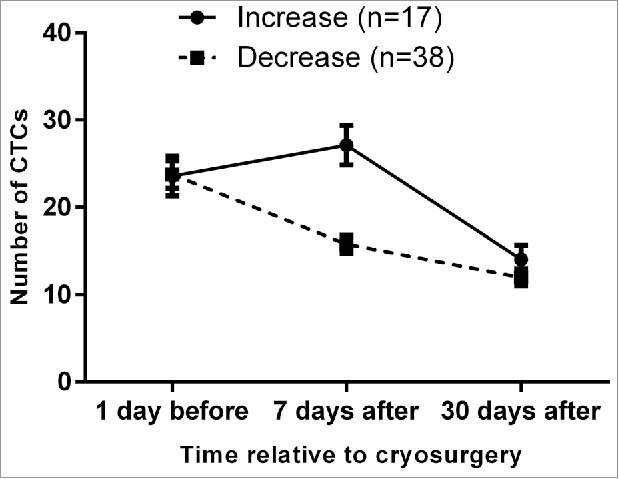
Short term increase in CTC numbers at 7 d after cryoablation therapy followed by a decrease at 30 d after treatment.
RT-qPCR is a commonly used and effective method for measuring gene expression and detecting CTCs. It is highly sensitive, quantitative, rapid and non-polluting, and enables monitoring in real time. RT-qPCR also overcomes the high rate of false positive findings that can be a problem with traditional PCR-based methods. In the present study, we used RT-qPCR to measure expression of the reference gene GADPH along with the metastasis-associated markers MAGE-3, Survivin and CEA in CTCs from 55 patients with locally advanced hepatocellular carcinoma before and after cryotherapy. CTC gene expression in peripheral blood was decreased following cryosurgery (P < 0.01), indicating effective control of recurrence and/or metastasis following this therapeutic intervention. Patients with high expression of these CTC markers are likely to have a poor prognosis, with progression of recurrence and/or metastasis.
Both immunocytochemistry and RT-qPCR have limitations in the detection and characterization of CTCs and neither approach leads to direct isolation of CTCs. These methods are typically inadequate for any type of functional characterization because they require a cell fixation step; thus, it is impossible to collect viable CTCs and explore their function. Moreover, surface markers of cancer cells vary with the processes of metastasis such as epithelial–mesenchymal transition,44 so the methods of enrichment and detection need to be improved to investigate the total amount and characterization of CTCs in peripheral blood.
How to monitor the progress of disease and treat it in a timely manner in patients with CRML is a challenging task. Many tumor treatments involve local surgical excision of lesions; the initial results are often promising, but recurrence and/or metastasis can occur and eradication of all cancerous cells can be very difficult to achieve. Like all existing cancer treatments, cryosurgery is not perfect, but it provides a method for the treatment of CRML. The possible immune system changes following cryosurgery could influence the therapeutic outcome if this approach were to be combined with an auxiliary treatment such as immunotherapy.45 In the future, evaluation of CTCs may become a new, noninvasive method for the early detection of cancers and/or re-examination following surgery and postoperative radio- and chemotherapy; it may even become as common and reliable a method of examination as radiology. Survival analysis of patients after follow-up may help in predicting prognosis.
In conclusion, dynamic monitoring of CTCs may be a useful biomarker for evaluating the efficacy of cryosurgery for CRML.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all participating patients for their involvement in this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Funding
This study was supported by the Hai Zhu District Scientific and Technological Plan, (No. 2013-CG-19), Guang Zhou City Hai Zhu District Science and Technology Industry and Information Bureau.
References
- 1.Valastyan S, Weinberg Robert A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011; 147:275-92; PMID:22000009; http://dx.doi.org/ 10.1016/j.cell.2011.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19:59-71; PMID:7740812; http://dx.doi.org/ 10.1007/BF00316981 [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19:1893-907; PMID:20647400; http://dx.doi.org/ 10.1158/1055-9965.EPI-10-0437 [DOI] [PubMed] [Google Scholar]
- 4.Tzeng CW, Aloia TA. Colorectal liver metastases. J Gastrointest Surg 2013; 17:195-201; quiz p.201-2; PMID:23054896; http://dx.doi.org/ 10.1007/s11605-012-2022-3 [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230:309-18; discussion 318-21; PMID:10493478; http://dx.doi.org/ 10.1097/00000658-199909000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutcliffe RP, Bhattacharya S. Colorectal liver metastases. Br Med Bull 2011; 99:107-24; PMID:21813558; http://dx.doi.org/ 10.1093/bmb/ldr034 [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Conlon K, Bodniewicz J, Dougherty E, DeMatteo RP, Blumgart LH, Fong Y. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 2001; 91:1121-8; PMID:11267957; http://dx.doi.org/ 10.1002/1097-0142(20010315)91:6%3c1121::AID-CNCR1108%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 8.Rivoire M, De Cian F, Meeus P, Gignoux B, Frering B, Kaemmerlen P. Cryosurgery as a means to improve surgical treatment of patients with multiple unresectable liver metastases. Anticancer Res 2000; 20:3785-90; PMID:11268455 [PubMed] [Google Scholar]
- 9.Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, Lu D. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin 2009; 59:145-70; PMID:19364702; http://dx.doi.org/ 10.3322/caac.20013 [DOI] [PubMed] [Google Scholar]
- 10.Kerkar S, Carlin AM, Sohn RL, Steffes C, Tyburski J, Littrup P, Weaver D. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery 2004; 136:770-9; PMID:15467661; http://dx.doi.org/ 10.1016/j.surg.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Alpantaki K, Datsis G, Zoras O, Kampouroglou A, Drositis I, Halkiadakis G, Katonis P. The value of cryosurgery in treating a case of thoracic chondrosarcoma. Case Rep Med 2011; 2011:243243; PMID:21629795; http://dx.doi.org/ 10.1155/2011/243243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert JK, Junginger T. Cryotherapy for liver tumors: current status, perspectives, clinical results, and review of literature. Tech Cancer Res Treat 2004; 3:151-63; PMID:15059021; http://dx.doi.org/ 10.1177/153303460400300208 [DOI] [PubMed] [Google Scholar]
- 13.Mala T, Edwin B, Samset E, Gladhaug I, Hol PK, Fosse E, Mathisen O, Bergan A, Soreide O. Magnetic-resonance-guided percutaneous cryoablation of hepatic tumours. Eur J Surg 2001; 167:610-7; PMID:11716448; http://dx.doi.org/ 10.1080/110241501753171227 [DOI] [PubMed] [Google Scholar]
- 14.Jungraithmayr W, Burger D, Olschewski M, Eggstein S. Cryoablation of malignant liver tumors: results of a single center study. Hepatobiliary Pancreat Dis Int 2005; 4:554-60; PMID:NOT_FOUND [PubMed] [Google Scholar]
- 15.Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist 1999; 4:197-208; PMID:10394588 [PubMed] [Google Scholar]
- 16.van der Pool AE, Lalmahomed ZS, de Wilt JH, Eggermont AM, Ijzermans JN, Verhoef C. Trends in treatment for synchronous colorectal liver metastases: differences in outcome before and after 2000. J Surg Oncol 2010; 102:413-8; PMID:20544718; http://dx.doi.org/ 10.1002/jso.21618 [DOI] [PubMed] [Google Scholar]
- 17.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013; 59:110-8; PMID:23014601; http://dx.doi.org/ 10.1373/clinchem.2012.194258 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9:274-84; PMID:19308067; http://dx.doi.org/ 10.1038/nrc2622 [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 2013; 23:573-81; PMID:23680145; http://dx.doi.org/ 10.1016/j.ccr.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother 2013; 62:931-9; PMID:23314304; http://dx.doi.org/ 10.1007/s00262-012-1387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lianidou ES, Markou A, Strati A. Molecular characterization of circulating tumor cells in breast cancer: challenges and promises for individualized cancer treatment. Cancer Metastasis Rev 2012; 31:663-71; PMID:22692478; http://dx.doi.org/ 10.1007/s10555-012-9366-8 [DOI] [PubMed] [Google Scholar]
- 22.Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A, et al.. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009; 175:808-16; PMID:19628770; http://dx.doi.org/ 10.2353/ajpath.2009.090078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, et al.. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29:1556-63; PMID:21422424; http://dx.doi.org/ 10.1200/JCO.2010.28.7045 [DOI] [PubMed] [Google Scholar]
- 24.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al.. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781-91; PMID:15317891; http://dx.doi.org/ 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 25.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006; 12:4218-24; PMID:16857794; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2821 [DOI] [PubMed] [Google Scholar]
- 26.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al.. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:3213-21; PMID:18591556; http://dx.doi.org/ 10.1200/JCO.2007.15.8923 [DOI] [PubMed] [Google Scholar]
- 27.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14:6302-9; PMID:18829513; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 28.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008; 8:329-40; PMID:18404148; http://dx.doi.org/ 10.1038/nrc2375 [DOI] [PubMed] [Google Scholar]
- 29.Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 2006; 33:S9-14; PMID:16797376; http://dx.doi.org/ 10.1053/j.seminoncol.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 30.Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 2008; 14:1430-6; PMID:18322961; http://dx.doi.org/ 10.3748/wjg.14.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu LZ, Li JL, Xu KC. Percutaneous Cryoablation for Liver Cancer. J Clin Transl Hepatol 2014; 2:182-8; PMID:26355719; http://dx.doi.org/ 10.14218/JCTH.2014.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Fan L, Zheng J, Cui R, Liu W, He Y, Li X, Huang S. Detection of circulating tumor cells in breast cancer patients utilizing multiparameter flow cytometry and assessment of the prognosis of patients in different CTCs levels. Cytometry A 2010; 77:213-9; PMID:20169594; http://dx.doi.org/ 10.1002/cyto.a.20838 [DOI] [PubMed] [Google Scholar]
- 33.Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 2002; 235:499-506; PMID:11923605; http://dx.doi.org/ 10.1097/00000658-200204000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Li M, Zhang X, Bai T, Chi G, Liu JY, Li Y. Isolation, culture and phenotypic characterization of human sweat gland epithelial cells. Int J Mol Med 2014; 34:997-1003; PMID:25187692; http://dx.doi.org/ 10.3892/ijmm.2014.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, Lin SR. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis. World J Surg 2006; 30:1007-13; PMID:16736329; http://dx.doi.org/ 10.1007/s00268-005-0485-z [DOI] [PubMed] [Google Scholar]
- 36.Ring A, Smith IE, Dowsett M. Circulating tumour cells in breast cancer. Lancet Oncol 2004; 5:79-88; PMID:14761811; http://dx.doi.org/ 10.1016/S1470-2045(04)01381-6 [DOI] [PubMed] [Google Scholar]
- 37.Franke WW, Schmid E, Osborn M, Weber K. Intermediate-sized filaments of human endothelial cells. JJ Cell Biol 1979; 81:570-80; PMID:379021; http://dx.doi.org/ 10.1083/jcb.81.3.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 1998; 95:4589-94; PMID:9539782; http://dx.doi.org/ 10.1073/pnas.95.8.4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pachmann K, Heiss P, Demel U, Tilz G. Detection and quantification of small numbers of circulating tumour cells in peripheral blood using laser scanning cytometer (LSC). Clin Chem Lab Med 2001; 39:811-7; PMID:11601678; http://dx.doi.org/ 10.1515/CCLM.2001.134 [DOI] [PubMed] [Google Scholar]
- 40.Liao Y, Wang SY, Meng XY, Yang J, Shi MJ, Liu HL, Chen FF, Xiong B. Circulating tumor cells in breast cancer and its association with tumor clinicopathological characteristics: a meta-analysis. Med Oncol 2014; 31:343; PMID:25412938; http://dx.doi.org/ 10.1007/s12032-014-0343-7 [DOI] [PubMed] [Google Scholar]
- 41.Den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006; 95:896-905; PMID:16953240; http://dx.doi.org/ 10.1038/sj.bjc.6603341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009; 58:1-11; PMID:19007768; http://dx.doi.org/ 10.1016/j.cryobiol.2008.10.126 [DOI] [PubMed] [Google Scholar]
- 43.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol 2012; 22:327-34; PMID:22465232; http://dx.doi.org/ 10.1016/j.semcancer.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ, van Rijn CJ, Terstappen LW. The detection of EpCAM(+) and EpCAM(−) circulating tumor cells. Sci Rep 2015; 5:12270; PMID:26184843; http://dx.doi.org/ 10.1038/srep12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niu L, Chen J, He L, Liao M, Yuan Y, Zeng J, Li J, Zuo J, Xu K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 2013; 42:1143-9; PMID:23899940; http://dx.doi.org/ 10.1097/MPA.0b013e3182965dde [DOI] [PubMed] [Google Scholar]