ABSTRACT
Background: Several randomized phase III trials in neuroendocrine tumors (NETs) showed the clinical role of new targeted agents and their impact on tumor response and outcome of whose patients affected by advanced NET. In this study, we summarize the available clinical data related to clinical efficacy of targeted therapies in the treatment of advanced NETs. Methods: A meta-analysis of randomized studies in accordance with the PRISMA guidelines was performed after searching the databases of PubMed, the Cochrane Library, and the ASCO University Meeting for relevant publications. Results: One thousand 9 hundred and 8 cases were included in the meta-analysis; among these, 1012 were in the experimental arm and 896 were in the control arm. The pooled analysis of the use of target agents in NETs revealed significantly increased of progression free survival compared to control group (hazard ratio = 0.59, 95% CI:0.42-0.84; P = 0.003). Subgroup analysis of patients according to tumor site showed a difference in favor of pancreatic neuroendocrine tumors. Moreover, targeted therapies improved the overall survival (hazard ratio = 0.79, 95%CI: 0.63-0.98; P = 0.03), and response rate (hazard ratio = 3.33, 95% CI 2.02-5.49; P < 0.00001) in all types of NETs. Conclusion: Our analysis supports the routine use of targeted agents for treatment of neuroendocrine tumors with particular regards to the pancreatic neuroendocrine tumors.
KEYWORDS: Everolimus, bevacizumab, NET, sunitinb
Introduction
Neuroendocrine tumors (NET) arise from cells of the endocrine (hormonal) and nervous systems. NETs are considered a rare disease even their incidence appear to be increasing.1,2 The prognosis of NETs may vary widely, depending on stage, grade or primary tumor site.3 Surgical resection of the primary tumors usually offer the only change of a long curative treatment; however, only a low percentage of patients are candidate to surgery as more than 50% of patients with NET have regional or distant metastatic disease at diagnosis.3 In advanced diseases, the therapeutic options may include: a close surveillance for slowly progressive tumors; a liver-directed treatments such as transarterial embolization; systemic treatment with cytotoxic chemotherapy or radionuclide. However, none of these approaches has been directly compared in randomized clinical trials.4
In the armamentarium of systemic treatment, somatostatin analogs, such as octrotide and lanreotide, are currently indicated for the relief of symptoms in patients with functionally active NETs.5,6 In addition, octreotide long-acting repeatable (LAR) has significantly prolonged time to tumor progression compared with placebo in patients with functionally active and inactive metastatic midgut NETs,7 supporting their use as a primary approach in the NET treatment. More recently, due to a better understanding of the biological mechanisms driving the growth of tumor cells with neuroendocrine phenotype has led to the development of targeted anti-cancer agents.8,9 To date, 2 agents have been approved by the US. Food and Drug Administration (FDA) for the treatment of patients with progressive, well-differentiated pancreatic NET: sunitinib (a vascular endothelial growth factor receptor-tyrosine kinase inhibitor) and everolimus (a mammalian target of rapamycin (mTOR). Three phase III trials demonstrated an improved in progression-free survival (PFS) of both sunitinb and everolimus compared with placebo,10-12 supporting their role in clinical practice.
The aim of this study is to analyze the available clinical data from randomized controlled trials (RCTs) looking for efficacy of target agents in patients with advanced NETs.
Materials and methods
Data retrieval strategies
We conducted the meta-analysis of randomized studies in accordance with the PRISMA guidelines.13 The databases of PubMed/MEDLINE, the Cochrane Library, and the American society of clinical oncology (ASCO) Meeting were searched for relevant publications using the following terms: “Neuroendocrine tumor” or “NET” or “Carcinoid tumors” and “target therapy” or “sunitinib” or “everolimus” or “bevacizumab.” The publications that were available in these databases up to December 31, 2015, were analyzed. The search was restricted to human studies, and the search criteria were limited to phase II or phase III trials. The computer search was supplemented with manual searches of the references listed in all of the retrieved review articles, including primary studies. When the results of a study were reported in subsequent interim analysis, only the most recent or complete and updated version was included in the analysis.
Inclusion criteria
The studies were identified according to the following inclusion criteria: 1) human participants with a NET; 2) a targeted agent therapy alone or in combination for experimental arm; 3) the presence of a control for comparison (placebo or not); 4) a primary outcome of response expressed as the hazard ratio (HR) for either PFS or overall survival (OS), as well as the response rate expressed as relative risk (RR). The following exclusion criteria were used: 1) insufficient data were available to estimate the outcomes; 2) animal studies; 3) the size of each arm was fewer than 10 participants; and 4) the presence of a single-arm study.
Data extraction
LZ and SV independently extracted the relevant data of each trials, including the name of the first author, country, publication year, characteristics of the enrolled patients, median follow-up and information about the study design (i.e., the type of blinding, type of control, and methods for randomization allocation), survival outcomes expressed as HRs for OS and PFS, and the number of patients who experienced a response rate with a grade 3-4 adverse event. The arm with the targeted agent has been considered as the experimental. For the time-to-event variables (OS or PFS), HRs with the 95% confidence intervals (95%CIs) were calculated for each study. For the dichotomous variables (partial or complete response rate and toxicity) RRs with the 95% CIs were calculated for each study.
Quality assessment and statistical analysis
The methodological quality of each included study was assessed by 2 independent researchers (GA and PC). The study quality was assessed using the Jadad 5-item scale. The final score ranged from 0 to 5.14 Disagreements were evaluated by the kappa test, and consensus was achieved in discussion with the corresponding author (GR).
Statistical analysis
Statistical analyses were performed using Revman 5.3. The summary estimates were generated using a fixed-effects model (Mantel–Haenszel method) or a random-effects model (DerSimonian–Laird method)15,16 depending on the absence or presence of heterogeneity. Statistical heterogeneity was assessed using the Q-test and the I2 statistic. I2 values of 25%, 50% and 75% were considered to indicate low, moderate and high heterogeneity, respectively.17 When P > 0.1 in the Q-test and I2< 50%, the fixed-effects model was used; otherwise, the random-effects model was used. Due to the small number of trials that were included, no publication bias was assessed. A sub-group analysis was performed to highlight any difference between studies with different tumor sites (pancreatic NETs versus other location). For all of the statistical analyses, a value of P < 0.05 was regarded as statistically significant, and all of the tests were 2-sided.
Results
Literature review, characteristics and quality of the included studies
The search yielded 8454 potentially relevant articles. Of these, 7744 studies were excluded as duplicates. After viewing the titles and abstracts of the 710 remaining studies, the full texts of 21 studies were retrieved and 7 studies 10-12,18-21 with 1012 cases in experimental arm and 896 cases in the control group were included in the meta-analysis according to the inclusion and exclusion criteria described in the materials and methods section (Fig. 1). One study was excluded because retracted by the authors.22 Among these studies, 3 studies10,12,18 investigated everolimus single agent as experimental arm, 3 studies sunitinib and bevacizumab respectively11,19,20 and one study the combination of everolimus and bevacizumab.21 Three studies enrolled patients with pancreatic NET10,11,21,22 only. The patients' characteristics were obtained for all studies. The characteristics of the studies included in the meta-analysis are summarized in Table 1. There were 2 phase II studies20,21 and 5 phase III studies.10-12,18,19 All studies had a comparator: in 310-12 the comparator was placebo, while in 1 was placebo plus octreotide LAR;18 in 2 studies the comparator was pegylated (PEG)-interferon alfa-2b and interferon respectively19,20 and everolimus in on trial.21 The median Jadad score was 5, showing a good quality of the included studies (Table 1).
Figure 1.
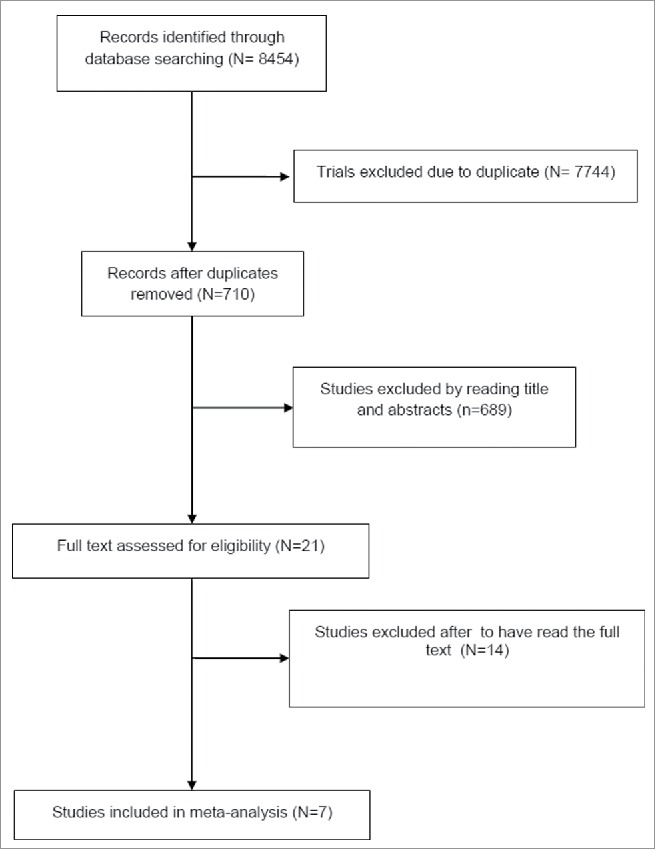
Trial selection flow chart.
Table 1.
Characteristics of the analyzed trials.
| Study | Design | Primary endpoint | Number of Patients Experimental Arm | Number of Patients Control Arm | Experimental drug | Control | Tumor location | Jaded score |
|---|---|---|---|---|---|---|---|---|
| Yao 201110 | III | PFS | 207 | 203 | Everolimus | Placebo | Pancreatic | 5 |
| Raymond11 | III | PFS | 86 | 85 | Sunitinib | Placebo | Pancreatic | 5 |
| Yao 201512 | III | PFS | 205 | 97 | Everolimus | Placebo | Non-pancreatic NETS | 5 |
| Pavel18 | III | PFS | 216 | 213 | Everolimus+Octreotide LAR | Placebo + octreotide LAR | Non-pancreatic NETS | 5 |
| Yao 2015 ASCO19 | III | PFS | 201 | 201 | Bevacizumab+Octereotide | Interferon α-2B+Octereotide | Non-pancreatic NETS | 4 |
| Yao 200820 | II | PFS | 22 | 22 | Bevacizumab | PEG interferon α−2B | Non-pancreatic NETS | 3 |
| Kulke 201521 | II | 75 | 75 | 75 | Everolimus+Bevacizumab | Everolimus | Pancreatic | 3 |
Efficacy data
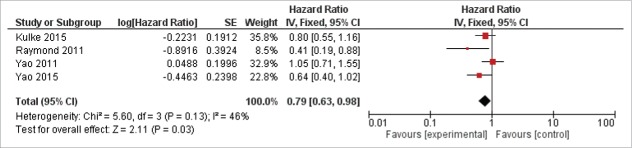
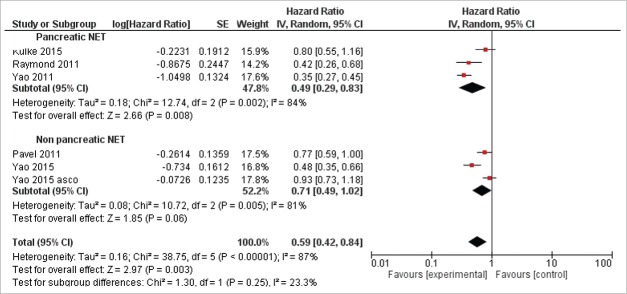
Data about OS and PFS were reported in Table 2. With regard to OS, data were obtained from 4 studies.10-12,21 The pooled analysis revealed that the new target therapies definitely improved the OS compared with control arm (0.79, 95%CI: 0.63–0.98; P = 0.03, Fig. 2). The analysis was performed using a fixed-effects model (I2 = 46%). In the experimental arm, a higher PFS has been observed respect control arm for all the included studies. All the studies reported HR for PFS, the pooled analysis revealed an improvement with new target therapies (HR = 0.59, 95% CI:0.42–0.84; P = 0.003 Fig. 3). The random-effects model was used for the analysis of the PFSs due to the presence of high heterogeneity (I2 = 87%) between the trials. In the subgroups analysis of the targeted agents in pancreatic and non-pancreatic NET, the results revealed the targeted therapies significantly improved the PFS to a greater extent in the pancreatic NET (HR = 0.49 95%CI: 0.29–0.83) than in non-pancreatic NET setting (HR = 0.71 95%CI: 0.49–1.02) (Fig. 3).
Table 2.
Data on overall survival, progression free survival, median treatment duration and median follow-up of the included studies.
| OS (months) |
PFS (months) |
Median treatment Duration (months) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Experimental drug | Control | p value | Experimental drug | Control | p value | Exp | Control | Median Follow-up (months) |
| Yao 201110 | NR | NR | 0.59 | 11.4* | 5.4* | <0.001 | 8.79 | 3.74 | 17 |
| Raymond11 | NR | NR | 0.02 | 11.4 | 5.5 | <0.001 | 4.6 | 3.7 | NR |
| Yao 201512 | NR | NR | 0.04 | 11 | 3.9 | <0.0001 | 40.4** | 19.6** | 21 |
| Pavel18 | NR | NR | NR | 16.4 | 11.3 | 0.026 | 9.25 | 9.15 | 28 |
| Yao 2015 ASCO19 | NR | NR | NR | 16.6* | 15.4* | 0.55 | NR | NR | NR |
| Yao 200820 | NR | NR | NR | 16.5 | 14 | 0.34 | 4.5 | 4.5 | 4.5 |
| Kulke 201521 | 36.7 | 35 | 0.16 | 16.7 | 14 | 0.12 | 13*** | 12*** | 25.9 |
OS: overall survival; PFS: progression free survival; NR: not reported.
according to central assessment;
Weeks;
Cycles
Figure 2.
Forest plots of hazard ratios (HRs) for overall survival (OS) comparing new target agents to control group. The Chi-squared test showed low heterogeneity between the trials. The fixed effects model was used.
Figure 3.
Forest plots of hazard ratios (HRs) for progression-free survival (PFS) comparing new target agents to control group. The Chi-squared test showed high heterogeneity between the trials. The random effects model was used.
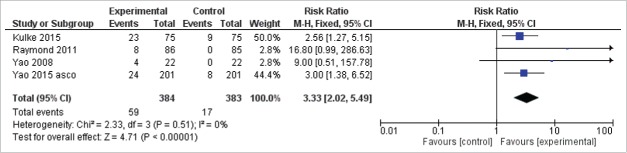
Five studies reported the RR of the target agents in NET.11,19-21 RR has been obtained in a total of 59/384 (15.3%) patients for the experimental group and in a total of 17/383 (4.4%) patients in the control group. Using the Mantel–Haenszel method for combining trials, the pooled RR for achieving an objective response with targeted agents vs. placebo or other therapies was 3.33 (95% CI 2.02–5.49; P < 0.00001; I2 = 0%) (Fig. 4). Regarding the toxicity, the data of the adverse events unfortunately were not homogeneously reported in the studies; therefore, an associated meta-analysis could not be performed.
Figure 4.
Forest plots of hazard ratios (HRs) for progression-free survival (PFS) comparing new target agents to control group in the subgroup of pancreatic NETs versus non pancreatic NETs. The Chi-squared test showed high heterogeneity between the trials. The random effects model was used.
Discussion
Although the molecular background of sporadic NETs is unknown, several studies suggested that the abnormal PI3K-AKT-mTOR pathway signaling is implicated in their pathogenesis.23 The mTOR protein is a serine-threonine kinase regulating cell growth, proliferation, metabolism, and angiogenesis and its autocrine activation, mediated mainly by the Insulin-like Growth Factor 1, has been associated with neuroendocrine tumor cell proliferation.24 The crucial role of mTOR pathway in NETs is also supported by the fact that NETs pathogenesis is frequently linked with familial cancer syndromes, such as neurofibromin 1 (NF-1) or tuberin (TSC). In particular, the chromosome arm 16p, which contains TSC-2, has been found to be lost in 37% of pancreatic NETs.25 The phosphatase and tensin homolog (PTEN) has been also detected mutated or deleted in the 10% up to 29% of patients with pancreatic NETs.25 All these tumor suppressor genes, if inactivated, led to an over-expression of the mTOR pathway.26 Moreover, mTOR pathway is linked to a progression of disease, in this context, Missiaglia et al showed that the down-regulation of the tumor suppressor of the Akt/mTOR pathway TSC-2 and PTEN which leads to deregulation of the mTOR pathway are linked to progression of pancreatic NETs to an increased rate of proliferation, and shortened PFS and OS.25 Although the crucial role of mTOR seems to be mainly confined in the subgroup of pancreatic NETs (in fact about the 14% of these last present mutations in genes in the mTOR pathway).26,27 The positive recent data from the RADIANT-4 trial 12 based on the use of mTOR targeting agent (everolimus) supported the leading role of mTOR axis in NETs not only in is pancreatic but also in lung or gastrointestinal tumors.
The angiogenesis process along with one of its “driver” such as the vascular endothelial growth factor (VEGF) play also an important role in NETs progression. Over expression of VEGF has been demonstrated in pancreatic or not pancreatic NETs and it has been associated to a worse outcome.26,28 Moreover the VEGF receptor-2 (VEGFR-2) is also generally over-expressed also in gastro-intestinal carcinoid tumors and carcinoid cancers.26,29 Thus, targeting these markers potentially arrest the NETs related-angiogenesis inducing, as a consequence, a tumor downstage and an improvement in PFS/OS.12,20,21
The present study is a systematic review and a meta-analysis of trials to assess the efficacy of targeted agents in patients with advanced NET. NETs are a heterogeneous disease arising from various primary sites such as the small intestine or other sites of the gastrointestinal tract, and the lung,30,31 therefore their management is complicated by a different clinical presentations, clinical disease course, symptoms and degree of aggressiveness.3 In our analysis, target agents improved the PFS and OS of advanced NETs patients compared with control group. Therefore, we may confirm the important role of targeted agents in treating the advanced NETs and in this setting, the completion of several randomized phase III studies has brought to the approval of 2 new sunitinib, and everolimus.32 Because of the observed long survival after progression of many patients, PFS is recommended as a feasible and relevant primary end point for both phase III studies and phase II studies,33 however we have been able to show a statistically significant improvement also in OS for patients with NETs treated with targeted therapies. In the subgroup analysis, we have reported a better PFS in patients with pancreatic NETs versus other site of NETs (HR = 0.49 and 0.71 respectively). However, it is well known for chemotherapy and targeted treatment than better response is observed for pancreatic NETs than non-pancreatic NETs. Therefore our study empowered this issue.
The identification of the patients who will benefit or will be resistant to targeted agents is mandatory in a clinical setting. Unfortunately, there are less established biomarkers able to predict the response of advanced NETs to targeted agents. In the near future, studies based on identification of biomarkers predicting response and clinical benefit to novel targeted agents in advanced NETs patients are needed.
It is worth of notes our meta-analysis presents several important limitations: 1) not all considered studies reported data on HRs of OS, PFS and RR, 2) different drugs with different mechanism of action have been analyzed with different impact on outcome-related variables, 3) only one study directly compared the combination of 2 targeted therapies, however an increase in toxicity in the combination arm was observed,21 4) this is not a meta-analysis performed from individual data from randomized studies, but from data available in literature, which is known to be much less robust, 5) data on toxicities were very heterogeneous and did not allow a reasonable pooling of the results, 6) although, we found a good global quality of examined studies (median Jadad score of 5), the quality and the design of clinical studies for advanced NETs are poor 34 due to several factors: the heterogeneity of the disease (different primary sites), very low incidence leading to a relative paucity of comparative and strategy studies. NET treatment strategy may include several options such as surgery, through liver-targeted therapies and several lines of systemic therapy.
Conclusions
NETs are a heterogeneous disease leading to a very difficult trial design and algorithm of therapy. Therefore, the optimal management of advanced NETs is still a challenge for medical oncologist. The recent success of phase III trials demonstrate that the novel agents such as sunitinib, and everolimus are an effective therapeutic options for patients with advanced NETs with particular regards to the pancreatic tumors. While, the combination of everolimus plus octreotide LAR improves PFS in patients with advanced NETs, no data are available on the antitumor activity of the combination of sunitinib and everolimus or sunitinb octreotide and LAR. Nowadays, new pathways with new targeted therapies are under investigation in advanced NETs, but it is still mandatory to design and conduct trials properly to solve the issue of the optimal management in any subgroups of advanced NETs, maybe thankful also the identification of “driver” biomarker for selected treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al.. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26:3063-6; PMID:18565894; http://dx.doi.org/ 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 2.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011; 40:1-18, vii; PMID:21349409; http://dx.doi.org/ 10.1016/j.ecl.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, et al.. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013; 42(4):557-77; PMID:23591432; http://dx.doi.org/ 10.1097/MPA.0b013e31828e34a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, et al.. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008; 9:61-72; PMID:18177818; http://dx.doi.org/ 10.1016/S1470-2045(07)70410-2 [DOI] [PubMed] [Google Scholar]
- 5.Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004; 15:966-973; PMID:15151956; http://dx.doi.org/ 10.1093/annonc/mdh216 [DOI] [PubMed] [Google Scholar]
- 6.Broder MS, Beenhouwer D, Strosberg JR, Neary MP, Cherepanov D. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: a systematic literature review. World J Gastroenterol 2015; 21(6):1945-55; PMID:25684964; http://dx.doi.org/ 10.3748/wjg.v21.i6.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, et al.. PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009; 27(28):4656-63; PMID:25684964; http://dx.doi.org/ 10.1200/JCO.2009.22.8510 [DOI] [PubMed] [Google Scholar]
- 8.Kulke MH, Bendell J, Kvols L, Neary MP, Cherepanov D. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol 2011; 4:29; PMID:21672194; http://dx.doi.org/ 10.1186/1756-8722-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capdevila J, Tabernero J. A shining light in the darkness for the treatment of pancreatic neuroendocrine tumors. Cancer Discov 2011; 1:213-21; PMID:22586573; http://dx.doi.org/ 10.1158/2159-8290.CD-11-0151 [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al.. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364(6):514-23; PMID:NOT_FOUND; http://dx.doi.org/ 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, et al.. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364:501-13; PMID:21306237; http://dx.doi.org/ 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 12.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al.. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2015; 387:968-77; PMID:26703889; http://dx.doi.org/ 10.1016/S0140-6736(15)00817-X.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . PRISMA Group. preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med 2009; 6:e1000097; PMID:19621072; http://dx.doi.org/ 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1-12; PMID:8721797; http://dx.doi.org/ 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177-88; PMID:3802833; http://dx.doi.org/ 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 16.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10:101-29; http://dx.doi.org/ 10.2307/3001666 [DOI] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60; PMID:12958120; http://dx.doi.org/ 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, et al.. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011; 378(9808):2005-12; PMID:22119496; http://dx.doi.org/ 10.1016/S0140-6736(11)61742-X [DOI] [PubMed] [Google Scholar]
- 19.Yao JC, Guthrie K, Moran C, Strosberg JR, Kulke MH, et al.. SWOG S0518: Phase III prospective randomized comparison of depot octreotide plus interferon alpha-2b versus depot octreotide plus bevacizumab (NSC #704865) in advanced, poor prognosis carcinoid patients (NCT00569127). J Clin Oncol 2015; 33 (suppl; abstr 4004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, Hess K, Ng C, Abbruzzese JL, Ajani JA. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008; 26(8):1316-23; PMID:18323556; http://dx.doi.org/ 10.1200/JCO.2007.13.6374 [DOI] [PubMed] [Google Scholar]
- 21.Kulke MH, Niedzwiecki D, Foster NR, Fruth B, et al.. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). J Clin Oncol 2015; 33 (suppl; abstr 4005); http://dx.doi.org/ 10.1200/JCO.2014.59.0927 [DOI] [Google Scholar]
- 22.Yao J, Wang JY, Liu Y, Wang B, Li YX, Zhang R, Wang LS, Liu L. A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med Oncol 2014; 31(12):251; PMID:25395378; http://dx.doi.org/ 10.1007/s12032-014-0251-x [DOI] [PubMed] [Google Scholar]
- 23.Pusceddu S, de Braud F, Concas L, Bregant C, Leuzzi L, Formisano B, Buzzoni R. Rationale and protocol of the MetNET-1 trial, a prospective, single center, phase II study to evaluate the activity and safety of everolimus in combination with octreotide LAR and metformin in patients with advanced pancreatic neuroendocrine tumors. Tumori 2014; 100(6):e286-9; PMID:25688512; http://dx.doi.org/ 10.1700/1778.19298 [DOI] [PubMed] [Google Scholar]
- 24.von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000; 60(16):4573-81; PMID:10969809 [PubMed] [Google Scholar]
- 25.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G, et al.. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2010; 28:245-55; PMID:19917848; http://dx.doi.org/ 10.1200/JCO.2008.21.5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JA, Kulke MH. Medical management of pancreatic neuroendocrine tumors: Current and future therapy. Surg Oncol Clin N Am 2016; 25(2):423-37; PMID:27013373; http://dx.doi.org/ 10.1016/j.soc.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 27.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, et al.. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011; 331(6021):1199-203; PMID:21252315; http://dx.doi.org/ 10.1126/science.1200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, Evans DB, Vauthey JN, Xie K, Yao JC. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007; 109(8):1478-86; PMID:17340592; http://dx.doi.org/ 10.1002/cncr.22554 [DOI] [PubMed] [Google Scholar]
- 29.Silva SR, Bowen KA, Rychahou PG, Jackson LN, Weiss HL, Lee EY, Townsend CM Jr, Evers BM. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer 2011; 128(5):1045-56; PMID:20473929; http://dx.doi.org/ 10.1002/ijc.25441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rindi G, Klimstra DS, Arnold R, et al.. Nomenclature and classifi cation of neuroendocrine neoplasm of the digestive system In: Bosman FT, Carniero F, Hruban RH, Theise ND, eds. WHO classification of tumours of the disgestive system, 4th edn. Lyon: International Agency for Research on Cancer, 2010:13-14 [Google Scholar]
- 31.Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, et al.. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006; 24:70-6; PMID:16382115; http://dx.doi.org/ 10.1200/JCO.2005.04.1202 [DOI] [PubMed] [Google Scholar]
- 32.Yao JC, Lagunes DR, Kulke MH. Targeted therapies in neuroendocrine tumors (NET): clinical trial challenges and lessons learned. Oncologist 2013; 18(5):525-32; PMID:23615698; http://dx.doi.org/ 10.1634/theoncologist.2012-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, Ellis LM, Benedetti JK, Bergsland EK, Hobday TJ, et al.. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011; 29(7):934-43; PMID:2126308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter T, Krzyzanowska MK. Quality of clinical trials in gastroenteropancreatic neuroendocrine tumours. Neuroendocrinology 2012; 96(3):238-48; PMID:22414794; http://dx.doi.org/ 10.1159/000337662 [DOI] [PubMed] [Google Scholar]