ABSTRACT
Inflammatory bowel diseases are an increasing phenomenon in western countries and in growing populations. The physiopathology of these conditions is linked to intestinal stem cells homeostasis and regenerative potential in a chronic inflammatory microenvironment. Patients with IBD present an increased risk of developing colorectal cancer (CRC), or colitis associated cancer (CAC). Conventional treatment for IBD target the inflammatory process (and include anti-inflammatory and immunosuppressive drugs) with biological agents emerging as a therapeutic approach for non-responders to traditional therapy. Conventional treatment provides scarce results and present severe complications. The intestinal environment may host incoming stem cells, able to engraft in the epithelial damaged sites and differentiate. Therefore, stem cell therapies represent an emerging alternative in inflammatory bowel diseases, with current investigations on the use of haematopoietic and mesenchymal stem cells, in particular adipose stem cells, apparently fundamental as regenerators and as immune-modulators. Here, we discuss stem cells in intestinal homeostasis and as therapeutic agents for the treatment of inflammatory bowel diseases.
KEYWORD: Biomarkers, chronic inflammation, cancer stem cells, cholangiocarcinoma, tumor microenvironment
Introduction
Inflammatory bowel diseases (IBD) include ulcerative colitis (UC) and Crohn's disease (CD), and may be classified as complex immune-mediated diseases developing in genetically susceptible organisms due to dysregulation of the immune response in the bacterial flora of the intestine. Although they present with a broad spectrum of clinical presentations, with onset emerging in young adults with alternating phases of relapse and remission.
The highest incidence rate of IBD is in the second to fourth decade of life. Furthermore, bimodal occurrences have been reported with a modest increase in the sixth and seventh decades of life.1,2 Adult-onset IBD represent 60-65% of cases with a higher prevalence of UC comprared to CD.
Nevertheless, IBD may develop in children and in the elderly with up to 25% of IBD cases occurring during childhood or adolescence and 10–15% of IBD patients being diagnosed at > 60 y of age. Furthermore, the CD:UC ratio is greater in the pediatric-onset group when compared to the adult-onset and elderly-onset groups. Moreover, very early-onset CD and elderly-onset CD are identified by preponderance of pure colonic disease, whereas ileocolonic disease is more frequently observed in older children and adults; complicated, extensive diseases are more commonly seen in pediatric-onset disease. In addition, UC patients show more extensive location in early-onset than in later-onset disease.3 The incidence rate of CD and UC is generally greater in North America and Western Europe with a reported increase in occurrence over the second half of the twentieth century. The annual incidence of UC is 0–19.2 per 100,000 in North America and 0.6–24.3 per 100,000 in Europe, with a prevalence of 37.5–248.6 per 100,000 and 4.9–505 per 100,000, respectively.4
However, the incidence of IBD is increasing in developing populations, which implies that environmental factors and genetic susceptibility contribute significantly to the pathogenesis of IBDs.5 Reports confirm that smoking and appendectomy increase the risk of CD but may offer protection from UC. Moreover, some studies indicate excess sugar consumption and oral contraceptives as risk factors for IBD in relation to associative factors such as genetic and environmental factors, previously described.
Genetic factors have been widely examined and 163 distinct risk loci have been individuated, and associated with numerous potentially associated genes. A large number of the individual genes act in the immune responses to pathogens (innate or adaptive), in maintenance of the integrity of the epithelial barrier, in injury repair and in response to oxidative stress. Most loci are found between UC and CD with analogous outcomes.6-8
Patients with IBD have a higher risk of developing colorectal cancer (CRC), or so-called colitis associated cancer (CAC). The annual incidence of CAC in UC, in fact, ranges from 0.051% to 0,16%, with a cumulative incidence from 2% to 7.5% at 30 y. An annual incidence rate of 0.05% has been observed concerning CD with an aggregate risk of 8.3% at 30 y.
Several risk factors may be considered for CAC including extensive disease, young age at diagnosis, family history of CRC, co-existing primary sclerosing cholangitis (PSC) and persistent inflammation of the colon.
Occurrences of impaired outcome of CRC have been reported in IBD patients with mortality increasing 2-fold compared to sporadic CRC cases. In relation to these conditions, we may note that at diagnosis of CD, the tumor is at an advanced stage. It is fundamental to stress that CAC is not always diagnosed before surgery. Young age and male sex are factors associated with poor outcome.9
Inflammatory bowel diseases are gradually increasing in western and developing countries with various factors contributing to their onset and development.. Recent literature has examined stem cells (SCs) in the inflammatory mechanism of IBDs and their potential therapeutic role versus traditional treatment. Moreover, the cancer stem cell model confirms that both normal tissues and cancers are arranged in a hierarchical manner, possessing tumor heterogeneity due to the production of various progenitor (medium proliferative) and differentiated (non-proliferative) cells via multipotent CSCs (high proliferative) cells.10 Cancer stem cells are multipotent cells, capable of self-renewal with vast proliferation potential as well as angiogenic and immune evasion properties. In addition, CSCs are able to develop high resistance to traditional treatment. Cancer stem cells may arise from normal SCs considering the prolonged life span altering their behavior subsequent to genetic and epigenetic modifications. Furthermore, investigations have hypothesized that progenitor, differentiated, or cells from outside the tumor as in cells deriving from the bone marrow, may also be antecedent to CSCs.11 Recent research on stem cell biology has provided an improved understanding of tumorigenesis as regards to IBDs which may lead to new discoveries of anti-cancer therapy.
The objective of this study is to present an overview of the physiologic implications of SCs in intestinal homeostasis with a perspective toward stem cell therapy in intestinal diseases.
Clinical features
IBD are recurring systemic diseases that mainly affect the gastrointestinal tract. The actual cause of IBD is still unclear, however the infiltration of leukocytes in the context of intestinal epithelium is considered fundamental to the development of mucosal injuries, owing to the growing proliferation of inflammatory cytokines and mediators12 (Fig. 1).
Figure 1.
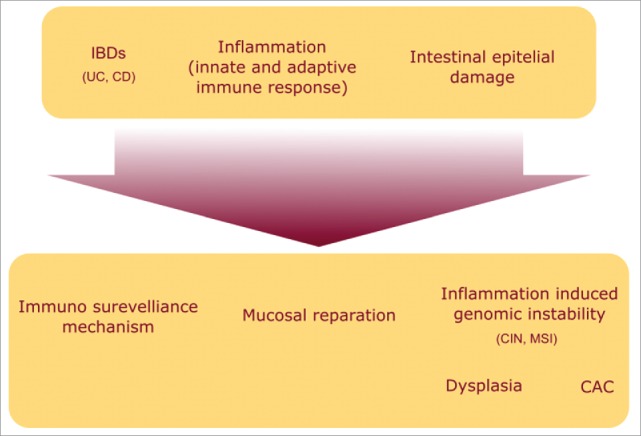
Pathophysiology of the inflammatory bowel diseases: inflammation occurring in IBD produces a damage to the mucosa which triggers the activation of immune-modulatory and reparatory mechanisms. In addition the inflammatory microenvironment deriving from these processes promotes carcinogenesis.
CD typically presents with development of ulcers, strictures, fistulas and granulomas in the mucosa. It primarily affects the ileocecal region, followed by the terminal ileum alone, diffuse small bowel, or isolated colonic disease, but can affect any part of the gastrointestinal tract from the oropharynx to the perineum. Diseased segments are commonly separated by “skip areas” of normal bowel. Inflammation can be transmural, often leading to fistula formation. Histologic examinations show aphthoid ulcers and focal chronic inflammation extending to the submucosa, occasionally related to granuloma formation.13
CD clinically presents diarrhea (with or without blood), malnutrition, abdominal pain, and weight loss. Extraintestinal manifestations, such as arthropathy or skin disorders, are rare and may otherwise prompt the discovery of occult intestinal disease.14 Generally, CD has a genetic component with a 7-fold greater risk of first-degree relatives developing the disorder.15
A polarized differentiation toward Th1 lymphocyte has been detected in CD which precedes the release of proinflammatory cytokines, including tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ). Moreover, Th1 cytokines induce the antigen-presenting cells to secrete a wider spectrum of inflammatory cytokines, giving rise to a self- sustained cycle.16
UC is a form of IBD characterized by superficial ulcerations, granularity, and a vascular pattern. Inflammation in UC is confined to the mucosal layer of the colon. The rectum is affected in 95% of patients, with variable degrees of proximal extension. Inflammation is mainly restricted to the mucosa and presents continuous complications of ulceration, edema, and hemorrhage along the length of the colon. Histologic examination often reveal acute and chronic inflammation of the mucosa by polymorphonuclear leukocytes and mononuclear cells, crypt abscesses, distortion of the mucosal glands, and goblet cell depletion.
UC clinically presents blood and mucus discharge, formation of granulation tissue and petechial hemorrhage, with presentation of a normal mucosa during remission. In most severe forms, there may be distension of the intestine, presenting deep ulceration and risk of perforation.17 In UC, secretion of IL-13 is significantly elevated. Despite Th1 involvement, UC patients present a Th2 response. The combination of these factors stimulate the translocation of specific bacterial species followed by activation of immune cells and mucosal inflammation.18 As in CD, Th17-related cytokines are also elevated in UC.19,20
The Montreal classification (2008) addresses the extent and behavior of CD and UC and is largely involved in research and clinical issues, however, its reliability is yet to be ascertained.21
The most significant IBD complication is the development of CAC. IBD-related tumors are often more extended than sporadic CRC and may involve the surrounding mucosa, thus confounding endoscopic investigations. IBD related tumors often develop in structured segments of colon and many are mucinous adenocarcinoma (up to 50%).22
A number of international gastrointestinal societies propose endoscopic monitoring for CAC and CRC. Endoscopic surveillance is fundamental in detection and resection of dysplastic lesions or in identifying CRC at an early and more curable stage in order to diminish morbidity and mortality rates. Patients with UC and CD colitis are encouraged to undergo a screening colonoscopy to verify disease extension and define endoscopic features that may determine increased risk of CAC.23-25
Intestinal stem cells (ISC)
The epithelium of the colonic mucosa is arranged into multiple crypts lined by a flat luminal surface. Unlike small intestinal architecture, the colon contains a high density of goblet cells but lacks in Paneth cells. ISC are undifferentiated, multipotent, self-renewable cells, engaged in tissue homeostasis and repair with a significant impact on the pathogenesis of IBD and CRC.26
In the process of crypt morphogenesis, early stem cell population rapidly expands via symmetric cell division then via asymmetric cell division to determine the adult SC pool which maintains the intestinal epithelium. This mechanism gradually allows dominance of the single progenitor cell resulting in a clonal pool of adult SC in each crypt (Fig. 2). Interestingly, clonality in adult intestinal crypts has been observed. However, single SC identity is not retained within the adult crypts. Therefore, the division of ISCs in adults is mostly asymmetrical (except during injury, disease, or cancer) and produces 2 different cells. One is identical to the original cell and the other with a capacity to differentiate. Progenitors are multipotent and do not proceed to immediate differentiation but are prone to rapid proliferation to establish a pool of transient amplifying (TA) cells. The TA cells are arranged along most of the crypt wall and develop into the differentiated intestinal lineages (enterocytes, goblet cells, neuroendocrine cells and Paneth cells). Paneth cells are seen at the base of the crypts and are detectable in a chronically inflamed colon. A fifth epithelial, so-called tuft-cell has been recently identified and appears to be Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) derived.27
Figure 2.

Anatomical location, differentiation capability and markers of intestinal stem cells: epithelial turnover occurs every 5–7 d in the colon and every 3–5 d in the small intestine. In the small bowel Lgr5 crypt base columnar stem cells coexist with Paneth cells at the crypt base and they generate transit-amplifying (TA) cells, occupying the rest of the crypt. TA cells differentiate into different functional cells on the villi, replacing the epithelial cells lost via anoikis. The +4 stem cells seem to be able of restoring the Lgr5+ CBC stem cell compartment following injury. In the colon Lgr5+ stem cells at the crypt base generate TA cells, which occupy the lower part of the crypt. TA cells differentiate into the mature lineages located on the surface epithelium. CBC cells and 4SC express different series of markers, which are listed on the right panel.
Two models of ISC have been identified: The ‘stem cell zone model’, by Cheng and Bjerknes,28-33 suggesting that the LGR5 positive and rapid cycling crypt base columnar (CBC) cells are the resident SC, and Potten's ‘+4 model’34-36 suggesting that slow cycling SC may be observed in a ring of 16 cells, closely located over the Paneth cells. A correct model has not been identified but rather a theory that includes the characteristics of both models. Initially B lymphoma Mo-MLV insertion region 1 homolog (BMI-1) was considered as a +4 SC marker, however this hypothesis was discarded by the identification of this marker throughout the proliferative zone of the crypt, including the LGR5+ CBC compartment. On the other hand, the LGR5-positive cells situated at the crypt base possess pluripotent and self-renewing properties, therefore are considered to be efficient markers for ISC.37-39
This model describes the intestinal crypt base as a specialized niche environment including both dedicated active SC (the CBC cells), which account for the epithelial homeostasis in normal conditions, and more quiescent BMI-1+ SC (known as +4 cells) that are activated in response to tissue injury to effect tissue repair. Furthermore, this model suggests that cells located in the lower positions of the TA cell compartment are able to acquire a stem cell phenotype to ensure epithelial homeostasis in case of a loss of stem cell populations residing at the base of the crypt. In fact, reconstitution of the intestinal architecture may occur on survival of the SC or progenitor zone after injury. However, the intestinal architecture will be irreversibly damaged in case of simultaneous destruction of both cell population.
The intestinal microenvironment has an important role in the advanced control of SC life. The intestinal SCs are situated in a specialized niche with adjacent epithelial cells, pericryptal stromal cells and the basement membrane. Paneth cells and pericryptal fibroblasts are fundamental components of the niche, which supplies essential signals of Wnt, Notch, bone morphogenetic proteins (BMP) and Hedgehog pathways to coordinate mechanism of self-renewal, proliferation and differentiation of intestinal SC.40 Constituents of the crypt lumen arising from epithelial cells or from bacteria may have an impact in intestinal SC. Moreover, intestinal subepithelial myofibroblasts mediate the crosstalk between epithelial and mesenchymal cells and secrete a wide range of morphogenetic factors involved in stem-cell homeostasis. This process will determine the intestinal architecture and the balance between proliferation and differentiation.41
Intestinal–derived mesenchymal stem cells (MSCs) are fundamental in intestinal homoestasis and have been isolated from human intestinal samples with a significant impact on differentiation and arrangement of the intestinal epithelial cells as well as on the immunomodulatory mechanisms.42
Several intestinal SC markers have been adopted to identify a molecular signature able to recognize ISC univocally. Lineage tracing experiments in mice confirmed the stem cell identity of LGR5+ CBC cells. These CBC cell originated clones included all the main epithelial cell lineages and were detectable over a long period. This observation validated the LGR5+ CBC cells as multipotent SC with self-renewing properties. Moreover, it has been demonstrated that single LGR5+ CBC cells from LGR5–EGFP mice could generate ‘organoids’ with the ability of self-renewal and capacity to restore the architecture and cellular composition of the intestinal epithelium. LGR5- cells were shown to be lacking in self-renewal activity.43
Musashi-1 (Msi-1) is a potential stem cell marker. In fact, some cells located in position 4-5 in the small intestine and a number of cells residing at the base of the crypt in the large intestine were found to be positive for Msi-1. Musashi-1 may also potentiate Notch signaling with a higher Msi-1 expression observed in developing crypts, in post-injury crypt regeneration and in some developing adenomas.44,45
Mouse telomerase reverse transcriptase (mTERT) has also been identified as an ISC marker. In the intestine, the analysis of mTERT expression in mice demonstrated that this marker is expressed by a subpopulation of ISC in the intestinal crypt cells.46
Another putative ISC marker is ALCAM (CD166).47 A recent study by Wang et al. identified the combination CD44+CD24loCD166+ and GRP78lo/- or c-Kit- as a combination of markers facilitating the identification of SC from the mouse small intestine or colon. This combination may also identify an enteroid forming ISC population not expressing LGR5. This combination of markers may be useful in monitoring human cells.48
Moreover, a strong Wnt signature has been observed. In fact, expression of ISC were reported in genes such as Sox9, Achaete–Scute homolog 2 (Ascl2), EphB2, Troy and Axin2. Some novel markers of the LGR5+ CBC SC, including olfactomedin 4 (Olfm4), SPARC-related modular calcium-binding 2 (Smoc2) and ring finger 43 (Rnf43) have been further identified using this criterium.40
The microtubule-associated doublecortin- like kinase 1 (DCLK-1) is expressed in Msi-1 positive cells near position 4 in intestinal crypts26 and label-retention data revealed stem cell characteristics in DCLK-1+ cells with self-renewal properties and the ability to form spheroids.49
Crypt dynamic is modified in IBD commonly leading to crypt distortion with hyperplasia, branching and fission. The repeated cycles of active inflammation are subsequent to wound healing and crypt regeneration, and are evidently dependent on the Wnt signaling pathway.
Events within the stem cell niche present a genetic removal of a specific stem cell which induces the formation of a reserve pool to counteract total phenotypic modification. Thus the ablation of LGR5+ cells in mice causes no alterations in the phenotype due to the compensatory effects of an expansion in the BMI-1+ cell population.50
Recent investigations reveal that disruption of the crypt microenvironment may lead to a return to stem cell function in cells that have withdrawn from the stem cell niche. The protein DCLK1 protein was expressed in tuft cells,51 representing a secretory population in the context of the intestinal epithelium. DCLK1 is also a putative marker of quiescent ISC.52 Abundant DCLK1+ tuft cells are observed in inflammation-induced carcinogenesis, which imply their potential role in the process.53 In addition, Westphalen et al. noted no tumors after 18 months of APC gene loss in the tuft cell population in Dclk1-CreERT x R26- LacZ mice, but observed occurrences of advanced CAC following APC loss and subsequent inflammatory stimulus.27
IBD is a chronic relapsing–remitting inflammatory disease of the intestinal tract, presenting with repeated cycles of epithelial injury and regeneration. Crypt dynamic is affected in IBD and in the context of these diseases crypt distortion is frequently observed54 and it has been demonstrated that adjacent crypts repopulate the injured area in a Wnt depending manner.55
ISCs are directly responsible for maintenance of intestinal homeostasis and regeneration following injury or inflammation and they are implicated in the pathogenesis of IBDs, which can be described as stem cell disorders in a susceptible host.56 Furthermore an attenuated induction of goblet cells differentiation factors in ISC has been observed in human colon biopsies from UC patients,57 while in CD patients and in experimental murine CD models, a defective differentiation from the ISC toward the Paneth cell compartment has been demonstrated.58
It has been proposed that the stem cell regenerative capacity is gradually decreased during the disease progression, resulting in long-term ulceration and malignant transformation.38 In this regard SCs could have a therapeutic role, in fact they appear to home to the injured intestinal areas as a response to factors produced in the context of inflamed tissues, and differentiate into epithelial and immune cells, such modulating tissue regeneration and inflammatory response.59
Current therapeutic strategies
Treatment for IBD has mainly focused on inhibiting the inflammatory process during recurrences and extending the duration of remission. Decision making regarding treatment of both UC and CD is dependent on the disease progression: patients with mild disease presentation are usually treated with aminosalicylates, whereas steroids are prescribed for moderate manifestations and cyclosporine is given to patients with severe disease. In CD, disease location and behavior are also considered. Maintenance therapies also include aminosalicylates, azathioprine, mercaptopurine, methotrexate, metronidazole, and associations of these.60,61 However, these treatments present a number of inconvenient side effects and scarce guarantees of clinical remission.62 This is true particularly of steroids and for this reason several attempts have been carried out in order to develop safer and more effective therapeutic options. Therefore an effective treatment should include an immune modulator capable of controlling inflammation without leading to exacerbated immunosuppression.
The application of biological therapy for the treatment of IBD aims at targeting directly and specifically various mechanisms involved in the immune response, such as growth factors for the immune cells, antibody production, and complement activation. A major challenge for antibody therapy is TNF-α and at present these drugs may be used both for non-responders to traditional treatment and employed in early onset cases.
Nevertheless biological therapies present a certain immunogenicity, inducing the generation of anti-drug antibodies, which are responsible for infusion reactions and reduction of the duration of the response to each infusion.63 As an alternative therapeutic strategy to TNF-α inhibitors, treatments that manipulate leukocyte adhesion, costimulatory signaling and cytokine receptors are emerging as potential treatments for IBD.
The current therapeutic approach for fistulising CD, in case of uncomplicated perianal fistulae, is based on seton placement, while abscesses and other complications require surgical intervention combined with medical therapy, which usually includes anti-TNF, antibiotics and thiopurines.13
To sum up, the conventional treatments for IBD have addressed the inflammation process and immunological modifications involved in the wound-healing process. An effective treatment approach should aim at stimulating epithelial proliferation and coordination of the remodeling process. In this context stem cell therapy may be considered a promising strategy for these patients.20
Adipose stem cells therapy for inflammatory bowel disease
Multipotent MSCs are being investigated to treat various human diseases. MSCs have a broad anatomical distribution and can be isolated from several human tissues such as bone marrow,64 adipose tissue,65 dental pulp,66 connective tissue,67 umbelical cord.68 MSCs commonly reside in “niches” of different tissues such as intestine69 and possess self-renewal potential. MSCs have no unique phenotypic marker and according to the minimal criteria by ISCT (International Society of Cellular Therapy) possess the following characteristics: the expression of CD105, CD73, CD90 and CD34 markers; the lack of expression of haematopoietic markers and the capacity to differentiate into osteoblasts, adipocytes and chondrocytes.70 MSCs are known to have immunosuppressive potential, which implies their therapeutic efficacy in immune-mediated disorders.71 In particular, MSCs obtained from adipose tissue have been shown to exert an immunosuppressive response in vitro and inhibit peripheral blood mononuclear cell proliferation and IFN-γ production while increasing IL-10 secretion72 (Fig. 3). The mechanism of action is based on cell contact and paracrine action involving the release of soluble factors. In particular, MSCs are known to provoke immune-modulating responses, such as IL-6, IL-10, prostaglandin, TGF-β, nitric oxide and others.73
Figure 3.
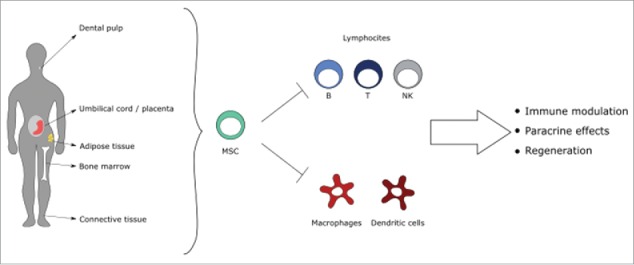
Tissue sources of mesenchymal stem cells and their mechanisms of action: MSC can be isolated from adult and fetal tissues. Their immune-regulatory properties address innate and adaptive immune cells, in the context of inflammatory bowel diseases microenvironment.
The use of MSCs depends on their ability to repair damaged tissues and inhibit inflammation and fibrosis.73 Regeneration of the intestinal lesions in IBD is a consequence of the inherent inflammatory process and the healing mechanisms that restore epithelial integrity and repair intestinal damage.
Several clinical trials regarding MSCs in IBD have investigated their role in fistulizing and in luminal disease,74 resulting in 2 major developments in MSC treatment: the local application of SCs to treat perianal CD and systemic infusion of SCs to treat luminal CD and UC. In ulcerative colitis, an anti-inflammatory effect and the capacity to home to inflammation sites have been observed.
In Crohn's Disease, adipose tissue derived MSCs (allogenic or autologous) are commonly used while in UC, bone-marrow derived MSCs are employed since myeloconditioning is not required. MSCs are, in fact, non-immunogenic adult stem cells, with the ability to evade immune recognition. These treatments have been evaluated in clinical trials on IBD patients with resistance to steroids and immunomodulating therapy.75
MSCs are able to close the fistula tract due to their ability to differentiate and may be locally administered to treat those patients with complex perianal fistulae who are unresponsive to infliximab.76 MSCs are effective in luminal disease because of their immunosuppressive potential. In this case, Duijvestein and colleagues have shown that in vitro MSC activation by IFN-γ increases the immunosuppressive capacity of cells and their therapeutic efficacy when administered in vivo to mice with colitis.77 In particular, MSCs from adipose tissue (ASCs) are able to suppress immune responses and provide a highly effective treatment for colitis induced by trinitrobenzene sulfonic acid,78 ameliorating the clinical signs of colitis. There are several potential mechanisms mediated by ASCs. ASCs strongly reduce acute mucosal inflammation with anti-inflammatory efficacy comparable to most immunosuppressive drugs. ASCs exert their action by down-regulating the production of a wide panel of mediators involved in the local and systemic inflammatory response. Therefore, in addition to the reduction in inflammatory infiltration, ASCs deactivated mucosal inflammatory responses. In this sense, Gonzalez and others79,80 found that ASCs enter rapidly into the inflamed colon after their injection without targeting the non-inflammed gut segments. This homing is correlated with the expression of active chemokine receptors (ie, CCR1, CCR5) present into inflamed colon. In addition to its potent anti-inflammatory effect, ASCs treatment down-regulates the expression of the Th1-type cytokines IFN-γ and TNF-α and increases the number of CD4 T cells producing IL10. The therapeutic effect of SC from adipose tissue may be attributed to an immunomodulatory action via suppression or inhibition of the inflammatory process and via angiogenesis stimulation.
Stem cell-based therapy with expanded adipose-derived SC (ASCs) has been tested for expediency and safety in a phase I and phase II clinical trial (Table 1). Fistulae are characteristic in CD patients and may cause devastating complications because of the effects of refractory to conventional therapy and high recurrence. In a phase I clinical trial,81-83 ASCs proved to be safe and effective in the treatment of fistulae associated with CD. In a phase II clinical trial83-85 patients were treated with ASCs in combination with fibrin glue. ASCs have been shown to possess 2 distinct biological characteristics: the capacity to differentiate and the ability to suppress proliferation of activated lymphocytes. No aberrant differentiation was detected in biopsies taken from patients in the phase I clinical trial. In conclusion, these clinical trials revealed a complete closure of the fistulae and no spontaneous or pressure suppuration in 75% of the cases. The remaining 25% showed a partial closure. No adverse effects related to the therapeutic use of ASCs were observed in any patient at the end of the follow-up period.86
Table 1.
Clinical trials investigating the outcome of adipose stem cells local therapy for the treatment of perianal fistulas in patients with Crohn's disease.
| Author | Type | Treatment | Source of ASC | Healing percentage (n. of CD patients) |
|---|---|---|---|---|
| Garcia Olmo et al. 2003 | Phase I | Local injection of stem cells | Autologus | 75% (4) |
| Garcia Olmo et al. 2005 | Phase II | Local injection of stem cells plus fibrin glue | Autologus | 71% (7) |
| de la Portilla et al. 2013 | Phase I/IIa | Local injection of stem cells | Allogenic | 28% (18) |
| Cho et al. 2013 | Phase I | Local injection of stem cells | Autologus | 30% (10) |
| Lee et al. 2013 | Phase II | Local injection of stem cells | Autologus | 82% (33) |
The aim of optimal therapy for IBD is to suppress inflammation, to stimulate proliferation and to coordinate remodeling during the healing process toward a complete recovery perspective. Moreover, ASCs possess other characteristics in addition to their immunomodulatory potential. These properties further determine their therapeutic action in treating IBD by way of their ability to home to damaged tissue sites and to secrete soluble factors toward the functional recovery of injured cells.
Treatments for IBD: Future perspectives
In UC, treatment generally depends on the stage of disease: patients presenting mild-moderate symptoms are usually treated with aminosalicylates and corticosteroids, whereas cyclosporine is commonly administered to patients with moderate-severe disease. In CD, treatment depends on both location and behavior of the disease ranging from aminosalicylates, antibiotics, corticosteroids to biological molecules to treat fistulizing disease.
The use of biological treatments in inflammatory disease is related to investigations that confirm the presence of proinflammatory cytokines in the gut lamina propria in IBD patients. These cytokines, in particular TNF-α, are fundamental in the maintenance of chronic inflammation. The use of monoclonal antibodies specific against TNF-α may be a suitable alternative. Moreover, long-term therapy with biological molecules may cause immunogenicity by generating anti-drug antibodies. These antibodies are able to stimulate acute and delayed infusion reactions and may lower the patient response rate to each infusion or injection. However, an optimal treatment still needs to be discovered despite the current endeavors to improve existing therapies. Approximately one-third of all IBD patients fail to respond to anti-TNF-α therapy and 10% of all IBD patients do not tolerate or are non-responders to existing medications for the treatment of the disease.87 Further studies are therefore necessary to discover treatment alternatives. In recent years, research has addressed the study and application of mesenchymal stem cells from adipose tissue (ASCs). Adult bone marrow has been extensively employed to acquire MSCs in a clinical context, despite the low expression in adult marrow, the difficulty of extraction and the risks of the donation process. A greater quantity of MSCs may be acquired via liposuction;88 cells may be assigned directly with similar characteristics to the MSCs derived from bone marrow.89 Various groups have devised equipment to automate cell isolation in order to simplify this mechanism.90,91 However, the current “ready to use” prototypes have not always produced satisfactory clinical and scientific results. Nevertheless, some investigators have devised a new clinical process of tissue regeneration, using an enzymatic92 and a mechanical disaggregation.93 The novel strategy permits a mechanical disaggregation of the tissue without manipulating the matrix. The mechanical isolating procedure is easier to use, safer, less expensive and more rapid proving it more advantageous compared to the current enzymatic procedures in use. Some case report investigations were studied to analyze the clinical outcomes of autologous micro-grafts obtained with a new “Rigenera” protocol94, which stimulates the generation of SCs during surgery. The subcutaneous adipose tissue is beneficial because of its accessibility and availability in isolating residing SCs. Carelli and colleagues (2014) report that microfragmented human lipoaspirated adipose tissue is a better stem cell source.95 The ASCs derived from microfragmented lipoaspirate tissue have been defined as multipotent with satisfactory differentiation potential. Rigenera protocol is generally applied to other types of connective tissues including dental pulp but may be suitable for adipose tissue. Extensive research is required regarding adipose stem cell treatment to improve current investigations and results from ongoing and future clinical trials will contribute significantly toward optimal IBD management and patient satisfaction.
Conclusion
In conclusion the recent understanding of stem cell role in IBD inflammatory process has endorsed the development of cellular therapy with SCs for the treatment of these conditions. The use of MSCs and in particular ASCs will overcome the ethical issues associated to the utilization of embryonic and placental SCs. Furthermore, MSCs are easily and safely accessible and their use under phase III clinical trial assessment is offering positive results.
Disclosure of potential of conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Prof. Giuseppina Caraglia B.A., English language expert and assistant for Department of Sciences and Environmental, Biological, Pharmaceutical Technologies, Caserta, Second University of Naples for providing excellent technical revision and support.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140:1785-94; PMID:21530745; http://dx.doi.org/ 10.1053/j.gastro.2011.01.055 [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004; 126:1504-17; PMID:15168363; http://dx.doi.org/ 10.1053/j.gastro.2004.01.063 [DOI] [PubMed] [Google Scholar]
- 3.Ruel J, Ruane D, Mehandru S, Gower-Rousseau C, Colombel JF. IBD across the age spectrum: is it the same disease? Nat Rev Gastroenterol Hepatol 2014; 11:88-98; PMID:24345891; http://dx.doi.org/ 10.1038/nrgastro.2013.240 [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al.. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142:46-54; PMID:22001864; http://dx.doi.org/ 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol 2006; 101:993-1002; PMID:16696783; http://dx.doi.org/ 10.1111/j.1572-0241.2006.00381.x [DOI] [PubMed] [Google Scholar]
- 6.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al.. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491:119-24; PMID:23128233; http://dx.doi.org/ 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011; 474:307-17; PMID:21677747; http://dx.doi.org/ 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ananthakrishnan A. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015; 12:205-17; PMID:25732745; http://dx.doi.org/ 10.1038/nrgastro.2015.34 [DOI] [PubMed] [Google Scholar]
- 9.Sebastian S, Hernández V, Myrelid P, Kariv R, Tsianos E, Toruner M, Marti-Gallostra M, Spinelli A, van der Meulen-de Jong AE, Yuksel ES, et al.. Colorectal cancer in inflammatory bowel disease: results of the 3rd ECCO pathogenesis scientific workshop (I). J Crohns Colitis 2014; 8:5-18; PMID:23664897; http://dx.doi.org/ 10.1016/j.crohns.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med 2007; 58:267-284; PMID:17002552; http://dx.doi.org/ 10.1146/annurev.med.58.062105.204854 [DOI] [PubMed] [Google Scholar]
- 11.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology 2010; 138:2151-62; PMID:20420952; http://dx.doi.org/ 10.1053/j.gastro.2009.12.063 [DOI] [PubMed] [Google Scholar]
- 12.Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol 2008; 43:1-17; PMID:18297430; http://dx.doi.org/ 10.1007/s00535-007-2111-3 [DOI] [PubMed] [Google Scholar]
- 13.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012; 380:1590-605; PMID:22914295; http://dx.doi.org/ 10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- 14.Freeman HJ. Natural history and long-term clinical course of Crohn's disease. World J Gastroenterol 2014; 20:31-36; PMID:24415855; http://dx.doi.org/ 10.3748/wjg.v20.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol 2015; 110:564-71; PMID:25803400; http://dx.doi.org/ 10.1038/ajg.2015.50 [DOI] [PubMed] [Google Scholar]
- 16.Plevy S. The immunology of inflammatory bowel disease. Gastroenterol Clin North Am 2002; 31:77-92; PMID:12122745; http://dx.doi.org/ 10.1016/S0889-8553(01)00006-1 [DOI] [PubMed] [Google Scholar]
- 17.Sherbaniuk RW. Ulcerative colitis: disease patterns and medical management. CMAJ 1964; 91:30-6; PMID:14182561 [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlach K, Hwang Y, Nikolaevetal A. H9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 2014; 15:676-86; PMID:24908389; http://dx.doi.org/ 10.1038/ni.2920 [DOI] [PubMed] [Google Scholar]
- 19.Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2009; 15:5784-88; PMID:19998498; http://dx.doi.org/ 10.3748/wjg.15.5784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Mattos BR, Garcia MP, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM, Simioni PU. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediators Inflamm 2015; 2015:493012; PMID:26339135; http://dx.doi.org/ 10.1155/2015/493012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al.. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl A):5A-36A; PMID:16151544; http://dx.doi.org/ 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 22.Bressenot A, Cahn V, Danese S, Peyrin-Biroulet L. Microscopic features of colorectal neoplasia in inflammatory bowel diseases. World J Gastroenterol 2014; 20:3164-72; PMID:24696602; http://dx.doi.org/ 10.3748/wjg.v20.i12.3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shergill AK, Farraye FA. Toward a Consensus on Endoscopic Surveillance of Patients with Colonic Inflammatory Bowel Disease. Gastrointest Endoscopy Clin N Am 2014; 24:469-81; PMID:24975537; http://dx.doi.org/ 10.1016/j.giec.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 24.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010; 138:746-74; PMID:20141809; http://dx.doi.org/ 10.1053/j.gastro.2009.12.035 [DOI] [PubMed] [Google Scholar]
- 25.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 2005; 11:314-21, Leighton JA, Shen B, Baron TH, et al.. ASGE guideline: endoscopy in the diag- nosis and treatment of inflammatory bowel disease. Gastrointest Endosc 2006; 63: 558-65; PMID:16564852; http://dx.doi.org/ 10.1097/01.MIB.0000160811.76729.d5 [DOI] [PubMed] [Google Scholar]
- 26.Hughes KR, Gândara RM, Javkar T, Sablitzky F, Hock H, Potten CS, Mahida YR. Heterogeneity in histone 2B-green fluorescent protein-retaining putative small intestinal stem cells at cell position 4 and their absence in the colon. Am J Physiol Gastrointest Liver Physiol 2012; 303:G1188-201; PMID:22997199; http://dx.doi.org/ 10.1152/ajpgi.00080.2012 [DOI] [PubMed] [Google Scholar]
- 27.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al.. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 2014. March;124(3):1283-95; PMID: 24487592; http://dx.doi.org/ 10.1172/JCI73434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat 1981; 160:51-63; PMID:7211716; http://dx.doi.org/ 10.1002/aja.1001600105 [DOI] [PubMed] [Google Scholar]
- 29.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. II. Evidence from Paneth cells in the newborn mouse. Am J Anat 1981; 160:65-75; PMID:7211717; http://dx.doi.org/ 10.1002/aja.1001600106 [DOI] [PubMed] [Google Scholar]
- 30.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 1981; 160:77-91; PMID:7211718; http://dx.doi.org/ 10.1002/aja.1001600107 [DOI] [PubMed] [Google Scholar]
- 31.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. IV. Effects of resectng 30% of the small intestine. Am J Anat 1981; 160:93-103; PMID:7211719; http://dx.doi.org/ 10.1002/aja.1001600108 [DOI] [PubMed] [Google Scholar]
- 32.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. V. Evidence for controls over orientation of boundaries between the stem-cell zone, proliferative zone, and the maturation zone. Am J Anat 1981; 160:105-12; PMID:7211714; http://dx.doi.org/ 10.1002/aja.1001600109 [DOI] [PubMed] [Google Scholar]
- 33.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol 2006; 419:337-83; PMID:17141062; http://dx.doi.org/ 10.1016/S0076-6879(06)19014-X [DOI] [PubMed] [Google Scholar]
- 34.Potten CS, Chadwick C, Ijiri K, Tsubouchi S, Hanson WR. The recruitability and cell-cycle state of intestinal stem cells. Int J Cell Cloning 1984; 2:126-40; PMID:6707492; http://dx.doi.org/ 10.1002/stem.5530020206 [DOI] [PubMed] [Google Scholar]
- 35.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990; 110:1001-20; PMID:2100251 [DOI] [PubMed] [Google Scholar]
- 36.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 1997; 78:219-43; PMID:9505935; http://dx.doi.org/ 10.1046/j.1365-2613.1997.280362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al.. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449:1003-7; PMID:17934449; http://dx.doi.org/ 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 38.Moossavi S, Zhang H, Sun J, Rezaei N. Host-microbiota interaction and intestinal stem cells in chronic inflammation and colorectal cancer. Expert Rev Clin Immunol 2013; 9:409-22; PMID:23634736; http://dx.doi.org/ 10.1586/eci.13.27 [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al.. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459:262-5; PMID:19329995; http://dx.doi.org/ 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 40.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014; 15:19-33; PMID:24326621; http://dx.doi.org/ 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- 41.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 2011; 474:318-326; PMID:21677748; http://dx.doi.org/ 10.1038/nature10212 [DOI] [PubMed] [Google Scholar]
- 42.Lanzoni G, Alviano F, Marchionni C, Bonsi L, Costa R, Foroni L, Roda G, Belluzzi A, Caponi A, Ricci F, et al.. Isolation of stem cell populations with trophic and immunoregulatory functions from human intestinal tissues: potential for cell therapy in inflammatory bowel disease. Cytotherapy 2009; 11:1020-31; PMID:19929466; http://dx.doi.org/ 10.3109/14653240903253840 [DOI] [PubMed] [Google Scholar]
- 43.Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: where are they? Cell Prolif 2009; 42:731-50; PMID:19788585; http://dx.doi.org/ 10.1111/j.1365-2184.2009.00642.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol 2001; 21:3888-900; PMID:11359897; http://dx.doi.org/ 10.1128/MCB.21.12.3888-3900.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 2003; 71:28-41; PMID:12558601; http://dx.doi.org/ 10.1046/j.1432-0436.2003.700603.x [DOI] [PubMed] [Google Scholar]
- 46.Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci U S A 2008; 105:10420-5; PMID:18650388; http://dx.doi.org/ 10.1073/pnas.0804800105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, Swain JR, Wong MH. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 2010; 139:2072-82; PMID:20826154; http://dx.doi.org/ 10.1053/j.gastro.2010.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, et al.. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 2013; 145:383-95; PMID:23644405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009; 87:1097-104; PMID:19727638 [DOI] [PubMed] [Google Scholar]
- 50.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011; 478:255-59; PMID:21927002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 2009; 137:2179-80; PMID:19879217 [DOI] [PubMed] [Google Scholar]
- 52.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 2008; 26:630-637; PMID:18055444 [DOI] [PubMed] [Google Scholar]
- 53.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 2011; 136:191-204; PMID:21688022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng H, Bjerknes M, Amar J, Gardiner G. Crypt production in normal and diseased human colonic epithelium. Anat. Rec 1986; 216:44-48; PMID:3094402 [DOI] [PubMed] [Google Scholar]
- 55.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012; 338:108-113; PMID:22956684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gersemann M, Stange EF, Wehkamp J. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol 2011; 17:3198-203; PMID:21912468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation 2009; 77:84-94; PMID:19281767 [DOI] [PubMed] [Google Scholar]
- 58.Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M. The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol 2007; 179:3109-18; PMID:17709525 [DOI] [PubMed] [Google Scholar]
- 59.Brittan M, Alison MR, Schier S, Wright NA. Bone marrow stem cell-mediated regeneration in IBD: where do we go from here? Gastroenterology 2007; 132:1171-73; PMID:17383436 [DOI] [PubMed] [Google Scholar]
- 60.Carter MJ, Lobo AJ, Travis SPL. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004; 53:V1-V16; PMID:15306569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D'Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis 2014; 8:726-34; PMID:24742736; http://dx.doi.org/ 10.1016/j.crohns.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 62.Reichel C, Streit J, Ott K, Wunsch S. Appropriateness of Crohn's disease therapy in gastroenterological rehabilitation. Digestion 2010; 82:239-45; PMID:20588039; http://dx.doi.org/ 10.1159/000291859 [DOI] [PubMed] [Google Scholar]
- 63.Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J. Immunogenicity to biologics: mechanisms, prediction and reduction. Arch Immunol Ther Exp 2012; 60:331-44; PMID:22930363; http://dx.doi.org/ 10.1007/s00005-012-0189-7 [DOI] [PubMed] [Google Scholar]
- 64.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol 2008; 449:27-44; PMID:18370081; http://dx.doi.org/ 10.1007/978-1-60327-169-1_2 [DOI] [PubMed] [Google Scholar]
- 65.De Francesco F, Ricci G, D'Andrea F, Nicoletti GF, Ferraro GA. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev 2015; 21:572-84; PMID:25953464; http://dx.doi.org/ 10.1089/ten.teb.2014.0608 [DOI] [PubMed] [Google Scholar]
- 66.Ledesma-Martinez E, Mendoza-Nunez VM, Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int 2016; 2016:4709572; PMID:26779263; http://dx.doi.org/ 10.1155/2016/4709572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vapniarsky N, Arzi B, Hu JC, Nolta JA, Athanasiou KA. Concise review: Human dermis as an autologous source of stem cells for tissue engineering and regenerative medicine. Stem Cells Transl Med 2015; 4:1187-98; PMID:26253713; http://dx.doi.org/ 10.5966/sctm.2015-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalaszczynska I, Ferdyn K. Wharton's jelly mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int 2015; 2015:430847; PMID:25861624; http://dx.doi.org/ 10.1155/2015/430847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol 2014; 107:77-107; PMID:24439803; http://dx.doi.org/ 10.1016/B978-0-12-416022-4.00003-2 [DOI] [PubMed] [Google Scholar]
- 70.Sousa BR, Parreira RC, Fonseca EA, Amaya MJ, Tonelli FM, Lacerda SM, Lalwani P, Santos AK, Gomes KN, Ulrich H, et al.. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014; 85:43-77; PMID:24700575; http://dx.doi.org/ 10.1002/cyto.a.22402 [DOI] [PubMed] [Google Scholar]
- 71.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, et al.. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016; 18:151-9; PMID:26724220; http://dx.doi.org/ 10.1016/j.jcyt.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, Shan F, Meng Y, Yuan Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue and Wharton's jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother 2016; 12:85-96; PMID:26186552; http://dx.doi.org/ 10.1080/21645515.2015.1030549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 2014; 15:1009-16; PMID:25329189; http://dx.doi.org/ 10.1038/ni.3002 [DOI] [PubMed] [Google Scholar]
- 74.Molendijk L, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ, et al.. Allogenic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with crohn's disease. Gastroenterology 2015; 149:918-27; PMID:26116801; http://dx.doi.org/ 10.1053/j.gastro.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 75.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA Jr. Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2015; 21:2696-707; PMID:26230863; http://dx.doi.org/ 10.1097/MIB.0000000000000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogenic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014; 12:64-71; PMID:23872668; http://dx.doi.org/ 10.1016/j.cgh.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 77.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, et al.. Pretreatment with interferon-g enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011; 29:1549-58; PMID:21898680; http://dx.doi.org/ 10.1002/stem.698 [DOI] [PubMed] [Google Scholar]
- 78.Ando Y, Inaba M, Sakaguchi Y, Tsuda M, Quan GK, Omae M, Okazaki K, Ikehara S. Subcutaneous adipose tissue-derived stem cells facilitate colonic mucosal recovery from 2,4,6-trinitrobenzene sulfonic acid (TNBS)- induced colitis in rats. Inflamm Bowel Dis 2008; 14:826-38; PMID:18253953; http://dx.doi.org/ 10.1002/ibd.20382 [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delfado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009; 58:929-39; PMID:19136511; http://dx.doi.org/ 10.1136/gut.2008.168534 [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009; 136:978-89; PMID:19135996; http://dx.doi.org/ 10.1053/j.gastro.2008.11.041 [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005; 48:1416-23; PMID:15933795; http://dx.doi.org/ 10.1007/s10350-005-0052-6 [DOI] [PubMed] [Google Scholar]
- 82.Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn's fistula: a phase I clinical study. Cell Transplant 2013; 22:279-85; PMID:23006344; http://dx.doi.org/ 10.3727/096368912X656045 [DOI] [PubMed] [Google Scholar]
- 83.de la Portilla F, Alba F, Garcia-Olmo D, Herrerias JM, Gonzalez FX, Galindo A. Expanded allogenic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 2013; 28:313-23; PMID:23053677; http://dx.doi.org/ 10.1007/s00384-012-1581-9 [DOI] [PubMed] [Google Scholar]
- 84.Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim do S, Jung SH, Kim M, Yoo HW, Kim I, et al.. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells 2013; 31:2575-81; PMID:23404825; http://dx.doi.org/ 10.1002/stem.1357 [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009; 52:79-86; PMID:19273960; http://dx.doi.org/ 10.1007/DCR.0b013e3181973487 [DOI] [PubMed] [Google Scholar]
- 86.Herreros M, Guadalajara H, De-La-Quintana P, Pascual I, Trebol J, Georgiev-Hristov T, Gonzalez-Gomez C, Garcia-Arranz M, Del-Valle E, Zorrilla J, et al.. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long term evaluation. Dis Colon Rectum 2012; 55:762-72; PMID:22706128; http://dx.doi.org/ 10.1097/DCR.0b013e318255364a [DOI] [PubMed] [Google Scholar]
- 87.Csontos AA, Molnar A, Piri Z, Katona B, Dako S, Palfi E, Miheller P. The effect of anti TNFa induction therapy on the nutritional status and dietary intake in inflammatory bowel disease. J Gastrointestin Liver Dis 2016; 25:49-56; PMID:27014753; http://dx.doi.org/ 10.15403/jgld.2014.1121.251.tnf [DOI] [PubMed] [Google Scholar]
- 88.Wang WZ, Fang XH, Williams SJ, Stephenson LL, Baynosa RC, Wong N, Khiabani KT, Zamboni WA. Analysis for apoptosis and necrosis on adipocytes, stromal vascular fraction, and adipose adipose-derived stem cells in human lipoaspirates after liposuction. Plast Reconstr Surg 2013; 131:77e-85e; PMID:23271558; http://dx.doi.org/ 10.1097/PRS.0b013e3182729ff7 [DOI] [PubMed] [Google Scholar]
- 89.Busser H, Najar M, Raicevic G, Pieters K, Velez Pombo R, Philippart P, Meuleman N, Bron D, Lagneaux L. Isolation and characterization of human mesenchymal stromal cell subpopulations: comparison of bone marrow and adipose tissue. Stem Cells Dev 2015; 24:2142-57; PMID:26086188; http://dx.doi.org/ 10.1089/scd.2015.0172 [DOI] [PubMed] [Google Scholar]
- 90.Hicok KC, Hedrick MH. Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol Biol 2011; 702:87-105; PMID:21082397; http://dx.doi.org/ 10.1007/978-1-61737-960-4_8 [DOI] [PubMed] [Google Scholar]
- 91.Güven S, Karagianni M, Schwalbe M, Schreiner S, Farhadi J, Bula S, Bieback K, Martin I, Scherberich A. Validation of an automated procedure to isolate human adipose tissue-drived cells using the Sepax technology. Tissue Eng Part C Methods 2012; 18:572-82; PMID:22372873; 10.1089/ten.TEC.2011.0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicoletti GF, De Francesco F, D'Andrea F, Ferraro GA. Methods and procedures in adipose stem cells: state of the art and perspective for translation medicine. J Cell Physiol 2015; 230:489-95; PMID:25294367; http://dx.doi.org/ 10.1002/jcp.24837 [DOI] [PubMed] [Google Scholar]
- 93.Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L, Redi CA, Perotti C, Kankuri E, Ambrosio G, et al.. A New medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol 2015; 230:2299-303; PMID:25728337; http://dx.doi.org/ 10.1002/jcp.24973 [DOI] [PubMed] [Google Scholar]
- 94.Purpura V, Bondioli E, Graziano A, Trovato L, Melandri D, Ghetti M, Marchesini A, Cusella De Angelis MG, Benedetti L, Ceccarelli G, et al.. Tissue Characterization after a new disaggregation method for skin micro-grafts generation. J Vis Exp 2016; PMID:26967938; http://dx.doi.org/ 10.3791/53579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, Latorre E, Grimoldi MG, Colli M, Bulfamante G, Tremolada C, et al.. Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant 2015; 24:1233-52; PMID:24806078; http://dx.doi.org/ 10.3727/096368914X681603 [DOI] [PubMed] [Google Scholar]


