Abstract
Purpose
To characterize the presenting characteristics, pre-operative clinical activity score (CAS), surgical approach, and visual outcomes in patients with thyroid eye disease undergoing repeat orbital decompression for recurrent or recalcitrant compressive optic neuropathy.
Methods
The medical records of patients with recurrent or recalcitrant compressive optic neuropathy (CON) undergoing repeat orbital decompressions were retrospectively reviewed. The primary outcome measures included pre- and post-operative Humphrey visual field mean deviation, visual acuity measured in logarithm of the minimal angle of resolution, color vision measured by Ishihara plates, and presence of relative afferent pupillary defect. Details of the surgical procedure and each patient’s CAS at presentation were also recorded.
Results
Six patients, 9 orbits, with a mean pre-operative CAS of 3.8 were included in this review. The mean time between initial decompression and presentation to our center for recurrent or persistent CON symptoms was 8.6 years (range, 1–15 years). At presentation, the average HVF mean deviation was −16.5 (standard deviation (SD): 8.8), improving to −3.8 (2.4) post-operatively with a mean of 9.3 months follow-up (mean improvement of 75%). Pre-operative visual acuity was 0.34 (0.23) LogMAR, improving to 0.05 (0.10) logMAR with a mean follow up of 10.4 months. Pre- to post-operative comparisons of clinical measures all showed statistically significant improvement (p<0.05). Eight eyes presented with decreased visual acuity (any VA < 20/20), 4 with decreased color vision (any color vision < 11), and 1 with a relative afferent pupillary defect and all of these patients demonstrated improvement following repeat orbital decompression.
Conclusions
In patients with thyroid eye disease, symptoms of recurrent compressive optic neuropathy occurred up to 15 years following initial orbital decompression underscoring the smoldering, progressive nature of the disease. Repeat decompression which focused on the orbital apex resulted in visual improvement in all 6 patients. Despite clinical evidence of compressive optic neuropathy, the mean CAS of these patients at presentation was only 3.8, highlighting the importance of close monitoring of patients with thyroid eye disease following decompression regardless of the external manifestations of disease activity.
Précis
In a series of nine orbits requiring repeat decompression for persistent or recurrent compressive optic neuropathy (CON), visual outcomes were excellent. The surgical approach and challenges are described.
Thyroid eye disease (TED), also known as Graves’ ophthalmopathy or orbitopathy, is a form of T-cell mediated autoimmune orbital inflammation seen in some patients with Graves’ disease (GD). TED is a two phase process, with an initial active, progressive phase followed by a fibrotic or quiescent phase.1 Autoimmune mediated expansion of the extraocular muscles and orbital soft tissues occurs during the active phase of TED, with resulting orbital and periorbital inflammation that may last up to 2 years. This is followed by a chronic fibrotic phase that is characterized by tissue thickening secondary to deposition of collagen and other connective tissue proteins. During this time, the inflammation-associated clinical findings become less robust and slowly stabilize.2,3 The clinical signs of TED are well established and include lid retraction, lagophthalmos, exophthalmos, strabismus, exposure keratopathy, and compressive optic neuropathy (CON).4 CON, the most feared complication of TED, occurs in approximately 3–5% of patients with TED and manifests with pupillary abnormalities, decreased visual acuity (VA), visual field defects, optic disc edema, and impairment of color vision.5 The pathology of CON is likely related to a combination of direct nerve compression by expanding orbital tissue at the orbital apex, axonal stretching, and compromised vascular supply to the optic nerve.6–10
There are several treatment modalities for CON, including oral or intravenous corticosteroids, orbital radiation, and surgical decompression.3,11–13 Surgical decompression for CON is performed by removal of fat, bony walls of the orbit, or a combination of these techniques. Surgical therapy can often lead to restoration or maintenance of visual function.14–19,20,21 The clinical activity score (CAS) was initially developed as a tool to identify active disease and predict patients’ response to immunosuppressive treatment; however, it has subsequently been used as a primary outcome measure for several different TED therapies.22–26 The score is derived from a clinical exam that assesses eyelid swelling and erythema, conjunctival redness and chemosis, caruncular inflammation, and orbital pain at rest or with eye movement.
The majority of current literature focuses on the treatment of the active phase of TED, with emphasis on preventing or reversing the effects of CON.12,27–29 There are few reports of patients with a relapse or progression of their thyroid orbitopathy following initially successful orbital decompression.30 The treatment of recurrent CON poses unique clinical and surgical challenges. The fibrotic response of chronic TED complicates surgical therapy and also limits the efficacy of medical treatment for recurrent CON. Furthermore, the relative rarity of recurrent disease has resulted in a lack of objective data guiding therapy. The use of the CAS for the evaluation of smoldering or recurrent disease has not been validated, and there are no clinical tools currently available to assess recurrent TED.
The purpose of this report is to describe the initial presenting characteristics, pre-operative CAS, surgical approach, and surgical outcomes in patients with TED and recurrent or recalcitrant CON referred to a quaternary care academic medical center specializing in thyroid eye disease, to undergo repeat orbital decompression.
METHODS
The medical records of patients undergoing repeat orbital decompression at the University of Michigan Kellogg Eye Center for recurrent or persistent CON secondary to TED orbitopathy from November 2008 to March 2013 were retrospectively reviewed.
This study was approved by the Institutional Review Board at the University of Michigan Health System and was carried out in accordance with the Declaration of Helsinki. Data collected included age, gender, race, date and surgical details of the previous decompression(s), symptoms of recurrent or persistent CON, laterality of involvement, smoking status, and thyroid status. Time between prior decompression and presentation with recurrent CON was recorded, and total follow-up, including baseline evaluation, and time after repeat surgical therapy, were also reported.
Outcome measures
Clinical outcome measures included mean deviation on Humphrey visual field (HVF) testing (HFA II, Carl Zeiss Meditec Inc, Dublin, CA, U.S.A.), visual acuity (Snellen types), color vision (Ishihara plates), presence or absence of relative afferent pupillary defect, and Hertel exophthalmometry measurements. These measures were obtained pre- and post-operatively. For patients with bilateral involvement, information was recorded for each orbit independently.
The diagnosis of CON was based on assessment of field defects, decreased visual acuity, reduced color vision, or relative afferent pupillary defects and exclusion of other potential causes of optic neuropathy. Outcome measures were repeated at each postoperative visit. Preoperative non-contrast orbital computed tomography (CT) scans were obtained prior to repeat decompression for all patients and were analyzed to determine the level of previous decompression performed and to assess orbital crowding, aiding in surgical planning. Repeat decompression was deemed successful if there was improvement of the mean deviation on HVF testing.
Snellen visual acuity data were converted logarithmically to the minimal angle of resolution (logMAR) equivalents. Descriptive statistics were utilized to describe the data. Preoperative and postoperative data including mean deviation of Humphrey visual field test results, visual acuity, and exophthalmometry were analyzed using paired samples t-test and Wilcoxon signed-rank test.
RESULTS
All 6 patients, 9 orbits, in the cohort had previously undergone orbital decompression for compressive optic neuropathy and presented at various time intervals with recurrent compressive optic neuropathy for which repeat orbital decompression was performed. 3 patients had bilateral involvement and 3 patients had unilateral involvement. Four patients had their prior decompression performed at an outside institution and were then referred to our institution when relapse of compressive optic neuropathy occurred. One patient had persistence of compressive optic neuropathy symptoms following initial decompression and was later referred to our institution. One patient had both their initial and repeat decompressions performed at our institution. The time interval from initial decompression to presentation for symptoms of CON ranged from 1 to at least 15 years (patient 2 had persistent CON after the initial decompression at an outside hospital, but was not referred to our center for another year). All patients were euthyroid and three patients were current smokers at the time of presentation to our facility. Data regarding patient characteristics are summarized in (Table 1).
Table 1.
Patient demographics and characteristics.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Gender | F | F | F | M | F | F |
| Age at repeat decompression (years) | 62 | 62 | 50 | 55 | 58 | 63 |
| Race | Caucasian | Caucasian | Caucasian | African- American |
African- American |
Caucasian |
| Smoker status at time of decompression | N | N | Y | Y | Y | N |
|
Thyroid disease status at time of presentation |
Euthyroid | Euthyroid | Euthyroid | Euthyroid | Euthyroid | Euthyroid |
|
Duration of GO1
at repeat decompression (years) |
25 | 2 | 8 | 15 | 15 | 16 |
|
Time between initial decompression and presentation for recurrent/persistent CON2 symptoms (years) |
5.5 | 1 | 7 | 15 | 11 | 12 |
|
Duration of CON2
symptoms at presentation (months) |
6 | 12 | 6 | 2–3 | 12 | 1 |
| CAS3 at presentation | 4/10 | 5/10 | 3/10 | 1/10 | 6/10 | 3/10 |
| Prior or present steroid | No | No | Yes | Yes | No | No |
| Laterality of repeat CON2 | Bilateral | Bilateral | Bilateral | Unilateral | Unilateral | Unilateral |
Abbreviations: Dt, date; BCVA, best corrected visual acuity; RAPD, relative afferent pupillary defect; MD, mean deviation.
Graves' Ophthalmopathy
Compressive Optic Neuropathy
Clinical Activity Score
Eight (89%) eyes presented with decreased visual acuity (any VA <20/20), four (44%) eyes presented with decreased color vision (Ishihara score <11), one (11%) eye presented with a relative afferent pupillary defect, and all patients (100%) presented with a visual field defect on Humphrey visual field testing.
Clinical signs of active inflammatory orbitopathy were also assessed using ten parameters of the CAS.22 Parameters measured included worsening pain behind the globe, pain with eye movement, redness of eyelids, conjunctival injection, eyelid swelling, chemosis, caruncular edema, increased proptosis, restricted extraocular motility, and worsening visual acuity. Based on these measures, 3 (50%) patients had a clinical activity score of 4 or higher preoperatively.
Preoperatively, mean visual acuity was 0.34 (+/− 0.23) LogMAR (Snellen 20/44) and average Hertel exophthalmometry measurement was 25.9 mm (+/− 6.8 mm). The average preoperative mean deviation (MD) on HVF testing was −16.5 (+/− 8.8).
(Table 2) shows prior (evidenced by documentation or CT findings) and repeat decompressions performed for each orbit.
Table 2.
Surgical approach for the initial and repeat orbital decompressions performed for each patient.
| Patient | Orbit | Initial decompression | Repeat decompression |
|---|---|---|---|
| 1 | OD | medial, inferior | medial, lateral |
| OS | medial, inferior | medial, lateral | |
| 2 | OD | medial, inferior | medial, lateral |
| OS | inferior | medial, lateral | |
| 3 | OD | medial, inferior | medial, lateral |
| OS | medial, inferior | medial, lateral | |
| 4 | OS | medial, inferior, anterolateral | medial, deep lateral |
| 5 | OD | medial, deep lateral | medial, inferior |
| 6 | OS | medial, inferior | lateral |
Abbreviations: GO, Graves ophthalmopathy; CON, compressive optic neuropathy; PO, per os (by mouth); CAS, clinical activity score; yrs, years.
In all cases, CT scan of the orbits obtained prior to repeat decompression revealed orbital fat stranding and thickening of the extraocular muscles, especially in the area of the orbital apex, causing a significant compression of the optic nerve. Areas of previous bony decompression were evident on CT, and in all cases the area of bony expansion did not extend to the orbital apex, providing a target for additional decompression.
Two (33%) patients (patients 3 and 4) received a course of preoperative oral steroids after baseline measurements were obtained, for initial management of CON. Patient 3 received 60mg for 1 week prior to decompression. At time of initial presentation, patient 4 received prednisone 100 mg for 3 days followed by 80 mg daily until time of decompression.
All patients showed resolution of compressive optic neuropathy at 1st postoperative follow up, which was on average at 0.5 months (range 0.2–1.6 months) following the repeat decompression. This was demonstrated by improvement among those with baseline-reduced visual acuity in 8 (100%) eyes, reduced color vision in 4 (100%), and resolution of relative afferent pupillary defect in 1 (100%). All patients had improvement of visual field defect on HVF testing.
At the first postoperative follow-up visit after repeat decompression, the average exophthalmometry (Hertel) measurement was 23.8 (+/− 7.2 mm) and the average MD on HVF testing was −7.0 (+/− 3.9). These measures were significantly improved from pre-decompression measurement (p=0.0204 and p=0.0082, respectively; paired t-tests). At last follow-up after repeat decompression, the average postoperative Hertel measurement was 22.9 (+/− 7.6 mm), with an average change from preoperative to last postoperative follow-up exophthalmometry of 3.06 mm (p=0.0056; paired t-test). At mean time of 9.3 months for last postoperative follow-up, the average HVF MD was −3.8 dB (+/− 2.4), with an average change of HVF MD of 12.72 dB (mean improvement of 75%). This improvement in MD was statistically significant (p=0.0007). Visual acuity improved to 0.05 (0.10) LogMAR (Snellen 20/22) from a pre-decompression measure of 0.34 (Snellen 20/44; p=0.0059; paired t-test) at a mean postoperative follow up time of 10.4 months. Non-parametric Wilcoxon signed-rank tests showed similarly significant findings. Figures 1 and 2 show the interval improvement of VA and HVF MD over time from initial presentation to last follow-up after repeat decompression.
Figure 1.
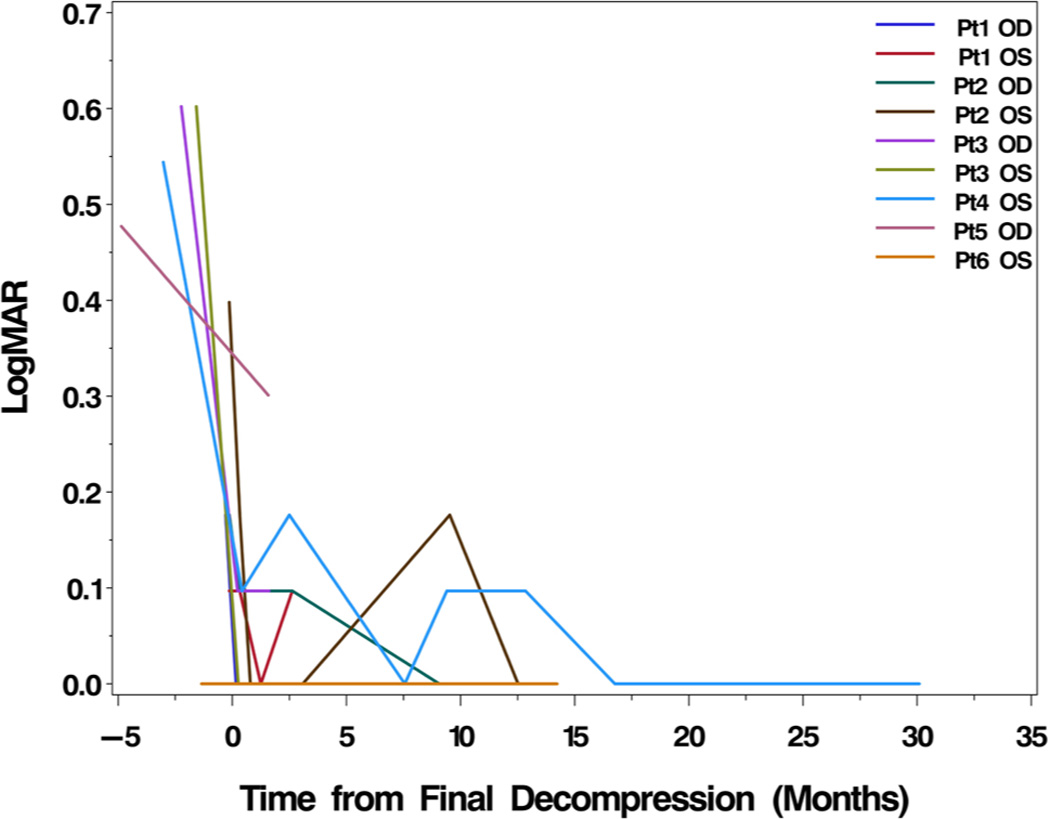
Change in LogMAR visual acuity (VA) from initial presentation to last followup. Every eye experienced improved VA following repeat decompression with attention to the orbital apex.
Figure 2.
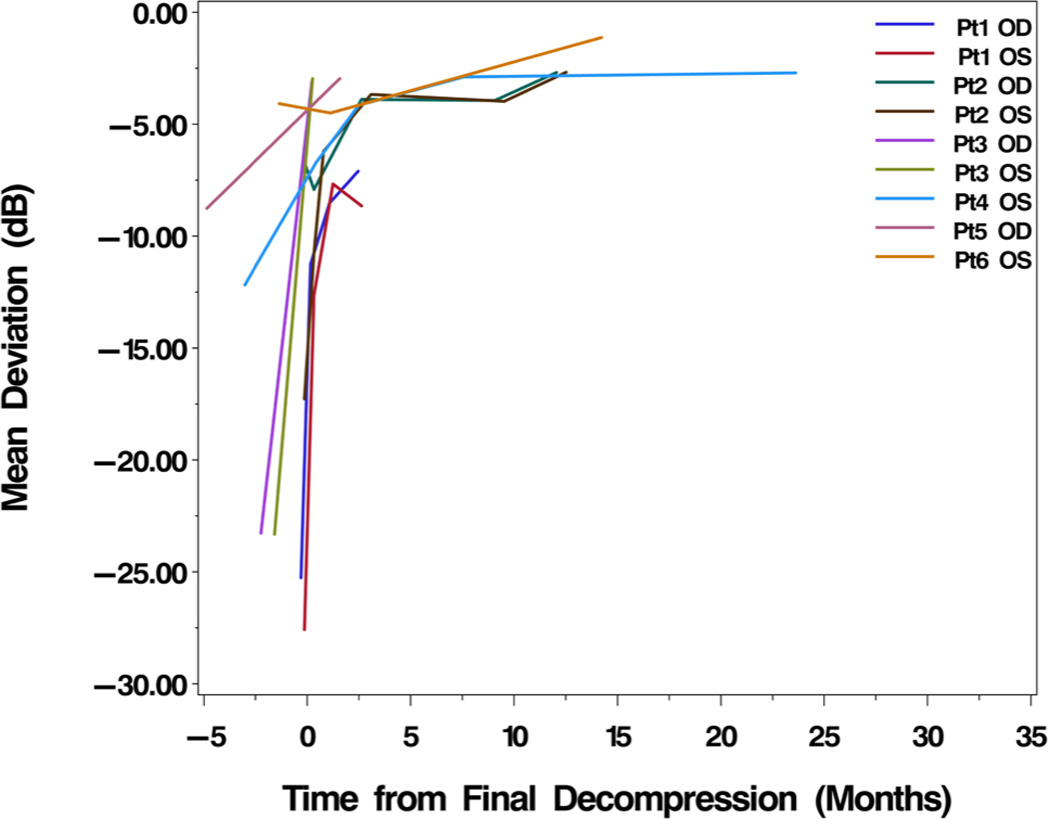
Change in mean deviation (MD) of Humphrey visual field (HVF) from initial presentation to last followup. Every eye experienced improved visual field following repeat decompression with attention to the orbital apex.
We present 4 illustrative cases:
Case 1
A 62-year old Caucasian woman, non-smoker with euthyroid status, had undergone previous right orbital fat decompression and left maxillary antrostomy with complete left ethmoidectomy and endoscopic left medial decompression, in addition to, right endoscopic medial decompression with left ethmoidectomy revision, these were done at an outside institution in 2006 and 2007, respectively. She presented to Surgeon 1 with bilateral decreased vision for the past 6 months. Her best-corrected visual acuity was found to be 20/30, right and 20/25, left. HVF MD was −25, right and −27, left. CT scan demonstrated enlarged EOM bilaterally, extensive soft tissue stranding at the intraconal lateral orbits with resultant soft tissue crowding at the orbital apices.
Four days after presentation, Surgeon 1 performed a left lateral decompression via a lid crease incision, along with further left medial decompression via a retrocaruncular approach. During surgery, it was observed that the superior medial wall had been left intact and the lateral wall of the sphenoid was untouched by prior surgeries. These areas were exposed and removed along with the remaining ethmoid air cells in addition to focus on removal of the palatine bone tip. Significant scarring from previous surgeries was encountered which required meticulous dissection.
Five days later she underwent the same surgical procedure on the right side without complication. Five days later she was seen for her first postoperative follow-up visit. Her visual acuity had improved to 20/20 on the right and was 20/25 on the left. HVF MD improved to − 11.25 on the right, and to −12.64 on the left. Her most recent follow-up visit, which was 6 weeks postoperative for the right side and 5 weeks postoperative for the left, revealed HVF MD of −7.10 on the right, (72% improvement) and −8.65 on the left (67% improvement). There was no change in visual acuity from the first post-operative follow-up visit.
Case 2
A 62-year old Caucasian woman, non-smoker with euthyroid status, presented to Surgeon 1. She had been urgently referred by an outside ophthalmologist for evaluation of bilateral compressive optic neuropathy refractory to orbital decompression performed by another ophthalmologist. The patient had been diagnosed with Graves’ disease 2 years prior and underwent radioactive iodine ablation. The following year, she underwent cataract extraction, after which her decreasing vision suggested thyroid eye disease. She was diagnosed with bilateral compressive optic neuropathy for which she had medial and inferior decompression performed on the right side and inferior decompression performed on the left side. Following this, she did not have improvement of her vision, and one year later she was referred to our institution.
At initial exam, her best-corrected visual acuities were 20/20 on the right and 20/50 on the left. Pupillary exam revealed no relative afferent pupillary defect. Color vision testing was full on the right and limited to 1/11 on the left. She had trace optic disc edema, right and significant disc edema, left. HVF MD was −9.31, right and −17.27, left. CT scan revealed enlarged extraocular muscles with crowing at the orbital apices, and areas of previous decompressions were visible.
Four days after initial presentation, she underwent left lateral and medial decompression. Lateral decompression was performed in a routine manner. After extensive bone removal, the periorbita was opened and fat released. The infero-lateral fat was noted to be “woody and hard” allowing only 1 mL of fat removal. A medial decompression was performed through a transcaruncular approach with dissection to the posterior lacrimal crest. The lamina papyrecea was removed and residual thick medial bone with scar was removed. A total external ethmoidectomy was performed, extended posteriorly, and the sphenoid sinus was entered. Attention was then directed to the inferior orbitotomy through the inferior fornix. Here, extensive scarring was encountered and the inferior oblique muscle was found to be very scarred down to periorbita. It was released with periorbita to expose the inferomedial strut and connect with the transcaruncular exposure. Next, the superior tip of the palatine bone and posterior strut was removed to decompress the nerve. The periorbita was opened allowing herniation of orbital fat into the newly created space.
Two weeks later, right medial and lateral decompressions were performed. The superior tip of the posterior strut was removed through the medial approach and no inferior orbitotomy was performed. At first postoperative visit for the left side, VA improved to 20/30 and there was restoration of full color vision. HVF MD was −10.78 (38% improvement). At first postoperative visit for the right side, 2 weeks later, VA was 20/25 with full color vision and HVF MD of −7.92 (15% improvement). At last postoperative visit 1 year later, her VA was 20/20 bilaterally with full color vision and HVF MD of −2.68 left (71% improvement), and −2.69, right (84% improvement).
Case 3
A 50-year old man, smoker, had undergone bilateral infero-medial decompression in 2005 at an outside institution for bilateral compressive optic neuropathy. He presented to Surgeon 2 with complaints of diplopia, bilateral pain and decreased vision for the past 6 months. His best- corrected visual acuity was 20/80 in both eyes. Color vision testing with Ishihara plates was 7/11 in the right and 7.5/11 in the left. HVF MD was −23.27, right, and −23.32, left.
One month later, Surgeon 2 performed a left medial and lateral decompression. Of note, the patient was initially treated with a preoperative course of oral steroids starting 1 week prior to repeat decompression. The lateral decompression was performed in a routine manner. Medially, significant scar tissue was encountered and periosteal dissection around the scar tissue had to be done to expose the medial orbital wall. The posterior ethmoid cavity was removed and this extended into the anterior portion of the sphenoid cavity. The ethmoid wall was removed posteriorly all the way back to the ethmoid-sphenoid junction. Next, the orbital portion of the palatine bone was moved allowing access into the maxillary sinus and posterior portion of the ethmoid. The periosteum was then opened allowing fat and muscle herniation into the maxillary, ethmoid, and sphenoid sinuses. Three weeks later, the same approach was undertaken on the right side.
On postoperative day 8 for the left orbit, the patient reported significantly improved visual acuity and resolution of orbital pain. The follow-up exam revealed improved visual acuity of 20/20 and color vision testing was 11/11 Ishihara plates. HVF MD improved to −2.96 (87% improvement). On postoperative day 7 visit for the right orbit, he reported improved vision and resolved orbital pain. His visual acuity had improved to 20/25 and color vision was full. HVF MD improved to −3.08 (87% improvement). These results remained stable at 2 month follow up visit.
Case 4
A 55-year old man, smoker with euthyroid status, had undergone left and right medial, anterolateral and inferior decompression 15 and 14 years prior to presentation, respectively, for bilateral CON. He presented 15 years later to Surgeon 3 with complaints of 2 to 3 months of blurred vision and decreased color vision in the left eye. His best-corrected visual acuity in the left eye was 20/50, he had a left afferent pupillary defect, color vision testing with Ishihara plates was 0/11, and fundus exam showed mild optic disc edema. HVF MD was −12.18.
He was initially treated with a course of oral prednisone. Three months later he underwent left medial and lateral decompression. Left lateral orbitotomy was performed through the prior scar. The prior decompression had removed the anterolateral orbital wall, leaving the deep lateral orbital wall and posterior orbital wall intact. Meticulous periorbital dissection was performed to avoid injuring orbital contents, which were fused to the temporalis muscle. Next, the lateral orbital rim was removed and residual sphenoid bone removed. Dissection was noted to be difficult due to scarring from previous surgery, dense bony callus, and persistent hemorrhage from small vessels in the scar tissue.
Medial decompression was performed through a transcaruncular approach and the medial wall was exposed just posterior to the posterior lacrimal crest. Dissection was limited due to extensive scarring of orbital contents that herniated on either side of the orbital strut in the ethmoid and maxillary sinuses. The remaining medial bony orbital wall was removed in piecemeal fashion, to include the orbital strut and bone immediately superior and inferior to the strut. Dissection was extended posteriorly to the posterior wall of the maxillary sinus with removal of all visible ethmoidal air cells.
On postoperative week 2, his follow-up exam revealed improved left visual acuity of 20/25, he had full color vision, and only a trace left afferent pupillary defect. HVF MD was −6.72 (45% improvement). By the time of postoperative month 8, his visual acuity was 20/20, color vision was intact, his afferent pupillary defect had resolved, and fundus exam showed resolution of disc edema. HVF MD at that visit was −2.89 (76% improvement).
DISCUSSION
Compressive optic neuropathy is a vision-threatening condition that affects approximately 3.4% of patients with Graves’ disease.31 In the primary, active phase of the disease, optic neuropathy typically occurs within 3–6 months of the onset of Graves’ ophthalmopathy.32 It is mainly attributed to crowding at the orbital apex and compression of the optic nerve. In these patients, surgical decompression may be beneficial as a first-line therapy or when medical therapy fails.33,34 The active phase of TED typically “burns-out” or stabilizes within 1–3 years following the onset of ophthalmopathy.1
It is rare for reactivation of orbitopathy to occur following the “burn-out” period. However, Chou et al. described cases in which patients developed late onset of optic neuropathy 7 to 12 years following stabilization of ophthalmopathy.35 In addition, a study by Baldeschi et al. observed a 1.3% incidence of delayed decompression related reactivation occurring several weeks after receiving bony decompression for aesthetic rehabilitation in patients with stable orbitopathy.36 Wenz et al. described 3 patients who had orbital decompression for compressive optic neuropathy and subsequently had a relapse of compressive optic neuropathy. These patients developed clinical signs of relapse from a few weeks up to 1 year following decompression.30 It is important to underscore that patients in our study had a prior decompression performed not for rehabilitative reasons, but for CON. In addition, our patients presented for a relapse or persistence of compressive optic neuropathy ranging from 1 to 15 years following prior decompression. These patients did not have correlative changes in risk factors with onset of recurrent CON symptoms (i.e. smoking behavior unchanged, thyroid medications unchanged). All patients in this study had stable thyroid levels at the time of their presentation, and half the patients had a CAS score, which is based on principles of inflammation, of less than 4. CT scans obtained at presentation to our institution demonstrated that all patients had marked enlargement of their extraocular muscles, most prominently at the orbital apex. Of note, based on the proposed mechanisms of action, it is our practice to avoid prescribing steroids in the absence of orbital inflammatory signs, or orbital radiation in the absence of “active phase” orbital fibroblast proliferation. Orbital apex compartment syndrome is viewed as an anatomic/mechanical problem that requires an anatomic/mechanical intervention (i.e. decompression surgery that adequately addressed the apex).
The range in time from initial decompression to presentation of CON in our patients is large, likely reflecting varying etiologies. The possible causes include reactivation of thyroid orbitopathy, continued activity at the time of prior decompression surgery, or progressive or reactive enlargement of the extraocular muscles following decompression.37
Treatment for CON secondary to thyroid eye disease has included steroids, immunosuppressants, radiotherapy, and orbital fat and bony decompression.14,20,21,27 The treatment for repeat CON is less established, as the mechanisms causing the repeat CON may be unclear.
In the population of thyroid eye disease patients for whom repeat orbital decompression is needed, there are numerous surgical complexities that are present. It has been suggested that degree of proptosis is proportional to the quantitative change in orbital soft tissue and may serve as a clinical indicator of risk of optic neuropathy as well as a guide for degree of decompression performed.38–40 However, other studies have shown that patients with compressive optic neuropathy may not present with severe proptosis or signs of orbital inflammation and therefore these markers may not be reliable clinical indicators for risk of optic neuropathy.7,41 Our findings are consistent with these studies, highlighting the fact that CAS was developed to score disease severity in the active inflammatory phase, and was never intended for use during the chronic, fibrotic phase. Hence, despite its name, it is actually not a true measure of disease activity. In addition, patients who presented with proptosis, the degree of postoperative change measured by exophthalmometry was highly variable. Some patients experienced significant reduction in proptosis, while others experienced no change. Regardless of amount of change in proptosis, all patients had improvement in symptoms of optic neuropathy. A likely explanation for the variable change in exophthalmometry for these patients is the varying degrees of extraocular muscle fibrosis, thus limiting the amount of retro-displacement of the globe despite adequate posterior decompression. This result demonstrates the limitation of exophthalmometry in assessing orbital volume, orbital apex syndrome and success of decompression surgery.
Other challenges encountered with repeat decompression include obliteration of the typical landmarks utilized during the initial decompression and increased difficulty with hemostasis.
With compressive optic neuropathy, the goal of decompression should be to always achieve a more extensive posterior decompression rather than anterior because the most important clinical outcome is prevention and/or resolution of optic nerve compression. If not initially or adequately performed, secondary decompression of the posterior orbit will present with many technical difficulties given the presence of scar and obliteration of anatomical landmarks. With congestive disease and CON, it is critical to maximize volume at the apex by removing the tip of the palatine bone and posterior strut. With medial decompression, one should start with an infero-medial approach, posterior to the anterior ethmoidal neurovascular bundle, as this offers better visualization and a safer approach. From here, one may proceed with dissection superiorly. In addition, when removing the lamina papyrecea, leaving the anterior portion intact and focusing on removal of the posterior bone may still offer adequate decompression while reducing the risk of post-operative complex strabismus.
Our small series demonstrates that regardless of the cause of the relapsed or persistent CON, length of time of CON, time from onset of Graves’ ophthalmopathy or interval from initial to repeat decompression, repeat decompression performed by 3 different surgeons in our institution was successful in resolution of CON.
While it is entirely possible that our patients’ compressive optic neuropathy was a result of progressive enlarging of extra-ocular muscles following initial decompression, CT scans prior to and immediately following the initial decompression were not available for these patients. Thus, we are unable to evaluate the immediate postoperative change in muscle size. In addition, we cannot accurately assess how much the apical crowding improved following the initial decompression. Interestingly, 4/6 patients presented with symptoms of CON at an onset of greater than 7 years following initial decompression, demonstrating the continued smoldering progression of the disease, many years after the typical range of 1–3 years of “active” disease.
Additionally, although inflammatory peri-ocular soft tissue symptoms and signs have been utilized through the CAS to identify active disease, this may not be an appropriate measure for monitoring disease activity or risk of developing significant complications. Despite having CON, only half the patients in our study had a CAS score of 4 or above. This demonstrates that CON may still occur in the setting of an externally quiescent orbit, and therefore a high index of suspicion should be maintained in Graves’ patients with changes in visual status. This study, and others, highlights the limitation of the CAS in the evaluation of patients with TED.
Our study is limited by several factors, particularly its retrospective nature. The study has a small cohort; also since the patients had different surgeons, there was not a fixed follow up period for which measurements could be obtained. Ideally, prospective studies would be conducted to investigate the specific causes and management of recurrent optic neuropathy in thyroid-related orbitopathy and to propose clear guidelines in the management of initial optic neuropathy to prevent relapse.
Acknowledgments
Financial Support: This work was supported in part by the Vision Research Core (P30 EY007003) and an unrestricted grant from Vision to Prevent Blindness. AK is supported by the Helmut F. Stern Career Development Professorship in Ophthalmology and Visual Sciences, the A. Alfred Taubman Medical Research Institute Emerging Scholars program, and the Physician Scientist Award from Research to Prevent Blindness. VME is supported by the Ravitz Foundation Professorship in Ophthalmology and Visual Sciences. CCN is supported by the Bartley R. Frueh, M.D. and Frueh Family Collegiate Professorship in Ophthalmology and Visual Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
This article was presented at the American Society of Ophthalmic Plastic and Reconstructive Surgery Fall Scientific Symposium, in New Orleans, LA, November 2013
The authors have no conflicts of interest to disclose
REFERENCES
- 1.Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves' disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5:177–194. [PubMed] [Google Scholar]
- 2.Bhatti MT, Dutton JJ. Thyroid eye disease: therapy in the active phase. J Neuroophthalmol. 2014;34:186–197. doi: 10.1097/WNO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21:168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS. Graves' Ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye (Lond) 2013;27:308–319. doi: 10.1038/eye.2012.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett L, Glatt HJ, Burde RM, Gado MH. Optic nerve dysfunction in thyroid eye disease: CT. Radiology. 1988;167:503–507. doi: 10.1148/radiology.167.2.3357962. [DOI] [PubMed] [Google Scholar]
- 7.Feldon SE, Muramatsu S, Weiner JM. Clinical classification of Graves' ophthalmopathy. Identification of risk factors for optic neuropathy. Arch Ophthalmol. 1984;102:1469–1472. doi: 10.1001/archopht.1984.01040031189015. [DOI] [PubMed] [Google Scholar]
- 8.Feldon SE, Lee CP, Muramatsu SK, Weiner JM. Quantitative computed tomography of Graves' ophthalmopathy. Extraocular muscle and orbital fat in development of optic neuropathy. Arch Ophthalmol. 1985;103:213–215. doi: 10.1001/archopht.1985.01050020065021. [DOI] [PubMed] [Google Scholar]
- 9.Kennerdell JS, Rosenbaum AE, El-Hoshy MH. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol. 1981;99:807–809. doi: 10.1001/archopht.1981.03930010807002. [DOI] [PubMed] [Google Scholar]
- 10.Nugent RA, Belkin RI, Neigel JM, et al. Graves orbitopathy: correlation of CT and clinical findings. Radiology. 1990;177:675–682. doi: 10.1148/radiology.177.3.2243967. [DOI] [PubMed] [Google Scholar]
- 11.Wiersinga WM, Smit T, Schuster-Uittenhoeve AL, et al. Therapeutic outcome of prednisone medication and of orbital irradiation in patients with Graves' ophthalmopathy. Ophthalmologica. 1988;197:75–84. doi: 10.1159/000309924. [DOI] [PubMed] [Google Scholar]
- 12.Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol. 1978;96:1199–1209. doi: 10.1001/archopht.1978.03910060033007. [DOI] [PubMed] [Google Scholar]
- 13.Wiersinga WM, Prummel MF. Graves' ophthalmopathy: a rational approach to treatment. Trends Endocrinol Metab. 2002;13:280–287. doi: 10.1016/s1043-2760(02)00622-7. [DOI] [PubMed] [Google Scholar]
- 14.Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol. 2000;84:600–605. doi: 10.1136/bjo.84.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao SL, Chang TC, Lin LL-K. Transcaruncular orbital decompression: an alternate procedure for Graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol. 2006;141:810–818. doi: 10.1016/j.ajo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.McCann JD, Goldberg RA, Anderson RL, et al. Medial wall decompression for optic neuropathy but lateral wall decompression with fat removal for non vision-threatening indications. Am J Ophthalmol. 2006;141:916–917. doi: 10.1016/j.ajo.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 17.Choe CH, Cho RI, Elner VM. Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthal Plast Reconstr Surg. 2011;27:4–11. doi: 10.1097/IOP.0b013e3181df6a87. [DOI] [PubMed] [Google Scholar]
- 18.Oh S-R, Tung JD, Priel A, et al. Reduction of Orbital Inflammation following Decompression for Thyroid-Related Orbitopathy. Biomed Res Int. 2013;2013:794984. doi: 10.1155/2013/794984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartalena L, Marcocci C, Bogazzi F, et al. Orbital decompression for severe Graves' ophthalmopathy. Results of a three-wall operative technique. J Neurosurg Sci. 1989;33:323–327. [PubMed] [Google Scholar]
- 20.Girod DA, Orcutt JC, Cummings CW. Orbital decompression for preservation of vision in Graves' ophthalmopathy. Arch Otolaryngol Head Neck Surg. 1993;119:229–233. doi: 10.1001/archotol.1993.01880140119019. [DOI] [PubMed] [Google Scholar]
- 21.Chang EL, Bernardino CR, Rubin PA. Transcaruncular orbital decompression for management of compressive optic neuropathy in thyroid- related orbitopathy. Plast Reconstr Surg. 2003;112:739–747. doi: 10.1097/01.PRS.0000069708.70121.67. [DOI] [PubMed] [Google Scholar]
- 22.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 23.Kouloulias V, Kouvaris J, Zygogianni A, et al. Efficacy and toxicity of radiotherapy for Graves' ophthalmopathy: the University of Athens experience. Head Neck Oncol. 2013;5:12. [Google Scholar]
- 24.Savino G, Balia L, Colucci D, et al. Intraorbital injection of rituximab: a new approach for active thyroid-associated orbitopathy, a prospective case series. Minerva Endocrinol. 2013;38:173–179. [PubMed] [Google Scholar]
- 25.Wakelkamp IM, Baldeschi L, Saeed P, et al. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves' ophthalmopathy? A randomized controlled trial. Clin Endocrinol (Oxf) 2005;63:323–328. doi: 10.1111/j.1365-2265.2005.02345.x. [DOI] [PubMed] [Google Scholar]
- 26.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon C, Shin JH, Woo KI, Kim Y-D. Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol. 2012;26:73–79. doi: 10.3341/kjo.2012.26.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcocci C, Bartalena L, Tanda ML, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–3567. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 29.Day RM, Carroll FD. Corticosteroids in the treatment of optic nerve involvement associated with thyroid dysfunction. Arch Ophthalmol. 1968;79:279–282. doi: 10.1001/archopht.1968.03850040281009. [DOI] [PubMed] [Google Scholar]
- 30.Wenz R, Levine MR, Putterman A, et al. Extraocular muscle enlargement after orbital decompression for Graves' ophthalmopathy. Ophthal Plast Reconstr Surg. 1994;10:34–41. doi: 10.1097/00002341-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ben Simon GJ, Syed HM, Syed H, et al. Clinical manifestations and treatment outcome of optic neuropathy in thyroid-related orbitopathy. Ophthalmic Surg Lasers Imaging. 2006;37:284–290. doi: 10.3928/15428877-20060701-04. [DOI] [PubMed] [Google Scholar]
- 32.Bunting H, Creten O, Muhtaseb M, Shuttleworth G. Late reactivation of thyroid associated ophthalmopathy causing optic neuropathy. Postgrad Med J. 2008;84:388–390. doi: 10.1136/pgmj.2007.065342. [DOI] [PubMed] [Google Scholar]
- 33.Clauser LC, Tieghi R, Galie M, et al. Surgical decompression in endocrine orbitopathy. Visual evoked potential evaluation and effect on the optic nerve. J Craniomaxillofac Surg. 2012;40:621–625. doi: 10.1016/j.jcms.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Cascone P, Rinna C, Reale G, et al. Compression and stretching in Graves orbitopathy: emergency orbital decompression techniques. J Craniofac Surg. 2012;23:1430–1433. doi: 10.1097/SCS.0b013e31825e3acf. [DOI] [PubMed] [Google Scholar]
- 35.Chou PI, Feldon SE. Late onset dysthyroid optic neuropathy. Thyroid. 1994;4:213–216. doi: 10.1089/thy.1994.4.213. [DOI] [PubMed] [Google Scholar]
- 36.Baldeschi L, Lupetti A, Vu P, et al. Reactivation of Graves' orbitopathy after rehabilitative orbital decompression. Ophthalmology. 2007;114:1395–1402. doi: 10.1016/j.ophtha.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Hu WD, Annunziata CC, Chokthaweesak W, et al. Radiographic analysis of extraocular muscle volumetric changes in thyroid-related orbitopathy following orbital decompression. Ophthal Plast Reconstr Surg. 2010;26:1–6. doi: 10.1097/IOP.0b013e3181b80fae. [DOI] [PubMed] [Google Scholar]
- 38.Gorman CA. The measurement of change in Graves' ophthalmopathy. Thyroid. 1998;8:539–543. doi: 10.1089/thy.1998.8.539. [DOI] [PubMed] [Google Scholar]
- 39.Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–1521. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 40.Kikkawa DO, Pornpanich K, Cruz RC, Levi L, Granet DB. Graded orbital decompression based on severity of proptosis. Ophthalmology. 2002;109:1219–1224. doi: 10.1016/s0161-6420(02)01068-0. [DOI] [PubMed] [Google Scholar]
- 41.McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves' Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–458. doi: 10.1136/bjo.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]


