What is Stentor coeruleus?
Stentor coeruleus is an astoundingly large (~1 mm long) single celled pond organism with a distinct trumpet shape and a well-defined morphology (Figure 1). The name stentor is a reference to its trumpet shape and the herald in Greek mythology known for having a loud voice, while coeruleus describes the blue-green pigment specific to the species. Abraham Trembley, who thought it was a type of hydra, first identified Stentor in 1744; but actually Stentor is a member of the Ciliate phylum in the class Heterotrichae.
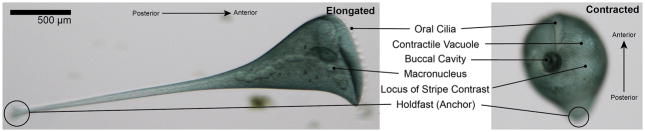
Figure 1. Anatomy of a Stentor coeruleus cell.
Brightfield images of both an extended cell (left) and a contracted cell (right) highlighting a few key features. The oral cilia are at the anterior and constantly beat to create a vortex to capture food particles in the buccal cavity. The contractile vacuole can be seen beneath the cell membrane and is also associated with scars in the membrane called contractile vacuole pores. The cell’s macronucleus is stretched along its length and packaged into distinct nodes, giving it the appearance of ‘beads on a string’. The cortical stripes are also visible; the clear stripes represent microtubule bundles known as Km fibers and the blue/green stripes contain dense fields of pigment granules. Asymmetric spacing of the rows results in a point at which the wide stripes meet the narrow stripes and is called the locus of stripe contrast. The posterior of the cell is defined by an anchoring structure known as the holdfast.
Stentor isn’t just big and blue. It has a highly complex body plan and a rich repertoire of behaviors, including the ability to learn. But Stentor is most famous for its amazing regenerative abilities. If a Stentor cell is cut in half, each half regenerates a half-sized cell with normal anatomy. Even if a single cell is cut into multiple small fragments, each fragment will generate into a normal-looking cell. The fact that a single cell could rebuild its complex anatomy while displaying many of the same developmental processes as animal embryos, including axiation and induction, grabbed the attention of the leading embryologists around 1900, including Balbiani, F. R. Lillie, and even Thomas Hunt Morgan. Following the lead of these early luminaries, Vance Tartar and Noël De Terra developed an astounding variety of microsurgical procedures designed to probe the mechanism of regeneration. Perhaps the most interesting classical discovery is that Stentor cells possess a region of their cortex known as the locus of stripe contrast, where widely spaced cortical rows are adjacent to narrowly spaced rows, which can induce the formation of additional body axes similar to an organizer region in metazoan development. But despite the challenges that these surgical observations raise for basic cell biology, Stentor was never developed as a molecular model system until now, and the mechanism of pattern formation and regeneration in Stentor remains unknown.
How can fragments of a single celled organism regenerate into whole organisms?
One of the most fascinating unanswered questions about Stentor is how the cell is able to heal and regenerate after injury. Why doesn’t the cytoplasm leak out after the cell is cut open? How are multiple cells able to regenerate from different fragments of an original cell? How does a cell know which structures are missing and then specifically and properly regenerate them? There must be molecular pathways that determine which structures are present, and which are missing and thus need to be replaced, but how this works is a complete mystery that challenges our fundamental conception of what a cell can and can’t do.
What are the limitations to regeneration?
Almost any piece of Stentor can regenerate as long as it contains part of the macronucleus and a small portion of the original cell membrane/cortex. The macronucleus in Stentor is highly polyploid and extends along the length of the whole cell. Due to the high ploidy, even a fraction of the macronucleus will contain many copies of the entire genome, which is one of the reasons this cell can regenerate after being cut into small pieces. As for the quantity of cytoplasm needed to support regeneration, Lillie and Weisz both found that surgically produced cell fragments must be at least 70–80 μm in diameter in order to regenerate given the presence of a macronuclear node. Also, grafting multiple cells together is possible and in some cases the two cells can maintain a fused state and divide as a stable doublet cell with two mouths and a single tail in the proper orientation. These experiments highlight the phenomenon of cortical inheritance often seen in ciliates, and has been well studied by Joseph Frankel in Tetrahymena thermophila.
Why isn’t Stentor more widely studied?
Although Stentor coeruleus was quite well studied through the mid-1900s, the inability to grow cells at high densities and the inability to perform genetic crosses due to low mating frequencies likely persuaded scientists to turn to better biochemical and genetic models for study. In addition, the vast majority of the microsurgical work was performed by just two scientists, Vance Tartar and Noël De Terra, both of whom unfortunately passed away before the development of many technologies that have made Stentor a more tractable system, and much of their expertise was lost with them.
What can we learn from studying Stentor?
Stentor coeruleus is a great model for studying complex morphogenesis at the level of a single cell without needing to worry about external influences from neighboring cells that are present in metazoan development. Whether or not any of the mechanisms that regulate Stentor morphogenesis are conserved in metazoans remains an open question but further research might shed light on how complex single-cells are organized. Stentor may also be useful for studying wound healing within cells, as it has the ability to maintain its integrity even after severe surgical manipulations. As a final example, Stentor could be useful as a model for memory at the level of a single cell. Work from David Wood has shown that Stentor possesses the ability to habituate to mechanical stimuli and can remain habituated over the course of hours, although no molecular mechanism for this phenomenon has been determined.
What tools are available for studying Stentor?
Stentor coeruleus is easily imaged on even the most basic microscopes at low magnification, where the majority of the cell structures can be resolved. As discussed previously, Stentor is amenable to microsurgical manipulation, including surgical removal of specific regions of the cell and even grafting of cell fragments onto other cells. Recently, RNA interference methodology has been adapted and shown to be an effective method for probing gene function in Stentor. This can be achieved by feeding the Stentor with bacteria expressing long double-stranded RNA corresponding to a gene of interest for 3–5 days.
What about classical genetics?
Ciliate genetics has been developed to a high level in Tetrahymena and Paramecium, but has never been developed as a tool for Stentor, owing to the low frequency of mating under standard laboratory conditions. There are a handful of studies that describe mating is Stentor coeruleus, and show that isolates from different locations have the ability to form mating pairs that look similar to mating pairs from other ciliates. Previous studies on Stentor, and work from other ciliates, suggest that stressful conditions such as starvation or temperature shifts can induce conjugation but these protocols do not seem to be successful in our lab strain. We have seen cells with altered morphologies consistent with descriptions of pre-conjugation but mating pairs have never been obtained, possibly because standard laboratory cultures only contain cells of a single mating type. Interestingly, mating pairs have been seen in two different species of Stentor obtained from the wild after being isolated for 24–48 hours without additional food, so there is potential for conjugation in Stentor coeruleus by isolating cells from natural sources. Development of classical genetics in Stentor could be a very powerful tool for furthering Stentor coeruleus as a useful model organism.
Is there a Stentor coeruleus genome project?
The macronuclear genome of Stentor coeruleus has been sequenced and assembled, and is currently being annotated in our lab. Once completed, the genome will be publicly accessible on the stentor.ciliate.org server.
Where can I find out more?
- Lillie FR. On the smallest parts of Stentor capable of regeneration. J Morphol. 1896;12:239–249. [Google Scholar]
- Morgan TH. Regeneration of proportionate structures in Stentor. The Biological Bulletin 1901 [Google Scholar]
- Tartar V. International Series of Monographs on Pure and Applied Biology. Vol. 5. Pergamon Press; 1961. The Biology of Stentor; pp. 1–434. [Google Scholar]
- De Terra N. Cytoskeletal discontinuities in the cell body cortex initiate basal body assembly and oral development in the ciliate Stentor. J Embryol Exp Morph. 1985;87:249–257. [PubMed] [Google Scholar]
- Frankel J. What do genic mutations tell us about the structural patterning of complex single-celled organism? Euk Cell. 2008;7(10):1617–1639. doi: 10.1128/EC.00161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genome Database Wiki. stentor.ciliate.org.
- Burchill BR. Conjugation in Stentor coeruleus. J Protozool. 1967;14(4):683–687. [Google Scholar]
- Webb TL, Francis D. Mating types in Stentor coeruleus. J Protozool. 1969;16(4):758–763. doi: 10.1111/j.1550-7408.1969.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Slabodnick, et al. Visualizing cytoplasmic flow during single-cell wound healing in Stentor coeruleus. JoVE. 2013;82:e50848. doi: 10.3791/50848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabodnick, et al. The kinase regulator Mob1 acts as a patterning protein during Stentor morphogenesis. PLoS Biology. 2014;12(5):e1001861. doi: 10.1371/journal.pbio.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DC. Parametric studies of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. J Neurobiology. 1969;1(3):345–360. doi: 10.1002/neu.480010309. [DOI] [PubMed] [Google Scholar]
- Frankel, Whitely Vance Tartar: A Unique Biologist. J Euk Microbiol. 1993;40(1):1–9. doi: 10.1111/j.1550-7408.1993.tb04873.x. [DOI] [PubMed] [Google Scholar]