Abstract
Fullerene nanowhiskers (FNWs) are thin crystalline fibers composed of fullerene molecules, including C60, C70, endohedral, or functionalized fullerenes. FNWs display n-type semiconducting behavior and are used in a diverse range of applications, including field-effect transistors, solar cells, chemical sensors, and photocatalysts. Alkali metal-doped C60 (fullerene) nanowhiskers (C60NWs) exhibit superconducting behavior. Potassium-doped C60NWs have realized the highest superconducting volume fraction of the alkali metal-doped C60 crystals and display a high critical current density (Jc) under a high magnetic field of 50 kOe. The growth control of FNWs is important for their success in practical applications. This paper reviews recent FNWs research focusing on their mechanical, electrical and superconducting properties and growth mechanisms in the liquid–liquid interfacial precipitation method.
Keywords: fullerene nanowhisker, fullerene nanotube, fullerene nanosheet, fullerene nanofiber, LLIP method, superconductor
1. Introduction
Fullerene molecules consist of closed cage-type structures that are composed of carbon atoms. The best-known fullerene is C60, which was discovered by Kroto et al in 1985 [1]. The second well-known molecule is C70, which was also identified in [1]. The C60 molecule is analogous to a soccer ball with 12 pentagons and 60 vertices where carbon atoms are located, and has 30 six-membered ring/six-membered ring joints with double bonds of carbon and 60 five-membered ring/six-membered ring joints with single bond of carbon.
Polymerization of C60 molecules can occur via [2 + 2] cycloaddition reactions, which form four-membered rings between adjacent C60 molecules. This cycloaddition mechanism involves a change of carbon hybridization from sp2 to sp3 [2].
Various properties of C60 have been studied by forming thin films on suitable substrates. Bulk samples can also be prepared by sintering at high temperatures. The [2 + 2] cycloaddition polymerization of C60 molecules is known to occur in the presence of ultraviolet or visible light illumination [3, 4], high-pressure sintering [5–8], and electron beam irradiation [9, 10]. The hardness of high-pressure sintered C60 reaches 200–300 GPa [11, 12].
However, fine needle-like crystals (whiskers) comprising C60, ‘C60 (fullerene) nanowhiskers (C60NWs)’, were found in a colloidal solution of lead zirconate titanate (PZT) with C60 added [13–15].
Fullerene nanofibers are linear and thin, with diameters less than 1000 nm [16, 17]. Fullerene nanosheets are thin two-dimensional substances. In this paper, we define fullerene nanosheets to be less than 1000 nm in thickness. Fullerene nanofibers and nanosheets can include a variety of fullerene molecules and their derivatives including C60, C70, Sc3N@C80 [18], C60[C(COOC2H5)2] [19–21] and (η2-C60)Pt(PPh3)2 [22].
The aspect ratio of fullerene nanofibers is defined to be greater than three [16]. Fullerene nanofibers are described as either non-tubular or tubular [23–27]. Non-tubular crystalline fullerene nanofibers are called fullerene nanowhiskers (FNWs). FNWs with both single-crystal and polycrystalline structures have been reported [53].
Fullerene nanofibers can incorporate either one or multiple types of fullerenes. This enables formation of both monocomponent and multicomponent structures. Examples of monocomponent structures include C60NWs, C70 (fullerene) nanowhiskers (C70NWs), C60 or C70 (fullerene) nanotubes (C60NTs or C70NTs), [23, 25, 28], and FNWs composed of C60[C(COOC2H5)2] molecules (C60[C(COOC2H5)2]NWs). Examples of multicomponent fullerene nanofibers include two-component C60–C70 NWs [29], two-component C60–C70 NTs [23], two-component C60–C60[C(COOC2H5)2] NWs [19], and two-component C60–(η2-C60)Pt(PPh3)2 NWs [22]. Figure 1 shows the classification of fullerene nanofibers.
Figure 1.

Classification of fullerene nanofibers. (a) single-crystal fullerene nanowhisker, (b) polycrystal fullerene nanowhisker, (c) single-crystal fullerene nanotube, (d) polycrystal fullerene nanotube, (e) amorphous fullerene nanotube.
Fullerene nanofibers and nanosheets can be synthesized using the ‘liquid–liquid interfacial precipitation (LLIP) method’ [30], which has been widely applied [31–38]. In this review, we discuss the LLIP method to synthesize fullerene nanofibers and nanosheets and the applications in which these materials have been investigated.
The terminology ‘FNW’ represents all needle-like crystals comprising fullerene molecules with diameters less than 1000 nm. The words ‘nanorod’ and ‘nanowire’ are replaced with ‘nanowhisker’ to avoid confusion as was described in review paper [16].
2. Synthesis of FNWs
2.1. LLIP method
The LLIP method is commonly used to synthesize fullerene nanofibers and nanosheets [30]. This method relies on diffusion of a poor solvent of fullerenes such as isopropyl alcohol (IPA) into a fullerene-saturated toluene solution. An aliquot of a C60-saturated toluene solution is added to a glass bottle. Following this, an appropriate amount of IPA is added gently to the solution to form a liquid–liquid interface [8]. The resulting mixture is kept at ambient temperatures, typically below 25 °C. During the slow mixing of toluene and IPA, the liquid–liquid interface becomes supersaturated in C60 and allows nucleation of C60NWs to occur. This supersaturated state is maintained as IPA diffuses into toluene and assists in the growth of C60NWs. This procedure is named ‘static LLIP method’ [30, 39]. The glass bottle is kept still in an incubator, where the C60NWs self-assemble into a shape similar to a cotton ball. The LLIP method can also be used in combination with ultrasonic mixing, manual mixing, or injection [24, 39, 40]. Ultrasonication induces rapid mixing of good solvents and poor solvents, causing formation of fine fullerene nuclei that grow into fullerene nanofibers or nanosheets.
The static LLIP method can involve either layering a poor solvent onto a good solvent or vice versa, and can be combined with manual mixing, supersonic mixing, mixing by injection of liquid, or ultrasonic mixing of liquid droplets [41]. These methods are collectively named the ‘dynamic LLIP method’.
Cha et al and Miyazawa et al reported the diaphragm LLIP method (DLLIP method), which involves injecting a poor solvent for fullerene into a fullerene solution through a porous membrane [40, 42, 43]. As an example, if IPA is slowly injected into a C60-saturated toluene solution through an anodic aluminum oxide membrane with nanosized pores, vertically grown microtubes of C60 are produced. All methods that mix two solvents to form fullerene nanofibers and nanosheets can be classified as LLIP processes.
Using the DLLIP method, the influence of alcohol chain length (methanol, ethanol, and IPA) on the length of C60 whiskers was investigated using toluene as a good solvent for C60. Amer et al reported that the length of C60 whiskers decreased when the chain length of the alcohol (poor solvent) increased [44]. The temperatures at which the C60 whiskers were grown was not reported; however, the above result suggests that the chain length of the alcohol influences the desolvation energy of solvated C60 molecules that governs the rate-limiting process of surface reaction [45].
2.2. Growth mechanism of FNWs using the LLIP method
The Young modulus of C60NWs has been examined using a transmission electron microscope equipped with an atomic force microscope [46]. The Young modulus of C60NWs increases with decreasing diameter [46–49]. This phenomenon is thought to occur because C60NWs have a core–shell structure with a porous interior region and a dense surface region [48, 50]. Kizuka et al found that C70NWs containing solvent molecules had a higher density of lattice defects in their interior regions, which caused a reduction in the Young modulus [51]. Additionally, the Young modulus of C70NTs was found to increase with decreasing diameter [25]. These studies conclude that by decreasing the diameter of fullerene nanofibers, crystallinity is increased, which in turn leads to an increase in the Young modulus.
In the LLIP process, FNWs grow from seed crystals [52–54]. The size of the initial C60NW nuclei is influenced by the degree of supersaturation of C60 in solution, which is determined by the mixing ratio of both good and poor solvents [39]. C60NTs grow in both directions along their growth axis from the seed crystals [53, 54]. However, the seed crystals should disappear by the core dissolution mechanism to form a through-hole structure [55].
The re-growth of C60NTs was observed in ultrasonically pulverized C60NTs [53]. The ultrasonically fractured C60NTs have steep wall edges, on which C60 molecules accumulate and crystallize [53]. This preferential accumulation of C60 in areas with a small radius of curvature, such as the hexagonal vertices, is an important growth mechanism of fullerene nanotubes [53, 54].
The growth of C60NWs is influenced by numerous factors, including time, temperature, light, solvent species, the ratio between good and poor solvents, and contained impurity water [39, 56–59]. The growth mechanism of C60NWs in C60-saturated toluene and IPA has been studied closely. The activation energy of growth (52.8 kJ mol−1) was calculated by varying the temperature and measuring the length of C60NWs. This value is approximately four times greater than the value obtained for the diffusion of C60 in a mixed solution of toluene and acetonitrile (13.1 kJ mol−1, 4:1 v/v) [56, 60]. The high activation energy indicated that the growth of C60NWs is rate limited by the desolvation process of C60 molecules bonded with solvent molecules on the crystal surface.
The dynamic LLIP process involves a fullerene solution being forcibly mixed with a poor solvent for fullerene. This process generates microscopic liquid–liquid interfaces between the fullerene solution and the poor solvent of fullerene, where supersaturated solutions lead to rapid nucleation of fine fullerene crystals. The formation of granular, linear, or sheet fullerene crystal morphologies depends on the growth kinetics, which may be governed by the degree of supersaturation, solvent species, and temperature.
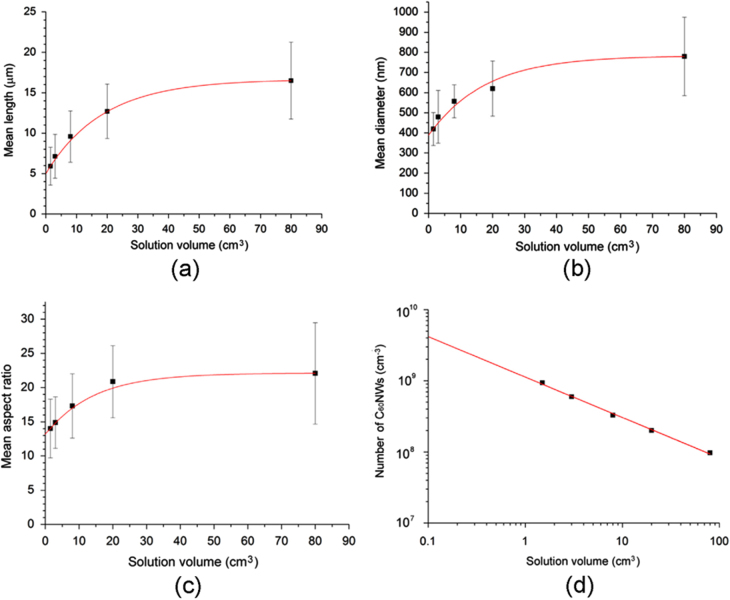
Size control of fullerene nanofibers is critical for practical applications. Wakahara et al reported that the diameter of C60NWs varied with the size of the glass bottles used in their synthesis. Linear relationships between the area of the liquid–liquid interface and the diameter of C60NWs were observed when the total volume of solution was fixed [61]. Changes in the lengths and diameters of C60NWs upon varying the solution volume have been examined [62]. These C60NWs were prepared by dynamic LLIP in a C60-saturated toluene and IPA system. After the initial formation of a liquid–liquid interface by layering an equal amount of IPA on a C60-saturated toluene solution, the solution was manually mixed by shaking 30 times. The relationships between solution volume and mean length, diameter and aspect ratio are shown in figures 2(a)–(c) [62]. The aspect ratio, as derived from the y-intercepts of figures 2(a) and (b) (5.02 μm/387 nm) yielded a value of 13.0, almost identical to the value derived from the y-intercept of figure 2(c) (13.1). Hence, it is reasonable to consider the size of C60NW nuclei can be estimated using the relationships shown in figures 2(a)–(c).
Figure 2.
(a) Relationship between solution volume and mean length of C60NWs. The equation fitted to the data is y = −11.6exp(−x/18.7) + 16.6. (b) Relationship between solution volume and mean diameter of C60NWs. The equation fitted to the data is y = −396.6exp(−x/17.6) + 783.7. (c) Relationship between solution volume and the mean aspect ratio of C60NWs. The equation fitted to the data is y = −9exp(−x/14.2) + 22.1. (d) Estimated number of C60NWs per unit volume plotted versus the solution volume. The equation fitted to the data is y = −1.12496 × 109 x−0.5674. Reprinted from [62], copyright 2014, with permission from Elsevier.
The relationship between the solution volume and number of C60NWs per unit volume is shown in figure 2(d). The number density, as calculated from the nominal content of C60 and the mean size of C60NWs in solution [62], increased as the solution volume decreased. This implies that the volume fraction of liquid–liquid interfaces increases when the solution volume is decreased. A power law relationship (y = 1.12 × 109x−0.567) was fitted to the data with an approximate index of −0.5, showing that the number density of C60NW nuclei in solution is inversely proportional to the square root of the solution volume.
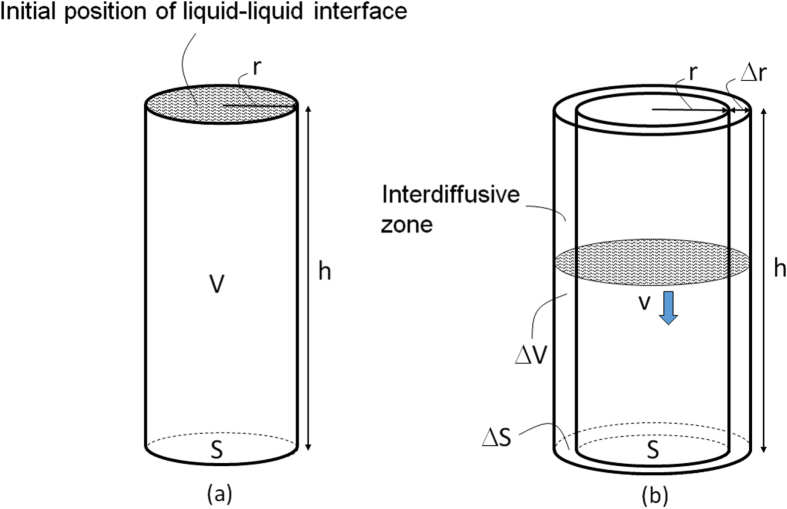
A model describing the changes in the liquid–liquid interface upon manual mixing is shown in figure 3. The initial layered interface (figure 3(a)) is assumed to form a sinusoidally modulated interface (figure 3(b)) upon the manual mixing. The amplitude of this interface increases along the height of the glass bottle, a section of this wavefront is highlighted by the blue rectangle (figure 3(c)). This highlighted section is modeled by a cylinder with height h, radius r, basal area S, and volume V (figure 4(a)). The front of the liquid–liquid interface travels vertically with a velocity v.
Figure 3.
Model showing the liquid–liquid interface (a) changing with manual mixing (b). The interface front between the C60-saturated toluene solution and IPA is assumed to move with a velocity v along the vertical direction of the glass bottle (c).
Figure 4.
Cylindrical model used to calculate the number density N of C60NW nuclei for the region of the liquid–liquid interface shown in figure 3(c).
The following equations hold.
| 1 |
| 2 |
| 3 |
The front of liquid–liquid interface is assumed to move along the height h with a time t.
| 4 |
As the liquid–liquid interface front moves, interdiffusion between C60-satulated toluene solution and IPA occurs (figure 4(b)). If the values of both t and Δr are assumed to be small, the area of the interdiffusion zone (ΔS) is approximated as follows:
| 5 |
The volume of interdiffusion ΔV is:
| 6 |
If Δr is assumed to be proportional to (Dt)1/2 with a coefficient of interdiffusion D [63], it is calculated as in equation (7) with a constant a,
| 7 |
Hence, combining (4), (5), and (7),
| 9 |
If N is defined as the number of C60NW nuclei per unit volume in the zone of interdiffusion, the mean number of C60NW nuclei contained in a unit volume of a cylinder (ρ) can be calculated by combining equations (1), (3), (6), and (9):
| 12 |
This model suggests that the mean number density of C60NW nuclei is inversely proportional to the square root of the solution volume, which was indeed confirmed experimentally (figure 2(d)).
3. Electrical and superconducting properties of C60NWs
C60NWs display n-type semiconducting behavior and are used in a diverse range of applications, including field effect transistors (FETs) [64], solar cells [65, 66], photocatalysts [67], chemical sensors [27], and photosensors [68]. However, Wakahara et al recently synthesized ambipolar FETs with C60/cobalt–porphyrin hybrid nanosheets using a LLIP method [92].
The carrier mobility of C60NWs in a FET was determined to be 2 × 10−2 cm2 V−1 s−1 under vacuum [64]. However, the as-synthesized solution-grown C60 needle-like crystals exhibited a very high mobility up to 11 cm2 V−1 s−1 [69]. As the measured carrier mobility of C60NWs, or needle-like crystals of C60, depends largely on the measurement conditions (solvent impurities, oxygen impurity, crystal structure, and lattice defects), electrical properties of the materials were investigated under controlled conditions. Only C60NWs with clearly defined chemical and structural properties were used.
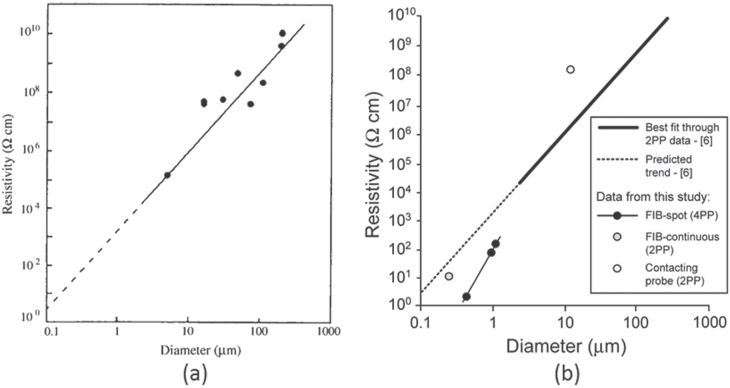
The electrical resistivity of C60 whiskers with diameters greater than 1 μm (∼10–a few hundred micrometers) was measured using a two-terminal method at ambient temperature [70]. The electrical resistivity of the C60 whiskers decreased dramatically with decreasing diameter (figure 5(a)). The resistivity of C60NWs is expected to be several Ohm centimeters (Ω cm), based on extrapolation of the curve-fitted data. Subsequently, Larsson et al measured the electrical resistivity using a four-point probe method [71]. Figure 5(b) summarizes their results including figure 5(a) [70]. The four-point probe method also showed a decrease in resistivity of C60 whiskers with decreasing diameter (FIB-spot (4PP)), figure 5(b)). A C60NW with a diameter of 650 nm showed a low resistivity of 3 Ω cm [71]. The decrease in resistivity with decreasing diameter suggested that C60NWs with smaller diameters and shorter C60 intermolecular distances are more crystalline and thus have a greater overlap of π electrons [70]. Recently, this fact was further confirmed by Barzegar et al using thinner C60NWs [93]. It was shown that the electrical mobility of as-grown C60NWs with diameters less than 300 nm increases with decreasing the diameter of C60NWs [64, 93–95].
Figure 5.
Electrical resistivity of C60 whiskers measured as a function of diameter. The resistivity measurement was performed by the two-point probe method (2PP) in (a) and by the four-point probe method (4PP) in (b). [6] in the inset of (b) is identical with [70]. FIB stands for focused ion beam. Part (a) reprinted with permission from [70], copyright © 2003 John Wiley & Sons, Ltd. Part (b) reproduced by permission of ECS—The Electrochemical Society from [71]
If the line measured using the four-point probe method is extrapolated to a diameter of 100 nm in figure 5(b), the resistivity will decrease to the order of 10−3 Ω cm. This result suggests that C60NWs may exhibit metallic conductivity when their diameters are sufficiently small. Xu et al showed that C60NWs are conductive only if the surface is not covered by oxygen [72].
To determine the electrical properties of a semiconductor, it is necessary to measure the temperature dependence of electrical conductivity. Ji et al performed these measurements using C60NWs with either a face-centered cubic (fcc) or a hexagonal closed packed (hcp) structure [73]. The fcc C60NW displayed higher electrical conductivity than did the hcp C60NW. This result confirms that the crystal structure influences the electrical properties of C60NWs. However, the effect of solvent molecules contained in the hcp C60NW is still under some debate. If C60 molecules adopt a closely packed structure, a greater overlap between π electrons would lead to higher electrical conductivity in these C60NWs [70, 73].
Carbon superconductors have been investigated for many years. The superconductivity of graphite (C8) specimens doped with alkali metals, including K (superconducting transition temperature (Tc) < 0.55 K [74], 0.128–0.198 K [75]), Cs (Tc = 0.020–0.135 K [74]), and Rb (Tc = 0.023–0.151 K [74]) has been reported. Graphite superconductors such as C6Ca (Tc = 11.5 K) and C6Yb (Tc = 6.5 K) were also synthesized [76]. C60NWs can be transformed into glassy carbon nanofibers by heat treatment [77–79]. When heated to 3000 °C, C60NWs transform into carbon nanofibers with up to 17 graphene layers [77]. The number of stacked graphene layers increases with increasing temperature between 2000 and 3000 °C [77]. Those C60NWs heated at high temperatures with developed graphitic ribbons are promising materials that may exhibit superconductivity if doped with alkali metals and alkaline-earth metals. In addition, the high-temperature-treated C60NWs become electron emission tips showing striped patterns that reflect the atomic structure of the crumpled graphitic layers [78, 79]. However, since long amorphous carbon nanofibers prepared by high-temperature heat treatment of C60NWs showed cytotoxicity like long multiwall carbon nanotubes [96], special care will be necessary in the practical uses of the glassy carbon nanofibers.
In 2004, boron-doped diamond was observed to exhibit superconductivity (Tc ≈ 4 K) [80]. Takano et al found that the Tc of a boron-doped diamond film was 7.4 K [81].
Hebard et al discovered that C60 doped with potassium (K) exhibited superconductivity [82]. A superconducting transition temperature (Tc) of 18 K was observed for both K-doped C60 films and bulk samples. Tanigaki et al reported the highest Tc value of 33 K in Cs2Rb1C60 powder [83].
Of the three known phases of K-doped C60 (fcc (K3C60), body-centered tetragonal (bct) (K4C60), and body-centered cubic (bcc) (K6C60)), only the fcc phase exhibits superconductivity [84]. Although C60NWs that are grown in solution display a solvated hexagonal structure, they transform into an fcc structure upon drying and removal of the internal solvent molecules [85]. Hence, these fcc C60NWs should be superconducting if doped with alkali metals [15]. C60 nanotubes were doped with Li, Na, and K, and the crystal structures were examined using Raman spectroscopy [86]. Superconductive C60NWs were also successfully fabricated by doping with K [87, 88]. Although the Tc value (17 K) of the K-doped C60NWs with a nominal composition of K3.3C60 was lower than the reported value of 18 K [82], the superconducting, shielding volume fraction was as high as 80%, and the critical current density Jc was more than 3 × 105 A cm−2 under 50 kOe [87, 88], although the doping was performed at 200 °C for 24 h. The shielding volume fraction of the K-doped C60 crystal powder was less than 1% when doped using the same process (figure 6). The high shielding volume fraction in the K-doped C60NWs may allow for light, flexible, and recyclable superconducting carbon cables. Initially, the superconducting shielding volume fraction of K-doped C60 crystals was at most 35%, even after prolonged heat treatment (20 days) at temperatures up to 250 °C [89].
Figure 6.

Shielding volume fractions in K-doped C60NWs and K-doped C60 crystal powder (reprinted from [87]).
Efforts to increase the Tc value of alkali-doped C60NWs are continuing. Values up to 26 K have been achieved by doping with Rb [90]. The volume fraction of Rb-doped C60NWs was approximately five times greater than that of Rb-doped C60 powder. As Rb is an abundant alkali metal like the other common metals such as copper, lead, or zinc [91], lightweight Rb-doped C60NWs are expected to find use in a variety of superconducting applications, including motor cars, cables for power delivery, and wind generators.
4. Summary
A variety of fullerene nanofibers and nanosheets have been synthesized using LLIP methods. These materials have found use in a wide range of applications, including solar cells, chemical sensors, photo sensors, photocatalysts, and ambipolar field-effect transistors. The synthesis of C60NWs using a dynamic LLIP method with a C60-saturated toluene solution and IPA suggests that nucleation is governed by the volume of the liquid–liquid interface produced by interdiffusion between the two solvents.
Alkali-metal-doped C60NWs are the first carbon fibers to display superconductivity while being lightweight and flexible. K- or Rb-doped C60NWs are promising superconductors with Tc values that are higher than those of any other practically used metal superconductors. Additionally, they are composed of non-toxic, abundant, and recyclable elements. Fullerene nanomaterials show great promise for a variety of applications in electrical and optical fields.
Acknowledgments
This research was supported by the Health and Labour Sciences Research Grants (H24-Chemistry-Shitei-009) from the Ministry of Health, Labour and Welfare of Japan, the JST Strategic Japanese-EU Cooperative Program ‘Study on managing the potential health and environmental risks of engineered nanomaterials’, the Center of Materials Research for Low Carbon Emission of the National Institute for Materials Science, and the Japan Society for the Promotion of Science KAKENHI Grant No. 26600007.
References
- Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E. 1985. C60: buckminsterfullerene Nature 318 162–163 162–3 10.1038/318162a0 [DOI] [Google Scholar]
- Okada S, Saito S, Oshiyama A. New metallic crystalline carbon: three dimensionally polymerized C60 fullerite. Phys. Rev. Lett. 1999;83:1986. doi: 10.1103/PhysRevLett.83.1986. [DOI] [PubMed] [Google Scholar]
- Rao A M. 1993. Photoinduced polymerization of solid C60 film Science 259 955–957 955–7 10.1126/science.259.5097.955 [DOI] [Google Scholar]
- Kato R, Miyazawa K. Raman laser polymerization of C60 nanowhiskers. J. Nanotechnol. 2012;2012:013502. doi: 10.1155/2012/101243. [DOI] [Google Scholar]
- Iwasa Y. New phases of C60 synthesized at high pressure. Science. 1994;264:1570. doi: 10.1126/science.264.5165.1570. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Satsuki H, Kuwabara M, Akaishi M. 2001. Microstructural analysis of high-pressure compressed C60 J. Mater. Res. 16 1960–1966 1960–6 10.1557/JMR.2001.0268 [DOI] [Google Scholar]
- Minato J, Miyazawa K, Suga T, Kanda H, Akaishi M, Yamaura K, Muromachi E, Kakisawa H. 2005. Characterization of high-pressure sintered C60 nanowhiskers and C60 powder J. Mater. Res. 20 742–746 742–6 10.1557/JMR.2005.0095 [DOI] [Google Scholar]
- Miyazawa K, Akaishi M, Kuwasaki Y, Suga T. 2003. Characterizing high-pressure compressed C60 whiskers and C60 powder J. Mater. Res. 18 166–172 166–72 10.1557/JMR.2003.0023 [DOI] [Google Scholar]
- Nakaya M, Nakayama T, Aono M. 2004. Fabrication and electron-beam-induced polymerization of C60 nanoribbon Thin Solid Films 464–5 327–330 327–30 10.1016/j.tsf.2004.06.064 [DOI] [Google Scholar]
- Miyazawa K, Minato J, Fujino M, Suga T. 2006. Structural investigation of heat-treated fullerene nanotubes and nanowhiskers Diam. Relat. Mater. 15 1143–1146 1143–6 10.1016/j.diamond.2005.10.027 [DOI] [Google Scholar]
- Popov M, Mordkovich V, Perfilov S, Kirichenko A, Kulnitskiy B, Perezhogin I, Blank V. 2014. Synthesis of ultrahard fullerite with a catalytic 3D polymerization reaction of C60 Carbon 76 250–256 250–6 10.1016/j.carbon.2014.04.075 [DOI] [Google Scholar]
- Blank V, Popov M, Pivovarov G, Lvova N, Gogolinsky K, Reshetov V. 1998. Ultrahard and superhard phases of fullerite C60: comparison with diamond on hardness and wear Diam. Relat. Mater. 7 427–431 427–31 10.1016/S0925-9635(97)00232-X [DOI] [Google Scholar]
- Miyazawa K, Obayashi A, Kuwabara M. 2001. C60 nanowhiskers in a mixture of lead zirconate titanate sol–C60 toluene solution J. Am. Ceram. Soc. 84 3037–3039 3037–9 10.1111/j.1151-2916.2001.tb01133.x [DOI] [Google Scholar]
- Rauwerdink K, Liu J, Kintigh J, Miller G P. 2007. Thermal, sonochemical, and mechanical behaviors of single crystal [60] fullerene nanotubes Microsc. Res. Tech. 70 513–521 513–21 10.1002/jemt.20480 [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Kuwabara M. Fine carbon wires and methods for producing the same. 6890505B2 US Patent. 2005
- Miyazawa K. 2009. Synthesis and properties of fullerene nanowhiskers and fullerene nanotubes J. Nanosci. Nanotechnol. 9 41–50 41–50 10.1166/jnn.2009.J013 [DOI] [PubMed] [Google Scholar]
- Miyazawa K, editor. Fullerene Nanowhiskers. Singapore: Pan Stanford Publishing Pte. Ltd; 2011. [Google Scholar]
- Wakahara T, Nemoto Y, Xu M, Miyazawa K, Fujita D. 2010. Preparation of endohedral metallofullerene nanowhiskers and nanosheets Carbon 48 3359–3363 3359–63 10.1016/j.carbon.2010.05.026 [DOI] [Google Scholar]
- Miyazawa K, Minato J, Mashino T, Nakamura S, Fujino M, Suga T. 2006. Structural characterization of room-temperature synthesized fullerene nanowhiskers Nukleonika 51 (Suppl. 1) S41–S48 S41–8 [Google Scholar]
- Miyazawa K, Mashino T, Suga T. 2004. Liquid phase synthesis of the nanowhiskers of fullerene derivatives Trans. Mater. Res. Soc. Japan 29 537–540 537–40 [Google Scholar]
- Miyazawa K, Mashino T, Suga T. 2003. Structural characterization of the C60[C(COOC2H5)2] whiskers prepared by the liquid–liquid interfacial precipitation method J. Mater. Res. 18 2730–2735 2730–5 10.1557/JMR.2003.0380 [DOI] [Google Scholar]
- Miyazawa K, Suga T. 2004. Transmission electron microscopy investigation of fullerene nanowhiskers and needle-like precipitates formed by using C60 and (η2-C60)Pt(PPh3)2 J. Mater. Res. 19 2410–2414 2410–4 10.1557/JMR.2004.0304 [DOI] [Google Scholar]
- Miyazawa K, Minato J, Yoshii T, Fujino M, Suga T. 2005. Structural characterization of the fullerene nanotubes prepared by the liquid–liquid interfacial precipitation method J. Mater. Res. 20 688–695 688–95 10.1557/JMR.2005.0091 [DOI] [Google Scholar]
- Miyazawa K, Ringor C. 2008. Platinum chloride deposition into C60 nanotubes Mater. Lett. 62 410–413 410–3 10.1016/j.matlet.2007.05.069 [DOI] [Google Scholar]
- Kizuka T, Miyazawa K, Tokumine T. Young’s modulus of single-crystal fullerene C70 nanotubes. J. Nanotechnol. 2012;2012:013502. doi: 10.1155/2012/969357. [DOI] [PubMed] [Google Scholar]
- Liu H. 2002. Imaging as-grown [60] fullerene nanotubes by template technique J. Am. Chem. Soc. 124 13370–13371 13370–1 10.1021/ja0280527 [DOI] [PubMed] [Google Scholar]
- Zhang X, Qu Y, Piao G, Zhao J, Jiao K. 2010. Reduced working electrode based on fullerene C60 nanotubes@DNA: characterization and application Mater. Sci. Eng. B 175 159–163 159–63 10.1016/j.mseb.2010.07.020 [DOI] [Google Scholar]
- Kizuka T, Miyazawa K, Tokumine T. 2012. Synthesis of oriented bundle fibers of fullerene C70 crystal nanotubes J. Nanosci. Nanotechnol. 12 2825–2828 2825–8 10.1166/jnn.2012.5803 [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Fujino M, Minato J, Yoshii T, Kizuka T, Suga T. Structure and properties of fullerene nanowhiskers prepared by the liquid–liquid interfacial precipitation method. Proc. SPIE; 2004. p. 224. [DOI] [Google Scholar]
- Miyazawa K, Kuwasaki Y, Obayashi A, Kuwabara M. 2002. C60 nanowhiskers formed by the liquid–liquid interfacial precipitation method J. Mater. Res. 17 83–88 83–8 10.1557/JMR.2002.0014 [DOI] [Google Scholar]
- Osonoe K, Kano R, Miyazawa K, Tachibana M. 2014. Synthesis of C70 two-dimensional nanosheets by liquid–liquid interfacial precipitation method J. Cryst. Growth 401 458–461 458- 461 10.1016/j.jcrysgro.2013.12.056 [DOI] [Google Scholar]
- Wakahara T, Sathish M, Miyazawa K, Hu C, Tateyama Y, Nemoto Y, Sasaki T, Ito O. 2009. Preparation and optical properties of fullerene/ferrocene hybrid hexagonal nanosheets and large-scale production of fullerene hexagonal nanosheets J. Am. Chem. Soc. 131 9940–9944 9940–4 10.1021/ja901032b [DOI] [PubMed] [Google Scholar]
- Shrestha L K, Sathish M, Hill J P, Miyazawa K, Tsuruoka T, Sanchez-Ballester N M, Honma I, Ji Q, Ariga K. 2013. Alcohol-induced decomposition of Olmstead’s crystalline Ag(I)-fullerene heteronanostructure yields‘bucky cubes’ J. Mater. Chem. C 1 1174–1181 1174–81 10.1039/c2tc00449f [DOI] [Google Scholar]
- Shrestha L K, Hill J P, Tsuruoka T, Miyazawa K, Ariga K. 2013. Surfactant-assisted assembly of fullerene (C60) nanorods and nanotubes formed at a liquid–liquid interface Langmuir 29 7195–7202 7195–202 10.1021/la304549v [DOI] [PubMed] [Google Scholar]
- Shrestha L K, Ji Q, Mori T, Miyazawa K, Yamauchi Y, Hill J P, Ariga K. 2013. Fullerene nanoarchitectonics: from zero to higher dimensions Chem. Asian J. 8 1662–1679 1662–79 10.1002/asia.201300247 [DOI] [PubMed] [Google Scholar]
- Shrestha L K, Yamauchi Y, Hill J P, Miyazawa K, Ariga K. 2013. Fullerene crystals with bimodal pore architectures consisting of macropores and mesopores J. Am. Chem. Soc. 135 586–589 586–9 10.1021/ja3108752 [DOI] [PubMed] [Google Scholar]
- Shrestha L K, Hill J P, Miyazawa K, Ariga K. 2012. Mixing antisolvents induced modulation in the morphology of crystalline C60 J. Nanosci. Nanotechnol. 12 6380–6384 6380–4 10.1166/jnn.2012.6220 [DOI] [PubMed] [Google Scholar]
- Sathish M, Miyazawa K. 2007. Size-tunable hexagonal fullerene (C60) nanosheets at liquid–liquid interface J. Am. Chem. Soc. 129 13816–13817 13816–7 10.1021/ja076251q [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Hotta K. 2010. The effect of solvent ratio and water on the growth of C60 nanowhiskers J. Cryst. Growth 312 2764–2770 2764–70 10.1016/j.jcrysgro.2010.06.020 [DOI] [Google Scholar]
- Cha S I, Miyazawa K, Kim J-D. 2008. Vertically well-aligned C60 micro-tube crystal array prepared using solution-based one step process Chem. Mater. 20 1667–1669 1667–9 10.1021/cm702986f [DOI] [Google Scholar]
- Miyazawa K, Minato J, Mashino T, Yoshii T, Kizuka T, Kato R, Tachibana M, Suga T. Characterization of the liquid-phase synthesized fullerene nanotubes and nanowhiskers. Proc. of the 2nd JSME/ASME Int. Conf. on Materials and Processing 2005—M&P2005: The 13th JSME Materials and Processing Conf.; Seattle, WA. 19–22 June; 2005. pp (SMS23)-1–4. [Google Scholar]
- Miyazawa K, Kuriyama R, Shimomura S, Wakahara T, Tachibana M. 2014. Growth and FIB-SEM analyses of C60 microtubes vertically synthesized on porous alumina membranes J. Cryst. Growth 388 5–11 5–11 10.1016/j.jcrysgro.2013.11.009 [DOI] [Google Scholar]
- Cha S I, Miyazawa K, Kim J. Substrate having fullerene thin wires and method for manufacture thereof. 8685160B2 United States Patent. 2014
- Amer M S, Todd T K, Busbee J D. 2011. Effect of linear alcohol molecular size on the self-assembly of fullerene whiskers Mater. Chem. Phys. 130 90–94 90–4 10.1016/j.matchemphys.2011.05.070 [DOI] [Google Scholar]
- Hotta K, Miyazawa K. 2008. Growth rate measurement of C60 fullerene nanowhiskers Nano 3 355–359 355–9 10.1142/S1793292008001192 [DOI] [Google Scholar]
- Asaka K, Kato R, Yoshizaki R, Miyazawa K, Kizuka T. 2007. Fracture surface and correlation of buckling force with aspect ratio of C60 crystalline whiskers Diam. Relat. Mater. 16 1936–1939 1936–9 10.1016/j.diamond.2007.08.005 [DOI] [Google Scholar]
- Kizuka T, Saito K, Miyazawa K. 2008. Young’s modulus of crystalline C60 nanotubes studied by in situ transmission electron microscopy Diam. Relat. Mater. 17 972–974 972–4 10.1016/j.diamond.2008.02.038 [DOI] [Google Scholar]
- Saito K, Miyazawa K, Kizuka T. Bending process and Young’s modulus of fullerene C60 nanowhiskers. Japan. J. Appl. Phys. 2009;48:013502. doi: 10.1143/JJAP.48.010217. [DOI] [Google Scholar]
- Asaka K, Kato R, Miyazawa K, Kizuka T. Buckling of C60 whiskers. Appl. Phys. Lett. 2006;89:013502. doi: 10.1063/1.2336590. [DOI] [Google Scholar]
- Kato R, Miyazawa K. 2011. Cross-sectional structural analysis of C60 nanowhiskers by transmission electron microscopy Diam. Relat. Mater. 20 299–303 299–303 10.1016/j.diamond.2011.01.016 [DOI] [Google Scholar]
- Kizuka T, Miyazawa K, Tokumine T. Solvation-assisted Young’s modulus control of single-crystal fullerene C70 nanowhiskers. J. Nanotechnol. 2012;2012:013502. doi: 10.1155/2012/583817. [DOI] [Google Scholar]
- Miyazawa K, Hamamoto K, Nagata S, Suga T. 2003. Structural investigation of the C60/C70 whiskers fabricated by forming liquid–liquid interfaces of toluene with dissolved C60/C70 and isopropyl alcohol J. Mater. Res. 18 1096–1103 1096–103 10.1557/JMR.2003.0151 [DOI] [Google Scholar]
- Minato J, Miyazawa K, Suga T. 2005. Morphology of C60 nanotubes fabricated by the liquid–liquid interfacial precipitation method Sci. Technol. Adv. Mater. 6 272–277 272–7 10.1016/j.stam.2005.02.006 [DOI] [Google Scholar]
- Ji H-X, Hu J-S, Tang Q-X, Song W-G, Wang C-R, Hu W-P, Wan L-J, Lee S-T. 2007. Controllable preparation of submicrometer single-crystal C60 rods and tubes through concentration depletion at the surfaces of seeds J. Phys. Chem. C 111 10498–10502 10498–502 10.1021/jp071912r [DOI] [Google Scholar]
- Ringor C L, Miyazawa K. 2009. Fabrication of solution grown C60 fullerene nanotubes with tunable diameter J. Nanosci. Nanotechnol. 9 6560–6564 6560–4 10.1166/jnn.2009.1307 [DOI] [PubMed] [Google Scholar]
- Hotta K, Miyazawa K. 2008. Growth rate measurement of C60 fullerene nanowhiskers Nano 3 355–359 355–9 10.1142/S1793292008001192 [DOI] [Google Scholar]
- Tachibana M, Kobayashi K, Uchida T, Kojima K, Tanimura M, Miyazawa K. 2003. Photo-assisted growth and polymerization of C60 ‘nano’ whiskers Chem. Phys. Lett. 374 279–285 279–85 10.1016/S0009-2614(03)00723-1 [DOI] [Google Scholar]
- Kobayashi K, Tachibana M, Kojima K. 2005. Photo-assisted growth of C60 nanowhiskers from solution J. Cryst. Growth 274 617–621 617–21 10.1016/j.jcrysgro.2004.10.020 [DOI] [Google Scholar]
- Miyazawa K, Hotta K. 2011. The effect of water on the stability of C60 fullerene nanowhiskers J. Nanopart. Res. 13 5739–5747 5739–47 10.1007/s11051-010-0132-y [DOI] [Google Scholar]
- Wei M, Luo H, Li N, Zhang S, Gan L. 2002. Study of electrochemical properties of pyrrolidinofullerenes by microelectrode voltammetry Microchem. J. 72 115–112 115–112 10.1016/S0026-265X(01)00096-0 [DOI] [Google Scholar]
- Wakahara T, Miyazawa K, Nemoto Y, Ito O. 2011. Diameter controlled growth of fullerene nanowhiskers and their optical properties Carbon 49 4644–4649 4644–9 10.1016/j.carbon.2011.06.041 [DOI] [Google Scholar]
- Miyazawa K, Hirata C, Wakahara T. 2014. Influence of the solution volume on the growth of C60 nanowhiskers J. Cryst. Growth 405 68–72 68–72 10.1016/j.jcrysgro.2014.07.036 [DOI] [Google Scholar]
- Kawasaki S, Sakai E. 1967. Measurement of diffusion of gold in copper by elastic scattering of deuteron J. Nucl. Sci. Technol. 4 273–277 273–7 10.1080/18811248.1967.9732743 [DOI] [Google Scholar]
- Ogawa K, Kato T, Ikegami A, Tsuji H, Aoki N, Ochiai Y. Electrical properties of field-effect transistors based on C60 nanowhiskers. Appl. Phys. Lett. 2006;88:013502. doi: 10.1063/1.2186519. [DOI] [Google Scholar]
- Somani P R, Somani S P, Umeno M. Toward organic thick film solar cells: three dimensional bulk heterojunction organic thick film solar cell using fullerene single crystal nanorods. Appl. Phys. Lett. 2007;91:013502. doi: 10.1063/1.2801624. [DOI] [Google Scholar]
- Shrestha R G, Shrestha L K, Khan A H, Kumar G S, Acharya S, Ariga K. 2014. Demonstration of ultrarapid interfacial formation of 1D fullerene nanorods with photovoltaic properties Appl. Mater. Interfaces 6 15597–15603 15597–603 10.1021/am5046235 [DOI] [PubMed] [Google Scholar]
- Cho B H, Lee K B, Miyazawa K, Ko W B. 2013. Preparation of fullerene (C60) nanowhisker-ZnO nanocomposites by heat treatment and photocatalytic degradation of methylene blue Asian J. Chem. 25 8027–8030 8027–30 10.14233/ajchem.2013.14974 [DOI] [Google Scholar]
- Yang J, Lim H, Choi H C, Shin H S. 2010. Wavelength-selective silencing of photocurrent in Au-coated C60 wire hybrid Chem. Commun. 46 2575–2577 2575–7 10.1039/b922223e [DOI] [PubMed] [Google Scholar]
- Li H, Tee B C-K, Cha J J, Cui Y, Chung J W, Lee S Y, Bao Z. 2012. High-mobility field-effect transistors from large-area solution-grown aligned C60 single crystals J. Am. Chem. Soc. 134 2760–2765 2760–5 10.1021/ja210430b [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Kuwasaki Y, Hamamoto K, Nagata S, Obayashi A, Kuwabara M. 2003. Structural characterization of the C60 nanowhiskers formed by the liquid–liquid interfacial precipitation method Surf. Interface Anal. 35 117–120 117–20 10.1002/sia.1506 [DOI] [Google Scholar]
- Larsson M P, Kjelstrup-Hansen J, Lucyszyn S. 2007. Dc characterisation of C60 whiskers and nanowhiskers ECS Trans. 2 27–38 27–38 10.1149/1.2408950 [DOI] [Google Scholar]
- Xu M S, Pathak Y, Fujita D, Ringor C, Miyazawa K. Covered conduction of individual C60 nanowhiskers. Nanotechnology. 2008;19:013502. doi: 10.1088/0957-4484/19/7/075712. [DOI] [PubMed] [Google Scholar]
- Ji H-X, Hu J-S, Wan L-J, Tang Q-X, Hu W-P. 2008. Controllable crystalline structure of fullerene nanorods and transport properties of an individual nanorod J. Mater. Chem. 18 328–332 328–32 10.1039/b712696d [DOI] [Google Scholar]
- Hannay N B, Geballe T H, Matthias B T, Andres K, Schmidt P, MacNair D. 1965. Superconductivity in graphitic compounds Phys. Rev. Lett. 14 225–226 225–6 10.1103/PhysRevLett.14.225 [DOI] [Google Scholar]
- Koike Y, Suematsu H, Higuchi K, Tanuma S. 1980. Superconductivity in graphite–alkali metal intercalation compounds Physica B 99 503–508 503–8 10.1016/0378-4363(80)90286-7 [DOI] [Google Scholar]
- Weller T E, Ellerby M, Saxena S S, Smith R P, Skipper N T. 2005. Superconductivity in the intercalated graphite compounds C6Yb and C6Ca Nat. Phys. 1 39–41 39–41 10.1038/nphys0010 [DOI] [Google Scholar]
- Kato R, Miyazawa K, Nishimura T, Wang Z M. High-resolution transmission electron microscopy of heat-treated C60 nanotubes. J. Phys.: Conf. Ser. 2009;159:013502. doi: 10.1088/1742-6596/159/1/012024. [DOI] [Google Scholar]
- Asaka K, Nakayama T, Miyazawa K, Saito Y. 2012. Study on structure of heat-treated fullerene nanowhiskers and their field electron emission characteristics Surf. Interface Anal. 44 780–783 780–3 10.1002/sia.3868 [DOI] [Google Scholar]
- Asaka K, Nakayama T, Miyazawa K, Saito Y. 2012. Structures and field emission properties of heat-treated C60 fullerene nanowhiskers Carbon 50 1209–1215 1209–15 10.1016/j.carbon.2011.10.035 [DOI] [Google Scholar]
- Ekimov E A, Sidorov V A, Bauer E D, Mel’nik N N, Curro N J, Thompson J D, Stishov S M. 2004. Superconductivity in diamond Nature 428 542–545 542–5 10.1038/nature02449 [DOI] [PubMed] [Google Scholar]
- Takano Y, Nagao M, Sakaguchi I, Tachiki M, Hatano T, Kobayashi K, Umezawa H, Kawarada H. 2004. Superconductivity in diamond thin films well above liquid helium temperature Appl. Phys. Lett. 85 2851–2853 2851–3 10.1063/1.1802389 [DOI] [Google Scholar]
- Hebard A F, Rosseinsky M J, Haddon R C, Murphy D W, Glarum S H, Palstra T T M, Ramirez A P, Kortan A R. Superconductivity at 18 K in potassium-doped C60. Nature. 1991;350:600. doi: 10.1038/350600a0. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Ebbesen T W, Saito S, Mizuki J, Tsai J S, Kubo Y, Kuroshima S. 1991. Superconductivity at 33 K in CsxRbyC60 Nature 352 222–223 222–3 10.1038/352222a0 [DOI] [Google Scholar]
- Haddon R C. 1992. Electronic structure, conductivity, and superconductivity of alkali metal doped C60 Acc. Chem. Res. 25 127–133 127–33 10.1021/ar00015a005 [DOI] [Google Scholar]
- Minato J, Miyazawa K. 2005. Solvated structure of C60 nanowhiskers Carbon 43 2837–2841 2837–41 10.1016/j.carbon.2005.06.013 [DOI] [Google Scholar]
- Cui W. 2011. Synthesis of alkali-metal-doped C60 nanotubes Diam. Relat. Mater. 20 93–96 93–6 10.1016/j.diamond.2010.10.006 [DOI] [Google Scholar]
- Takeya H, Miyazawa K, Kato R, Wakahara T, Ozaki T, Okazaki H, Yamaguchi T, Takano Y. 2012. Superconducting fullerene nanowhiskers Molecules 17 4851–4859 4851–9 10.3390/molecules17054851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya H, Kato R, Wakahara T, Miyazawa K, Yamaguchi T, Ozaki T, Okazaki H, Takano Y. 2013. Preparation and superconductivity of potassium-doped fullerene nanowhiskers Mater. Res. Bull. 48 343–345 343–5 10.1016/j.materresbull.2012.10.033 [DOI] [Google Scholar]
- Murphy D W, Rosseinsky M J, Haddon R C, Ramirez A P, Hebard A F, Tycko R, Fleming R M, Dabbagh G. 1991. Superconductivity in alkali metal fullerides Physica C 185–9 403–407 403–7 10.1016/0921-4534(91)92006-W [DOI] [Google Scholar]
- Takeya H, Miyazawa K, Takano Y. 2012. Development of alkali-metal doped superconducting fullerene nanowhiskers Mater. Integr. 25 38–44 38–44 (in Japanese) [Google Scholar]
- Butterman W C, Reese R G., Jr 2003 Mineral Commodity Profiles—Rubidium (US Geological Survey Open-File Report 03-045) [Google Scholar]
- Wakahara T, Angelo P D’, Miyazawa K, Nemoto Y, Ito O. 2012. Fullerene/cobalt porphyrin hybrid nanosheets with ambipolar charge transporting characteristics J. Am. Chem. Soc. 134 7204–7206 7204–6 10.1021/ja211951v [DOI] [PubMed] [Google Scholar]
- Barzegar H R, Larsen C, Edman L, Wågberg T. 2013. Solution-based phototransformation of C60 nanorods: towards improved electronic devices Part. Part. Syst. Charact. 30 715–720 715–20 10.1002/ppsc.201300016 [DOI] [Google Scholar]
- Larsen C, Barzegar H R, Nitze F, Wågberg T, Edman L. On the fabrication of crystalline C60 nanorod transistors from solution. Nanotechnology. 2012;23:013502. doi: 10.1088/0957-4484/23/34/344015. [DOI] [PubMed] [Google Scholar]
- Doi T, Koyama K, Chiba Y, Tsuji H, Ueno M, Chen S-R, Aoki N, Bird J P, Ochiai Y. Electron transport properties in photo and supersonic wave irradiated C60 fullerene nano-whisker field-effect transistors. Japan. J. Appl. Phys. 2010;49:013502. doi: 10.1143/JJAP.49.04DN12. [DOI] [Google Scholar]
- Cui H. 2014. High-temperature calcined fullerene nanowhiskers as well as long needle-like multi-wall carbon nanotubes have abilities to induce NLRP3-mediated IL-1β secretion Biochem. Biophys. Res. Commun. 452 593–599 593–9 10.1016/j.bbrc.2014.08.118 [DOI] [PubMed] [Google Scholar]