Abstract
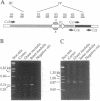
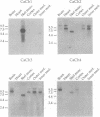
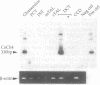
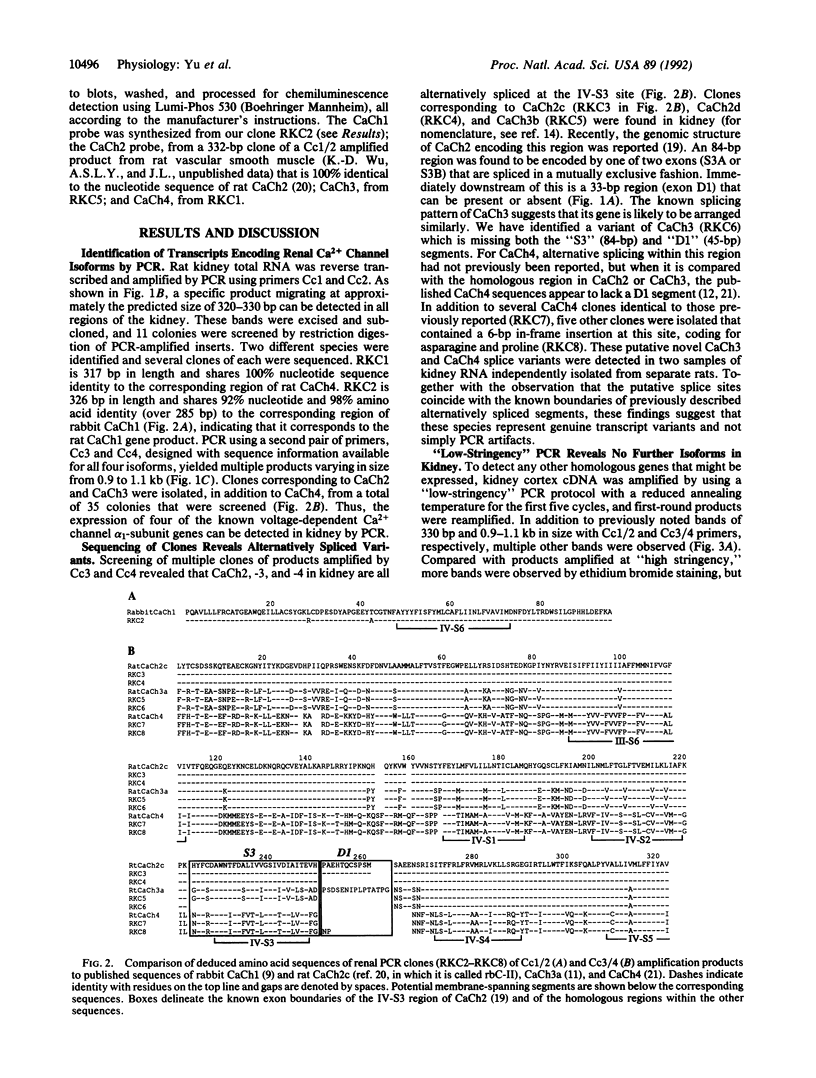
Active, transepithelial, Ca2+ reabsorption in kidney occurs primarily in the distal convoluted tubule. Recent evidence suggests that entry of Ca2+ at the apical membrane through channels bearing resemblance to those of the voltage-dependent L type may be the rate-determining step in Ca2+ reabsorption. To determine the molecular identity of the pore-forming subunit of voltage-dependent Ca2+ channel(s) in the kidney, a homology-based PCR cloning strategy was employed. Nondegenerate primers, based on conserved regions of the published cDNA sequences of voltage-dependent Ca2+ channel alpha 1 subunits, were used to amplify cDNA from rat kidney, and the products were subcloned and sequenced. A family of molecular species was identified, representing alternatively spliced transcripts of four known genes encoding these channel subunits. Northern blot analysis indicated that the expression of each of the genes exhibits a distinct spatial distribution within the kidney. One gene, CaCh4, is expressed primarily in the cortex, and by microdissected-tubule PCR was found predominantly in the distal convoluted tubule, consistent with a role in transepithelial Ca2+ reabsorption at this site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacskai B. J., Friedman P. A. Activation of latent Ca2+ channels in renal epithelial cells by parathyroid hormone. Nature. 1990 Sep 27;347(6291):388–391. doi: 10.1038/347388a0. [DOI] [PubMed] [Google Scholar]
- Costanzo L. S., Windhager E. E. Calcium and sodium transport by the distal convoluted tubule of the rat. Am J Physiol. 1978 Nov;235(5):F492–F506. doi: 10.1152/ajprenal.1978.235.5.F492. [DOI] [PubMed] [Google Scholar]
- Diebold R. J., Koch W. J., Ellinor P. T., Wang J. J., Muthuchamy M., Wieczorek D. F., Schwartz A. Mutually exclusive exon splicing of the cardiac calcium channel alpha 1 subunit gene generates developmentally regulated isoforms in the rat heart. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1497–1501. doi: 10.1073/pnas.89.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubel S. J., Starr T. V., Hell J., Ahlijanian M. K., Enyeart J. J., Catterall W. A., Snutch T. P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman P. A. Basal and hormone-activated calcium absorption in mouse renal thick ascending limbs. Am J Physiol. 1988 Jan;254(1 Pt 2):F62–F70. doi: 10.1152/ajprenal.1988.254.1.F62. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hui A., Ellinor P. T., Krizanova O., Wang J. J., Diebold R. J., Schwartz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the alpha 1 subunit of the voltage-dependent calcium channel. Neuron. 1991 Jul;7(1):35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf N. N., McMahon D. K., Hainsworth C. N., Kay B. K. A two-motif isoform of the major calcium channel subunit in skeletal muscle. Neuron. 1992 May;8(5):899–906. doi: 10.1016/0896-6273(92)90204-q. [DOI] [PubMed] [Google Scholar]
- Merot J., Poncet V., Bidet M., Tauc M., Poujeol P. Apical membrane ionic channels in the rabbit cortical thick ascending limb in primary culture. Biochim Biophys Acta. 1991 Dec 9;1070(2):387–400. doi: 10.1016/0005-2736(91)90079-n. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Murphy H. R., Martin B. M., Garcia-Perez A. Detection of specific mRNAs in single nephron segments by use of the polymerase chain reaction. Am J Physiol. 1990 May;258(5 Pt 2):F1470–F1474. doi: 10.1152/ajprenal.1990.258.5.F1470. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Wei X. Y., Castellano A., Birnbaumer L. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem. 1990 Nov 25;265(33):20430–20436. [PubMed] [Google Scholar]
- Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991 Jul;7(1):45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Starr T. V., Prystay W., Snutch T. P. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]