Abstract
Previous studies have found dysfunctional resting state functional connectivity (RSFC) in depressed patients. Examining RSFC might aid biomarker discovery for depression. However RSFC in young people at risk of depression has yet to be examined.
35 healthy adolescents (13–18 yrs old.) were recruited. 17 scoring high on the Mood and Feelings Questionnaire (MFQ > 27 (High Risk: HR), and 18 scoring low on the MFQ < 15 (Low Risk: LR) matched on age and gender. We selected seed regions in the salience network (SN: amygdala and pregenual anterior cingulate cortex (pgACC)) and the central executive network (CEN: dorsal medial prefrontal cortex (dmPFC)). Mood and anhedonia measures were correlated with brain connectivity.
We found decreased RSFC in the HR group between the amygdala and the pgACC and hippocampus and precuneus. We also found decreased RSFC in the HR group between the pgACC and the putamen and between the dmPFC and the precuneus. The pgACC RSFC with the insula/orbitofrontal cortex correlated inversely with the anticipation of pleasure in all subjects. Increased RSFC was observed between the pgACC and the prefrontal cortex and the amygdala and the temporal pole in the HR group compared to the LR group.
Our findings are the first to show that adolescents with depression symptoms have dysfunctional RSFC between seeds in the SN and CEN with nodes in the Default Mode Network. As increased connectivity between the pgACC and the insula correlated with decreased ability to anticipate pleasure, we suggest this might be mechanism underlying the risk of experiencing anhedonia, a suggested biomarker for depression.
Keywords: fMRI, Depression, Biomarker, Resting-state, Connectivity, Salience network, Adolescent, DMN
Highlights
-
•
This is the first study to examine RSFC in healthy adolescents with increased depression symptomatology.
-
•
We find RSFC between the pgACC and the insula/OFC correlates with ability to anticipate pleasure.
-
•
We suggest neural mechanisms for risk for clinical depression.
1. Introduction
Adolescence is a crucial developmental period where the incidence of depression increases significantly, and reports have emphasised that around 8% of adolescents are affected by depression by the age of 16 (Saluja et al., 2004). Identifying biomarkers such as dysfunctional neural networks could help develop preventative treatments for young people at increased risk of clinical depression.
It is been shown that patients with major depressive disorder (MDD) have abnormalities in their resting state functional connectivity (RSFC) in networks such as the Salience Network (SN) the Central Executive Network (CEN) and the Default Mode Network (DMN) (Sheline et al., 2010).
The Salience Network (SN) and the central executive network (CEN) show strong task-related activation and are less active at rest in healthy controls. The SN which consists of regions such as the anterior insula, pregenual anterior cingulate and amygdala are implicated in the processing of various aspects of salient stimuli whereas the CEN, which consists of regions such as the dorsolateral, dorsal medial prefrontal cortex and the posterior parietal cortex, is involved in cognitive functioning including attention and working memory (Bressler and Menon, 2010). In MDD, abnormalities in these networks are thought to reflect deficits in attentional control over emotional stimuli, difficulties with suppression of unwanted thoughts and difficulties with emotion recognition. However there have been inconsistencies in the direction of effects with some studies finding increased SN and CEN (Horn et al., 2010, Manoliu et al., 2014, Ramasubbu et al., 2014, Sheline et al., 2010), whilst others find reduced connectivity (Liston et al., 2014, Tahmasian et al., 2013, Ye et al., 2012) in these regions in MDD. These inconsistencies might be related to differences in the MDD population studied (adults, elderly, adolescents), medication history and depression severity.
There is small number of RSFC studies in adolescents with MDD. These studies have mostly focused on investigating the SN and they report decreased RSFC between the amygdala and the hippocampus, parahippocampus and brain stem which has also been shown to correlate with depression severity (Cullen et al., 2014). The same study however, also showed increased RSFC between the amygdala and the precuneus in depressed adolescents. Mixed results were also reported by Pannekoek et al. who found both increased RSFC between the amygdala and the parietal cortex in MDD adolescents and decreased RSFC between the amygdala and regions such as the pgACC, frontal pole and the paracingulate gyrus (Pannekoek et al., 2014).
The authors suggested that risk for depression may be related to dysfunction in brain areas involved in supporting self-relational processes and reward prediction. Another study examining familial risk for depression in adolescents found decreased RSFC between the prefrontal cortex and parts of the CEN, in those with a parent with depression which also correlated the parents depression severity (Clasen et al., 2014). The authors suggested that an increase in vulnerability to depression may thus be underpinned by altered development of the CEN in young people at risk.
Our current study aims to examine RSFC in another at risk group, adolescents with increased depression symptomatology but with no clinical diagnoses. Based on the previous literature, we selected seed regions that have been shown dysfunctional in depressed patients and adolescents at familial risk of depression, amygdala and pgACC seeds from the SN and the dmPFC highlighted as a key node of within the CEN and dysfunctional RSFC in depressed patients (Sheline et al., 2010). We hypothesised that adolescents at risk of depression would also have decreased RSFC between key brain regions implicated in the aetiology of depression, supporting the notion that dysfunctional resting state neural networks may be biomarkers for depression.
2. Materials and methods
2.1. Participants
35 healthy adolescents (13–18 yrs old.) were recruited. 17 healthy adolescents scoring high on the Mood and Feelings Questionnaire (Costello et al., 1998) (MFQ > 27 (HR), and 18 adolescents scoring low on the MFQ < 15 (LR) matched on age and gender. Participants completed the MFQ; a self-report questionnaire designed to assess recent (last 2 weeks) presence and severity of depressive symptoms as specified in DSM-IV. The measure is composed of 33 statements corresponding to how often the individual has experienced particular behaviours and feelings during this time. There is considerable psychometric data for this child version, including good test–retest reliability for a score of 27 and above indicating increased depression symptom severity (Pearson's r ¼ 0.78) (Wood et al., 1995) and below 15 indicating healthy controls (Kyte et al., 2005).
Participants who scored between 15 and 27 were excluded from the study. The University of Reading Ethics Committee provided ethical approval and the investigation was carried out in accordance with the latest version of the Declaration of Helsinki. We obtained written informed consent from all participants before screening and after giving the complete description of the study. Exclusion criteria for all subjects consisted of current or past history of alcohol or drug dependency, pregnancy and any contradictions to MRI, e.g. pacemaker, mechanical heart valve, hip replacement, metal implants. Further, both of the groups were determined to be free from current or past axis 1 disorder (including anxiety disorders, depression, eating disorders psychosis and substance abuse) on the structured clinical interview for DMS-IV (Spitzer et al., 2004). None of the participant took current medication apart from the contraceptive pill. All subjects were rated on the following questionnaires: Mood and Feeling Questionnaire (MFQ; (Angold et al., 1995)), Beck Depression Inventory (BDI; (Beck et al., 1961)), the Fawcett–Clarke Pleasure Scale (FCPS; (Fawcett et al., 1983)), and the Snaith–Hamilton Pleasure Scale (SHAPS; (Snaith et al., 1995)), the Temporal Experience of Pleasure Scale, consummatory subscale TEPS-C and anticipatory subscale TEPS-A (Gard et al., 2006), before scanning. Body mass index (BMI) for each individual was also calculated.
2.2. Overall design
MRI derived measures of brain function, based on blood-oxygenation-level-dependent (BOLD) contrast were used to compare brain responses at rest across the LR group and the HR group. The resting-state data were acquired before any other scans including the structural scan. Subjects were instructed to lie in dimmed light with their eyes open, think of nothing in particular, and not to fall asleep, similar to our previous studies (Cowdrey et al., 2012, McCabe and Mishor, 2011, McCabe et al., 2010, Rzepa et al., 2015) and a method found to have higher reliability than eyes closed (Patriat et al., 2013). To measure whether undergoing a scan had an effect on mood change, volunteers completed the Befindlischkeit scale on mood (BFS; (Hobi, 1985)) and an energy and affect Visual Analogue Scale (VAS) pre and post scan.
2.3. Image acquisition
A Siemens Magnetom Trio 3T whole body MRI scanner and a thirty-two-channel head coil were used. Multi-band accelerated echo planar imaging sequencing (Center for Magnetic Resonance Research, Minnesota) was used with an acceleration factor of 6 and iPAT acceleration factor of 2. T2*-weighted EPI slices were obtained every 0.7 s (TR = 0.7, TE = 0.03), these parameters were optimised given our scanner capability and used to increase sampling rates and increase our power to detect resting state networks as has been shown previously with multiband (Xu et al., 2013, Filippini et al., 2014). 54 transverse slices with in-plane resolution of 2.4 × 2.4 mm were attained and slice thickness was 2.4 mm. The matrix size was 96 × 96 and the field of view (FOV) were 230 × 230 mm. Acquisition was performed during resting-state scan, yielding 400 vol in total. Sagittal 3D MPRAGE images were also acquired with an isotropic in-plane resolution of 1 × 1 × 1 (TI = 0.9 s, TR = 2.02, flip angle 9°, FOV = 250 × 250 mm) yielding 192 slices.
2.4. fMRI data analysis
2.4.1. Pre-processing
Imaging data were pre-processed and analyzed using FSL tools (www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). fMRI data pre-processing was carried out using FEAT (FMRI Expert Analysis Tool, Version 6.0, a part of FSL software), and included the following steps: non-brain removal (Smith, 2002), motion correction using MCFLIRT(Jenkinson and Smith, 2001), spatial smoothing using a Gaussian kernel of full-width at half maximum (FWHM) of 5 mm, grand mean intensity normalization of the entire 4D dataset by a single multiplicative factor and high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 64.0s). fMRI volumes were registered to the individual's structural scan and the MNI-152 standard space image (Montreal Neurological Institute, Montreal, QC, Canada) using FMRIB's Linear Image Registration Tool (FLIRT).
2.4.2. Time series extraction and higher level analysis
To study resting-state functional connectivity, a seed-based correlation approach was used. Using the Harvard-Oxford subcortical structural atlas (Kennedy et al., 1998) we created a structural bilateral amygdala seed as the amygdala is a small structure and not suitable for a ROI sphere. To maximize the exact coverage, the masks of these seed regions were threshold by 20% to include voxels having at least 80% of probability of being in these particular regions. We also created seeds for the dmPFC (18 34 29; −24 35 28) (6 mm sphere so as to not cross into other brain regions) coordinates from (Sheline et al., 2010) and pgACC (8 mm sphere with a center at 0 38 0 so as to not cross into other brain regions). The dmPFC and pgACC seeds were created with Wake Forest University Pickatlas tool in SPM8 as in our previous study and can be seen in the figures (McCabe et al., 2011).
The mean time course within the left and right seeds of each ROI (except for the pgACC, only comprising one medial seed) was calculated and used as a regressor in a general linear model. In addition, white matter signal, cerebrospinal fluid signal, 6 motion parameters (3 translations and 3 rotations), and the global signal were used as nuisance regressors. We have obtained white matter and cerebrospinal fluid masks using FSL's FAST segmentation program. The resulting segmented images were then thresholded to ensure 80% tissue type probability. For each individual, the general linear model was analyzed by using the FMRI Expert Analysis Tool [version 5.4, part of FMRIB's Software Library (Smith et al., 2004)]. The resulting parameter estimate maps were then analyzed using higher level 1 sample t-tests for group averages and between samples t-tests for group differences. Clusters were determined by Z > 2.3 voxel-wise thresholding and a family-wise error-corrected cluster significance threshold of P < 0.05 (Worsley, 2001). From the results we then only report those that met the further correction for number of ROIs examined which gave P < 0.016 (i.e, P < 0.05 Bonferroni corrected for the 3 networks of interest: amygdala, dmPFC, pgACC (Davidson et al., 2003). Next, the % BOLD signal change data was extracted from the regions of significant effect (Table 2) using the FSL tool Featquery (www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004).
Table 2.
RSFC between seed regions and whole brain compared between groups.
| Brain region | MNI coordinates |
z-score | Cluster size | p-value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| LR > HR | ||||||
| L Amygdala seed | ||||||
| pgACC | 2 | 36 | −2 | 6.13 | 4447 | <0.001 |
| Precuneus | 4 | −68 | 20 | 3.5 | 476 | <0.001 |
| PCC | 0 | −40 | 24 | 3.21 | 476 | <0.001 |
| pgACC seed | ||||||
| Thalamus | 0 | −4 | 2 | 6.28 | 1150 | <0.001 |
| Pallidum | 16 | −4 | −6 | 4.17 | 1150 | <0.001* |
| Pallidum/Putamen | −16 | 6 | −2 | 3.11 | 1150 | <0.001 |
| R amygdala seed | ||||||
| Hippocampus | −14 | −10 | −18 | 4.05 | 357 | 0.00069 |
| R dmPFC seed | ||||||
| Cuneal Cortex/Precuneus | 8 | −76 | 36 | 3.15 | 401 | 0.0009 |
| Lateral Occipital Cortex | −18 | −82 | 24 | 3.1 | 401 | 0.0009 |
| HR > LR | ||||||
| L Amygdala seed | ||||||
| Temporal pole | −46 | 4 | −18 | 3.2 | 1473 | <0.001 |
| pgACC seed | ||||||
| Brain stem | 8 | 30 | 30 | 4.69 | 1156 | <0.001 |
| ACC | 6 | 32 | 26 | 3.85 | 2552 | <0.001 |
| vmPFC | −6 | 50 | −4 | 3.79 | 2552 | <0.001 |
| Lateral OFC | 40 | 22 | −16 | 3.57 | 369 | 0.0013 |
| Insula/OFC | 34 | 10 | −18 | 3.27 | 369 | 0.0013 |
2.4.3. Correlational analyses
To examine the relationship between the scores on behavioural questionnaires and RSFC we extracted the % BOLD signal change using FSL Featquery and correlated with scores on our questionnaires.
3. Results
3.1. Demographic and clinical data
Demographic data analysis (Table 1) revealed no significant age and gender differences between HR and the LR groups. Results for BMI scores were calculated and there was a significant BMI difference between the HR and the LR group. There were also significant differences between the two groups on measures of mood, depression and anhedonia (Table 1) (MFQ, BDI, SHAPS, FCPS, TEPS-A, TEPS-C) (Table 1).
Table 1.
Demographic and clinical characteristics.
| Measure | HR (n = 17) mean (SD) | LR (n = 18) mean (SD) | p-value |
|---|---|---|---|
| Age (years) | 16.59 (1.18) | 16.33 (1.6) | 0.598 |
| Gender (male) | 4/13 | 6/12 | 0.535 |
| BMI | 21.82 (2.72) | 20.1 (1.94) | 0.041 |
| MFQ | 40.41 (6.1) | 4.4 (5.1) | <0.001 |
| BDI | 29.82 (12.7) | 2.28 (4.13) | <0.001 |
| FCPS | 121.12 (18.7) | 137.89 (21.3) | 0.019 |
| SHAPS | 30.11 (5.56) | 20.77 (8) | <0.001 |
| TEPS-A | 37.29 (7.58) | 50.66 (5.14) | <0.001 |
| TEPS-C | 31.76 (5.98) | 36.66 (7.28) | <0.001 |
3.2. Mood, energy and affect scores
Repeated measures ANOVA with within subject factor of time (before and after scan) and between subject factor of group (HR and LR) was employed to examine whether there would be any differences on scores of mood as measured by the BFS. Results for BFS revealed that there was no significant main effect of time (F(1.33) = 0.011; p = 0.919) but there was a significant main effect of group (F(1.33) = 11.33; p = 0.002) and significant interaction between time and group (F(1.33) = 5.56; p = 0.025). Further paired sample t-test analysis revealed that there was a significant difference for time in the LR group (t(17) = −3.08; p = 0.007) meaning they felt worse after the scan and non significant difference for time in the at HR group (t(16) = 1.29; p = 0.216) (Table S1).
Repeated measures ANOVA with within subject factor of time on two levels (before and after scan) and within subject factor of Emotion on nine levels (alertness, disgust, drowsiness, sadness, happiness, anxiety, withdrawn, faint, nausea) and between subject factor of group (healthy controls and at risk) was employed to examine whether there will be any differences between the LR and HR groups scores of energy and affect, as measured by VAS. Results for VAS revealed that there was no significant main effect of time (F(1.33) = 0.385; p = 0.539) and no significant main effect of group (F(1.33) = 3.37; p = 0.075) but there was a significant main effect of Emotion (F(8.264) = 57.78; p < 0.001) yet no significant interaction between the time, emotion and group (F(8.264) = 1.28; p = 0.252). Further paired sample t-test analysis revealed that there was a significant difference for time in the LR group for disgust (t(17) = −2.949, p = 0.009) feeling faint (t(17) = −2.164, p = 0.045) and in the HR group for drowsiness (t(16) = 2.57; p = 0.02) and anxiety (t(16) = 2.14; p = 0.049) all increasing after the scan except anxiety (Table S1).
3.3. Main effects of stimuli on blood oxygen level-dependent responses
Table S2 provides a summary of the main effects, i.e. the brain regions that had RSFC with the seed regions (baseline) for the LR group only. Overall, the patterns of connectivity associated with each of the seed regions are consistent with resting-state and functional connectivity experiments in healthy controls, subjects at risk for depression and depressed patients (Bebko et al., 2015, Cullen et al., 2014, Guo et al., 2015, Sheline et al., 2009, Sheline et al., 2010, Shen et al., 2015, Clasen et al., 2014)
3.4. Effects of mood on RSFC
There was no main effect of age, gender, BMI or MFQ on RSFC.
3.5. Decreased functional connectivity in the HR group
3.5.1. Left amygdala seed
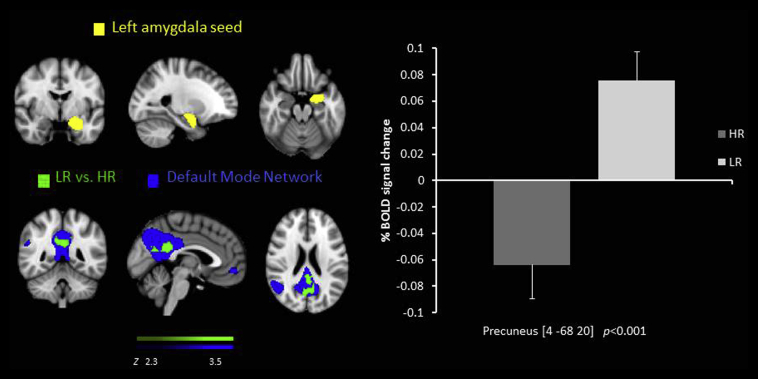
There was decreased RSFC in the HR group compared to the LR group between the left amygdala seed and the pgACC and the precuneus (Fig. 1) and posterior cingulate cortex (PCC) (Table 2).
Fig. 1.
Resting state functional connectivity between the left amygdala seed region  and the precuneus, lower in the HR group than the LR
and the precuneus, lower in the HR group than the LR  overlaid on the DMN
overlaid on the DMN  . % BOLD signal change extracted for connectivity for both of the groups.
. % BOLD signal change extracted for connectivity for both of the groups.
3.5.2. pgACC seed
There was decreased RSFC in the HR group compared to the LR group between the pgACC seed and the thalamus, palladium and the putamen (Table 2).
3.5.3. Right amygdala seed
There was decreased RSFC in the HR group compared to the LR group between the right amygdala seed and the hippocampus (Table 2).
3.5.4. Right dmPFC seed
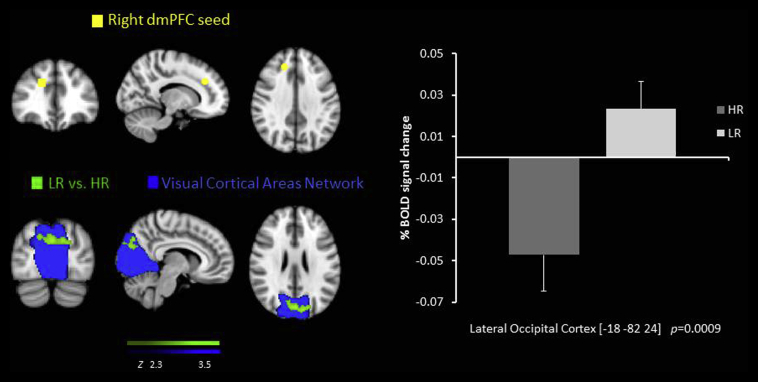
There was decreased RSFC in the HR group compared to the LR group between the right dmPFC seed and the precuneus/cuneal cortex and the lateral occipital cortex (Table 2, Fig. 2).
Fig. 2.
Resting state functional connectivity between the right dmPFC seed region  and the lateral occipital cortex, lower in the HR group than the LR
and the lateral occipital cortex, lower in the HR group than the LR  overlaid on the Visual Cortical Network
overlaid on the Visual Cortical Network  . % BOLD signal change extracted for both of the groups.
. % BOLD signal change extracted for both of the groups.
3.6. Correlational analysis with behaviour
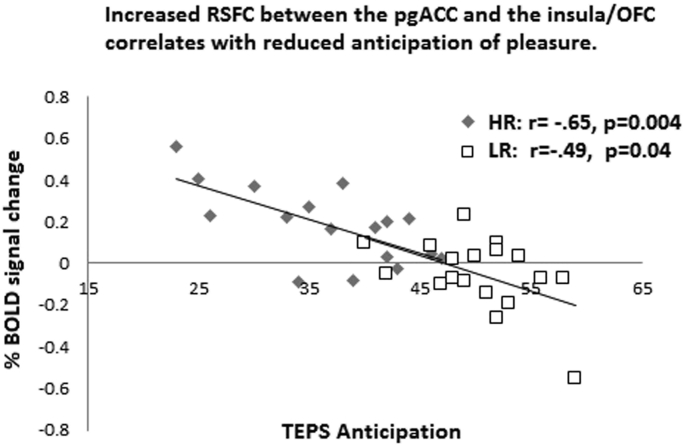
There was a negative correlation between increased RSFC of the pgACC seed and the insula/OFC and decreased anticipation of pleasure (TEPS-A) in both the HR (r = −0.65, p = 0.004) and LR groups (r = −0.48, p = 0.04) (Fig. 3).
Fig. 3.
Increased resting state functional connectivity between the pgACC seed region and the insula/LOFC correlates with reduced anticipation of pleasure.
3.7. Increased functional connectivity in the HR group
3.7.1. Left amygdala seed
There was increased RSFC in the HR group compared to the LR group between the left amygdala seed and the temporal pole (Table 2).
3.7.2. pgACC seed
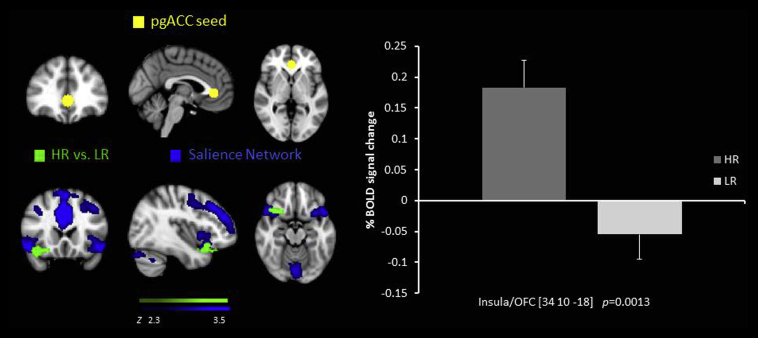
There was increased RSFC in the HR group compared to the LR group between the pgACC seed and the brain stem, the anterior cingulate cortex, the ventral medial prefrontal cortex and the OFC/insula (Table 2, Fig. 4).
Fig. 4.
Resting state functional connectivity between the pgACC seed region  and the insula/LOFC, higher in the HR group than the LR
and the insula/LOFC, higher in the HR group than the LR  overlaid on the Salience Network
overlaid on the Salience Network  . % BOLD signal change extracted for connectivity for both of the groups.
. % BOLD signal change extracted for connectivity for both of the groups.
All p-values for clusters were firstly determined by Z > 2.3 voxel-wise thresholding and a family-wise error-corrected cluster significance threshold of P < 0.05, then further Bonferroni corrected for number of ROIs examined which gave P < 0.016 (i.e, P < 0.05 (Davidson et al., 2003). OFC-orbitofrontal cortex, pgACC- pregenual anterior cingulate cortex, ACC-anterior cingulate cortex.
All data except * remained significant even when the global signal was not used as a nuisance regressor.
4. Discussion
The main aim of our study was to investigate RSFC in young people at risk of developing major depressive disorder by virtue of having increased depression symptomatology. We hypothesised deceased RSFC in key regions that have been found decreased in adults with depression such as regions in the SN and CEN.
Specifically we found decreased RSFC in the HR group compared to the LR group between the amygdala seed and the pgACC. The pgACC is claimed to be a node of communicating between the dorsal ACC important for error detection and attention and the more ventral ACC implicated in emotion processing, regulation and salience detection (Ball et al., 2014). Further, studies in depressed patients, remitted depressed patients and young people with depression symptoms find dysfunctional ACC activity during tasks involved in processing emotions and rewards (Ho et al., 2014, Fitzgerald et al., 2008, McCabe et al., 2009; Rzepa et al., in press), and recently in a RSFC study in depressed adolescents (Pannekoek et al., 2014). Thus it has been suggested that reduced RSFC between the amygdala and the pgACC may reflect problems in integrating inputs of positive information and influencing affect regulation, resulting in decreased hedonic responses and increased feelings of negativity. We found decreased RSFC between the pgACC seed and the thalamus, palladium and the putamen. These are areas of the brain involved in reward and emotion processing and also found dysfunctional in depression (Alexander et al., 1990, Phillips et al., 2003). As our results are in line with the decreased pgACC to amygdala, pallidum and thalamus RSFC found in depressed patients (Anand et al., 2005) it suggests that dysfunctional cortico-limbic connectivity in young people at risk of depression might be a biomarker underpinning problems with the control and regulation of emotional processes.
We also found decreased RSFC in the HR group compared to the LR group between the amygdala seed region and the PCC and the precuneus which have been implicated in self-referential processing in fMRI tasks and thought to underlie maladaptive rumination in depression (Nejad et al., 2013). Further these regions are key nodes of the default mode Network (DMN). The DMN is a network of distributed brain regions that show prominent activation during rest, and deactivation during the performance of cognitive tasks (Whitfield-Gabrieli and Ford, 2012). Studies have revealed that in healthy subjects, the DMN is associated with rumination and self-reflection and that greater suppression of DMN is related to a better performance on attention demanding tasks. Whilst some studies find the DMN overactive in MDD patients when compared with healthy controls, which has been suggested to underlie the symptom of negative rumination in depression (Whitfield-Gabrieli and Ford, 2012) others find decreased connectivity between the DMN and the ventral striatum and the sensorimotor cortex which also correlate negatively with behavioural inhibition in young people at increased familial risk of depression (Frost Bellgowan et al., 2015). However, reports of decreased amygdala RSFC with the precuneus in children at familial risk for depression and in depressed adolescents have been shown (Luking et al., 2011). Our results are in line with Luking at al. and suggest, that if adolescents with increased risk of depression have difficulty in emotion regulation this may be due to decreased amygdala-precuneus connectivity. To further explore this, future studies should examine the relationship between emotion processing and RSFC in young people at risk of depression.
We also found decreased RSFC in the HR group compared to the LR group between the amygdala seed and the hippocampus, similar to that found previously in MDD adolescents and in children with a family history of depression (Cullen et al., 2014, Luking et al., 2011). The amygdala possesses strong connections with the hippocampus which plays a crucial role in encoding and retrieval of emotional stimuli (Smith et al., 2006). Thus it could be suggested that decreased connectivity between the amygdala and the hippocampus might lead to less ability to suppress negative memories which in turn could be a risk factor for developing depression.
Our results also revealed decreased RSFC between the dmPFC seed and the precuneus part of the DMN and the lateral occipital cortex part of the visual network. The dmPFC is a key node in the CEN and is a structure implicated in many cognitive and emotional processes (Janssen et al., 2015, Jaworska et al., 2014). Similarly, recent studies have shown that RSFC between the CEN and the DMN is decreased in MDD (Abbott et al., 2013, Manoliu et al., 2013, Sheline et al., 2010) which has also been associated with patients’ difficulties to disengage from self-referential processes that may lead to negative thoughts (Manoliu et al., 2013). Thus our results are an extension of this, as we also see a similar pattern in young people at high risk but not currently diagnosed. Further, the decreased connectivity between the dmPFC and the occipital cortex in the HR group, found in our study, may indicate a mechanism by which compromised control over emotional images in adolescents could increase the risk of depression.
Interestingly, we also found increased RSFC between the pgACC seed and the insula, brain stem and frontal regions such as the ACC, vmPFC, lateral OFC and the amygdala seed and the temporal pole. As described above the pgACC is a key node for emotion processing, regulation and salience detection (Ball et al., 2014) and a previous study by Horn et al. also found altered pgACC RSFC with the anterior insula which was also related to depression severity and glutamate levels (Horn et al., 2010). The insula is thought to be an integration center for emotion, visceromotor, autonomic and interoceptive information and is also key node in switching between the CEN and the DMN during task performance (Guo et al., 2015, Sridharan et al., 2008). Given that we found that increased connectivity between the pgACC and the insula correlated with decreased ability to anticipate pleasure, we suggest this might be mechanism underlying the experience of anhedonia and therefore a possible further biomarker for depression in adolescents.
Increased RSFC between the amygdala and the temporal pole in the HR group may also be important given that the temporal pole has been implicated in studies examining Theory of Mind (the ability to predict other people's behaviour by attributing mental states such as believes and desires). For example a previous study in MDD patients reported increased amygdala to Temporal pole RSFC which also correlated inversely with depression severity (Ramasubbu et al., 2014). Interestingly, the authors argued that because the connectivity between these regions increased as depression severity decreased this may represent a compensatory mechanism by which subjects are more likely to maintain a balance in processing socially and emotionally relevant information. Intriguingly finding a similar pattern in our sample of young people at risk might also be related to resilience and protection against future depression development. Thus future studies would benefit from larger sample sizes and longitudinal designs to address this directly. Furthermore, our analysis examined the entire amygdala, but prior work has shown that amygdala subregions have known dissociable functional networks (Roy et al., 2009). Therefore future research should investigate how RSFC patterns in adolescents at risk of depression vary across amygdala subregions; interestingly such research would also benefit from the implementation of multiband sequencing that we have used in this study (Ugurbil et al., 2013).
Taken together we have shown that even in young people who are not currently depressed but who are at risk, due to depression symptomatology, there are decreased RSFC between key regions involved in the processing of salient stimuli and decision making. Further increased connectivity between amygdala and the temporal pole may also be an indicator of resilience to clinical depression in the future, we believe this is certainly worth investigating further.
Conflict of interest
The authors have no conflict of interest.
Author contributions
C McCabe and E Rzepa declare having materially participated in the research and/or article preparation. C McCabe and E Rzepa collected the data and analyzed the data and wrote the paper. Both authors have approved the final article.
Funding and source
Funded by the University of Reading. The University had no role in collecting, analysing of writing up of the data.
Acknowledgments
We thank Prof Steve Smith at fMRIB Oxford University for his help with multiband EPI sequence parameter settings.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2016.07.013.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abbott C.C., Lemke N.T., Gopal S., Thoma R.J., Bustillo J., Calhoun V.D., Turner J.A. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front. Psychiatry. 2013;4(10) doi: 10.3389/fpsyt.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D., DeLong M.R. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Wu J., Gao S., Bukhari L., Mathews V.P., Kalnin A., Lowe M.J. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Angold A., Costello E.j., Messer S.C., Pickles A., Winder F., Silver D. The development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int. J. Methods Psychiatri. Res. 1995;5:237–249. [Google Scholar]
- Ball T.M., Stein M.B., Paulus M.P. Toward the application of functional neuroimaging to individualized treatment for anxiety and depression. Depress. anxiety. 2014;31(11):920–933. doi: 10.1002/da.22299. [DOI] [PubMed] [Google Scholar]
- Bebko G., Bertocci M., Chase H., Dwojak A., Bonar L., Almeida J., Perlman S.B., Versace A., Schirda C., Travis M., Gill M.K., Demeter C., Diwadkar V., Sunshine J., Holland S., Kowatch R., Birmaher B., Axelson D., Horwitz S., Frazier T., Arnold L.E., Fristad M., Youngstrom E., Findling R., Phillips M.L. Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res. 2015;231(1):77–86. doi: 10.1016/j.pscychresns.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Archives General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bressler S.L., Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cognitive Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Clasen P.C., Beevers C.G., Mumford J.A., Schnyer D.M. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev. Cogn. Neurosci. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Angold A., March J., Fairbank J. Life events and post-traumatic stress: the development of a new measure for children and adolescents. Psychol. Med. 1998;28(6):1275–1288. doi: 10.1017/s0033291798007569. [DOI] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014 Feb;35(2):483–491. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Westlund M.K., Klimes-Dougan B., Mueller B.A., Houri A., Eberly L.E., Lim K.O. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71(10):1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W., Anderle M.J., Kalin N.H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am. J. Psychiatry. 2003;160(1):64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Fawcett J., Clark D.C., Scheftner W.A., Gibbons R.D. 1983. Assessing Anhedonia in Psychiatric Patients. [DOI] [PubMed] [Google Scholar]
- Filippini N., Zsoldos E., Haapakoski R., Sexton C.E., Mahmood A., Allan C.L., Topiwala A., Valkanova V., Brunner E.J., Shipley M.J. Study protocol: the Whitehall II imaging sub-study. BMC Psychiatry. 2014;14(1):159. doi: 10.1186/1471-244X-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.B., Laird A.R., Maller J., Daskalakis Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008;29(6):683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost Bellgowan, Molfese J., Marx P., Thomason M., Glen M., Santiago D., Gotlib J., Drevets I.H., Hamilton W.C., J.P A neural substrate for behavioral inhibition in the risk for major depressive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(10):841–848. doi: 10.1016/j.jaac.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.E., G.M.G., Kring A.M., John O.P. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Personal. 2006;40:1086–1102. [Google Scholar]
- Guo W., Liu F., Xiao C., Zhang Z., Liu J., Yu M., Zhang J., Zhao J. Decreased insular connectivity in drug-naive major depressive disorder at rest. J. Affect. Disord. 2015;179:31–37. doi: 10.1016/j.jad.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Ho T.C., Yang G., Wu J., Cassey P., Brown S.D., Hoang N., Chan M., Connolly C.G., Henje-Blom E., Duncan L.G., Chesney M.A., Paulus M.P., Max J.E., Patel R., Simmons A.N., Yang T.T. Functional connectivity of negative emotional processing in adolescent depression. J. Affect. Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobi V. 1985. Basler Befindlichkeitsskala. Manual. Weinheim: Beltz. [Google Scholar]
- Horn D.I., Yu C., Steiner J., Buchmann J., Kaufmann J., Osoba A., Eckert U., Zierhut K.C., Schiltz K., He H., Biswal B., Bogerts B., Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen T.W., Heslenfeld D.J., Mourik R.V., Logan G.D., Oosterlaan J. Neural correlates of response inhibition in children with attention-deficit/hyperactivity disorder: a controlled version of the stop-signal task. Psychiatry Res. 2015 Aug 30;233(2):278–284. doi: 10.1016/j.pscychresns.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Jaworska N., Yang X.R., Knott V., Macqueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J. Biol. Psychiatry. 2015 Oct;16(7):448–471. doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kennedy D.N., Lange N., Makris N., Bates J., Meyer J., Caviness V.S., Jr. Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb. Cortex. 1998;8(4):372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Kyte Z.A., Goodyer I.M., Sahakian B.J. Selected executive skills in adolescents with recent first episode major depression. J. Child Psychol. Psychiatry. 2005;46(9):995–1005. doi: 10.1111/j.1469-7610.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., Dubin M.J. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Repovs G., Belden A.C., Gaffrey M.S., Botteron K.N., Luby J.L., Barch D.M. Functional connectivity of the amygdala in early-childhood-onset depression. J. Am. Acad. Child. Adolesc. Psychiatry. 2011;50(10):1027–1041. doi: 10.1016/j.jaac.2011.07.019. e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthoffer D., Zimmer C., Forstl H., Bauml J., Riedl V., Wohlschlager A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthoffer D., Zimmer C., Forstl H., Bauml J., Riedl V., Wohlschlager A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2014;7 doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C., Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57(4):1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C., Cowen P.J., Harmer C.J. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C., Mishor Z., Filippini N., Cowen P.J., Taylor M.J., Harmer C.J. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol. Psychiatry. 2011 Jun;16(6):592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek J.N., van der Werff S.J., Meens P.H., van den Bulk B.G., Jolles D.D., Veer I.M., van Lang N.D., Rombouts S.A., van der Wee N.J., Vermeiren R.R. Aberrant resting-state functional connectivity in limbic and salience networks in treatment–naive clinically depressed adolescents. J. Child Psychol. psychiatry, allied Discip. 2014;55(12):1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Patriat R., Molloy E.K., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V., RM B. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage Sep. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Ramasubbu R., Konduru N., Cortese F., Bray S., Gaxiola-Valdez I., Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front. Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009 Apr 1;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepa E., McCabe C. Blunted neural response to anticipation, effort and consummation of reward and aversion in adolescents with depression symptomatology. J. Psychopharmacol. 2016 doi: 10.1177/0269881116681416. (in press) [DOI] [PubMed] [Google Scholar]
- Rzepa E., Tudge L., McCabe C. The CB1 neutral antagonist tetrahydrocannabivarin reduces default mode network and increases executive control network resting state functional connectivity in healthy volunteers. Int. J. Neuropsychopharmacol. 2015;19(2) doi: 10.1093/ijnp/pyv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja G., Iachan R., Scheidt P.C., Overpeck M.D., Sun W., Giedd J.N. Prevalence of and risk factors for depressive symptoms among young adolescents. Archives Pediatr. Adolesc. Med. 2004;158(8):760–765. doi: 10.1001/archpedi.158.8.760. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T., Li C., Wang B., Yang W.M., Zhang C., Wu Z., Qiu M.H., Liu J., Xu Y.F., Peng D.H. Increased cognition connectivity network in major depression disorder: a FMRI study. Psychiatry Investig. 2015;12(2):227–234. doi: 10.4306/pi.2015.12.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith A.P., Stephan K.E., Rugg M.D., Dolan R.J. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49(4):631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Snaith R.P., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br. J. Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. 2004. Structured Clinical Interview for the DSM–IV (SCID–I/P) [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M., Knight D.C., Manoliu A., Schwerthoffer D., Scherr M., Meng C., Shao J., Peters H., Doll A., Khazaie H., Drzezga A., Bauml J., Zimmer C., Forstl H., Wohlschlager A.M., Riedl V., Sorg C. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front. Hum. Neurosci. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Xu J., Auerbach E.J. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage. 2013 Oct 15;80:80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wood A., Kroll L., Moore A., Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. J. Child Psychol. Psychiatry. 1995;36(2):327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Worsley K. Statistical analysis of activation images. Funct. MRI An Introd. methods. 2001;14:251–270. [Google Scholar]
- Xu J., Moeller S., Auerbach E.J., Strupp J., Smith S.M., Feinberg D.A., Yacoub E., Uğurbil K. Evaluation of slice accelerations using multiband echo planar imaging at 3T. Neuroimage. 2013;83:991–1001. doi: 10.1016/j.neuroimage.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T., Peng J., Nie B.B., Gao J., Liu J.T., Li Y., Wang G., Ma X., Li K.C., Shan B.C. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur. J. Radiol. 2012;81(12):4035–4040. doi: 10.1016/j.ejrad.2011.04.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.