Abstract
Recent work continues to place cholinergic circuits at center stage for normal executive and mnemonic functioning, and provides compelling evidence that the loss of cholinergic signaling and cognitive decline are inextricably linked. This review focuses on the last few years of studies on the mechanisms by which cholinergic signaling contributes to circuit activity related to cognition. We attempt to identify areas of controversy, as well as consensus, on what is and is not yet known about how cholinergic signaling in the CNS contributes to normal cognitive processes. In addition, we delineate the findings from recent work on the extent to which dysfunction of cholinergic circuits contributes to cognitive decline associated with neurodegenerative disorders.
INTRODUCTION
Cholinergic signaling in the CNS provides important control over circuit dynamics underlying cognitive processing. Since 1906, when Alois Alzheimer delineated the symptomatology of the disease that bears his name, many have tested the hypothesis that failures of cholinergic circuitry of the basal forebrain are responsible for the cognitive impairments associated with neurodegenerative disorders (Bartus et al., 1982; Drachman and Leavitt, 1974). Recent studies further implicate alterations in cholinergic signaling in disorders of attention and cognitive control (Higley and Picciotto, 2014; Wallace et al., 2011). There remains active debate about the fundamental mechanisms of cholinergic signaling that, until recently, were beyond the grasp of direct experimental testing. As discussed below, technical advances for selective stimulation, higher temporal and spatial resolution of chemical detection, and in vivo recording in awake behaving animals have opened the door to deeper investigation of the role of cholinergic circuits in attention and memory.
The majority of cholinergic neurons in the mammalian brain are found in 4 regions. These include (1) the brainstem pedunculo-pontine and lateral dorsal tegmental nuclei; (2) a subset of thalamic nuclei; (3) the striatum, where cholinergic neurons serve as local interneurons (CIN); and (4) the basal forebrain nuclei, which collectively serve as the major sources of cholinergic projection neurons to neocortex, hippocampus and amygdala (Mesulam et al., 1983; Woolf, 1991). A small and species variable number of CINs are also found in cortex and hippocampus (Frotscher et al., 2000), although the cholinergic identity of the latter group is in dispute (Blusztajn and Rinnofner, 2016; Yi et al., 2015). The essentials of the organization of cholinergic neurons in these brain regions are evident in a wide range of vertebrate species from fish to primates (Giraldez-Perez et al., 2013; Hong et al., 2013; Mesulam et al., 1983; Woolf, 1991). Recent studies even find parallels to cholinergic circuits that are essential to memory encoding in invertebrates (Barnstedt et al., 2016).
This review will focus on the cholinergic neurons of the basal forebrain that provide the predominant cholinergic projections directly engaged in cognitive processing in mammals. For a recent discussion of the emergent role of projection neurons from the brainstem cholinergic groups are fundamental to aspects of sleep, wakefulness, and autonomic control, we refer the reader to Mena-Segovia (2016), Beierlein (2014), and Sarter and Bruno (2000). For a more detailed consideration of recent work on cholinergic signaling in both the dorsal and ventral striatum we refer the reader to Goldberg et al. (2012), Gonzales and Smith, (2015) and Pisani et al. (2007).
1. Cholinergic neurons and cholinergic signaling mechanisms in the CNS
1a. Functional organization of cholinergic neurons & their projections
1a.i. Overview of cholinergic neurons & projections
The vast majority of cholinergic input to cortical and subcortical structures engaged in cognition arises from distal projection neurons whose cell bodies reside in the basal forebrain (Fig 1A). The basal forebrain cholinergic projection neurons elaborate highly extensive, multiply branched inputs to neocortex, archeocortex and other subcortical structures (Woolf, 1991). The cell bodies of the basal forebrain cholinergic neurons are interspersed with non-cholinergic neurons and distributed in a series of nuclei, including the medial septal (MS) nucleus, the diagonal band (DB) nuclei – with vertical and horizontal and domains - the preoptic nucleus, the nucleus basalis (NB), and the substantia innominata (SI; Fig 1 & Woolf, 1991). In primates, the cholinergic nuclear groups are referred to somewhat differently: Ch1 = MS, Ch2 = vertical limb of the Diagonal Band of Broca (DBB), Ch3 = horizontal limb of DBB, Ch4 = the basal magnocellular complex that includes the SI, the Nucleus Basalis of Meynert (NBM), the magnocellular preoptic nucleus and the ventral pallidum (Mesulam et al., 1983).
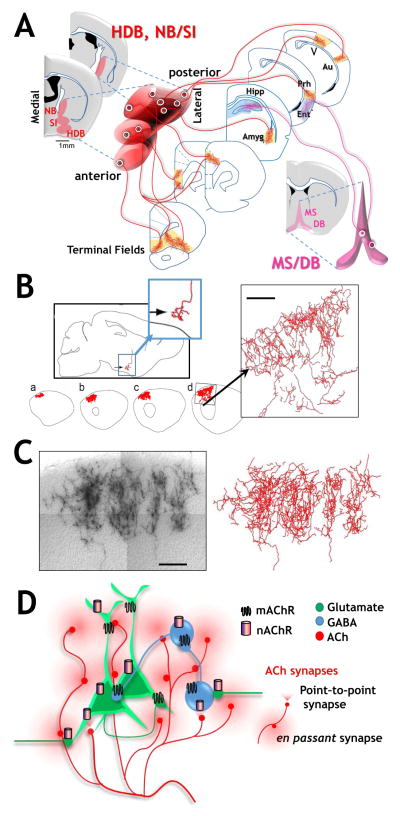
Figure 1. Functionally modular projection patterns, exotic axonal morphologies and diverse ACh release-receptor interactions contribute to complex spatio-temporal dynamics of ACh signaling by basal forebrain cholinergic neurons.
(see text and Reviews by Munoz & Rudy, 2014; Picciotto et al., 2012; Sarter, 2016; Zaborzsky et al., 2015).
A. Schematic of projection patterns of basal forebrain cholinergic neurons. Left hand side: schematic of coronal sections indicating the approximate anterior to posterior and medial to lateral distribution of the HDB (horizontal limb of the diagonal band) and NB/SI (Nucleus Basalis/Substantia innominata). Anterior medial BFCNs within these nuclear groups project to medial frontal cortical targets whereas posterior located cholinergic neurons project to more posterior targets such as the BLA and perirhinal cortex. Right hand side: Medial septal (MS) and vertical limb of the diagonal band (VDB) neurons provide cholinergic input to the hippocampus and entorhinal cortex.
B. and C. Axonal morphology of fully reconstructed basal forebrain cholinergic neurons and the extensive terminal arborization formed in cortex. Adapted with permission from Wu et al., 2014 https://creativecommons.org/licenses/by/3.0/.
D. Schematic representation of both point-to-point (focused, triangular) and en passant (broad circular) mechanisms by which ACh is released in the CNS, thereby effecting both glutamatergic (green) and GABAergic (blue) neuronal excitability. Such release profiles may correspond to the more rapid and transient responses and the slower, longer lasting modulatory effects of ACh, respectively (see text for discussion). Also shown are representative distributions of both muscarinic (depicted as 7 TM squiggles) and nicotinic (represented as single tubes) AChRs at pre, post and peri synaptic sites. Both mAChR and nAChR subtypes at each of these locations contribute to the direct and indirect mechanisms by which ACh can alter synaptic excitability (see text for discussion).
1a.ii. Birth and migration of BFCNs
The basal forebrain cholinergic neurons (BFCN) are born in the neurogenic zones of the ventral telencephalon, including the medial ganglionic eminence (MGE) and the preoptic area. The newborn neurons migrate radially, and organize into distinct clusters along the rostral-caudal axis of the forebrain (Marin et al., 2000). An alternative path for a sub population of cholinergic neurons in Ch3 (hDB) and Ch4 (NB/SI) that appear to originate in the ventral pallium has also been described (Pombero et al., 2011). BFCN identity is determined by the sequential expression of distinct combinations of transcription factors. In contrast, very little is known about the developmental programs that guide axonal projections through distinct routes and to distinct target fields. Analyses of the consequence of genetic deletion of various transcription factors have defined early events that distinguish major subpopulations of MGE/POA derived neurons, e.g. GABAergic vs cholinergic (Sussel et al., 1999; Zhao et al., 2003), striatal cholinergic interneuron vs. BF cholinergic projection neurons (Chen et al., 2010b). These studies, along with those probing the function of cell surface receptors (Boskovic et al., 2014; Cui et al., 2011; Elshatory and Gan, 2008; Jia et al., 2014; Sanchez-Ortiz et al., 2012) reveal subtle differences in the response of distinct populations of neurons within the BFCNs. These differences raise the likelihood that there is an as yet under-appreciated heterogeneity of BFCNs. How, or if, this heterogeneity relates to differential BFCN innervation patterns, firing profiles or loss in neurodegenerative disorders is not clear. A full appreciation for the complexity of BFCN awaits the use of higher resolution molecular technologies including single cell transcriptome analyses.
1a.iii. Projection fields of Basal Forebrain Cholinergic Neurons
The MS and DB form a functional cluster that sends cholinergic projections primarily to the hippocampus, parahippocampus olfactory bulb and midline cortical structures (Knox and Keller, 2015). In contrast, the NB (or NBM) and SI provide the majority of cholinergic projections to neocortex and to the amygdala (Woolf, 1991). Each of these nuclei is heterogeneous in phenotype: the cholinergic neurons are intermingled with neurons of distinct transmitter and peptide content (Gritti et al., 2006; Mufson et al., 2006).
Although the concept that there is a rough topographical organization of the basal forebrain has been discussed since the 1980’s (Mesulam et al., 1983; Saper, 1984; Zaborszky et al., 1999) the cholinergic system has more typically been viewed as both spatially and functionally “diffuse” (Saper, 1984, Woolf, 1991). Recent studies paint a somewhat different picture by combining a rich array of immunological and genetic techniques to efficiently label neurons expressing choline acetyltransferase (ChAT), the biosynthetic enzyme for acetylcholine (ACh) and the vesicular ACh transporter (VAChT). These genetic approaches have been united with anterograde, retrograde and transynaptic labels to optimize the comprehensive mapping of cholinergic cell bodies and their projection fields (although see Yi et al. 2015 for a consideration of the caveats associated with purely transgenic probes). Functional connectivity is further explored using photoactivatable, genetically targeted probes that permit selective excitation or inhibition of ChAT+ neurons down to select branches of their terminal arbors (Figure 1A; Jiang et al., 2016; Unal et al., 2015). Alternatively, intentionally sparse genetic labeling has been used to provide unprecedented insight into the morphology of single BFCNs, revealing that these neurons have a considerably more extensive and complex axonal architecture than previously appreciated (Fig 1B, C; Wu et al., 2014).
Overall, these new studies support the idea that the connectivity of cholinergic projection neurons to their cortical targets has sufficient specificity to subserve functionally and spatially selective signaling. Perhaps the most important generalization is the predominant role of a functionally based topographical organization of the BFCNs as suggested by Zaborsky & colleagues.
The projections from basal forebrain cholinergic neurons to frontal cortical targets are the most completely described (Bloem et al., 2014; Chandler and Waterhouse, 2012; Chandler et al., 2013). Medial frontal cortex receives projections from more medial and anterior located cholinergic neurons within the basal forebrain nuclear groups. The dorsal regions of prefrontal cortical areas receive projections from medially located NB/SI and DB neurons, whereas more ventral regions of prefrontal cortex receive projections from more laterally located BFCNs (Fig 1A & Bloem et al., 2014). More rostrally positioned BFCNs appear to project to both superficial and deep layers of frontal cortex, while more caudally placed BFCNs preferentially project to deep layers of cortex (Bloem et al., 2014).
Lateral portions of cortical and subcortical areas, for the most part, receive projections from more lateral and more posterior located cholinergic neurons (Fig 1A & Kondo and Zaborszky, 2016; Zaborszky et al., 2015a; Zaborszky et al., 2015b). There are at least two exceptions to this general topography: the hippocampus and the entorhinal cortex (in contrast to the adjacent perirhinal/ectorhinal cortices) receive the majority of their cholinergic input from the MS/VDB cholinergic groups (Kondo and Zaborszky, 2016; Woolf, 1991).
Simultaneous retrograde BF labeling from multiple cortical regions reveals several organizing principles. First, although there is overlap within the basal forebrain of cholinergic neurons that project to non-adjacent cortical areas, the degree of overlap is dependent upon the interconnectivity of the cortical projection areas in question. These findings suggest a level of organization of the BFCN neurons that might facilitate coordinate control of functionally linked, although spatially distinct, cortical projection areas (Figure 1A; Zaborszky et al., 2015a; Zaborszky et al., 2015b). Second, axons from very distinct BF areas can innervate immediately adjacent cortical regions, such as the perirhinal (NB/SI) and entorhinal cortices (MS/vDB). Whether this latter example indicates a hierarchy of coordinate functional modulation or is the result of developmental differences between archeo and neocortex remains to be seen.
1a.iv. Axonal morphology of basal forebrain cholinergic neurons
Axons from individual cholinergic neurons form collaterals that innervate multiple cortical regions that are functionally related (Chandler and Waterhouse, 2012; Chandler et al., 2013; Chavez and Zaborszky, 2016), but do not appear to have collaterals to functionally distinct regions of neocortex (e.g. A1 vs V1; Kim et al., 2016). Recent experiments employing retrograde labeling of BFCNs neurons demonstrate that as many as 20% of labeled NBM/SI neurons have axons that terminate in 3 different prefrontal domains, including the medial PFC, anterior cingulate cortex and the orbitofrontal cortex (Chandler and Waterhouse, 2012; Chandler et al., 2013).
The ability of single BFCN neurons to target large territories of cortex clearly requires extensive collateral formation. Just how elaborate individual cholinergic axon arbors can be was demonstrated - rather elegantly - by sparse genetic labeling (Wu et al., 2014; and see Fig 1B & 1C, taken from Wu et al., 2014 with permission). Based on their measurements the calculated average length of a single axon of a basal forebrain cholinergic neuron in a mouse brain, including all of its terminal branches, is ~30 cm. Calculations based on human post mortem data are consistent with single cholinergic axons of >100 meters! Clearly the BF cholinergic neurons have the capacity to modulate activity across multiple cortical areas or columns within specific cortices – but likely at an enormous metabolic cost. Indeed, metabolic demand to maintain function over the lifespan has been raised as a possible basis for the sensitivity of the BF cholinergic system in neurodegenerative disorders (Wu et al., 2014).
1a.v. Summary
Taken together the more recent mapping and morphological studies of BFCNs demonstrate that (a) these cholinergic projection neurons can be extremely elaborate in both the extent of axonal arbors and the number of axonal branches, (b) there is topographic, rather than diffuse, organization of BFCNs and their target fields along anterior to posterior, ventral to dorsal and medial to lateral axes (Fig 1; Kim et al., 2016), (c) in frontal cortex some cholinergic projection neurons are “dedicated” i.e. there are BFCNs that project to a single region of orbitofrontal, anterior cingulate or prefrontal cortex, but the majority of BFCNs project to more than one frontal domain. These multiply projecting BFCNs appear to innervate functionally connected structures and might mediate co-ordinate cholinergic signaling in behaviorally related targets. Consistent with this idea, BFCN input to operationally distinct areas of sensory cortex are segregated from one another along the anterior posterior axis of BFCN (Kim et al., 2016). Overall, the concept of cholinergic signaling occurring in functional modules has received robust support in this new era of brain mapping.
An area of study that has received relatively little scrutiny with the modern mapping techniques is the architecture of cholinergic projections to deeper structures, such as the hippocampus and amygdala. Given that the basal lateral amygdala (BLA) appears to receive the densest cholinergic innervation of any structure besides the striatum, and cholinergic input to the BLA exerts an important influence on acquisition and retention of emotional memories (Knox, 2016), detailed single neuron mapping of the cholinergic neurons that project to the amygdala should be very revealing.
1b. Multiple temporal & spatial profiles of ACh release & ACh actions
1b.i. Overview of ACh release mechanisms
In the peripheral nervous system (PNS), ACh is typically released in excess and in close apposition to the post synaptic target. Despite rapid hydrolysis by acetylcholinesterase (AChE), sufficient ACh survives the transynaptic journey to elicit fast and robust signaling via activation of muscarinic and/or nicotinic receptors in a highly temporally & spatially restricted manner. In the CNS, cholinergic signaling is also initiated when ACh is released and is mediated by interaction with ACh receptors on target cells. This is where the similarities between PNS and CNS cholinergic signaling appear to end. ACh release in the brain has classically been conceptualized as slow and tonic (Descarries et al., 1997). This idea of a “volume” mode of transmission was supported by early views that the cholinergic system was anatomically diffuse (an idea that is increasingly being challenged, as per above), and early microdialysis experiments - with spatially and temporally limited probes – that documented ambient levels of ACh in the micromolar range tonically present in brain tissue (Descarries et al., 1997). This notion has now been substantially revised (Sarter & colleagues; see Sarter et al., 2014).
1.b. ii. Temporal profiles of ACh release: transient vs tonic
Our understanding of the spatiotemporal profiles of ACh release from basal forebrain cholinergic neurons has been transformed by the generation of new, high resolution stimulation and recording techniques. Based on more rapid assays for ACh release and on new approaches for selective activation of cholinergic neurons and specific BFCN terminal fields, we now know that ACh release and downstream signaling can be faster and more focal than previously appreciated (Munoz and Rudy, 2014; Sarter and Kim, 2015). The discovery of relatively fast increases (over seconds, rather than minutes) in ACh release was made possible by the development of choline oxidase coated microelectrodes that detect the choline formed following breakdown of ACh (Parikh et al., 2004). These microelectrodes are highly selective and offer efficient and accurate temporal and spatial resolution of ACh release.
Using electrochemical detection, Sarter & colleagues have convincingly demonstrated the existence of cholinergic “transients” - relatively rapid spikes of ACh - that begin within 200–500 msec of a behaviorally relevant stimulus and last several seconds (Sarter et al., 2014). By combining optogenetic stimulation of BFCN cell bodies or cholinergic terminal fields in mPFC with electrochemical detection, Gritton et al (2016) detected ACh release within 100 msec of the light stimulus. A recent study by Nelson & Mooney (2016) demonstrates even faster kinetics of cholinergic signaling: they report direct BFCN to pyramidal neuron (nicotinic) EPSCs with ~10 msec delay from optogenetic activation of terminal fields to evoked postsynaptic responses.
Despite the growing evidence for more temporally precise ACh release and cholinergic signaling, there is still strong support from experiments that support a slower and spatially broader change in ACh concentration. Runfeldt et al (2014) demonstrate profound effects of these slower, more extensive changes in ACh on microcircuit properties in the cortex that may be important for detection and encoding of information. Likewise, the time course of ACh responses following optogenetic stimulation of BF cholinergic projection neurons vs their terminal fields in BLA are consistent with relatively rapid effects of released ACh (detection within <20–100 msec), and yet appear to be entirely “modulatory” in nature, changing BLA principal neuron firing patterns and/or the efficacy of transmission at cortical-BLA synapses (Jiang et al., 2016; Unal et al., 2015).
Distinct cellular phenotypes of cholinergic neurons may provide the cellular basis for two different modes of signaling: fast and focal and slow and paracrine. Zaborszky and colleagues have performed detailed electrophysiological characterization of the cholinergic neurons in the NBM and identified two populations: a more excitable, early firing population that show spike frequency adaptation and a less excitable, late firing population that could maintain low frequency tonic firing (Unal et al., 2012). Overall, it appears that both more rapid/transient as well as less temporally and spatially focal modes of ACh release play important roles in different aspects of information processing (Picciotto et al., 2012; Sarter and Kim, 2015).
It is not yet clear whether cholinergic transients mediating relatively rapid responses are fundamentally different from the signaling that underlies longer lasting, neuromodulatory changes in circuit dynamics by ACh. The term “neuromodulation” has had variable definitions associated with “changes in state of a neuron or a group of neurons that alters the response to subsequent stimulation” (Picciotto et al., 2012). Neuromodulation by ACh has been shown to include changes in release probability, shifts in firing patterns or altered excitability through shifts in the input/output relationship (Picciotto et al., 2012). The spatial and temporal dynamics of ACh signaling underlying cholinergic modulation may vary depending on the system.
1b.iii. Summary
The cumulative evidence on ACh release appears to support multiple temporal modalities and spatial domains. In addition to tonic, low levels of ACh release, temporally and spatially discrete release of ACh may play a vital and specific role in mediating cognitive processes. In view of the potentially vast “synaptic space” covered by the axonal terminal arbors of single cholinergic neurons (Wu et al., 2014; Fig 1), the onset and duration of co-ordinate ACh effects may be very broad, despite focal release at each synaptic bouton. Alternatively, one might propose mechanisms for selective activation of a subset of boutons along a single BFCN terminal arbor. An interesting avenue for future research is how differential expression of acetylcholinesterase may influence the various temporal profiles of ACh release and signaling. Resolution of the details of the temporal and spatial dynamics of ACh signaling are within reach with new technologies for focal, selective stimulation and monitoring of resultant Ca signals within specific subcellular compartments.
1c. ACh receptors: still more diversity in receptor subtypes, cellular locales and downstream signaling mechanisms
1c.i. Overview: ACh receptors
Once released from the terminal axonal arbors, ACh signaling proceeds via its binding to acetylcholine receptors (AChRs) to exert a wide range of effects that are dependent on the AChR subtype(s) present on the target, the cellular localization of the AChRs and the multiplicity of downstream signaling cascades that can be activated. AChR mediated responses were originally categorized based on their sensitivity to plant alkaloids and subsequently on their binding capacity for muscarine or nicotine (mAChRs & nAChRs). Both classes of AChRs are distributed widely throughout the brain (Gotti et al., 2006; Thiele, 2013). Although the structural diversity of mAChR and nAChRs has been known for several decades, new evidence supports still more receptor subtypes and signaling mechanisms by which ACh can modulate network excitability than previously appreciated.
1c.ii. Muscarinic and Nicotinic AChRs
Muscarinic AChRs are metabotropic receptors that bind ACh and transduce their signaling via activation of heterotrimeric G proteins that in turn affect the opening, closing, and kinetics of (primarily) K, Ca and non-selective cation channels. There are 5 mAChR encoding genes (M1–M5) that are often considered in two broader groups: the M1 type which includes M1, M3 and M5 and signals via Gq/11, and the M2 type which includes M2 and M4 receptors, that signal via Gi/o (reviewed in Thiele, 2013). The effects of mAChR activation are typically somewhat slower and more long-lasting than those activated via the ionotropic nicotinic AChRs (Brown, 2010; Thiele, 2013).
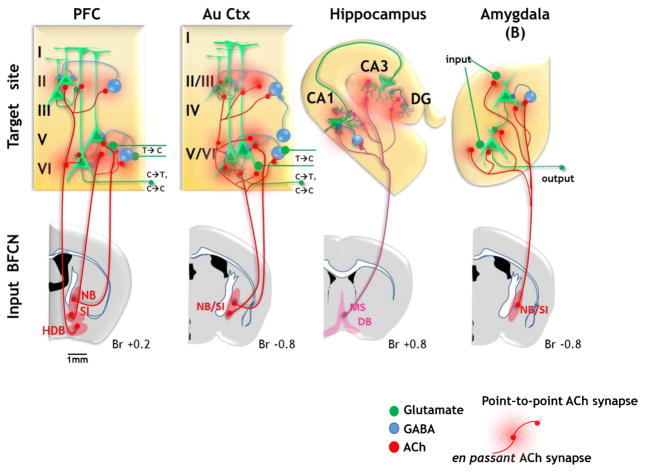
In cortical circuits, M1 receptors are widely expressed on somatodendritic domains of both interneurons and layer II/III and layer V pyramidal neurons, where they increase excitability. In contrast, M2 receptors are typically pre-synaptic, serving both as autoreceptors on cholinergic axons acting as a brake on ACh release, and as heterosynaptic receptors on local inhibitory GABAergic terminals to decrease GABA release. M4 receptors are expressed in somatodendritic domains of layer IV pyramidal neurons (Figure 1D; Thiele, 2013). The coordinate effect of ACh release, acting through these distinctly expressed and targeted mACh receptors, as well as nicotinic AChRs, is believed to contribute to gating of information flow through, and perhaps between, cortical columns.
A recent finding places M1 receptors on hippocampal dentate gyrus axons where their activation is coupled to increased T-type Ca channel activity. The resultant increase in intra-axonal Ca sustains increases in granule cell axonal excitability (Martinello et al., 2015). The authors propose that this previously unreported cellular signaling mechanism may be an important pathway for cholinergic enhancement of cognitive processing in hippocampus.
In another recent advance, Butcher et al. (2016) have developed antibodies that allow detection of activated M1 receptors in tissue (based on the connection between ligand dependent activation of M1 receptors, and phosphorylation of serine 228). Using this reagent, they demonstrate M1 activation in hippocampus in response to fear conditioning. The combination of these types of tools with those for the selective stimulation of BFCN terminal fields and assays of ACh release described above will certainly provide important insight into how and where mAChRs participate in complex cognitive tasks.
In contrast to ACh interactions with metabotropic mAChRs, ACh binding to nicotinic AChRs results in the direct gating of non-selective cation channels. Twelve different types of nAChR subunits have been identified in brain (α2–10 and β2–β4; Dani and Bertrand, 2007). In principle, all of these subunits could combine in different proportions and order around the receptor pore to form both homomeric and heteromeric pentamers (Dani and Bertrand, 2007). Mercifully, the actual set of combinations and permutations expressed in the brain is far fewer than the simple factorial calculation would suggest. The most common nAChR subtypes in the brain are α4β2* and α7* nAChRs receptors (where the asterisk indicates “containing”; Gotti et al., 2006). These differ in their permeability to calcium, affinity for ACh, profile of agonist induced desensitization, sensitivity to allosteric modulators and sensitivity to both endogenous and exogenous nAChRs antagonists (Dani and Bertrand, 2007; Miwa et al., 2012). The α7* nAChRs are renowned for their relatively high Ca conductance and relatively low agonist affinity. In contrast α4β2* nAChRs have lower Ca conductance and higher agonist affinity (Dani and Bertrand, 2007).
As is the case with mAChRs there are multiple types of nAChRs expressed on both pyramidal and interneuron populations in cortex whose activation by ACh contributes to attention circuits. Mice in which the β2 nAChR subunit gene has been disrupted perform poorly in a 5 choice serial reaction time task. Lentiviral mediated restoration of β2*nAChRs within the prelimbic, but not anterior cingulate cortex rescued attentional performance (Guillem et al., 2011). In a follow-up study this group first carefully mapped the individual neuronal responses to ACh via nAChRs in mPFC, demonstrating layer and nAChR subtype specific pyramidal neuron and interneuron responses. Both β2*nAChR and α7* nAChR mediated ACh currents were seen in interneurons at all levels, although increases in IPSC measured in pyramidal neurons were seen in layers II/III and V but not layer VI (Fig 2). ACh induced currents were also seen in layer V and layer VI pyramidal neurons – in the former case mediated both by pyramidal neuron α7* nAChR and presynaptic β2*nAChR. Layer VI responses were predominately from β2*nAChR. Bath application increased both interneuronal and pyramidal neuron firing at all levels except in layer II/III pyramidal neurons – a network response that required β2*nAChRs (Poorthuis et al., 2013). In sum, these studies begin to unravel the complex relationships between different nAChRs within mPFC circuits that relate to attention (Fig 2).
Figure 2. Schematic Representation of Cholinergic Inputs and Signaling in Cortical, Hippocampal and Amygdala circuits.
(see text) from Bloem et al., 2014.; Kim et al., 2016; Nelson & Mooney, 2016; Jiang et al., 2016; Munoz & Rudy, 2014; Gu & Yakel, 2011; Cheng & Yakel, 2016. Numerous studies now converge on specific mechanisms of ACh release and profiles of AChR activation in different brain areas. Each schematic represents a summary of recent studies of the cholinergic projection neurons (below) and consequent signaling effects of ACh in local circuit activity in (above; left to right) the Prefrontal Cortex (PFC), Auditory cortex (Au Ctx), hippocampus and (basal) amygdala.
The presence of nAChRs on a diverse set of cell types within and beyond the brain has ignited the search for endogenous small molecule ligands (other than ACh) and for proteins that interact with nAChRs. Three findings are potentially relevant to understanding the role of nAChRs in cognitive processes.
First, it is now clear that multiple members of the Ly6 family, a small gene family encoding GPI-anchored cell surface proteins that share a so-called three finger fold with snake toxins, bind to nAChRs. Lynx1 and Lynx2 were originally identified as proteins that modulated a range of nAChR channel properties, (especially α4β2*nAChRs) and were proposed to essentially serve as a brake on cholinergic signaling in the CNS. Additional family members have now been shown to interact with α4β2, as well as α7*nAChRs and to affect both channel functions and intracellular trafficking (Miwa et al., 2012; Nichols et al., 2014; Puddifoot et al., 2015; Wu et al., 2015).
Second, Kabbani and colleagues have demonstrated that α7*nAChR form complexes with heterotrimeric G proteins, providing a foundation for sustained α7* mediated effects in neurons (Nordman and Kabbani, 2012; Nordman et al., 2014; Zhong et al., 2008; Zhong et al., 2013). Thus, not only can α7*nAChR function as ionotropic receptors and sources of elevated Ca2+, they can also activate metabotropic-like second messenger signaling. It is not yet clear how extensive these signaling mechanisms are in vivo, but they force a reconsideration of the time scales and mechanisms by which α7*nAChRs can influence circuit excitability well beyond direct ionotropic receptor gating.
Third is the potential role of presynaptic α7* AChRs as sites for Abeta peptide binding and activation of increased glutamate release in circuits related to cognition (Hascup and Hascup, 2016; Koukouli and Maskos, 2015; Lilja et al., 2011). It should also be noted that until recently, Abeta was thought to only interact with α7 homo-pentameric nAChRs. After more than 20 years of speculation that endogenous α7* receptors may be heteromeric, α7β2* receptors have now been established as a potentially significant component of the brain nAChRs (Wu et al., 2016). Of particular interest is the presence of α7β2* in BFCNs and the fact that α7β2* receptors have up to 100-fold greater sensitivity to pathological Abeta peptides than do homomeric α7 nAChRs. These new findings raise the possibility that α7β2* AChRs contribute to the cholinergic loss in Alzheimer’s disease (see Part 3, below).
The debate continues as to the physiological impact of and mechanisms by which nAChRs contribute to the modulation of synaptic excitability. Examples abound demonstrating the presynaptic localization of nAChRs receptors in slice, but evidence from in vivo systems demonstrating that the presynaptic nAChRs are a requisite component of behavior are rare (Duffy et al., 2009; Fabian-Fine et al., 2001; Jiang et al., 2013; Lubin et al., 1999). The functional localization of α7* nAChRs to presynaptic sites, plus their high Ca permeability, supports the idea that activation of even a very few such receptors could profoundly affect the release of the neurotransmitters stored in the ACh-responsive synaptic bouton (Cheng and Yakel, 2015; Dickinson et al., 2008; Zhong et al., 2013). Such a scenario with presynaptic α7* receptors and downstream activation of Ca signaling has also been implicated – but not yet proven - as an essential component of prolonged increases in release probability induced by AChR activation in acute slice preparations of and in in vivo recordings from cortex, hippocampus and amygdala (Fig 1D; 2 and Cheng and Yakel, 2015; Dickinson et al., 2008; Jiang et al., 2013; Jiang et al., 2016; Zhong et al., 2013).
1c. iii. AChRs location, location, location
It has been appreciated for some time that various subtypes of both muscarinic and nicotinic AChRs are often located at pre and post synaptic sites, with some receptors located “extrasynaptically” at considerable distance from the origin(s) of ACh release (Fabian-Fine et al., 2001; MacDermott et al., 1999; Mechawar et al., 2002; Picciotto et al., 2012; Yamasaki et al., 2010). The presence of both mAChRs and nAChRs on presynaptic terminals that release transmitters other than ACh is now considered a common mechanism for heterosynaptic regulation of synaptic plasticity (Picciotto et al., 2012). Nevertheless, evidence for precise apposition of BFCN terminals with cholinoceptive presynaptic structures that would be required as proof of such heterosynaptic modulation remains a serious challenge, one that hopefully can be addressed by application of new high resolution 3D imaging. Indeed, the observation that AChRs are often at some distance from the presumptive sites of ACh release has been cited as evidence for a broader spatial distribution of AChR ligands than would be predicted by focal ACh release and robust AChE activity (Descarries and Mechawar, 2000; Yamasaki et al., 2010).
1c.iv. Summary
New sites and mechanisms of cholinergic signaling have come to the fore, adding to the already rich diversity of means by which ACh can contribute to the mediation and modulation of activity in neural networks. Overall, recent findings converge on the idea that both mAChRs and nAChRs are distributed to contribute to multiple modes of ACh signaling. The real challenge lies with discerning which of the myriad signaling mechanisms available are actually employed when ACh is released in vivo. The latter challenge has been significantly advanced with the advent of selective stimulation and recording techniques that allow the assessment of endogenously released ACh.
There are increasing numbers of reports consistent with cholinergic transmission being mediated by fast synaptic release and consequent activation of postsynaptic nicotinic and/or muscarinic AChRs (e.g. Nelson and Mooney, 2016). On the other hand, the paucity of morphological evidence for point-to-point cholinergic contacts and the prolonged temporal profile of ACh effects are consistent with more modulatory actions (Mechawar et al., 2002; Umbriaco et al., 1995; Umbriaco et al., 1994). The latter include examples of long lasting modulatory changes in excitability due to presynaptic changes mediated by nicotinic and/or muscarinic AChRs. Recent optogenetic studies further demonstrate that ACh release from BFCNs can elicit LTP and STD in hippocampus (Gu and Yakel, 2011) and LTP at cortical- BLA synapses (Jiang et al., 2016). Likewise, durable changes in post synaptic excitability are also documented that may engage AChRs coupled to G protein signaling and/or changes in Ca signaling networks.
Although the multiplicity of potential mechanisms and the complexity of cholinergic signaling may be inconvenient to study, the data are, in fact, consistent with multiple temporal and spatial mechanisms by which ACh can interact with its cognate receptors in vivo. We can now combine the increased resolution afforded by significant technological advances, in both selective cholinergic stimulation and release assays, with advancing methodologies for electrophysiological and imaging based recording in awake, behaving animals. These combinatorial approaches to examine cholinergic signaling dynamics in vivo bring us closer to physiologically and behaviorally relevant answers to the question of HOW cholinergic signaling influences the excitability of specific circuits and networks to alter cognitive processing.
2. Cholinergic signaling and circuits involved in attention and memory
A wealth of prior physiological, lesion, pharmacological and genetic studies converge on the idea that ACh is involved in cognitive processes, including attention and memory (Hasselmo and Sarter, 2011; Micheau and Marighetto, 2011; Picciotto et al., 2012). Here we will briefly review the evidence that acetylcholine signaling is involved in specific aspects of cognitive processing, focusing on recent work on circuit mechanisms underlying cognition and beginning with the best studied cognitive domain with respect to ACh: attention.
2a. Cholinergic signaling and circuits in attention
2a.i. Overview of the role of ACh in mediating attention
Pharmacological exposure to exogenous substances sparked early work into the potential involvement of AChR signaling in attention. Nicotine has been widely reported to improve performance in specific attention tasks and exposure to nicotine during development can lead to lasting impairments in attention performance and in the brain areas thought to mediate attention (Bloem et al., 2014; Jung et al., 2016). Studies of polymorphisms, mutations, and deletions of various cholinergic genes further link alterations in cholinergic signaling to modified attention performance (Sarter et al., 2016a; Sarter et al., 2016b). Overall, the last 5 years have greatly sharpened our understanding of how cholinergic circuits co-ordinate with both prefrontal and sensory cortices to shape behavior in response to attentional tasks.
Attention has been conceptualized as consisting of two separate processing streams: goal or cue driven attention is termed “top down” while sensory driven attention is termed “bottom up” (Katsuki and Constantinidis, 2014; Sarter et al., 2016b). Essentially, top down attention may be thought of as voluntary, or “feed-back” driven - whereby incoming sensory information is modulated by higher cortical areas. In contrast, bottom up attention is thought of as involuntary, or “feedforward” - whereby sensory information is fed forward and up to the cortex (Katsuki and Constantinidis, 2014; Sarter et al., 2016b). Here we will focus on cholinergic circuit mechanisms of “top down” aspects of attention, as it is the best studied and most pertinent to cognitive performance.
2a.ii. Cholinergic modulation of prefrontal cortex related to attention
The prefrontal cortex (PFC) is an integral node in circuits underlying attention, exerting top down control over sensory cortical areas to enhance detection of task relevant cues. The PFC is also a significant target of cholinergic modulation: the organization of cholinergic inputs to the PFC from the NBM and the DB has now been mapped in detail (Fig 2; Bloem et al., 2014, Chandler and Waterhouse, 2012, Chandler et al., 2013).
Sarter and his colleagues have used their enzyme selective microelectrodes to document the presence of transient (subsecond to seconds) increases in ACh in the PFC in response to attention task related cues (Howe et al., 2013; Parikh et al., 2007). Based on the speed and relatively short lived duration of the choline transients, they propose that, at least in PFC, ACh mediates, rather than modulates, cue detection and cue triggered changes in goal oriented behavior (Howe et al., 2013, Parikh et al., 2007). Gritton et al. (2016) demonstrated that enhancing the cue associated cholinergic transient with optogenetic stimulation improved cue detection and that stimulation of a cholinergic transient during a non-cued trial led to a “false positive” behavioral response from the animal, presumably due to a mistaken detection of a cue. Blocking the ACh transients with optogenetic inhibition of BFCNs caused the animals to “miss” many of the cues, consistent with the idea that ACh release is a requisite signal for cue detection (Gritton et al., 2016). As such, all of the pertinent arguments are now in place: the relevant PFC circuits are cholinoceptive, ACh is released in the PFC during execution of goal-directed attention tasks and the release of ACh is both necessary and sufficient to mediate task related cue detection.
Together these data support the idea that ACh signaling in the PFC is positioned at both the beginning and the end of the attention loop, initiating top down control over downstream sensory cortical areas to enhance detection of behavior relevant cues and also signaling cue detection to the animal via transient cholinergic release/signaling. Recent findings suggest that the downstream mechanisms by which PFC circuits are coordinated with appropriate sensory cortical areas may also engage BF cholinergic signaling (Nguyen et al., 2015).
2a.iii. Cholinergic modulation of sensory cortex related to attention
Detection of behaviorally relevant stimuli in sensory cortices during attentional performance is thought be facilitated by an increase in signal to noise ratio due to increased firing rate of task relevant sensory cortical neurons, along with a decorrelation of intra cortical noise, often measured as “desynchronization”, or decreased power of low frequency LFP. During the actual performance of a behavioral task, decorrelation increases the response reliability of sensory cortex neurons to the appropriate stimuli (Cohen and Maunsell, 2009; Mitchell et al., 2009). Stimulation of cholinergic signaling from the basal forebrain to sensory cortex (S1, A1 or V1) has now been definitively demonstrated to mediate decorrelation of neuronal activity (Chen et al., 2015; Goard and Dan, 2009; Kalmbach et al., 2012; Kalmbach and Waters, 2014; Kim et al., 2016; Pinto et al., 2013). Chen et al. (2015) identified the micro-circuitry that is likely responsible for the cholinergic control of desynchronization during visual attention tasks. Optogenetic stimulation of cholinergic signaling in V1 elicits IPSCs in PV+ interneurons and in pyramidal neurons receiving input from SOM+ interneurons. The cholinergic stimulation of SOM+ interneuron activity appears to be both necessary and sufficient to mediate desynchronization (Chen et al., 2015). Optogenetic inhibition of cholinergic neurons in the basal forebrain leads to increased synchronization of cortical neurons and decreased response reliability of cortical sensory neurons, again consistent with the idea that cholinergic signaling is both necessary and sufficient for mediating cortical correlates of attention (Pinto et al., 2013). ACh application in sensory cortices also improves the ability of the cortex to discriminate between stimuli and increases modularity of microcircuit activity by modulating the response to thalamic inputs, thereby seeming to facilitate the processing of specific stimuli (Runfeldt et al., 2014; Thiele et al., 2012). However, at least in auditory cortex, ACh actually seems to broaden tuning curves (Nelson and Mooney, 2016).
2a.vi. Summary
Recent studies delineate the multiple contributions of basal forebrain cholinergic signaling to the synaptic and circuit mechanisms engaged in attentional processing in PFC and sensory cortex (Fig 2). Although we don’t yet know the mechanisms underlying the integration of these cortical processes of attention at the level of network interactions, the central role of BF cholinergic signaling in cue detection and attentional processing is becoming clear.
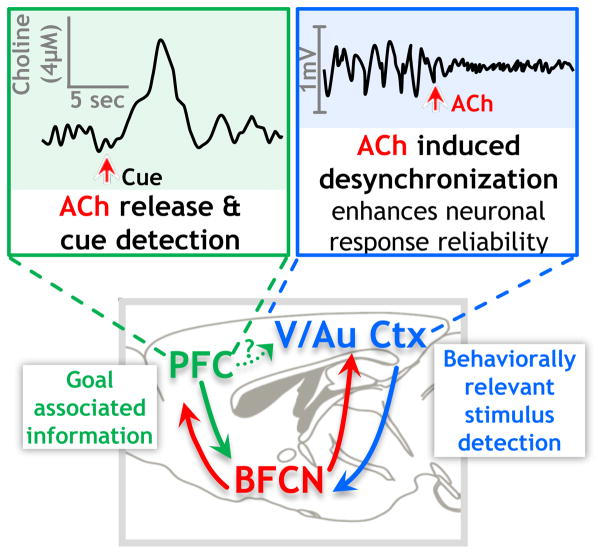
Figure 3 presents a schematic of potential cholinergic interactions with networks engaged in selective attention. In this schematic, task relevant information would be “filtered” by PFC and integrated with modality specific encoding in sensory cortices via the cholinergic basal forebrain relay. Cholinergic modulation of circuits within the sensory cortices induces decorrelation of intracortical noise, which contributes to increasing the signal to noise ratio and facilitating response reliability to behaviorally relevant stimuli. If the PFC exerts top down control over sensory cortices during attentional performance, then the attention related activity changes observed in sensory areas may be due, at least in part, to indirect PFC signaling via cholinergic basal forebrain relays (Nguyen et al., 2015). Detection of a behaviorally relevant cue is then communicated back to the PFC by the sensory cortex, via circuitry including the cholinergic basal forebrain which triggers a transient release of ACh in the PFC, signaling cue detection to the animal.
Figure 3. Schematic of BFCN interaction with attention related circuitry.
Task oriented information from the PFC is transmitted to the basal forebrain, which signals to sensory cortex where cholinergic signaling causes decorrelation (Chen et al., 2015; Goard & Dan, 2009; Kalmbach et al., 2012; Kalmbach & Waters, 2014; Pinto et al., 2013; Runfeldt et al., 2014; Thiele et al., 2012; Kim et al., 2016) and enhances response reliability (Cohen & Maunsell, 2009; Mitchell et al. 2009). Once a task relevant stimulus is detected in the sensory cortex, cholinergic signaling from the basal forebrain to the PFC is stimulated and transient ACh release within the PFC signals cue detection (Sarter et al., 2016a; Parikh et al., 2007; Howe et al., 2013).
There are, of course, several unresolved issues with this conceptual framework. In particular, the pathway(s) by which proposed PFC-stimulation influences sensory cortical activity remains to be directly demonstrated. In fact, the top down effects of PFC on visual cortex are only partially blocked by lesion of the cholinergic basal forebrain relay (Nguyen et al., 2015). In addition, although the enhanced signal to noise ratio in V1 is known to be mediated by cholinergic mechanisms of decorrelation, the mechanisms by which cholinergic signaling elicits increased excitability in V1 are less clear. Recent work by Nelson and Mooney (2016) in auditory cortex reveals that stimulating BF cholinergic input increased both excitation and inhibition via fast synaptic activation of nAChRs and that the net effect of BFCN input to auditory cortex is to broaden the bandwidth of individual neurons while restricting the dynamic range of the response strength.
More work is needed to resolve the contribution of cholinergic signaling to both the enhanced signal to noise ratio and to the increased excitability. Recent studies stressing the importance of M1 type receptors in mediating top down attention (Gould et al., 2015) and the considerable literature on ACh-mediated synaptic enhancement in PFC (Poorthuis et al., 2013) will be important to pursue to better understand how cholinergic signaling participates in PFC/sensory cortical interactions.
Finally, it should be noted that we have focused on cholinergic mechanisms of “top down” attention. Nicotine administration enhances ERP indices of both top down and bottom up attention and cholinergic mediated decorrelation of neuronal activity in V1 increases response reliability of neurons under both goal oriented, trained paradigms as well as during observation of naturalistic movies (Goard and Dan, 2009; Knott et al., 2014; Pinto et al., 2013). Enhancing cholinergic tone may therefore increase signal to noise ratios and facilitate stimulus detection whether or not performance is goal-oriented. Clearly more work is needed to determine the contribution of basal forebrain cholinergic inputs in the modulation of bottom up attention. Genetic manipulation of different cholinergic receptors appears to differentially influence performance on top down vs bottom up attention, with M1 mAChRs and α7* nAChRs being particularly important for top down, while α4* nAChRs are implicated in both attentional streams (Gould et al., 2015; Guillem et al., 2011; Hyde et al., 2016).
2b. Cholinergic signaling & circuits related to memory
2b.i. Overview of ACh in mediating memory
Memory is perhaps the most complex of cognitive functions, engaging a multiplicity of brain regions and a vast array of circuit and synaptic mechanisms for the initial acquisition, short and long term storage, recall, and/or extinction of a single memory. The following discussion is limited to potential contributions of acetylcholine to memory encoded in only two brain regions. Specifically, we discuss recent work on the role of cholinergic signaling in spatial memory, which heavily engages the hippocampus, and in emotionally salient memories, with a focus on studies in the amygdala (Burgess et al., 2002; Janak and Tye, 2015).
2b.ii. Basal Forebrain Cholinergic regulation of Hippocampal circuits related to memory
Cholinergic signaling from the MS and DB to the hippocampus is certainly important for formation of spatial memories: ACh has consistently been shown by microdialysis to be elevated in the hippocampus during performance of various memory tasks (Mitsushima et al., 2013; Roland et al., 2014; Stanley et al., 2012), and numerous studies have probed the effects of exogenous – and more recently endogenous - ACh on hippocampal plasticity and performance in spatial memory tasks (Cobb and Davies, 2005; Kutlu and Gould, 2016). Blockade of mAChR signaling locally in the hippocampus impairs memory (Carli et al., 1997; Wallenstein and Vago, 2001). The potential role for nAChRs-mediated signaling in spatial memory and context-dependent conditioning is more complex with some recent studies showing nAChRs upregulated in the hippocampus following spatial memory task acquisition (Kutlu and Gould, 2016; Shanmugasundaram et al., 2015; Subramaniyan et al., 2014), while other studies show that nicotine administration can variably enhance, depress or have little effect on short vs long term hippocampal based memories (Gould et al., 2015; Kutlu and Gould, 2016).
2b.ii.a. Cellular and Synaptic Mechanisms of ACh in the Hippocampus
Hippocampal circuits are renowned for their susceptibility to activity dependent synaptic plasticity, commonly considered a cellular substrate of memory. Recent work has demonstrated that cholinergic signaling, and specifically signaling via α7* nAChRs and M1-type mAChRs, plays an important role in long term potentiation (LTP) and plasticity at hippocampal synapses, providing a potential cellular level mechanism by which ACh may mediate memory (Fig 2.; Cheng and Yakel, 2015; Gu et al., 2012; Gu and Yakel, 2011). Exogenously applied or endogenously released ACh induces significant changes in synaptic plasticity in the hippocampus in a manner that is precisely controlled by the timing between the activation of cholinergic signaling and the activation of glutamatergic inputs to CA1 (Gu et al., 2012; Gu and Yakel, 2011). Changing the temporal relationship between optogenetic stimulation of cholinergic signaling and electrical stimulation of the CA3 → CA1 input, switches the type of synaptic plasticity induced from LTP to short term depression (STD), indicating that temporally specific cholinergic signaling is extremely important in determining how information in the hippocampus is encoded (Gu et al., 2012). Cholinergic stimulation at 100 ms before CA3 → CA1 input elicited an LTP that was entirely dependent on α7* nAChRs, whereas if cholinergic stimulation occurred 10 ms after the CA3 → CA1 stimulation, the LTP that was elicited was blocked (only) by muscarinic antagonists (Gu and Yakel, 2011).
Nicotinic AChR-mediated LTP in hippocampus depends on α7* nAChR expression in both the pre and post synaptic neurons and is accompanied by long lasting increases in calcium signals in both CA3 and CA1 neurons (Gu et al., 2012). Cholinergic-facilitated-STD was accompanied by decreases in calcium currents (Gu et al., 2012). Taken together, these data are consistent with the idea that endogenous cholinergic signaling modulates CA3 → CA1 synaptic plasticity in the hippocampus via α7* nAChR triggered calcium signals (Gu et al., 2012) as well as via M1 mAChRs, presumably by inactivating SK channels (Buchanan et al., 2010; Gu and Yakel, 2011). α7* nAChR selective agonists have also been shown to potentiate transmission and strengthen synapses in the hippocampus independent of presynaptic activity (Cheng and Yakel, 2014). Last, but not least, recent reports describe an entirely new cellular mechanism for hippocampal LTP by M1-type mAChR enhancement of axonal excitability (Martinello et al., 2015).
Nicotinic AChR-dependent strengthening of hippocampal synapses can be mediated by stabilizing GluA1 receptors on dendritic spines, an effect which is dependent upon α7* nAChR expression at hippocampal synapses (Halff et al., 2014). Galvez et al. (2016) studied the stabilization, rather than induction, of LTP and showed that that stabilization of LTP within the hippocampus is dependent upon cholinergic signaling, and specifically upon α7* nAChR signaling as well. Lesion of hippocampal cholinergic input or blockade of nAChRs rendered previously potentiated synapses vulnerable to “depotentiation” by low frequency stimulation. This effect was mediated by increased stabilization of f-actin and dendritic spines and independent of effects on AMPAR internalization. It therefore seems that nAChR signaling is critically important for both inducing and maintaining synaptic plasticity in the hippocampus. On the other hand, mAChR blockade prevents learning induced increases in AMPA/NMDA receptor ratio in the hippocampus, indicating an important role for mAChRs in mediating hippocampal synaptic plasticity (Mitsushima et al., 2013).
2b.ii.b. Circuit and Network Mechanisms of ACh in the Hippocampus
At the network level, the balance between gamma and theta band oscillations in hippocampal activity has been shown to be important for learning and memory (Duzel et al., 2010; Hasselmo and Stern, 2014). Cholinergic signaling can both induce these oscillations, and modulate their strength, perhaps providing an integrated electrophysiological substrate by which cholinergic signaling improves memory (Dannenberg et al., 2015; Douchamps et al., 2013; Lu and Henderson, 2010; Newman et al., 2013; Zhang et al., 2015).
Theta band oscillations are proposed to mediate the balance between encoding and retrieval of memory, with encoding occurring at theta peak and retrieval at theta trough (Hasselmo, 2014; Kunec et al., 2005). The separation between encoding and retrieval is considered vital for formation of accurate associations (Easton et al., 2012; Hasselmo and Stern, 2014; Kunec et al., 2005). In support of an essential role for cholinergic signaling in facilitating encoding, muscarinic blockade has been shown both to shift CA1 pyramidal cell firing towards theta trough (thus away from the encoding peak) during exploration of a novel environment and to impair the encoding of an experimental environment (Douchamps et al., 2013; Newman et al., 2014). Theta oscillations in CA3 are partially dependent on α7* nAChR signaling and theta oscillations in CA1 are completely abolished by knockout of the α7 gene (Lu and Henderson, 2010). Taken together, these studies indicate that both nAChR and mAChR signaling are important for induction and maintenance of theta oscillations as well as for memory encoding. Recent work has also shown that stimulation of cholinergic neurons in the MS led both to signaling via a direct, cholinergic basal forebrain - hippocampal projection and to the recruitment of an indirect, cholinergic to GABAergic basal forebrain to hippocampal pathway. The two pathways worked synergistically to maximize hippocampal firing synchrony with theta oscillations (Dannenberg et al., 2015).
Gamma oscillations, on the other hand, are thought to be an index of gating of information flow through the hippocampus: high frequency CA1 gamma is associated with gamma phase locking between CA1 and medial entorhinal cortex (MEC) and low frequency CA1 gamma is associated with gamma locking between CA3 and CA1 (Colgin et al., 2009). As the MEC provides the majority of information input to CA1, and CA3 is a critical area for information storage, gamma oscillations may be another mechanism to separate encoding from retrieval. Systemic cholinergic blockade reduces theta - gamma locking in the MEC, and reduces encoding of an enclosure (Douchamps et al., 2013). Gamma oscillations in CA3 are dependent upon α4β2 nAChRs (Zhang et al., 2015).
2b.iii. Basal Forebrain Cholinergic regulation of Amygdala circuits related to memory
In contrast to hippocampal-dependent spatial memory, the consolidation of emotionally salient memories is mediated in large part by the amygdala, a subcortical limbic structure that receives a dense cholinergic projection (Janak and Tye, 2015; Woolf, 1991). Cholinergic signaling specifically within the amygdala is vital for encoding emotionally salient memories (Fig 2; Knox, 2016). Optogenetic stimulation of cholinergic signaling in the amygdala strengthens emotionally salient memories, and optogenetic inhibition weakens them: both nAChRs and mAChRs appear to be involved (Jiang et al., 2016). Stimulation of cholinergic signaling in the amygdala can induce LTP under the same conditions that strengthen memory retention in vivo, perhaps providing a mechanism by which ACh mediates the formation of emotional memories (Jiang et al., 2016). These findings are consistent with other work demonstrating that cholinergic signaling via α7* nAChRs is necessary to induce activity dependent LTP in amygdala - paralleling results from the hippocampus showing that some forms of cholinergic LTP were uniquely dependent on α7* nAChR receptor expression (Gu et al., 2012; Jiang et al., 2013).
Other recent studies have emphasized the importance of inhibitory effects of cholinergic signaling in the amygdala, perhaps pertaining to spike timing dependent LTD (Gu and Yakel, 2011; Unal et al., 2015). Unal et al. (2015) report that optogenetic stimulation of cholinergic signaling in the amygdala has a state dependent, and largely inhibitory, effect on pyramidal cell firing. They also showed that the effects of endogenous ACh release by optogenetics contrasted with pharmacological stimulation of cholinergic receptors, which resulted in long lasting depolarization of pyramidal cells.
Taken together the optogenetic studies of cholinergic signaling in the amygdala and hippocampal circuits highlight a few key points. First, it appears that the effects of endogenous ACh release may not be adequately modeled by the application of exogenous agonists. Second, cholinergic signaling is exquisitely time sensitive and temporally specific: subtle differences in ACh stimulation paradigms can yield very different circuit effects. As such it is especially important that going forward we focus on defining stimulation paradigms for the examination of cholinergic signaling that are as behaviorally and physiologically relevant as possible. Such an approach will facilitate moving the field from demonstrating what ACh can do to what ACh does do in subserving cognitive task performance.
2b. iv. Summary
The most consistent finding in circuit level studies of memory is that endogenous release of ACh, likely acting via both nicotinic and muscarinic AChRs, plays an important role in the induction of LTP, a synaptic substrate of memory. In the amygdala this effect appears to mediate the retention of emotional memories. In the hippocampus cholinergic signaling both facilitates LTP and modulates cognition associated oscillatory activity. Theta rhythm phase can both modulate the likelihood that LTP is induced and determine whether stimulation will induce synaptic potentiation or depression (Hasselmo and Stern, 2014). Oscillations within the hippocampus seem to signal separation of encoding from retrieval processes, a distinction that is essential for memory as the status of these oscillations at the beginning of a behavioral task predicts learning success (Backus et al., 2016). Overall, recent work reinforces the idea that specific patterns of cholinergic signaling in memory related brain regions plays important roles in state dependent optimization of learning and memory. Another exciting new avenue for exploration in the modulation of hippocampal based memory not discussed above is the influence of cholinergic signaling on newborn hippocampal neurons.
Many of the recent studies that have demonstrated a central role of cholinergic signaling in both attentional and memory related circuits and behaviors have been made possible by the advent of optogenetics for the selective stimulation of BF cholinergic neurons and their terminal fields (Jiang et al., 2014; Luchicchi et al., 2014). An additional benefit of the technology is its illumination (pun intended) of the extent to which many transmitters – including ACh - are co-stored and, under some stimulation conditions, may be co-released with other transmitters (e.g. GABA, glutamate) (Granger et al., 2016). Of course such issues are readily addressed by combining a bit of modern pharmacology with the optogenetics, as has been done in the reports summarized here on the role of endogenous ACh signaling in attention and learning.
3. Cholinergic circuit alterations in cognitive decline
Given its prominent role in regulating circuits of attention and memory, it is no surprise that cholinergic signaling is a key player in mediating cognitive performance. In fact, boosting cholinergic signaling enhances attention and memory performance in naturally occurring poor performers (Galloway et al., 2014; Knott et al., 2014; Knott et al., 2015; Niemegeers et al., 2014; Paolone et al., 2013). Cholinergic signaling has therefore long been suspected as a key player in neurodegenerative disorders, which are characterized by cognitive decline.
The cholinergic hypothesis of perhaps the prototypical neurodegenerative disorder, Alzheimer’s disease (AD) emerged from influential studies done in the 1970s and 1980s (Bartus et al., 1982) that provided evidence of loss of cholinergic markers in the cortex (Geula and Mesulam, 1989), and loss of the number of neurons in the BF (Whitehouse et al., 1982). Soon after multiple groups found that the memory deficits in AD could be recapitulated by administering muscarinic antagonists (Drachman and Leavitt, 1974; Newhouse et al., 1988), and critically that procholinergic drugs could slow cognitive decline. What has followed has been a series of conflicting reports that challenge the cholinergic hypothesis of AD and the subsequent demonstration that pro-cholinergic drugs have modest effects on cognition, and are unable to affect the outcome of neurodegeneration.
Evidence of the involvement of the cholinergic system in PD (PD) also has been around since the 1980s. Interestingly, the first observations of loss of NBM neurons were in patients with PD, though the connection between the NBM and cognition was not yet understood. Some investigators consider the cholinergic lesion of PD, or perhaps more importantly for the focus of this review PD with dementia (PDD), to be more severe than that seen in AD (Perry et al., 1995). Given this, it is not surprising that some of the first effective treatments for PD were actually anticholinesterase medications (Aarsland et al., 2004; Bohnen et al., 2012).
It is clear that AD and PD both present with substantial cholinergic deficits. What can be gathered reliably from this is that the role of the cholinergic system in mediating cognition is complex, and that the vulnerability of BFCNs to metabolic demands remains a likely causal factor in cognitive decline. In fact, the last few years have witnessed a distinct “comeback” for cholinergic hypotheses of cognitive dysfunction. Here we attempt to bring together some of the key issues raised in the context of the role of abnormal cholinergic signaling in the etiology of attention and memory deficits in these two disorders, as they are the most common neurodegenerative diseases and therefore represent one of the most urgent problems facing the scientific community today.
3a. Alzheimer’s Disease
3a.i. Cholinergic dysfunction and cognitive consequences in Alzheimer’s disease
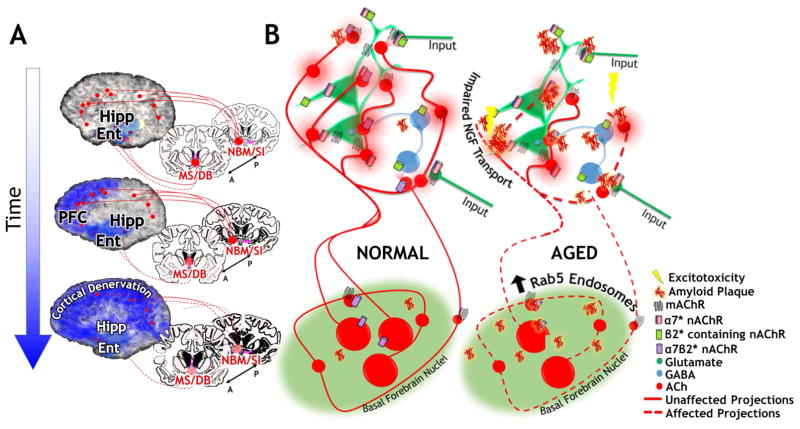
Reductions in BF volume, widespread reductions in AChE expression and reductions in both α4β2* and α7* receptors have all been documented in patients with AD (Grothe et al., 2012; Hanyu et al., 2002; Rinne et al., 2003; Sabri et al., 2015; Teipel et al., 2011; Wu et al., 2010). BF degeneration is a reliable biomarker for AD, and in fact BF atrophy is seen in mild cognitive decline (MCI), the putative prodrome of AD (Grothe et al., 2012; Grothe et al., 2010; Teipel et al., 2011). Age related decreases in BF volume have also been demonstrated (Grothe et al., 2010; Hanyu et al., 2002), leading Grothe et al. to suggest that BF atrophy in AD occurs against a background of normal age-related atrophy, which is aggravated in the disease state. Ray et al. (2015) found that preservation of the BF among individuals with MCI correlated with a shift of memory recall from the fornix-hippocampal tract to the parahippocampal cingulum tract. They suggest that BF aids in adaptation to disease related damage in MCI. Kuhl et al (1996) found that VAChT binding in MCI differed according to age of onset: those with earlier cognitive impairment showed reduced binding throughout cortical and hippocampal areas; while those with later cognitive impairment showed reduced binding only in the temporal cortex and hippocampus. Thus cholinergic signaling may serve to adaptively mitigate cognitive effects of the disease and delay clinical cognitive manifestations. Studies in animal models have shown that only the unique combination of AD-like pathology and severe cholinergic deficits results in memory impairments (Laursen et al., 2013).
Although AD is most classically associated with memory deficits, such deficits are typically conflated with attention issues (Romberg et al., 2013). Recently some effort has been exerted in assessing the degree to which attention issues contribute to cognitive decline in AD. Impaired attention in individuals with AD is associated with worsened performance on activities of daily living, and thus greatly affects the psychosocial impact of the disease (Miloyan et al., 2013). (Bracco et al., 2014) showed that treatment with cholinesterase inhibitors stabilized attention performance in individuals with AD, while memory performance continued to decline. This raises the interesting possibility that cholinesterase inhibitor treatment chiefly targets the attention deficit, rather than the memory deficit. Indeed, attention task related responses in attention related areas such as the PFC have previously been shown to be decreased in patients, and this deficit is improved by treatment with a cholinesterase inhibitor (Bentley et al., 2008). Teasing apart the relative contributions of attention deficits from memory deficits to overall cognitive impairment may be important to determining the relevant time period for intervention and prevention of the disease.
3a.ii. Potential mechanisms underlying cholinergic dysfunction in Alzheimer’s disease
Although cholinergic signaling is clearly an important mediator of the cognitive degeneration characteristic of AD, the exact mechanism by which the cholinergic system is affected is unknown. Recent work on animal models has explored the possibility that one or more AChRs are direct targets for Abeta toxicity. In one animal model, in which mice carry three transgenes encoding familial AD mutations (3xTg-AD), there were age-dependent decreases in α7 nAChR expression that correlated with Abeta plaque deposition as well as cognitive impairments (Oddo et al., 2005). Additional studies demonstrate that Abeta oligomers are ligands for nAChRs, and in the case of α7*nAChRs, low concentrations of Abeta (in the pM range) serve as agonist, whereas in the nM range Abeta is an antagonist (Puzzo et al., 2008). A recent report suggests that Abeta induced neuronal glutamate release in the hippocampus by activating α7* nAChRs (Hascup and Hascup, 2016). In primary cortical neuronal cultures, activation of α4 β2* nAChR protects against Aβ peptide induced cytotoxicity (Kihara et al., 1998), and in hippocampal slice recordings, an α4β2*nAChR selective agonist reversed Abeta impairment of LTP induction (Wu et al., 2008).
Mobley and colleagues report that triplication of the APP gene, which encodes the precursor of Abeta, results in enlarged early endosomes in cholinergic axons and defective retrograde transport of NGF (Salehi et al., 2006; Xu et al., 2016). The early endosomal pathology is associated with elevated activation of the Rab5 GTPase and this activation results from elevated levels of either the full length APP protein or the APP C-terminal fragment. This provides a possible mechanism for selective sensitivity of cholinergic neurons, which are uniquely dependent on NGF signaling, to APP pathology that is distinct from generation of Abeta aggregates. In the single BFCN tracing studies discussed above, Wu et al. (2014) described fragmentation of cholinergic arbors in a FAD transgenic mouse model and postulated that the metabolic demand of maintaining these enormous axonal arbors makes the cholinergic neurons especially sensitive to degenerative insults. Whether axonal fragmentation is secondary to deficits in endocytic trafficking is not known. Similarly, the relative contribution of this effect and direct A beta effects on cholinergic receptors toward inciting the cholinergic lesion in AD remains unclear. However, circumventing potential endosome disruptions to provide NGF directly has provided some promising avenues for treatment: an exciting recent trial delivered NGF chronically to the cholinergic basal forebrain by implanting encapsulated NGF producing cells into the basal forebrain of patients with AD (Eyjolfsdottir et al., 2016). This treatment seemed to show promise for stabilizing cholinergic markers and cognitive function (Eyjolfsdottir et al., 2016).
3b. Parkinson’s Disease
3b.i. Cholinergic dysfunction and cognitive consequences in Parkinson’s disease
Although classically considered a dopaminergic disorder, cholinergic lesions were actually among the first to be documented in Parkinson’s Disease (PD) (Nakano and Hirano, 1984). Both post mortem studies and in vivo imaging have documented loss of BFCN’s, as well as profound cholinergic denervation of the cortex and thalamus in patients with PD (although interestingly, ACh in the striatum appears to be increased) (Bohnen et al., 2015; Bohnen et al., 2012; Celebi et al., 2012; Choi et al., 2012; Hall et al., 2014; Klein et al., 2010; Kuhl et al., 1996; Muller et al., 2015; Nejad-Davarani et al., 2016; Shin et al., 2012; Yarnall et al., 2013). Indeed when cholinergic lesions are seen in Parkinson’s, they are at least as severe as those found in AD (Celebi et al., 2012; Kuhl et al., 1996; Park et al., 2015). A meta-analysis of multiple trials of AChE inhibitor treatment found that patients with PD actually show greater response than patients with AD, which the authors assert is consistent with a greater cholinergic lesion in Parkinson’s (Weintraub et al., 2011).
As in AD, the cholinergic lesion in PD seems to mediate the decrease in cognitive performance, as reduced BF volume and reduced AChE binding are consistently associated with impaired cognition (Bohnen et al., 2015; Bohnen et al., 2012; Choi et al., 2012; Muller et al., 2015). Use of anticholinergic drugs in patients with PD worsens their cognitive performance while use of cholinesterase inhibitors improves cognition, possibly by restoring aberrant spontaneous brain activity patterns to control levels (Dubois et al., 2012; Possin et al., 2013; Weintraub et al., 2011).
Cholinergic dysfunction might be responsible for the transition from PD to PD with dementia (PDD) - reductions in cholinergic markers are consistently more severe in PDD than in PD (Choi et al., 2012; Hall et al., 2014; Klein et al., 2010; Lee et al., 2013; Shin et al., 2012). In contrast, cortical ChAT activity is often mildly reduced in patients with PD without dementia. These results suggest that cholinergic fiber degeneration precedes cholinergic cell loss, as patients with milder forms of PD have only limited fiber loss in the absence of a decrease in NBM cholinergic neurons, while patients with PDD have more extensive fiber loss coupled with a 60–65% reduction of ChAT positive neurons in NBM (Hall et al., 2014). In fact reduced BF volume in patients with PD with MCI has been shown to predict subsequent progression to PDD (Lee et al., 2013).
Cognitive measures, especially of attention, are consistently significant covariates in determining the relationship between reduced walking speed, a central motor feature of PD, and cortical cholinergic denervation (Bohnen et al., 2012; Muller et al., 2015; Rochester et al., 2012). Recent animal model studies have provided insight into the mechanisms by which cholinergic signaling in circuits of attention may be responsible for another key motor symptom of PD: frequent falls. Simultaneous lesion of both cholinergic and dopaminergic signaling induces both memory and attention deficits and leads to significantly more frequent falls than either selective cholinergic or dopaminergic lesions alone (Kucinski et al., 2013; Wisman et al., 2008; Zurkovsky et al., 2013). This effect is specific to the basal forebrain, as triple lesioned rats who received an additional cholinergic selective lesion in the PPT do not show a further increase in falls (Kucinski and Sarter, 2015). In these studies, attentional performance was correlated with the rate of falls in double lesion animals, suggesting that attentional impairment caused by cholinergic denervation “unmasks” dopaminergic deficits and increases falls (Kucinski et al., 2013).
Patients with PD do not typically present clinically until the dopaminergic lesion is quite severe. It is possible that in early stages of the disease intact ACh dependent mechanisms mask minor dopaminergic deficits; as the disease progresses a threshold of cholinergic dysfunction is passed beyond which these mechanisms can no longer compensate and the dopaminergic lesion is “unmasked”. This model is supported in animal models in which the dopaminergic system has been partially lesioned. In these animals activity dependence of dopaminergic release was blunted, but only when nicotinic cholinergic signaling is blocked. With nicotinic signaling intact, the blunting effect of dopaminergic lesion is masked and dopamine release is again exquisitely activity dependent (Jennings et al., 2015).
3bii. Potential mechanisms underlying cholinergic dysfunction in Parkinson’s disease
It seems clear that cholinergic signaling is an important mediator of both PDD and the more classical motor symptoms of PD. However, the exact mechanism by which the cholinergic system is affected in PD remains elusive. Recent studies of animal models of PD have illuminated an intricate interplay between the cholinergic lesion and the more classical dopaminergic lesion in PD.
Interestingly, administration of nicotine in animal models protects against dopaminergic cell loss (Liu et al., 2007; Liu et al., 2004; Quik et al., 2012). These effects of nicotine require α7*nAChR signaling and can be mimicked using α7*nAChR selective agonists. α7 receptor signaling appears to mediate these effects by blocking activation of astrocytes and microglia, thereby attenuating the neuroinflammatory response and cell death. In keeping with this, a history of smoking has been shown to lower the risk of PD, likely due to nicotine’s protective effects (Chen et al., 2010a; Louis et al., 2008; Magen et al., 2012). However, lesioning the dopaminergic system experimentally can lead to selective loss of cholinergic signaling (Hu et al., 2011; Knol et al., 2014; Szegö et al., 2011; Szego et al., 2013). A recent study documented basal forebrain degeneration in patients with late stage PD, but not in patients with early stage PD (Ziegler et al., 2013). Patients at all stages of the disease showed degeneration of the dopaminergic cells in the SN, leading the authors to conclude that the dopaminergic lesion precedes the cholinergic lesion in PD (Ziegler et al., 2013). Decreased cholinergic signaling and dopaminergic cell loss may therefore be conjoined in PD in a “snowball effect” whereby damage to one system leads to increased damage to the other and vice versa.
Rare, familial cases of PD have been associated with overexpression of alpha synuclein, a protein which aggregates into Lewy Bodies, a major neuropathological finding in PD. Transgenic mice that over-express alpha-synuclein, show cognitive deficits, loss of MS/DB cholinergic neurons, reduced cortical ACh and reduced cholinergic fibers in the hippocampus (Magen et al., 2012; Szegö et al., 2011; Szego et al., 2013). Alpha-synuclein accumulates in cholinergic cells, and given the exorbitant metabolic load of these neurons with their highly collatoralized axons this may result in disruption of cortical ACh via impaired synthesis, vesicular trafficking, and/or release (Magen et al., 2012)
3c. Summary and future directions
Recent evidence converges on the idea that cholinergic circuits of attention and memory are affected in both of the most common neurodegenerative disorders: AD and PD. Among these disorders, the lesion of cholinergic circuits subserving cognition appears to be an important modulator of disease severity. In AD, preservation of cholinergic circuits may mitigate disease onset and severity of the cognitive deficit. In PD attentional capacity influences the severity of many of the more classical motor phenotypes and may serve a “masking” effect and is therefore a key determinant of clinical progression and severity. Understanding the pathogenesis of cholinergic lesions is therefore vital to determining the best course for intervention in cognitive decline.
In both disorders attentional impairment is difficult to separate from memory impairment. Furthermore there is emerging evidence that selective populations of cholinergic neurons and their projections are affected in different conditions. How different populations are targeted and if these differences correlate with attentional vs memory dysfunctions remains to be determined.
Many of the above studies have been facilitated by important advances in human neuroimaging techniques seen over the last decade. Magnetic resonance imaging (MRI), positron emission tomography (PET), and now the combination of the two, allow studies of structure, function and the intersection between these modalities in humans in normal and in disease states. With the added advantage of repeated scanning, neuroimaging can provide a temporal profile of a disease state. Considerable effort has been made to delineate the cholinergic nuclei Ch1–Ch4 in human neuroimaging studies. Using localization maps created from postmortem tissue, several groups have developed largely overlapping cholinergic atlases that allow direct examination of structural changes to the cholinergic system (Teipel et al., 2005; Zaborszky et al., 2008). By combining more refined cognitive batteries that better separate attention and memory with the growing power of brain imaging we should be in a position to gain insight into the progression of disruptions in cholinergic signaling and the severity of attentional and memory impairments. The development of new generations of PET tracers that report on different aspects of cholinergic signaling is one area of great excitement (Padakanti et al., 2014; Petrou et al., 2014).
CONCLUSION
The challenges to understanding how cholinergic signaling participates in cognitive processing have been significant: cholinergic neurons are few and far between, are not neatly clustered in compact, homogenous brain nuclei, or bundled in their projection paths to distal targets. The transmitter itself is difficult to assay, the cellular effects of ACh numerous and varied and the network interactions difficult to gauge in view of remarkably complex axonal morphologies and extensive projection fields. Nevertheless, the fundamental importance of cholinergic signaling in normal cognitive function and in cognitive impairments – from mild to severe - that manifest in a steadily rising percentage of the world’s population, has driven the basic neuroscience to its ingenious best.
Thanks to new approaches ranging from intersectional genetics to multisite in vivo recording in awake behaving animals, to development of novel human imaging probes, the last 5 years has brought new perspectives to some basic tenets of cholinergic signaling mechanisms. Perhaps most surprising is our new-found appreciation of the exotic morphology of individual cholinergic neuronal axons, which span centimeters of territory in mice and cover meters of real estate in the human brain. Brain mapping technology reveals new principles of the functional organization of the cholinergic projections engaged in distinct aspects of attention and memory-related behaviors. Also surprising, for a system with more than the usual number of cellular and molecular mechanisms for eliciting short term and long lasting changes in neuronal excitability, we continue to find additional synaptic and circuit functions that are influenced by ACh receptors and uncover to novel cellular and intracellular signaling mechanisms affected by ACh. The field has finally broken through the selective stimulation barriers to begin dissecting how different neural networks are differentially affected by cholinergic inputs. Our ability to address major challenges in assessing what goes wrong with cholinergic signaling in human pathologies of cognitive decline and in devising new strategies to correct them, may not be so far away.
Figure 4. Alterations to Cholinergic Projections and Circuits in Dementia.
A. Trajectory of Alzheimer’s Disease Dementia from early to late stage (see text). Schematic representation of current concepts of cholinergic axonal retrograde death, superimposed on schematic that indicates the increasing extent of affected brain areas in blue.
B. Cellular mechanisms of cholinergic neuronal loss and subsequent disruption of target hippocampal & cortical domains. Possible mechanisms thought to underlie early loss of cholinergic projections in an animal model of AD-like dementia: Aβ induced excitotoxicity mediated by interaction with α7* nAChRs (Hascup & Hascup, 2016; Liu et al., 2016; Wu et al., 2016), and impaired neurotrophin–induced retrograde transport via Rab5 endosome enlargement (Xu et al., 2015). Images for A adapted from human brain atlas (Hawrylycz et al., 2012)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Ballinger, Email: Elizabeth.Ballinger@stonybrookmedicine.edu, Medical Scientist Training Program, Program in Neuroscience, Department of Neurobiology & Behavior Stony Brook University, NY 11794.
Mala Ananth, Email: Mala.Ananth@stonybrook.edu, Program in Neuroscience, Department of Neurobiology & Behavior, Department of Psychiatry & Behavioral science, Stony Brook University, NY 11794.
David A. Talmage, Email: David.Talmage@stonybrook.edu, Professor, Department of Pharmacological Sciences, CNS Disorders Center, Center for Molecular Medicine, Stony Brook University, NY 11794
Lorna Role, Email: Lorna.Role@stonybrook.edu, Distinguished Professor & Chair Department of Neurobiology & Behavior Co-director, Neurosciences Institute, CNS Disorders Center, Center for Molecular Medicine, Stony Brook University, NY 11794.
LITERATURE CITED
- Aarsland D, Mosimann UP, McKeith IG. Role of cholinesterase inhibitors in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2004;17:164–171. doi: 10.1177/0891988704267463. [DOI] [PubMed] [Google Scholar]
- Backus AR, Schoffelen JM, Szebenyi S, Hanslmayr S, Doeller CF. Hippocampal-Prefrontal Theta Oscillations Support Memory Integration. Curr Biol. 2016;26:450–457. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Barnstedt O, Owald D, Felsenberg J, Brain R, Moszynski JP, Talbot CB, Perrat PN, Waddell S. Memory-Relevant Mushroom Body Output Synapses Are Cholinergic. Neuron. 2016;89:1237–1247. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Beierlein M. Synaptic properties and functional consequences of cholinergic signalling in the mammalian CNS. J Physiol. 2014;592:4129–4130. doi: 10.1113/jphysiol.2014.278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131:409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem B, Schoppink L, Rotaru DC, Faiz A, Hendriks P, Mansvelder HD, van de Berg WD, Wouterlood FG. Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J Neurosci. 2014;34:16234–16246. doi: 10.1523/JNEUROSCI.3011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK, Rinnofner J. Intrinsic Cholinergic Neurons in the Hippocampus: Fact or Artifact? Front Synaptic Neurosci. 2016;8:6. doi: 10.3389/fnsyn.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Muller ML, Petrou M, Kotagal V, Koeppe RA, Scott PJ, Frey KA. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol. 2015;72:194–200. doi: 10.1001/jamaneurol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovic Z, Alfonsi F, Rumballe BA, Fonseka S, Windels F, Coulson EJ. The role of p75NTR in cholinergic basal forebrain structure and function. J Neurosci. 2014;34:13033–13038. doi: 10.1523/JNEUROSCI.2364-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracco L, Bessi V, Padiglioni S, Marini S, Pepeu G. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer’s disease patients. J Alzheimers Dis. 2014;40:737–742. doi: 10.3233/JAD-131154. [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci. 2010;41:340–346. doi: 10.1007/s12031-010-9377-2. [DOI] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels. Neuron. 2010;68:948–963. doi: 10.1016/j.neuron.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The Human Hippocampus and Spatial and Episodic Memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Butcher AJ, Bradley SJ, Prihandoko R, Brooke SM, Mogg A, Bourgognon JM, Macedo-Hatch T, Edwards JM, Bottrill AR, Challiss RA, et al. An Antibody Biosensor Establishes the Activation of the M1 Muscarinic Acetylcholine Receptor during Learning and Memory. J Biol Chem. 2016;291:8862–8875. doi: 10.1074/jbc.M115.681726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Luschi R, Samanin R. Dose-related impairment of spatial learning by intrahippocampal scopolamine: antagonism by ondansetron, a 5-HT3 receptor antagonist. Behavioural Brain Research. 1997;82:185–194. doi: 10.1016/s0166-4328(97)80988-6. [DOI] [PubMed] [Google Scholar]
- Celebi O, Temucin CM, Elibol B, Saka E. Short latency afferent inhibition in Parkinson’s disease patients with dementia. Mov Disord. 2012;27:1052–1055. doi: 10.1002/mds.25040. [DOI] [PubMed] [Google Scholar]
- Chandler D, Waterhouse BD. Evidence for broad versus segregated projections from cholinergic and noradrenergic nuclei to functionally and anatomically discrete subregions of prefrontal cortex. Front Behav Neurosci. 2012;6:20. doi: 10.3389/fnbeh.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Lamperski CS, Waterhouse BD. Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 2013;1522:38–58. doi: 10.1016/j.brainres.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C, Zaborszky L. Basal Forebrain Cholinergic-Auditory Cortical Network: Primary Versus Nonprimary Auditory Cortical Areas. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010a;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chatterjee M, Li JY. The mouse homeobox gene Gbx2 is required for the development of cholinergic interneurons in the striatum. J Neurosci. 2010b;30:14824–14834. doi: 10.1523/JNEUROSCI.3742-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sur M. An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci. 2015;18:892–902. doi: 10.1038/nn.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J Neurosci. 2014;34:124–133. doi: 10.1523/JNEUROSCI.2973-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. The effect of alpha7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem Pharmacol. 2015 doi: 10.1016/j.bcp.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Jung TM, Lee JE, Lee SK, Sohn YH, Lee PH. Volumetric analysis of the substantia innominata in patients with Parkinson’s disease according to cognitive status. Neurobiol Aging. 2012;33:1265–1272. doi: 10.1016/j.neurobiolaging.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol. 2005;562:81–88. doi: 10.1113/jphysiol.2004.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Cui X, Weng YQ, Frappe I, Burgess A, Girao da Cruz MT, Schachner M, Aubert I. The cell adhesion molecule L1 regulates the expression of choline acetyltransferase and the development of septal cholinergic neurons. Brain Behav. 2011;1:73–86. doi: 10.1002/brb3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J Neurosci. 2015;35:8394–8410. doi: 10.1523/JNEUROSCI.4460-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Progress in Neurobiology. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Progress in Brain Research (Elsevier) 2000:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Douchamps V, Jeewajee A, Blundell P, Burgess N, Lever C. Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. J Neurosci. 2013;33:8689–8704. doi: 10.1523/JNEUROSCI.4483-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Dubois B, Tolosa E, Katzenschlager R, Emre M, Lees AJ, Schumann G, Pourcher E, Gray J, Thomas G, Swartz J, et al. Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27:1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Zhou P, Milner TA, Pickel VM. Spatial and intracellular relationships between the alpha7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse. Neuroscience. 2009;161:1091–1103. doi: 10.1016/j.neuroscience.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Penny WD, Burgess N. Brain oscillations and memory. Curr Opin Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Easton A, Douchamps V, Eacott M, Lever C. A specific role for septohippocampal acetylcholine in memory? Neuropsychologia. 2012;50:3156–3168. doi: 10.1016/j.neuropsychologia.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Gan L. The LIM-homeobox gene Islet-1 is required for the development of restricted forebrain cholinergic neurons. J Neurosci. 2008;28:3291–3297. doi: 10.1523/JNEUROSCI.5730-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyjolfsdottir H, Eriksdotter M, Linderoth B, Lind G, Juliusson B, Kusk P, Almkvist O, Andreasen N, Blennow K, Ferreira D. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alzheimer’s Research & Therapy. 2016;8:1. doi: 10.1186/s13195-016-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31. doi: 10.1016/s0306-4522(99)00525-4. [DOI] [PubMed] [Google Scholar]
- Galloway CR, Lebois EP, Shagarabi SL, Hernandez NA, Manns JR. Effects of selective activation of M1 and M4 muscarinic receptors on object recognition memory performance in rats. Pharmacology. 2014;93:57–64. doi: 10.1159/000357682. [DOI] [PubMed] [Google Scholar]
- Galvez B, Gross N, Sumikawa K. Activation of alpha7 nicotinic acetylcholine receptors protects potentiated synapses from depotentiation during theta pattern stimulation in the hippocampal CA1 region of rats. Neuropharmacology. 2016;105:378–387. doi: 10.1016/j.neuropharm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Cortical cholinergic fibers in aging and Alzheimer’s disease: a morphometric study. Neuroscience. 1989;33:469–481. doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Giraldez-Perez RM, Gaytan SP, Pasaro R. Cholinergic and nitrergic neuronal networks in the goldfish telencephalon. Acta Neurobiol Exp (Wars) 2013;73:338–353. doi: 10.55782/ane-2013-1941. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 2015;1349:1–45. doi: 10.1111/nyas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, et al. Role for the M Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci U S A. 2016;113:E1089–1097. doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic Basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, Amunts K, Suarez-Gonzalez A, Cantero JL. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cereb Cortex. 2010;20:1685–1695. doi: 10.1093/cercor/bhp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Lamb PW, Yakel JL. Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. J Neurosci. 2012;32:12337–12348. doi: 10.1523/JNEUROSCI.2129-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Halff AW, Gomez-Varela D, John D, Berg DK. A novel mechanism for nicotinic potentiation of glutamatergic synapses. J Neurosci. 2014;34:2051–2064. doi: 10.1523/JNEUROSCI.2795-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014;137:2493–2508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K. MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. AJNR Am J Neuroradiol. 2002;23:27–32. [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER. Soluble Amyloid-beta42 Stimulates Glutamate Release through Activation of the alpha7 Nicotinic Acetylcholine Receptor. J Alzheimers Dis. 2016 doi: 10.3233/JAD-160041. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120523. doi: 10.1098/rstb.2012.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Theta rhythm and the encoding and retrieval of space and time. Neuroimage. 2014;85(Pt 2):656–666. doi: 10.1016/j.neuroimage.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Picciotto MR. Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr Opin Neurobiol. 2014;29:88–95. doi: 10.1016/j.conb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E, Santhakumar K, Akitake CA, Ahn SJ, Thisse C, Thisse B, Wyart C, Mangin JM, Halpern ME. Cholinergic left-right asymmetry in the habenulo-interpeduncular pathway. Proc Natl Acad Sci U S A. 2013;110:21171–21176. doi: 10.1073/pnas.1319566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhu C, Liu Y, Wang F, Huang Z, Fan W, Wu J. Dynamic alterations of gene expression of nicotinic acetylcholine receptor alpha 7, alpha 4 and beta 2 subunits in an acute MPTP-lesioned mouse model. Neuroscience Letters. 2011;494:232–236. doi: 10.1016/j.neulet.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Hyde M, Choueiry J, Smith D, de la Salle S, Nelson R, Impey D, Baddeley A, Aidelbaum R, Millar A, Knott V. Cholinergic modulation of auditory P3 event-related potentials as indexed by CHRNA4 and CHRNA7 genotype variation in healthy volunteers. Neurosci Lett. 2016;623:36–41. doi: 10.1016/j.neulet.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings KA, Platt NJ, Cragg SJ. The impact of a parkinsonian lesion on dynamic striatal dopamine transmission depends on nicotinic receptor activation. Neurobiol Dis. 2015;82:262–268. doi: 10.1016/j.nbd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Guo Y, Tang Y, Xu Q, Li B, Wu Q. Regulation of the protocadherin Celsr3 gene and its role in globus pallidus development and connectivity. Mol Cell Biol. 2014;34:3895–3910. doi: 10.1128/MCB.00760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Emmetsberger J, Talmage DA, Role LW. Type III neuregulin 1 is required for multiple forms of excitatory synaptic plasticity of mouse cortico-amygdala circuits. J Neurosci. 2013;33:9655–9666. doi: 10.1523/JNEUROSCI.2888-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, Lopez-Hernandez GY, Ballinger EC, Wang S, Talmage DA, Role LW. Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Neuron. 2016;90:1057–1070. doi: 10.1016/j.neuron.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Lopez-Hernandez GY, Lederman J, Talmage DA, Role LW. Optogenetic studies of nicotinic contributions to cholinergic signaling in the central nervous system. Rev Neurosci. 2014;25:755–771. doi: 10.1515/revneuro-2014-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Hsieh LS, Lee AM, Zhou Z, Coman D, Heath CJ, Hyder F, Mineur YS, Yuan Q, Goldman D, et al. An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nature Neuroscience. 2016 doi: 10.1038/nn.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach A, Hedrick T, Waters J. Selective optogenetic stimulation of cholinergic axons in neocortex. J Neurophysiol. 2012;107:2008–2019. doi: 10.1152/jn.00870.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach A, Waters J. Modulation of high- and low-frequency components of the cortical local field potential via nicotinic and muscarinic acetylcholine receptors in anesthetized mice. J Neurophysiol. 2014;111:258–272. doi: 10.1152/jn.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C. Bottom-up and top-down attention: different processes and overlapping neural systems. Neuroscientist. 2014;20:509–521. doi: 10.1177/1073858413514136. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Res. 1998;792:331–334. doi: 10.1016/s0006-8993(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jung AH, Jeong D, Choi I, Kim K, Shin S, Kim SJ, Lee SH. Selectivity of Neuromodulatory Projections from the Basal Forebrain and Locus Ceruleus to Primary Sensory Cortices. J Neurosci. 2016;36:5314–5327. doi: 10.1523/JNEUROSCI.4333-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Knol RJ, de Bruin K, Opmeer B, Voorn P, Jonker AJ, van Eck-Smit BL, Booij J. Decreased ipsilateral [123 I] iododexetimide binding to cortical muscarinic receptors in unilaterally 6-hydroxydopamine lesioned rats. Nuclear medicine and biology. 2014;41:90–95. doi: 10.1016/j.nucmedbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Knott V, Choueiry J, Dort H, Smith D, Impey D, de la Salle S, Philippe T. Baseline-dependent modulating effects of nicotine on voluntary and involuntary attention measured with brain event-related P3 potentials. Pharmacol Biochem Behav. 2014;122:107–117. doi: 10.1016/j.pbb.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Knott V, de la Salle S, Choueiry J, Impey D, Smith D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A. Neurocognitive effects of acute choline supplementation in low, medium and high performer healthy volunteers. Pharmacology Biochemistry and Behavior. 2015;131:119–129. doi: 10.1016/j.pbb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Knox D. The Role Of Basal Forebrain Cholinergic Neurons In Fear and Extinction Memory. Neurobiology of Learning and Memory. 2016 doi: 10.1016/j.nlm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Keller SM. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus. 2015 doi: 10.1002/hipo.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Zaborszky L. Topographic organization of the basal forebrain projections to the perirhinal, postrhinal, and entorhinal cortex in rats. J Comp Neurol. 2016 doi: 10.1002/cne.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukouli F, Maskos U. The multiple roles of the alpha7 nicotinic acetylcholine receptor in modulating glutamatergic systems in the normal and diseased nervous system. Biochem Pharmacol. 2015;97:378–387. doi: 10.1016/j.bcp.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M. Modeling fall propensity in Parkinson’s disease: deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci. 2013;33:16522–16539. doi: 10.1523/JNEUROSCI.2545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Sarter M. Modeling Parkinson’s disease falls associated with brainstem cholinergic systems decline. Behav Neurosci. 2015;129:96–104. doi: 10.1037/bne0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, Wieland DM, Koeppe RA. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Kunec S, Hasselmo ME, Kopell N. Encoding and retrieval in the CA3 region of the hippocampus: a model of theta-phase separation. J Neurophysiol. 2005;94:70–82. doi: 10.1152/jn.00731.2004. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol Behav. 2016;155:162–171. doi: 10.1016/j.physbeh.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen B, Mork A, Plath N, Kristiansen U, Bastlund JF. Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav Brain Res. 2013;240:146–152. doi: 10.1016/j.bbr.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Lee JE, Cho KH, Song SK, Kim HJ, Lee HS, Sohn YH, Lee PH. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2013 doi: 10.1136/jnnp-2013-305062. jnnp-2013–305062. [DOI] [PubMed] [Google Scholar]
- Lilja AM, Porras O, Storelli E, Nordberg A, Marutle A. Functional interactions of fibrillar and oligomeric amyloid-beta with alpha7 nicotinic receptors in Alzheimer’s disease. J Alzheimers Dis. 2011;23:335–347. doi: 10.3233/JAD-2010-101242. [DOI] [PubMed] [Google Scholar]
- Liu S, Fa M, Ninan I, Trinchese F, Dauer W, Arancio O. Alpha-synuclein involvement in hippocampal synaptic plasticity: role of NO, cGMP, cGK and CaMKII. Eur J Neurosci. 2007;25:3583–3596. doi: 10.1111/j.1460-9568.2007.05569.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Benito-Leon J, Bermejo-Pareja F Neurological Disorders in Central Spain Study G. Population-based prospective study of cigarette smoking and risk of incident essential tremor. Neurology. 2008;70:1682–1687. doi: 10.1212/01.wnl.0000311271.42596.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CB, Henderson Z. Nicotine induction of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience. 2010;166:84–93. doi: 10.1016/j.neuroscience.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Lubin M, Erisir A, Aoki C. Ultrastructural immunolocalization of the alpha 7 nAChR subunit in guinea pig medial prefrontal cortex. Ann N Y Acad Sci. 1999;868:628–632. doi: 10.1111/j.1749-6632.1999.tb11337.x. [DOI] [PubMed] [Google Scholar]
- Luchicchi A, Bloem B, Viana JN, Mansvelder HD, Role LW. Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front Synaptic Neurosci. 2014;6:24. doi: 10.3389/fnsyn.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Magen I, Fleming SM, Zhu C, Garcia EC, Cardiff KM, Dinh D, De La Rosa K, Sanchez M, Torres ER, Masliah E, et al. Cognitive deficits in a mouse model of pre-manifest Parkinson’s disease. Eur J Neurosci. 2012;35:870–882. doi: 10.1111/j.1460-9568.2012.08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello K, Huang Z, Lujan R, Tran B, Watanabe M, Cooper EC, Brown DA, Shah MM. Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron. 2015;85:346–363. doi: 10.1016/j.neuron.2014.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Watkins KC, Descarries L. Ultrastructural features of the acetylcholine innervation in the developing parietal cortex of rat. The Journal of Comparative Neurology. 2002;443:250–258. doi: 10.1002/cne.10114. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J. Structural and functional considerations of the cholinergic brainstem. J Neural Transm (Vienna) 2016 doi: 10.1007/s00702-016-1530-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Micheau J, Marighetto A. Acetylcholine and memory: a long, complex and chaotic but still living relationship. Behav Brain Res. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Miloyan BH, Razani J, Larco A, Avila J, Chung J. Aspects of Attention Predict Real-World Task Performance in Alzheimer’s Disease. Appl Neuropsychol Adult. 2013 doi: 10.1080/09084282.2012.685133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Sano A, Takahashi T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun. 2013;4:2760. doi: 10.1038/ncomms3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Lester HA, Walz A. Optimizing cholinergic tone through lynx modulators of nicotinic receptors: implications for plasticity and nicotine addiction. Physiology (Bethesda) 2012;27:187–199. doi: 10.1152/physiol.00002.2012. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Che S, Ginsberg SD. Neuronal gene expression profiling: uncovering the molecular biology of neurodegenerative disease. Prog Brain Res. 2006;158:197–222. doi: 10.1016/S0079-6123(06)58010-0. [DOI] [PubMed] [Google Scholar]
- Muller ML, Bohnen NI, Kotagal V, Scott PJ, Koeppe RA, Frey KA, Albin RL. Clinical markers for identifying cholinergic deficits in Parkinson’s disease. Mov Disord. 2015;30:269–273. doi: 10.1002/mds.26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz W, Rudy B. Spatiotemporal specificity in cholinergic control of neocortical function. Curr Opin Neurobiol. 2014;26:149–160. doi: 10.1016/j.conb.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Hirano A. Parkinson’s disease: neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann Neurol. 1984;15:415–418. doi: 10.1002/ana.410150503. [DOI] [PubMed] [Google Scholar]
- Nejad-Davarani S, Muller M, Koeppe R, Scott P, Dauer W, Albin R, Frey K, Bohnen N. Clinical correlation of multivariate spatial covariance analysis of 18F-FEOBV VAChT PET in Parkinson disease. Journal of Nuclear Medicine. 2016;57:1832–1832. [Google Scholar]
- Nelson A, Mooney R. The Basal Forebrain and Motor Cortex Provide Convergent yet Distinct Movement-Related Inputs to the Auditory Cortex. Neuron. 2016;90:635–648. doi: 10.1016/j.neuron.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, Cohen RM, Murphy DL. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- Newman EL, Climer JR, Hasselmo ME. Grid cell spatial tuning reduced following systemic muscarinic receptor blockade. Hippocampus. 2014;24:643–655. doi: 10.1002/hipo.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J Neurosci. 2013;33:19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Huppe-Gourgues F, Vaucher E. Activation of the mouse primary visual cortex by medial prefrontal subregion stimulation is not mediated by cholinergic basalo-cortical projections. Front Syst Neurosci. 2015;9:1. doi: 10.3389/fnsys.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WA, Henderson BJ, Yu C, Parker RL, Richards CI, Lester HA, Miwa JM. Lynx1 shifts alpha4beta2 nicotinic receptor subunit stoichiometry by affecting assembly in the endoplasmic reticulum. J Biol Chem. 2014;289:31423–31432. doi: 10.1074/jbc.M114.573667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemegeers P, Dumont GJ, Quisenaerts C, Morrens M, Boonzaier J, Fransen E, de Bruijn ER, Hulstijn W, Sabbe BG. The effects of nicotine on cognition are dependent on baseline performance. Eur Neuropsychopharmacol. 2014;24:1015–1023. doi: 10.1016/j.euroneuro.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Kabbani N. An interaction between alpha7 nicotinic receptors and a G-protein pathway complex regulates neurite growth in neural cells. J Cell Sci. 2012;125:5502–5513. doi: 10.1242/jcs.110379. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Phillips WS, Kodama N, Clark SG, Del Negro CA, Kabbani N. Axon targeting of the alpha 7 nicotinic receptor in developing hippocampal neurons by Gprin1 regulates growth. J Neurochem. 2014;129:649–662. doi: 10.1111/jnc.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102:3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padakanti PK, Zhang X, Jin H, Cui J, Wang R, Li J, Flores HP, Parsons SM, Perlmutter JS, Tu Z. In vitro and in vivo characterization of two C-11-labeled pet tracers for vesicular acetylcholine transporter. Mol Imaging Biol. 2014;16:773–780. doi: 10.1007/s11307-014-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Park HE, Park IS, Oh YS, Yang DW, Lee KS, Choi HS, Ahn KJ, Kim JS. Subcortical whiter matter hyperintensities within the cholinergic pathways of patients with dementia and parkinsonism. J Neurol Sci. 2015;353:44–48. doi: 10.1016/j.jns.2015.03.046. [DOI] [PubMed] [Google Scholar]
- Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, Irving D, Brown A, Perry RH. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuroscience. 1995;64:385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, Muller ML, Albin RL, Koeppe RA. In vivo imaging of human cholinergic nerve terminals with (−)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med. 2014;55:396–404. doi: 10.2967/jnumed.113.124792. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pombero A, Bueno C, Saglietti L, Rodenas M, Guimera J, Bulfone A, Martinez S. Pallial origin of basal forebrain cholinergic neurons in the nucleus basalis of Meynert and horizontal limb of the diagonal band nucleus. Development. 2011;138:4315–4326. doi: 10.1242/dev.069534. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Kang GA, Guo C, Fine EM, Trujillo AJ, Racine CA, Wilheim R, Johnson ET, Witt JL, Seeley WW, et al. Rivastigmine is associated with restoration of left frontal brain activity in Parkinson’s disease. Mov Disord. 2013;28:1384–1390. doi: 10.1002/mds.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddifoot CA, Wu M, Sung RJ, Joiner WJ. Ly6h regulates trafficking of alpha7 nicotinic acetylcholine receptors and nicotine-induced potentiation of glutamatergic signaling. J Neurosci. 2015;35:3420–3430. doi: 10.1523/JNEUROSCI.3630-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Metzler-Baddeley C, Khondoker MR, Grothe MJ, Teipel S, Wright P, Heinsen H, Jones DK, Aggleton JP, O’Sullivan MJ. Cholinergic basal forebrain structure influences the reconfiguration of white matter connections to support residual memory in mild cognitive impairment. J Neurosci. 2015;35:739–747. doi: 10.1523/JNEUROSCI.3617-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Kaasinen V, Jarvenpaa T, Nagren K, Roivainen A, Yu M, Oikonen V, Kurki T. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:113–115. doi: 10.1136/jnnp.74.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 2012;135:2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Stewart AL, Janke KL, Gielow MR, Kostek JA, Savage LM, Servatius RJ, Pang KC. Medial septum-diagonal band of Broca (MSDB) GABAergic regulation of hippocampal acetylcholine efflux is dependent on cognitive demands. J Neurosci. 2014;34:506–514. doi: 10.1523/JNEUROSCI.2352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C, Bussey TJ, Saksida LM. Paying more attention to attention: towards more comprehensive cognitive translation using mouse models of Alzheimer’s disease. Brain Res Bull. 2013;92:49–55. doi: 10.1016/j.brainresbull.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Runfeldt MJ, Sadovsky AJ, MacLean JN. Acetylcholine functionally reorganizes neocortical microcircuits. J Neurophysiol. 2014;112:1205–1216. doi: 10.1152/jn.00071.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Becker GA, Meyer PM, Hesse S, Wilke S, Graef S, Patt M, Luthardt J, Wagenknecht G, Hoepping A, et al. First-in-human PET quantification study of cerebral alpha4beta2* nicotinic acetylcholine receptors using the novel specific radioligand (−)-[F]Flubatine. Neuroimage. 2015;118:199–208. doi: 10.1016/j.neuroimage.2015.05.065. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortiz E, Yui D, Song D, Li Y, Rubenstein JL, Reichardt LF, Parada LF. TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J Neurosci. 2012;32:4065–4079. doi: 10.1523/JNEUROSCI.6314-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Kim Y. Interpreting chemical neurotransmission in vivo: techniques, time scales, and theories. ACS Chem Neurosci. 2015;6:8–10. doi: 10.1021/cn500319m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Berry AS, Gritton H, Howe WM, Parikh V. What do phasic cholinergic signals do? Neurobiol Learn Mem. 2016a;130:135–141. doi: 10.1016/j.nlm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Blakely RD, Cherian AK. Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. Journal of Physiology-Paris. 2016b doi: 10.1016/j.jphysparis.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39:1912–1920. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundaram B, Sase A, Miklosi AG, Sialana FJ, Subramaniyan S, Aher YD, Groger M, Hoger H, Bennett KL, Lubec G. Frontal cortex and hippocampus neurotransmitter receptor complex level parallels spatial memory performance in the radial arm maze. Behav Brain Res. 2015;289:157–168. doi: 10.1016/j.bbr.2015.04.043. [DOI] [PubMed] [Google Scholar]
- Shin J, Choi S, Lee JE, Lee HS, Sohn YH, Lee PH. Subcortical white matter hyperintensities within the cholinergic pathways of Parkinson’s disease patients according to cognitive status. J Neurol Neurosurg Psychiatry. 2012;83:315–321. doi: 10.1136/jnnp-2011-300872. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Wilson MA, Fadel JR. Hippocampal neurotransmitter efflux during one-trial novel object recognition in rats. Neurosci Lett. 2012;511:38–42. doi: 10.1016/j.neulet.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan S, Heo S, Patil S, Li L, Hoger H, Pollak A, Lubec G. A hippocampal nicotinic acetylcholine alpha 7-containing receptor complex is linked to memory retrieval in the multiple-T-maze in C57BL/6j mice. Behav Brain Res. 2014;270:137–145. doi: 10.1016/j.bbr.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Szegö ÉM, Gerhardt E, Outeiro TF, Kermer P. Dopamine-depletion and increased α-synuclein load induce degeneration of cortical cholinergic fibers in mice. Journal of the neurological sciences. 2011;310:90–95. doi: 10.1016/j.jns.2011.06.048. [DOI] [PubMed] [Google Scholar]
- Szego EM, Outeiro TF, Kermer P, Schulz JB. Impairment of the septal cholinergic neurons in MPTP-treated A30P alpha-synuclein mice. Neurobiol Aging. 2013;34:589–601. doi: 10.1016/j.neurobiolaging.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain. 2005;128:2626–2644. doi: 10.1093/brain/awh589. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, Moller HJ, Heinsen H, Hampel H. The cholinergic system in mild cognitive impairment and Alzheimer’s disease: an in vivo MRI and DTI study. Hum Brain Mapp. 2011;32:1349–1362. doi: 10.1002/hbm.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A. Muscarinic signaling in the brain. Annu Rev Neurosci. 2013;36:271–294. doi: 10.1146/annurev-neuro-062012-170433. [DOI] [PubMed] [Google Scholar]
- Thiele A, Herrero JL, Distler C, Hoffmann KP. Contribution of cholinergic and GABAergic mechanisms to direction tuning, discriminability, response reliability, and neuronal rate correlations in macaque middle temporal area. J Neurosci. 2012;32:16602–16615. doi: 10.1523/JNEUROSCI.0554-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1) Hippocampus. 1995;5:605–620. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol. 1994;348:351–373. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Unal CT, Golowasch JP, Zaborszky L. Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front Behav Neurosci. 2012;6:21. doi: 10.3389/fnbeh.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35:853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Somogyi M, Meng X. Rivastigmine in Alzheimer’s disease and Parkinson’s disease dementia: an ADAS-cog factor analysis. Am J Alzheimers Dis Other Demen. 2011;26:443–449. doi: 10.1177/1533317511424892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Struble RG, Clark AW, Price DL. Alzheimer disease: plaques, tangles, and the basal forebrain. Ann Neurol. 1982;12:494. doi: 10.1002/ana.410120517. [DOI] [PubMed] [Google Scholar]
- Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci. 2008;28:7797–7807. doi: 10.1523/JNEUROSCI.1885-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic Systems in Mammalian Brain and Spinal Cord. Progress in Neurobiology. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Wu H, Williams J, Nathans J. Complete morphologies of basal forebrain cholinergic neurons in the mouse. Elife. 2014;3:e02444. doi: 10.7554/eLife.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ishikawa M, Zhang J, Hashimoto K. Brain imaging of nicotinic receptors in Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010:548913. doi: 10.4061/2010/548913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P, Yakel JL. Heteromeric alpha7beta2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharmacol Sci. 2016;37:562–574. doi: 10.1016/j.tips.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Puddifoot CA, Taylor P, Joiner WJ. Mechanisms of inhibition and potentiation of alpha4beta2 nicotinic acetylcholine receptors by members of the Ly6 protein family. J Biol Chem. 2015;290:24509–24518. doi: 10.1074/jbc.M115.647248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, He YX, Guo F, Qi JS. Alpha4beta2 nicotinic acetylcholine receptors are required for the amyloid beta protein-induced suppression of long-term potentiation in rat hippocampal CA1 region in vivo. Brain Res Bull. 2008;77:84–90. doi: 10.1016/j.brainresbull.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Xu W, Weissmiller AM, White JA, 2nd, Fang F, Wang X, Wu Y, Pearn ML, Zhao X, Sawa M, Chen S, et al. Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration. J Clin Invest. 2016;126:1815–1833. doi: 10.1172/JCI82409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 2010;30:4408–4418. doi: 10.1523/JNEUROSCI.5719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Baker MR, David R, Khoo TK, Duncan GW, Galna B, Burn DJ. Short latency afferent inhibition: a biomarker for mild cognitive impairment in Parkinson’s disease? Mov Disord. 2013;28:1285–1288. doi: 10.1002/mds.25360. [DOI] [PubMed] [Google Scholar]
- Yi F, Catudio-Garrett E, Gabriel R, Wilhelm M, Erdelyi F, Szabo G, Deisseroth K, Lawrence J. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front Synaptic Neurosci. 2015;7:4. doi: 10.3389/fnsyn.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015a;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J. Organization of the Basal Forebrain Cholinergic Projection System. 2015b:491–507. [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ge XY, Wang JG, Wang YL, Wang Y, Yu Y, Li PP, Lu CB. Induction of long-term oscillations in the gamma frequency band by nAChR activation in rat hippocampal CA3 area. Neuroscience. 2015;301:49–60. doi: 10.1016/j.neuroscience.2015.05.060. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2008;28:9111–9116. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Talmage DA, Role LW. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS One. 2013;8:e82719. doi: 10.1371/journal.pone.0082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Wonderlick JS, Ashourian P, Hansen LA, Young JC, Murphy AJ, Koppuzha CK, Growdon JH, Corkin S. Substantia nigra volume loss before basal forebrain degeneration in early Parkinson disease. JAMA neurology. 2013;70:241–247. doi: 10.1001/jamaneurol.2013.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Bychkov E, Tsakem EL, Siedlecki C, Blakely RD, Gurevich EV. Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Dis Model Mech. 2013;6:171–183. doi: 10.1242/dmm.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]