Abstract
Weak and ineffective antitumor cytotoxic T lymphocyte (CTL) responses can be rescued by immunomodulatory monoclonal antibodies (mAbs) targeting PD-1 or CD137. Using Batf3-/- mice, which are defective for cross-presentation of cell-associated antigens, we show that Batf3-dependent dendritic cells (DCs) are essential for the response to therapy with anti-CD137 or anti-PD-1 mAbs. Batf3-/- mice failed to prime an endogenous CTL-mediated immune response toward tumor-associated antigens, including neoantigens. As a result, the immunomodulatory mAbs could not amplify any therapeutically functional immune response in these mice. Moreover, administration of systemic sFlt3L and local poly-ICLC enhanced DC-mediated cross-priming and synergized with anti-CD137- and anti-PD-1-mediated immunostimulation in tumor therapy against B16-OVA-derived melanomas, whereas this function was lost in Batf3-/- mice. These experiments show that cross-priming of tumor antigens by Flt3L- and Batf3-dependent DCs is crucial to the efficacy of immunostimulatory mAbs and represents a very attractive point of intervention to enhance their clinical antitumor effects.
Keywords: CD137, PD-1, Batf3-dependent dendritic cells, cancer immunotherapy
Introduction
Tumor cells are antigenic as a result of abundant mutated sequences in their exomes (1). However, they are poorly immunogenic to prime cytotoxic T lymphocyte (CTL) responses because antigen presentation takes place in the absence of appropriate costimulation, and in a strongly immunosuppressive environment (2). The immune response to cell-associated antigens requires the interplay of specialized and professional antigen presenting cells called dendritic cells (DCs). Among the variety of DC subsets, certain DCs excel at redirecting cell-associated phagocytosed proteins to the MHC class I antigen presentation pathway (3), a process termed cross-presentation, or cross-priming if it results in CD8 T cell activation. There is evidence that tumor antigens are efficiently cross-presented in vivo (4).
Two DC subsets have been identified in mice as the most efficient at cross-priming in vivo: lymphoid-tissue resident CD11c+ CD8α+ Clec9a/DNGR-1+ XCR1+ DCs and migratory CD11c+ CD103+ Clec9a/DNGR-1+ XCR1+ DCs (5). Differentiation of both DC subsets shows an absolute requirement for Flt3L, and is largely affected by the absence of Batf3 (6). Notably, the absence of Batf3 not only impairs numbers, but also functional responses in the remaining CD11c+ Clec9a/DNGR1+ XCR1+ DCs, such as cell-associated cross-presentation or IL-12 production (7, 8). Notably, Batf3-/- mice show impaired immunity against syngeneic immunogenic fibrosarcomas (6) and regulate T cell infiltration in models of melanoma (9). However, other Batf3-independent DC subsets mediate the immune system-dependent antitumor activity of anthracyclines (10) and mediate tumor rejection under activating conditions in Batf3-deficient mice (11). Recent reports further support an important role for intratumoral Batf3-dependent CD103+ DCs in priming a CTL response through IL-12 production (12, 13). In humans, an equivalent Batf3-dependent DC subset characterized by expression of CD11c, CD141, Clec9a/DNGR-1 and XCR1 has been identified in peripheral blood and lymphoid organs (14).
Immunotherapy of cancer is currently being revolutionized by the use of immunomodulatory monoclonal antibodies (mAbs). Interaction of PD-1 (CD279), on activated and exhausted lymphocytes, with its ligands (PD-L1 or PD-L2, expressed on antigen-presenting DCs and tumor cells) downmodulates T cell signaling (15, 16). Interference with these interactions using mAbs to PD-1 or PD-L1 has proved effective in cancer patients with metastatic melanoma, renal cell carcinoma, non-small lung cancer, bladder cancer, head and neck cancer, and other malignancies (17). In addition, stimulation of the costimulatory receptor on activated T lymphocytes CD137 (4-1BB) (18) results in complete tumor rejection in some transplantable tumor models (19). These promising findings have led to the clinical development of two anti-CD137 agents mainly for refractory lymphoma (BMS-663513/Urelumab and PF-05082566; NCT01775631, NCT02253992, NCT01307267).
The anti-PD-1 and anti-CD137 mAbs both induce tumor rejection by synergizing with vaccines (20), indicating that their function relies on a preexisting suboptimal CTL immune response that, if boosted, results in synergistic effects (1). Herein, we find an absolute need for Batf3-dependent DCs in cross-priming of tumor antigens to CTLs that subsequently upregulate PD-1 and CD137. This antitumor response can thus be manipulated with exogenous immunostimulatory mAbs. In consequence, expansion and activation of Batf3-dependent DCs concomitant with anti-CD137 mAb or anti-PD-1 treatment results in a suitable combined antitumor therapy.
Materials and Methods
Mice
Mice were bred at the CNIC and the CIMA in specific pathogen-free conditions. Batf3-/- on C57BL/6 background (kindly provided by Dr. Kenneth M. Murphy, Washington University, MO, USA) were further backcrossed with C57BL/6 mice at the CNIC to establish WT and Batf3-/- cousin colonies from the heterozygotes. Animal studies (protocol approval 150/12) were approved by the local ethics committee. All animal procedures conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes, enforced in Spanish law under Real Decreto 1201/2005.
Cell lines, culture conditions and tissue processing
MC38, MC38-OVA, B16F10 and B16-OVA cells were cultured in RPMI medium (Gibco) supplemented with 10% decomplemented and filtered fetal bovine serum (Sigma Aldrich) containing 50 µM β-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin (all from Gibco). MC38-OVA cells were kindly provided by Kees Melief (Leiden University Medical Center, Netherlands). All cell lines were cultured at 37ºC with 5% CO2. Isolated lymph nodes (LN) were incubated in collagenase/DNase for 15 minutes at 37ºC, followed by mechanical disaggregation using frosted slides. Single cell suspensions were then stained for flow cytometry.
Flow cytometry
Acquisition was performed using a FACS Canto II flow cytometer (BD Biosciences). The antibodies used included FITC-conjugated αPD-1 (29F.1A12) and αCD40 (3/23); PE-conjugated αCD11b (M1/70), αCD137 (17B5), and αIFNγ (XMG1.2); PrCPCy5.5-conjugated αCD103 (2E7) and αCD11c (N418); APC-conjugated αCD11b (M1/70), αPDL1 (10F.9G2), αCD8 (53-6.7) and αXCR1 (ZET); BV570-conjugated αCD8 (53-6.7); and BV421-conjugated αCD4 (RM4-5). For identification of epitope-specific T cells, PE or Alexa Fluor 647-conjugated H-2Kb-OVA257-264 tetramer (MBL and NIH Tetramer Facility), H-2Kb-KSPWFTTL pentamer (gp70, Proimmune) or H2-Db-ASMTNMELM dextramer (Adpgk, Immudex) were used. For intracellular staining, cells were fixed and permeabilized using Cytofix/Cytoperm buffer and then incubated with fluorochrome-conjugated antibodies in PermWash buffer (BD Biosciences).
In vivo tumor experiments
Cultured tumor cells were trypsinized before reaching confluence and suspended in phosphate buffered saline (PBS). Unless specified otherwise, 5 x 105 cells in 50 µl PBS were used for inoculation. Cells were injected subcutaneously (s.c.) using 29G syringes into the shaved right flank of 8-12 week-old C57Bl/6 Batf3-/- and WT mice. Tumor size was measured twice weekly and calculated as the product of orthogonal diameters.
Anti-CD137 (1D8) antibody was produced as described (19). Anti-PD-1 (RMP1-14) antibody was purchased from BioXcell. Antibodies (100 µg) were administered intraperitoneally (i.p.) in PBS on days 4, 7 and 10 after tumor inoculation. Recombinant mouse IL-12 (25 ng/dose) (Miltenyi) was administered intratumorally (i.t.) on days 7, 9 and 11. In experiments involving injection of IL-12, anti-CD137 was administered on days 7, 10 and 13. For in vivo DC expansion, 10 µg of sFlt3L-coding plasmid (pUMVC3-mFLex, Aldevron) or a control empty plasmid were injected i.v. to achieve hydrodynamic liver gene transfer. For in vivo stimulation of DCs, 100 µg poly-ICLC (Hiltonol, Oncovir) were injected i.t. on day 7 or when tumors reached 25-50 mm2. PBS was injected as control.
Ex vivo cross-presentation of surrogate tumor antigen
To test the ex vivo cross-presentation capacity of LN DCs, sFlt3L plasmid-injected mice were bilaterally inoculated s.c. with 2 x 106 MC38-OVA cells. LNs were extracted 48h later. CD11c+ cells were magnetically sorted with CD11c microbeads in an AutoMACS Pro Separator (Miltenyi) and further FACS-sorted where indicated. OT-I CD8 T lymphocytes were magnetically sorted from the spleens of C57Bl/6 mice using CD8 microbeads (Miltenyi). Cell Violet-labeled (Thermo Fisher) OT-I lymphocytes were cocultured with Batf3-/- and WT LN-derived CD11c+ or FACS-sorted CD11c+ subsets over a range of ratios. SIINFEKL peptide-pulsed DCs served as positive controls. After 72 h, culture supernatants were collected and OVA-reactive T cells were restimulated ex-vivo with 1 µg/ml SIINFEKL peptide for 5 h, being Brefeldin A (10 µg/ml; Sigma-Aldrich) added for the last 4h. Cells were then stained for membrane markers before being fixed and permeabilized for staining of intracellular IFN-γ. Secreted IFN-γ was measured in culture supernatants with the BD Biosciences OptEIA Mouse IFN-γ ELISA kit.
Analysis of T cell priming by tumor antigens
WT and Batf3-/- mice were inoculated s.c. with 2 x 106 MC38-OVA cells. Mice were injected i.p. with 100 µg anti-CD137 or an isotype control at days 5 and 7 after tumor inoculation. LNs and tumors were extracted at day 9. LNs were incubated at 37ºC in Liberase TL (Roche, 20 minutes) and tumors in Liberase TL/DNase I (30 minutes). Then, both LN and tumors were mechanically dissociated through a 70 µm cell strainer (Fisher Scientific). Single cell suspensions were stained and analyzed by flow cytometry.
For OVA- or Adpgk-specific T cell restimulation ex-vivo, single cell suspensions from LNs were cultured for 2 h in 10% FBS RPMI medium containing 1 µg/ml SIINFEKL or ASMTNMELM peptide. Then Brefeldin A was added at a final concentration of 10 µg/ml and cells were incubated for 10 h. Cells were stained for surface markers, fixed and permeabilized for intracellular IFN-γ staining. Samples were analyzed by flow cytometry.
Statistical analysis
Tumor growth data were analyzed with Prism software (GraphPad Software, Inc.). Mean diameters of tumors over time were fitted using the formula y = A x e (t-t0) / (1 + e(t-t0)/B), where t represents time, A the maximum size reached by the tumor and B its growth rate. Treatments were compared using the extra sum-of-squares F test. Tumor survival was compared with log-rank (Mantel-Cox) tests. All other analyses among groups were performed as described in figure legends.
Results
Ineffective antitumor therapy with immunomodulatory mAbs in Batf3-/- mice
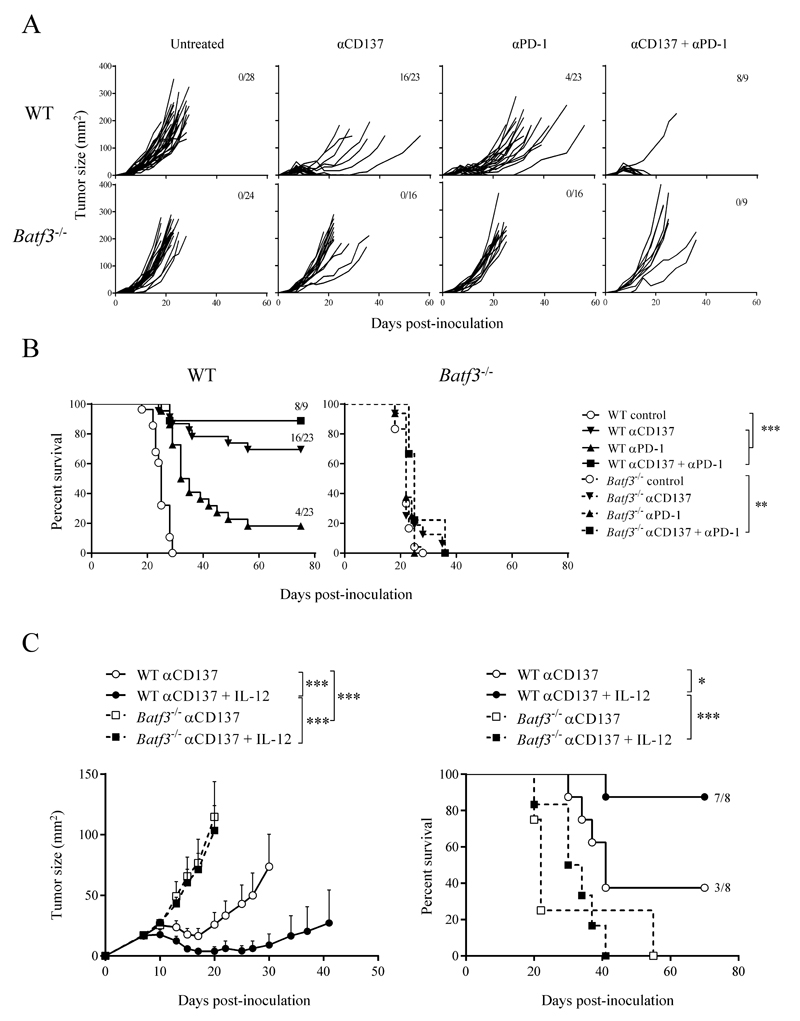
The absence of Batf3 affects the ontogeny and function of CD8α+ DCs in lymphoid organs and CD103+ DCs in the periphery, impairing cell-associated cross-presentation and the ability to produce IL-12 in response to infectious challenge. The antitumor effects of immunostimulatory anti-PD-1 and anti-CD137 mAbs are contingent on an already present baseline immune response, which is rescued and amplified by treatment. Based on the proposed role for Batf3-dependent DCs in immune-surveillance (6), we hypothesized that the preexisting immune response rescued by the immunostimulatory mAbs might be mediated by Batf3-dependent cross-priming. Grafted MC38-derived tumors were lethal in C57Bl/6 WT and Batf3-deficient mice, with slightly faster progression in Batf3-/- mice (Fig. 1A). In WT mice, tumor growth was delayed or curtailed by a course of treatment with anti-PD-1 or anti-CD137 mAbs, starting on day 4 after tumor cell inoculation. Combination treatment with both mAbs had a synergistic effect on their antitumor action (Fig. 1A and 1B), as previously reported in in other tumor models (21). The antitumor efficacy of anti-CD137 and anti-PD-1 mAbs, used alone or in combination, was abolished in Batf3-/- mice (Fig. 1A and 1B), suggesting that Batf3-dependent DCs are responsible for the baseline immune response that is potentiated by immunostimulatory mAbs, as Batf3-/- mice only present some functional defects in CD8α+ resident DC or CD103+ migratory DC (6, 7, 12).
Fig. 1. Antitumor therapy with immunomodulatory mAbs is abrogated in Batf3-/- mice and is not rescued by IL-12 administration.
WT or Batf3-/- mice were s.c. inoculated with 5 x 105 MC38 cells. (A, B) Mice were injected i.p. with 100 µg anti-PD-1 and anti-CD137 mAbs, alone or in combination (100 µg each), or with vehicle (untreated) on days 4, 7 and 10 after tumor cell inoculation. (A) Growth plots of individual tumors. (B) Overall survival charts show pooled results from 3 independent experiments with similar results. (C) Tumor-inoculated mice were injected i.p. with 100 µg anti-CD137 mAb on days 7, 10 and 13. The indicated groups of mice additionally received i.t. injections of recombinant mouse IL-12 or saline on days 7, 9 and 11. IL-12 was injected at 25 ng/dose into the tumor nodules. On the left, tumor area (mean ± SEM); on the right, overall survival. Fractions indicate the number of animals surviving at the end of the protocol. * p < 0.05; ** p < 0.01; *** p < 0.001.
We explored whether the ability of Batf3-dependent DCs to specifically provide IL-12 that boosts CTL function (8, 13) could underlie the advantage of Batf3-dependent DCs to mediate basal antitumor response. We analyzed the ability of intratumorally injected IL-12 to rescue the antitumor effect of systemic anti-CD137 mAb in the absence of Batf3. Repetitive injections of recombinant IL-12 in tumor lesions clearly potentiated the antitumor effects of systemic anti-CD137 mAb in WT mice, leading to rejection of most of the tumors (Fig. 1C). In stark contrast, no therapeutic effect was seen in identically treated Batf3-/- mice (Fig. 1C). Administration of IL-12 is thus unable to compensate the loss of a key function of Batf3-dependent DCs in the synergy with immunostimulatory anti-CD137 mAb.
Impaired ability of Batf3-/- DCs to cross-prime CTLs against tumor antigens
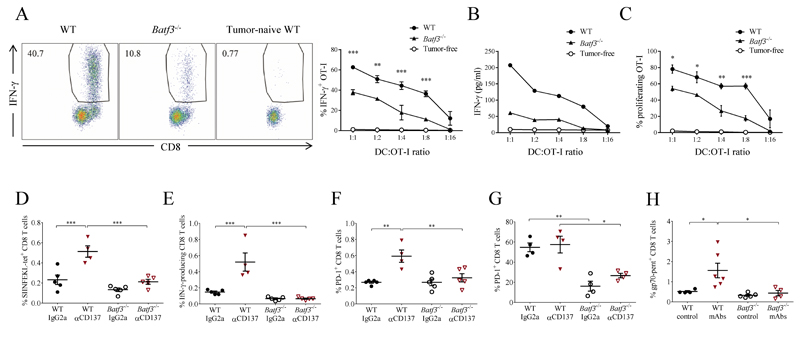
To investigate the possible involvement of deficient cross-presentation in the non-responsiveness of Batf3-/- mice to anti-PD-1 and anti-CD137 mAbs, we analyzed the ability of CD11c+ DCs to cross-present tumor-associated antigens to CD8+ T cells ex vivo. For these experiments, we used MC38 cells transfected to express ovalbumin (OVA) as a surrogate tumor antigen (22). Two days after tumor-cell grafting, CD11c+ DCs from tumor-draining LNs were magnetically sorted and cocultured at different ratios with OT-I OVA-specific CD8+ T cells. At all ratios tested, OT-I T cells cocultured with DCs from Batf3-/- mice produced markedly lower levels of intracellular and secreted IFNγ than cells cocultured with WT DCs (Fig. 2, A and B), and also showed impaired proliferation (Fig. 2C), although there was some remaining cross-priming activity by Batf3-/- DC.
Fig. 2. Reduced ability of Batf3-/- DC to cross-prime CTLs against tumor antigens both in steady state and after treatment with anti-CD137 and anti-PD-1 mAbs.
(A-C) CD11c+ DCs from WT and Batf3-/- mice bearing MC38-OVA tumors were magnetically sorted from tumor-draining LNs and cocultured (see Methods) with purified naïve CD8+ OT-I TCR transgenic T cells over a range of DC:T cell ratios. (A) Left: representative flow cytometry dot plots of intracellular IFN-γ staining in OT-I T cells cultured at a 1:4 DC:T cell ratio. Right: percentages of IFN-γ-positive OT-I T cells at all ratios tested. (B) IFN-γ concentrations in the culture supernatants. (C) Percentages of proliferating OT-I cells by dilution of Cell Violet dye (D-F) WT and Batf3-/- mice grafted with MC38-OVA cells were treated with anti-CD137 (days 5 and 7) and tumor-draining LN analyzed on day 9 (see Methods). (D) Frequency of H-2Kb-OVA-tetramer+ cells among CD8+ T cells. (E) Intracellular IFN-γ production induced by restimulation with OVA257-264 peptide in CD8+ T cells from tumor-draining LN. (F) PD-1 surface staining on tumor-draining LN CD8+ T cells. (G) Frequency of PD-1+ lymphocytes among CD8+ TILs in mice treated as in D. (H) WT and Batf3-/- mice grafted with MC38 cells were treated with anti-CD137 and anti-PD-1 mAbs on days 12 and 14 and tumor-infiltrating lymphocytes were analyzed on day 16 to detect CD8 T lymphocytes specific for gp70 antigen (A-C) Two-way and (D-H) one-way ANOVA with Bonferroni post-hoc test. * p < 0.05; ** p< 0.01; *** p < 0.001
To further inquire the DC subsets responsible for tumor cross-priming in WT and Batf3-/- mice, we FACS-sorted DC subsets from MC38-OVA tumor-draining LNs into resident CD11chiMHC-IIintCD11b+ and CD11chiMHC-IIintCD8α+, and migratory CD11cintMHC-IIhiCD103+ and CD11cintMHC-IIhiCD103- DCs and co-cultured them with purified OT-I T cells as above. Notably, only migratory DCs were able to cross-present and, among these, migratory CD103+ DCs demonstrated better ability for cross-presentation of tumor-associated antigens in a Batf3-dependent fashion (Supplementary Fig. S1).
We next tested whether deficiency in cross-presentation in the absence of Batf3 results in impaired cross-priming to tumor antigens in vivo. We analyzed priming of CD8+ T cells from the endogenous repertoire to grafted MC38-OVA tumors in WT and Batf3-/- mice treated or not with anti-CD137. In WT mice, treatment with anti-CD137 mAb increased the frequency and numbers of tumor antigen-specific CD8+ T cells from the endogenous repertoire in the tumor-draining LN (Fig. 2D), correlating with an increased effector response upon restimulation with tumor-antigen peptide (Fig. 2E). These effects were blocked in the absence of Batf3 (Fig. 2, D and E). Notably, priming of CD8+ T cells resulted in upregulation of surface PD-1 in CD8+ T cells at the tumor-draining LN in WT mice, and this was impaired in Batf3-/- mice (Fig. 2F). Tumor-infiltrating lymphocytes (TILs) were basally activated and expressed high PD-1 levels that were not further increased by anti-CD137 treatment (Fig. 2G). However, TILs expressed much lower levels of PD-1 in Batf3-/- mice (Fig. 2G), which correlates with their reduced potential to respond to immunomodulatory mAb therapy. These results show that Batf3-dependent DCs are crucial for the priming and concomitant induction of targets for immunostimulatory mAbs by tumor-specific CD8+ T cells.
We further analyzed the response against gp70, a well-described endogenous antigen in MC38 colon cancer cells (23). Notably, CD8+ TILs specific for gp70 were increased in a Batf3-dependent fashion upon anti-CD137 and anti-PD-1 mAb treatment, as detected by pentamer staining (Figure 2H). A similar analysis of the response to the Adpgk mutated neoantigen (24) showed some positive responses in WT but not Batf3-deficient mice (Supplementary Fig. S2).
Priming of CD137+ PD1+ antigen-specific TILs by activated Batf3-dependent DCs
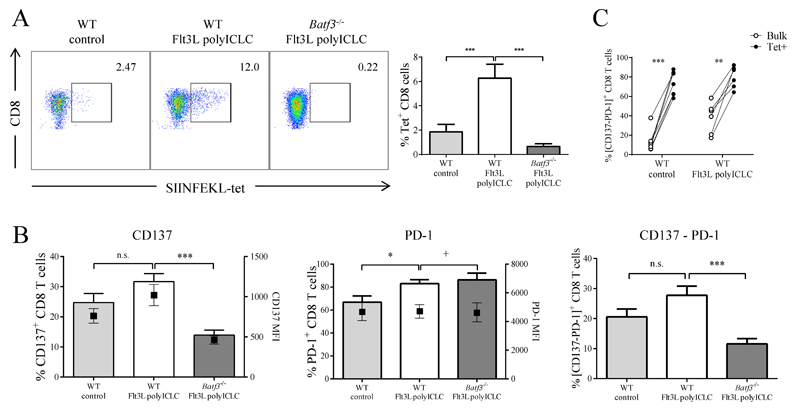
We hypothesized that expansion and activation of Batf3-dependent DCs with sFlt3L and the TLR3 adjuvant poly-ICLC would synergize with immunostimulatory mAbs to enhance priming of tumor-specific CD8+ T cells. To extend our results to an alternative tumor model, we used B16-OVA melanoma cells grafted subcutaneously. Hydrodynamic injection of a plasmid expressing sFlt3L markedly promoted the expansion of cross-presenting DCs (Supplementary Fig. S3A). Intratumoral administration of poly-ICLC increased some activation markers including CD40 and PD-L1 in DCs from the in spleen, tumor and tumor-draining LN, particularly in the TLR3-expressing CD103+ DCs (Supplementary Fig. S3B-S3D). Immunity to B16-OVA was estimated from the number of TILs detected by OVA-MHC-tetramer staining, and was almost undetectable in control mice treated with empty vector and intratumoral saline buffer (Fig. 3A). Systemic hydrodynamic injection of sFlt3L combined with intratumoral injection of poly-ICLC raised a specific antitumor CTL response, and this induction was blocked in Batf3-/- mice (Fig. 3A). These events were paralleled by an increased frequency of CD137+ CD8+ T cells in WT mice treated with sFlt3L and poly-ICLC, and the impairment of this effect in Batf3-/- mice (Fig. 3B). Notably, antigen-specific TILs showed higher surface expression of PD-1 and CD137 compared with the bulk of CD8 infiltrating T cells (Fig. 3C). These results show that expansion and activation of Batf3-dependent DCs increases the frequency of primed CD8+ T cells that upregulate markers of activation and exhaustion and are sensitive to immunostimulatory mAb treatment because of the expression of the targets for such agents.
Figure 3. sFlt3L and poly-ICLC induce a Batf3-dependent increase in the numbers of tumor-antigen-specific TILs expressing CD137 and PD-1.
WT or Batf3-/- mice were inoculated with B16-OVA melanoma cells on day 0, concomitant with hydrodynamic gene transfer of sFlt3L or control empty plasmid. On day 7, tumors were injected with poly-ICLC or control. Tumors were retrieved and TILs analyzed on day 10. (A) H2Kb-OVA257-264 tetramer staining in CD8+ TILs. Left: Representative plots. Right: Graphs corresponding to a representative experiment (n = 3) (B) Surface CD137 and PD-1 immunostaining in CD8 TILs. (C) PD-1 and CD137 surface immunostaining in SIINFEKL tetramer+ gated T cells. One-way ANOVA with Bonferroni post-hoc test, * p < 0.05; ** p < 0.01; *** p < 0.001.
Batf3-dependent DC activation enhances antitumor ability of immunomodulatory mAbs
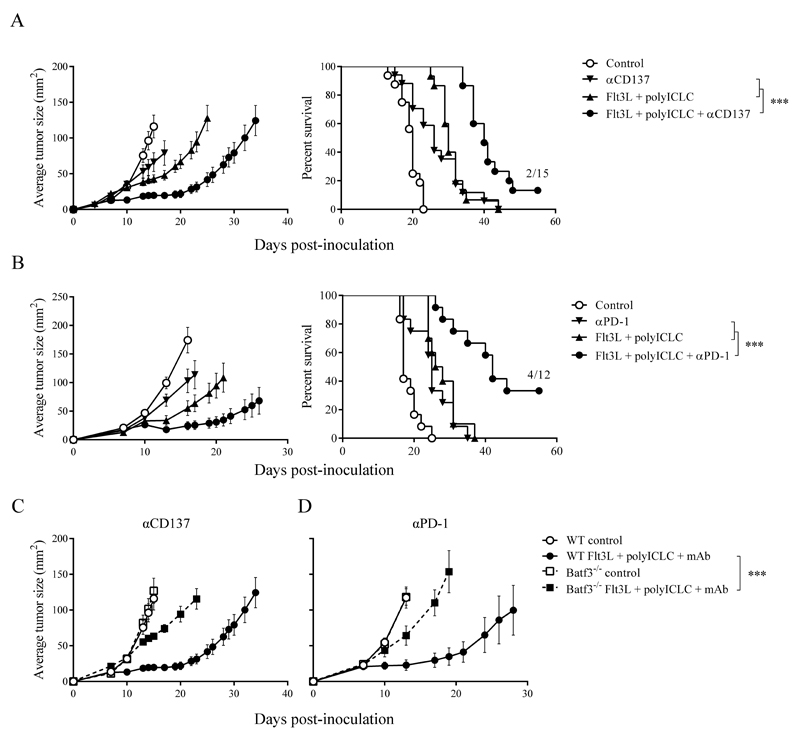
We next sought to establish how Flt3L- and poly-ICLC-enhanced priming of CD8+ T cells affects the antitumor efficacy of anti-CD137 and anti-PD-1 mAbs. For this analysis we used the B16-OVA model, which in our hands responds weakly or not at all to anti-PD-1 or anti-CD137 mAb treatment (Fig. 4, A and B). Hydrodynamic injection of sFlt3L was concomitant with tumor inoculation, and intratumoral injection of poly-ICLC at day 7 was administered with or without anti-PD-1 or anti-CD137 mAbs at days 4, 7 and 10 after tumor inoculation. The triple combinations retarded tumor progression and significantly extended overall survival in WT mice (Fig. 4, A and B) but had no significant effect in Batf3-/- mice (Fig. 4, C and D). Furthermore, we found that quadruple combination immunotherapy encompassing sFlt3L + poly-ICLC + anti-CD137 + anti-PD-1 mAbs exerted marked antitumor effects against parental B16F10-derived melanomas (Supplementary Fig. S4A), while completely eradicated B16-OVA-derived tumors (Supplementary Fig. S4B). Functional enhancement of Batf3-dependent DCs thus cooperates synergistically with anti-CD137 and anti-PD-1 mAbs, indicating that baseline Batf3-dependent cross-priming is a key limiting factor that can be targeted to enhance antitumor immunity.
Figure 4. sFlt3L and poly-ICLC do not control the progression of B16-OVA derived tumors in Batf3-/- mice.
WT B16-OVA-bearing mice administered with hydrodynamic gene transfer with sFlt3L or control empty plasmid received i.p. injections of anti-CD137 mAb (A) or anti-PD-1 mAb (B), controlled by vehicle buffer, on days 4, 7 and 10. Poly-ICLC or control was administered i.t. on day 7. On the left, tumor areas (mean ± SEM). On the right, overall survival. (C-D) Comparison of the combined efficacy of sFlt3L + poly-ICLC with anti-CD137 mAb (C) or anti-PD-1 (D) in WT and Batf3-/- mice. Graphs represent pooled data from 4 (A,C) or 2 (B,D) independent experiments with similar results, for a total of 10-15 mice per group. * p < 0.05; ** p < 0.01; *** p < 0.001
Discussion
This study shows the immunodynamic interactions between professional cross-priming DCs and immunostimulatory mAbs that target CD137 and PD-1. The observations are fully consistent with an essential presentation of tumor antigens to CD8+ T cells by Batf3-dependent DCs. Both migratory CD103+ DCs and LN-resident CD8α+ DCs are functionally or ontogenically impaired in Batf3-/- mice (6,7,12), as they are also in Irf8-/- mice (12). Our results support a model in which at least one of these DC subsets is crucial for the basal antitumor response that is amplified by immunostimulatory mAbs.
Batf3-dependent DC subsets have been identified in the tumor environment, where they are functional and even have positive prognostic significance (12). These DCs are effective at taking up antigen from tumor cell debris for MHC class I cross-presentation. We find that these DCs mediate CTL priming at the malignant tissue or migrate via lymphatic afferent vessels to reach the draining LNs and meet naive or central memory CD8+ T cells. These primed CTLs upregulate surface CD137 and PD-1, making them suitable targets for immunostimulatory mAbs. Our results show that expansion and activation of Batf3-dependent DCs result in increased antitumor priming and more effective tumor rejection in response to immunostimulatory mAbs. The dependency of anti-CD137 mAb treatment on DCs was suggested by the decreased efficacy of treatment upon depletion of CD11c cells (25). In the case of anti-PD-1 mAb, treatment synergizes with vaccines consisting of tumor cells transfected with GM-CSF or Flt3L, whose activity depends on attraction and differentiation of DC subsets (26).
Our data are consistent with the recent results from Gajewski and colleagues, elegantly showing that Batf3-dependent CD103+ DCs play an important role in regulating the infiltration of T cells in the tumor. Notably, intratumoral injection of cultured Flt3L-derived DCs rescues the response to anti-CTLA-4 and anti-PD-L1 immunomodulatory mAbs in terms of inducing antitumor CTLs and exerting antitumor activity (9). Previous studies from the same group had indicated a role for CD8α+ dendritic cells in the baseline CTL response to a transplantable melanoma model (27).
CD103+ DCs were recently shown to be responsible not only for priming in the draining LN, but also for IL-12-dependent promotion of a productive CD8+ T cell response locally in the tumor (12, 13), suggesting that expansion and activation of Batf3-dependent DCs might favor the generation of antitumor responses at several levels. Although professional cross-priming DCs have been characterized as key IL-12 producers in infections and also in the tumor environment (8, 12, 13), we find that treatment of tumor-bearing mice with exogenous IL-12 is unable to rescue a key Batf3-dependent function needed for synergy with immunostimulatory mAbs. Therefore, while IL-12 production might be involved in the action of Batf3-dependent DCs, other functions of cross-priming DCs are absolutely needed. It is becoming apparent that effective anti-CTLA-4 or anti-PD-1 mAb therapy requires the presence of a measurable pre-existent CTL response to the tumor mutatome epitopes, both in humans and mice (28). It is now crucial to identify whether such responses are caused by direct presentation of antigens by tumor cells or by cross-priming of tumor cell-associated antigens in the tumor or in the tumor-draining LNs. Our data suggest that basal antitumor responses that are amplified by immunostimulatory mAbs have a critical requirement for professional cross-priming by DCs.
The need for cross-priming in the antitumor immune response also indicates possible relationships with mechanisms of immunogenic tumor cell death (10). Recent results show a crucial role for Batf3-dependent CD103+ DCs in priming a CTL response through IL-12 production in the context of tumor cell death induced with paclitaxel (12, 13). However, doxorubicin-mediated immunogenicity against F244 sarcoma cells is Batf3-independent (10) and Batf3-deficient mice are able to reject tumors under conditions with exogenously provided IL-12 (11). Therefore the precise role of Batf3-dependent CD103+ DCs may depend on the context of the ongoing baseline immune response in the tumor, which will be eventually modulated by the treatment with immunostimulatory mAbs.
Each addition to our knowledge in this area of tumor antigen cross-priming has the potential to provide predictive biomarkers for the efficacy of immunostimulatory mAbs, since cross-priming against tumor neo-antigens seems to be a key determinant of the variable efficacy of these treatments in mice and humans (1, 12, 28). Moreover, more effective vaccines could be prepared by immune sorting or targeting these cross-priming DC populations or their differentiation in culture from precursors (29).
Overall, our results raise important pointers for improving therapy with immunostimulatory mAbs. The cross-priming function of DCs is essential for the therapeutic effect of immunostimulatory mAbs, but the baseline CTL-priming function is suboptimal. These observations suggest the potential to devise exogenous or in situ tumor vaccination therapies to enhance cross-priming of tumor antigens and thereby increase the efficacy of immunostimulatory mAbs.
Supplementary Material
Statement of significance.
Immunotherapy with immunostimulatory monoclonal antibodies (mAbs) is currently achieving durable clinical responses in different types of cancer. We show that cross-priming of tumor antigens by Batf3-dependent dendritic cells is a key limiting factor that can be exploited to enhance the antitumor efficacy of anti-PD-1 and anti-CD137 immunostimulatory mAbs.
Acknowledgments
We are grateful to the CIMA and CNIC facilities, technicians and assistants and to Simon Bartlett for editorial assistance. We are indebted to all the scientists that have shared reagents with us, as indicated in Methods, and in particular to K. Melief for the kind gift of MC38-OVA cells and Andres Salazar (Oncovir) for poly-ICLC (Hiltonol). We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC-I tetramers.
Financial support: Work at IM lab is funded by MICINN (SAF2008-03294, SAF2011-22831), Departamento de salud del Gobierno de Navarra, Redes temáticas de investigación cooperativa RETIC (RD06/0020/0065), European commission 7th framework program (ENCITE and IACT). Work in the DS laboratory is funded by the CNIC and grants from the Spanish Ministry of Economy and Competitiveness (SAF-2013-42920R), the European Research Council (ERC Starting Independent Researcher Grant 2010, ERC-2010-StG 260414). The CNIC is supported by the Spanish Ministry of Economy and Competitiveness and the Pro-CNIC Foundation. IM and DS are funded by the European Commission (635122-PROCROP H2020).
Footnotes
Conflicts of interest: Ignacio Melero has served in advisory boards of Bristol Myers-Squibb, Roche-Genentech, AstraZeneca, Merck Serono and Boehringer Ingelheim, and holds research grants by Pfizer, Bristol Myers and Alligator.
Author contributions: Alfonso R. Sánchez-Paulete performed experiments, analyzed data and wrote the manuscript; Francisco J. Cueto performed experiments, analyzed data and edited the manuscript; María Martínez-López performed experiments and edited the manuscript; Sara Labiano analyzed data; María Rodríguez analyzed data; Maria Jure-Kunkel provided key reagents and models; Arantza Azpilikueta performed experiments; M. Angela Aznar analyzed data; Aizea Morales-Kastresana analyzed data; José I. Quetglas performed experiments and analyzed data; David Sancho designed experiments, analyzed data and wrote the manuscript; Ignacio Melero designed experiments, analyzed data and wrote the manuscript.
References
- 1.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 4.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 5.Schraml BU, Reis e Sousa C. Defining dendritic cells. Curr Opin Immunol. 2015;32:13–20. doi: 10.1016/j.coi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seillet C, Jackson JT, Markey KA, Brady HJ, Hill GR, Macdonald KP, et al. CD8alpha+ DCs can be induced in the absence of transcription factors Id2, Nfil3, and Batf3. Blood. 2013;121:1574–83. doi: 10.1182/blood-2012-07-445650. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Lopez M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol. 2015;45:119–29. doi: 10.1002/eji.201444651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, Kc W, Albring JC, et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490:502–7. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–52. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 16.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Chan TA. Cancer: Antitumour immunity gets a boost. Nature. 2014;515:496–8. doi: 10.1038/515496a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–53. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 20.Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazon A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin Cancer Res. 2009;15:1507–9. doi: 10.1158/1078-0432.CCR-08-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palazon A, Martinez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, et al. The HIF-1alpha hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov. 2012;2:608–23. doi: 10.1158/2159-8290.CD-11-0314. [DOI] [PubMed] [Google Scholar]
- 22.Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJ. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res. 2013;19:5381–9. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 23.Yang JC, Perry-Lalley D. The envelope protein of an endogenous murine retrovirus is a tumor-associated T-cell antigen for multiple murine tumors. J Immunother. 2000;23:177–83. doi: 10.1097/00002371-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 25.Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–36. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 26.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–55. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer CT, Ghorbani P, Nandan A, Dudek M, Arnold-Schrauf C, Hesse C, et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood. 2014;124:3081–91. doi: 10.1182/blood-2013-12-545772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.