Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer death worldwide. HCC is usually asymptomatic at potential curative stages, and it has very poor prognosis if detected later. Thus, the identification of early biomarkers and novel therapies is essential to improve HCC patient survival. Ion channels have been proposed as potential tumor markers and therapeutic targets for several cancers including HCC. Especially, the ether à-go-go-1 (Eag1) voltage-gated potassium channel has been suggested as an early marker for HCC. Eag1 is overexpressed during HCC development from the cirrhotic and the preneoplastic lesions preceding HCC in a rat model. The channel is also overexpressed in human HCC. Astemizole has gained great interest as a potential anticancer drug because it targets several proteins involved in cancer including Eag1. Actually, in vivo studies have shown that astemizole may have clinical utility for HCC prevention and treatment. Here, we will review first some general aspects of HCC including the current biomarkers and therapies, and then we will focus on Eag1 channels as promising tools in the early diagnosis of HCC.
Keywords: ion channels, Eag1, hepatocellular carcinoma, astemizole, diethylnitrosamine
Introduction
Primary liver cancer is a major health problem,1 representing the second leading cause of cancer-related deaths in the world.2 Hepatocellular carcinoma (HCC) accounts for up to 90% of primary liver cancers.1 Other liver cancer types include childhood hepatoblastoma, adult cholangiocarcinoma (originating from the intrahepatic biliary ducts), and angiosarcoma (from the intrahepatic blood vessels).3
HCC affects men three times more frequently than women worldwide. It is the fifth most frequently diagnosed cancer in adult men, and the seventh most commonly diagnosed cancer in adult women.4 Interestingly, significant differences have been noted between the races. Asians are affected two times more than blacks, and Hispanics are affected two times more than whites. The ethnic variability reflects the contribution of specific causal factor differences among populations.4 Because of its poor prognosis, it is compulsory to find novel early markers of the disease as well as new therapeutic approaches for HCC prevention and treatment.
HCC pathophysiology
Liver cirrhosis is the strongest HCC predisposing factor. Actually, 80% of HCC cases formerly developed cirrhosis. Other well-defined risk factors comprise viral infections (chronic hepatitis B and C), toxics (alcohol and aflatoxin B1), and altered metabolic conditions (diabetes, nonalcoholic fatty liver disease, hereditary hemochromatosis).5–7 Recently, tobacco and obesity have also been proposed as potential risk factors for the development of HCC.8 The major risk factors for HCC are described in Table 1.
Table 1.
Main HCC risk factors
| Risk factor | Description |
|---|---|
| Liver cirrhosis | This is the most important clinical risk factor for HCC. The transition from chronic liver disease to cirrhosis involves inflammation and activation of hepatic stellate cells with ensuing fibrogenesis and angiogenesis. As many hepatocytes are lost, the liver loses the ability to metabolize bilirubin (which can result in an increased serum bilirubin and transaminase level). Liver cirrhosis is characterized by diffuse nodular regeneration surrounded by dense fibrotic septa with subsequent parenchymal extinction and liver structure collapse.58,59 |
| HBV infection | The hepatitis B virus is a DNA virus belonging to the Hepadnaviridae family. Many evidences support that HBv DNA is frequently integrated into the chromosomal DNA of hepatocytes in most HBV-infected patients. The mechanisms of carcinogenesis in HBV infection have been extensively studied, and a major factor is chronic necroinflammation with subsequent fibrosis and hepatocyte proliferation. However, HCC may occur in HBsAg carriers without cirrhosis. Therefore, viral factors are likely involved in HBV-related hepatocarcinogenesis. For instance, the HBx protein promotes cell cycle progression, inactivates negative growth regulators, and binds to and inhibits the expression of p53 and other tumor suppressor genes and senescence-related factors.5,6,60–62 |
| HCV infection | HCV is an enveloped, single-stranded, positive-sense RNA virus. Up to 80% of HCV-infected individuals fail to eliminate the virus acutely and progress to chronic HCV infection. Continuous inflammation and hepatocyte regeneration in the setting of chronic hepatitis and subsequent progression to cirrhosis are thought to lead to chromosomal damage and possibly to initiate hepatic carcinogenesis. HCV also induces steatosis; oxidative stress causes steatohepatitis and these pathways lead to liver injury or HCC in chronic HCv infection.6,63,64 |
| Aflatoxin (AFB1) | This is a difuranocoumarin-derivative mycotoxin from Aspergillus flavus and Aspergillus parasiticus. it is produced under certain climatic factors and storage techniques favoring the fungus growth and contaminating foods such as maize, groundnuts, rice, and sorghum. IARC ranked AFB1 as the most potent experimental hepatocarcinogen, and it is causally related to the development of HCC in humans. Hepatitis B virus infection may directly or indirectly sensitize hepatocytes to the carcinogenic effects of AFB1, raising the possibility of a synergistic hepatocarcinogenic interaction between these two agents, although the precise mechanism is still not well understood.6,65 |
| Alcohol | Alcohol use has definitely been recognized as a cause of HCC. This may be well related to the development of HCC due to direct (genotoxic) and indirect factors (cirrhosis development). ADH metabolizes ethanol to acetaldehyde; this metabolite is not only extremely toxic but also carcinogenic. Alcohol-related liver cirrhosis is most likely the main risk factor for HCC in groups of people with low rates of hepatitis B or C viral infection, such as the US and Northern europe. Some reports mention that 25–80 g/d of alcohol use for 10 years or more for men, and 12–20 g/d for women, increases the risk of developing cirrhosis.1,66 |
| DM | This metabolic disorder may predispose the liver to relative insulin resistance due to inadequate insulin secretion or receptor insensitivity to endogenous insulin. Several evidences showed that insulin resistance and DM induce the progression of NAFLD, including its most severe form, NASH, which has been identified as a cause of cirrhosis and HCC.1,67 |
| NAFLD | NAFLD defines liver abnormalities ranging from simple steatosis (abnormal hepatic fat accumulation) or nonalcoholic fatty liver to NASH with or without cirrhosis development. importantly, it is also closely linked to obesity and metabolic syndromes. Although there is significant progress in understanding carcinogenesis, the exact mechanism of HCC development from NAFLD has not yet been fully elucidated. However, a recent report suggests that the antiapoptotic protein survivin is differently expressed in NASH-HCC-related tissues compared with HCV-HCC-related samples. Besides, another study identified that mTOR was differently expressed in NAFLD-related cirrhosis compared with other causes of cirrhosis.68,69 |
| HH | HH is an inherited (genetic) disorder causing the body to absorb too much iron from the diet. The main storage sites of iron are the hepatocytes, and this metal is essential for their normal functioning, although iron is ubiquitous in human cells. The hepatotoxic and hepatocarcinogenic potential of excessive iron (>5 g) is due to its ability to generate ROS intermediates and oxidative stress (by the Fenton reaction). This stress damages DNA, lipids, and proteins, resulting in necrosis and apoptosis of hepatocytes. Patients with HH have an estimated 240-fold increased relative risk of developing HCC.1,70 |
| Tobacco use | Tobacco smoking has been suggested as a significant HCC risk factor. Nicotine, the main component of cigarette smoke, upregulates CYP2E1 activity in the liver of rodents and humans. CYP2E1 induction is associated with ROS generation and lipid peroxidation, which may be some mechanisms whereby tobacco smoke contributes to HCC.71 |
| Obesity | Obesity has been associated with HCC because lipid accumulation within hepatocytes leads to a chronic low-grade inflammation involving the release of proinflammatory cytokines and inhibition of anti-inflammatory cytokines, leading to hyperinsulinemia. increased levels of iGF-1 have important proliferative and antiapoptotic effects. This factor also promotes angiogenesis via increased vascular endothelial growth factor production, which in turn leads to proliferation of cancer cells.72 |
| OCs | OCs are combinations of estrogens and progestogens. Synthetic estrogens such as ethinyl estradiol and mestranol produce malignant liver tumors in rodents, probably by acting as promoting agents. Additionally, progestogens (norethisterone and norethynodrel) cause benign liver neoplasia in animals. OCs may cause liver cancer by mitogenesis because increased proliferation rates may enhance the rate of spontaneous mutations due to DNA polymerase errors.1,54 |
Abbreviations: ADH, alcohol dehydrogenase; DM, diabetes mellitus; HBsAg, hepatitis B surface antigen; HBv, hepatitis B virus; HCC, hepatocellular carcinoma; HCv, hepatitis C virus; HH, hereditary hemochromatosis; IARC, International Agency for Research on Cancer; IGF-1, insulin growth factor-1; mTOR, mechanistic target of rapamycin; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OCs, oral contraceptives; ROS, reactive oxygen species.
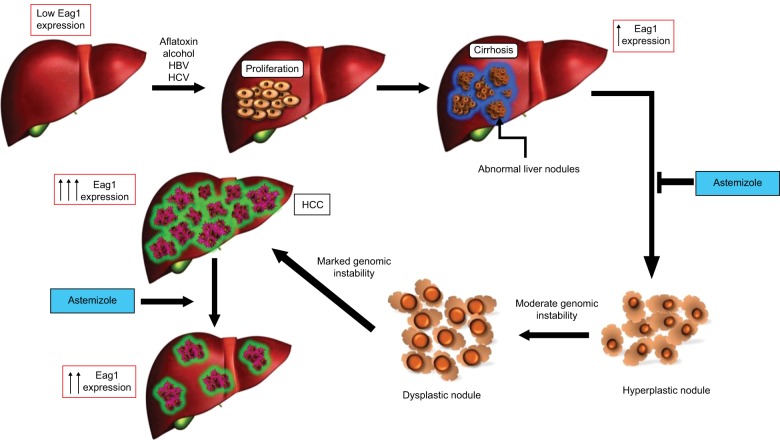
Hepatocarcinogenesis is a multistep process in which a number of genetic alterations accumulate in the cells. After hepatic injury due to major predisposing risk factors for HCC, necrosis arises followed by hepatocyte proliferation. This continuous process of destructive–regenerative cycles in the hepatocyte promotes chronic liver injury and progressive liver fibrosis resulting in cirrhosis. If the process continues, the next step is the progressive malignant transformation of cirrhotic nodules and premalignant lesions, which will finally lead to HCC9,10 (Figure 1).
Figure 1.
Eag1 expression in the progression of HCC.
Notes: The principal risk factors for the development of HCC induce serious liver damage, causing necrosis and proliferation in the hepatocyte. This phase is known as chronic liver disease and may continue to liver cirrhosis. Cirrhosis is characterized by the presence of fibrosis; during this process, the connective tissue separates the liver into multiple regeneration nodules, that is, the fibrosis surrounds the nodules completely. The hyperplastic nodules evolve to dysplastic ones and ultimately to HCC. The use of astemizole in the cirrhosis stage may prevent the development of HCC and even produce HCC regression. Eag1 channels have been shown to be expressed at early stages of HCC development.32
Abbreviations: Eag1, ether à-go-go-1; HCC, hepatocellular carcinoma.
Because most HCC cases are associated with chronic viral hepatitis, prevention of virus infection should lead to the prevention of HCC.11–13 Some preventive strategies already used include vaccination (the universal vaccination was introduced in all newborns and high-risk groups as routine immunization13–15), antiviral treatment (it has been shown that antiviral treatment of chronic hepatitis B virus and hepatitis C virus infections may reduce the risk of HCC13,16,17), and periodical surveillance in patients at risk of developing a disease (Figure 2).11,13,18
Figure 2.
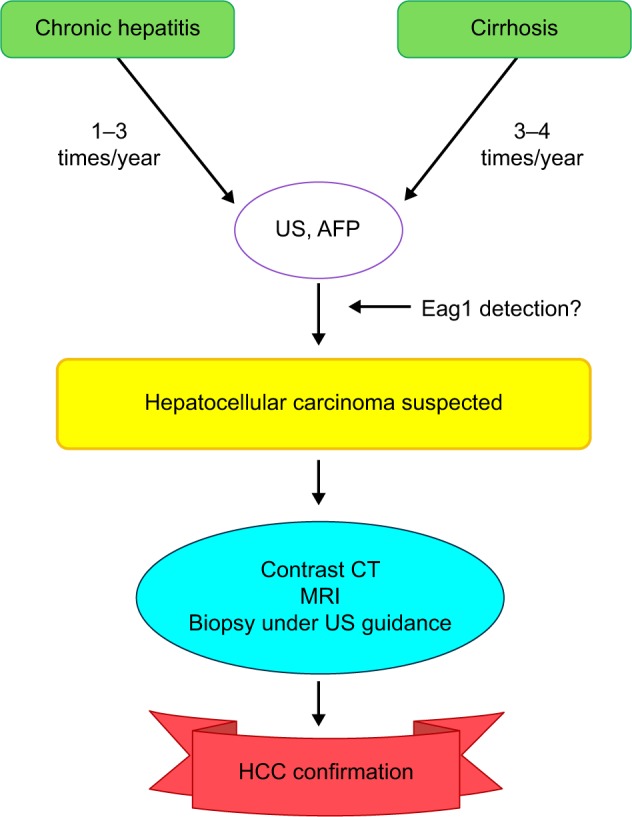
Follow-up for patients at risk of HCC.
Notes: The early screening should help to detect HCC when treatable. This screening should be performed, especially in patients with liver diseases at risk of developing HCC, and Eag1 detection may be also included in this screening.
Abbreviations: AFP, alfa-fetoprotein; CT, computed tomography; Eag1, ether à-go-go-1; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; US, ultrasound.
HCC diagnosis and treatment
Diagnosis
HCC is frequently diagnosed at an asymptomatic stage while the patients are being evaluated for liver transplantation or as part of routine screening in cirrhotic patients. The classic clinical features of HCC include right upper quadrant pain, weight loss, and worsening of liver function. Patients with chronic liver diseases belong to a high-risk group for HCC and a follow-up based on imaging and tumor marker levels11,19 should be regularly made for early diagnosis.
Current and emerging potential HCC biomarkers
Alfa-fetoprotein (AFP) is commonly used as an HCC marker; serum AFP levels may be helpful in the diagnosis and management of HCC. AFP is higher than 20 ng/mL in more than 70% of HCC patients. However, AFP levels from 10 to 500 ng/mL and even up to 1,000 ng/mL may be found in patients with other liver diseases who do not have HCC. AFP is useful mainly in monitoring the response to treatment and detecting recurrence after treatment of HCC, if the AFP levels were elevated before treatment.11,12,19,20
Despite that an ideal biomarker should have high sensitivity and specificity21 and be detectable in any of different samples such as blood, urine, or tissues,22 the coexistence of inflammation and cirrhosis in HCC makes the early diagnosis and prognostic assessment much more difficult. This complication highlights the need to identify valuable biomarkers for the diagnosis and treatment of HCC.22
Recent advances in genomics and proteomics have facilitated the identification of many biomarkers, including new HCC molecular markers. However, only a few biomarkers are acceptable for clinical utility because the rest have low predictive accuracy and/or high cost.23,24 The most relevant biomarkers in liver cancer are described in Table 2.
Table 2.
Principal and potential HCC biomarkers
| AFP | AFP is a fetal component protein produced in the yolk sac and liver of the developing fetus, it has been considered as the most useful serum protein for the diagnosis of patients at risk of HCC. High AFP serum levels have been found in 60%–70% of patients with HCC. Nevertheless, it is not HCC specific since elevated AFP levels are observed in liver cirrhosis and other tumors, including lung, biliary, gastric, and pancreatic cancer. AFP levels <20 ng/mL are considered normal, while high levels of AFP (> 400 ng/mL) are strongly predictive for HCC. This diagnostic test has sensitivity values of 41%–65% to diagnose HCC, but low specificity.24,73 |
| DKK1 | DKK1 is a serum protein marker for HCC and plays an important role in HCC progression through the promotion of cytoplasmic/nuclear accumulation of beta-catenin in HCC cells via the wnt/beta-catenin signaling. it has been proposed that DKK1 alone or in combination with AFP is better than AFP alone for HCC diagnosis, especially for patients with normal AFP values or at early stage of HCC.24,74–76 |
| GP73 | This is a type ii Golgi transmembrane protein. GP73 is expressed primarily in the bile duct of epithelial cells and rarely in the hepatocytes of the normal human liver. Its expression is significantly increased in liver diseases including HCC. Therefore, GP73 has been suggested as a potential HCC serum marker and also as a potentially useful marker of general liver disease progression.24,77 |
| PIVKA-II | PIVKA-II (also known as DCP) is an abnormal prothrombin protein that is increased in the sera of patients with HCC. Zakhary et al proved that PivKA-ii has the ability to discriminate between different histopathological grades of HCC (early and late stages of HCC); this confers PIVKA-II’s high sensitivity and specificity in comparison to αFP.24,78,79 |
| SCCA-IGM | Under physiological conditions, it is found in the spinous and granular layers of normal squamous epithelium. Recent findings have identified overexpression of SCCA variants (SCCA-1, SCCA-2, and SCCA-PD) in all surgically resected HCC specimens but no expression in the normal livers. Specifically, SCCA-1 and SCCA-2 isoforms protect neoplastic cells from apoptosis induced by several stimuli; in vivo experiments have demonstrated that SCCA-1 promotes tumor growth.80,81 |
| AFU | AFU is a liposomal enzyme present in all mammalian cells, blood, and body fluids, which is involved in the degradation of a variety of fructose-containing fucoglyco-conjugates. AFU-based detection is able to diagnose 85% of patients with HCC 6 months before the detection by ultrasonography. Consequently, AFU has been proposed as a promising tumor marker in the diagnosis of HCC.74,82,83 |
| GPC3 | This is a member of the heparan sulfate proteoglycan family. Glypicans regulate the activity of several signaling molecules including wnts. GPC3 can be detected in the serum as a secreted protein in a subset of patients with HCC, but it is undetectable in healthy individuals; patients with hepatitis and cirrhosis; or benign hepatic lesions. Therefore, GPC3 protein detection by immunohistochemistry in liver biopsies is currently being used in the clinic to confirm HCC diagnosis when the malignant nature of the lesion is difficult to establish.84,85 |
| IGF-II | IGF-II is a 67-amino-acid polypeptide growth factor that is mainly produced by liver cells and plays a crucial role in normal fetal growth. iGF-ii may play an important role in the development of HCC neovascularization. it has been observed that free-circulating IGF-II levels are significantly higher in HCC patients than in those with chronic liver disease.86,87 |
| OPN | OPN expression is found in normal bones and kidneys. Serum OPN levels are correlated with hepatic inflammation and fibrosis in heavy alcohol drinkers, Circulating levels of OPN are elevated in patients with liver lesions associated with HCV and HBV infections. Although data suggest a better performance of plasma OPN in the diagnosis of HCC, the role of this biomarker still needs validation.88 |
| miRNAs | miRNAs are a class of small, noncoding, ~22 nucleotide-long RNAs, which may function as posttranscriptional regulators of gene expression. Many miRNAs are expressed in a tissue- or organ-specific manner, suggesting them as highly specific biomarkers. in the case of liver, miRNAs may enter the serum passively through apoptosis and necrosis or actively through secretion of exosomes and viral particles.89 its detection in serum may provide a way to estimate miRNA activity in the liver. in addition to serum, miRNAs are detectable and remarkably stable in clinical samples such as blood, plasma, urine, and feces. Furthermore, frozen samples can be stored without substantial degradation because miRNAs are shown to be resistant to endogenous RNase activity, extreme pH, high temperature, and multiple freeze–thaw cycles.90 in the last years, the expression profile of miRNAs in different types of samples such as HCC, cirrhotic patients, and healthy controls has been investigated. Some studies have shown the potential of the serum microRNAs miR-19a, miR-296, miR-130a, miR-195, miR-192, miR-34a, miR-146a, miR-15b, miR-21, miR-130b, miR-183, miR-20a-5p, miR-320a, miR-324-3p, and miR-375 as early diagnostic HCC biomarkers.91–93 The new identification of panel of serum miRNAs (alone or combined with AFP) could be useful as early detection biomarkers for HCC screening with high accuracy in HCC diagnosis. it is important to mention that additional studies are required to confirm the clinical use of miRNAs in HCC diagnosis and prognosis.44,45 |
| Exosomes | Exosomes are cell-derived vesicles ranging from 30 to 100 nm that have been shown to affect gene expression in recipient cells. exosomes contain characteristic RNA transcripts, including miRNAs, transfer RNAs, other types of noncoding RNAs, and several types of proteins depending on the cell of origin. exosomes have gained great interest because they could serve as biomarkers for different types of cancer and could be easily detected in plasma. Thus, hepatocyte-derived exosomes may be also useful in HCC diagnosis.44–47 |
Abbreviations: AFP, alfa-fetoprotein; AFU, alfa-L-fucosidase; DCP, des-γ-carboxy-prothrombin; DKK1, dickkopf-1; GPC3; glypican 3; GP73, Golgi protein 73; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IGF, insulin-like growth factor; miRNAs, microRNAs; OPN, osteopontin; PIVKA-II, protein induced by vitamin K absence or antagonist ii; SCCA-IGM, serine protease inhibitor squamous cell carcinoma antigen-immunoglobulin M complex.
Most of these markers have been studied in a retrospective manner, and only a few prospective trials have evaluated their clinical significance. However, as HCC is a complex disease with multiple underlying pathogenic mechanisms caused by a variety of risk factors, it is difficult to characterize HCC with a single biomarker. In addition, most of the used biomarkers are associated with liver damage and not HCC itself. Therefore, novel HCC markers and diagnostic approaches are needed. The combination of biomarkers in a detection kit may be more valuable for the diagnosis and prognosis of HCC. It is also important to identify noninvasive and cost-effective biomarkers for early diagnosis.
Imaging
Ultrasound (US) studies still play an important role in detecting HCC because US is able to detect small liver lesions and has relatively high sensitivity and specificity. Its use in diagnosis is being replaced by computed tomography and magnetic resonance imaging, especially for those patients whose liver cannot be fully imaged by US because the tumors are very small or whose echograms do not show lesions clearly.11,12,19
Biopsy
If a tumor is detected by imaging, a biopsy can be obtained typically with a needle under US radiologic guidance. The material obtained by fine-needle aspiration is evaluated to determine HCC staging.11,12,19 Because HCC patients are diagnosed at advanced stages of the disease, it is advisable to screen patients who are at risk of developing the disease like those with chronic hepatitis and cirrhosis for early diagnosis (Figure 2). The most common classification of HCC depends on its stage (from early to terminal) and its differentiation grade (well, moderately, or poorly differentiated).13
Treatment
HCC treatment depends on the tumor stage, patient performance status, and liver function and requires a multidisciplinary approach. The treatment strategies of HCC are summarized in Table 3.
Table 3.
Therapies according to the HCC stage
| HCC stage | Treatment |
|---|---|
| Early | Hepatic resection, liver transplantation, and ablation by radiofrequency or percutaneous alcohol injection |
| Intermediate | Chemoembolization |
| Advanced | Chemotherapy; the multikinase inhibitor sorafenib is the most commonly used treatment option. |
| Terminal | Symptomatic treatment |
Unfortunately, HCC is among the most chemoresistant tumors. Chemotherapy with cytotoxic agents, such as doxorubicin, gemcitabine, cisplatin, and 5-fluorouracil, or combined regimen for palliative care is associated with low response rates. Interestingly, targeted chemotherapy with multikinase inhibitors (Table 4) seems to be an attractive alternative to conventional systemic chemotherapy.
Table 4.
Molecular targeted therapies for HCC
| Drug | Molecular mechanism |
|---|---|
| First line | |
| Sorafenib | Multikinase tyrosine kinase inhibitor (Raf, VEGFR, PDGFR)12,13,94–97 |
| Sunitinib | Multi-targeting receptor tyrosine kinase inhibitor12,13,95,96 |
| Brivanib | Multikinase tyrosine kinase inhibitor (VEGFR, FGFR)12,13,95 |
| Linifanib | PDGFR/VEGFR tyrosine kinase inhibitor12,13,95 |
| Lenvatinib | Multikinase tyrosine kinase inhibitor (VEGFR2 and VEGFR3)12 |
| Second line | |
| Brivanib post-sorafenib | Multikinase tyrosine kinase inhibitor (VEGFR, PDGFR)12,13,97 |
| Everolimus | mTOR inhibitor12,95 |
| Ramucirumab | Monoclonal anti-VEGFR2 antibody12,95 |
| Regorafenib | raf, VEGF, PDGF, Tie2 inhibitor12 |
| Tivantinib | c-Met inhibitor12,13 |
| Cabozantinib | c-Met inhibitor12 |
| Refametinib | MeK inhibitor12 |
Abbreviations: FGFR, fibroblast growth factor receptor; mTOR, mechanistic target of rapamycin; PDGFR, platelet-derived growth factor receptor; VEGFR; vascular endothelial growth factor receptor.
Electrochemotherapy is a treatment used in cutaneous and subcutaneous tumors with positive results. Its use in deep tumors has been studied. The study showed favorable results in the treatment of liver metastases. Therefore this treatment was proposed to be used in other types of liver tumors including HCC.25
Due to the very poor prognosis of HCC and its late detection, it is essential to find early tumor markers as well as new therapeutic targets and potential anticancer drugs. Ion channels seem to be promising alternative tools in oncology to reach these goals.
Eag1 potassium channels as potential early biomarkers and alternative tools for HCC prevention and treatment
Several reports have shown the abnormal expression of potassium channels in many tumors. Especially, the voltage-gated potassium channel ether à-go-go-1 (Eag1, KCNH1, Kv10.1) has gained enormous interest in cancer research because of its oncogenic properties.26–28 Eag1 is overexpressed in most human tumors, including liver, cervical, lung, breast, colon, and prostate cancer.26–32 Eag1 channels have been also proposed as early tumor biomarkers and therapeutic targets for different types of cancers.30–36 In accordance with its role in cell proliferation, inhibition of Eag1 reduces tumor cell proliferation in vitro and in vivo 26,28,32,33,35,37–40 Astemizole is an antihistamine that has gained great interest as a potential anticancer drug because it targets several proteins involved in cancer, including Eag1 channels, histamine receptors, and proteins involved in drug resistance41 Cells expressing Eag1 and treated with astemizole display lower cell proliferation; it is hypothesized that this cell proliferation inhibition may be due to astemizole’s blockage of the Eag1 channel.32,33,37,42 In vivo studies showed that astemizole administration reduced the growth rate of xenograft tumors in mice implanted with Eag1-expressing cells.42 Thus, Eag1 channels are inhibited by astemizole and may be potential targets for cancer therapy.26,27,32,41 Because Eag1 expression has been reported in human biopsies from liver tumors,30 astemizole may be used as an anticancer therapy in patients with liver cancer.
Considering the poor prognosis of HCC, our research group investigated if Eag1 channels could serve as early HCC markers by studying Eag1 expression during tumor development in vivo. We used the already established animal model of HCC development, where chronicle injection with diethylnitrosamine (DEN) induces liver injury in rats. This model recapitulates the sequential change from cirrhosis to HCC, as it occurs in humans. Interestingly, we observed high Eag1 mRNA expression in most of the DEN-treated groups, and strong Eag1 protein expression was observed at early stages of HCC development32 (Figure 1). Eag1 expression at the mRNA and protein level was clearly higher in cirrhotic tissues and preneoplastic lesions, in comparison to normal livers.32 Thus, our results showed the potential of Eag1 channels as HCC early biomarkers.
We also observed that astemizole clearly prevented the development of HCC; the antihistamine prevented tumor formation when administered either from the start of DEN treatment or from week 12 of DEN treatment, when cirrhosis is already present.32 These results suggest that astemizole might be used as a chemopreventive agent in patients at risk of developing liver cancer. Additionally, astemizole could be used as an antineoplastic because when administered at the end of the DEN treatment, the animals did not develop big tumors as those receiving astemizole,32 suggesting that this compound may induce tumor regression.
Eag1 mRNA and protein are expressed in the human HCC cell lines HepG2 and HuH7; accordingly, astemizole decreased their cell proliferation and induced apoptosis.32 Taken together, all these results suggest that astemizole may be a novel therapeutic approach for HCC patients.
Other types of channels and transporters also displayed a differential expression during HCC development, suggesting other channels as potential early markers and/or therapeutic targets of HCC.43
Future research on Eag1 channels as HCC biomarkers and challenges
The discovery of cancer biomarkers in recent years has become a major focus of cancer research. The development of new technologies, especially genomics and proteomics, has made possible the identification and discovery of potential biomarkers. In the recent years, the study of microRNAs and exosomes has gained great interest to diagnose HCC in at-risk patients.44–48 An ideal tumor marker would be DNA-, RNA-, protein-, or antibody-based measurable in serum, urine, or sputum.49,50
Because Eag1 may have clinical potential as an early biomarker for HCC,32 it would be very interesting to track Eag1 protein expression using sensitive imaging techniques in human livers. Actually, when Eag1-expressing cells were injected into mice, the channel was detected in nonpalpable tumors51 using dye-tagged antibodies and imaging techniques. This type of approach might also be used to screen Eag1 expression in the liver of patients at risk of developing HCC and in HCC animal models. It would be also very important to study exosomes containing Eag1 at different stages of HCC in liquid biopsies. Astemizole may be a very promising preventive option for patients at risk of developing HCC as well as a hopeful therapeutic option for HCC patients.32 Therefore, clinical trials testing the effect of astemizole alone or in combination with other antineoplastic drugs in patients either at risk of developing or with HCC should be performed. In addition, detection of Eag1 could be included in the surveillance scheme for patients who are at risk of developing HCC for timely diagnosis (Figure 2).
The precise role of Eag1 channels in HCC development remains to be elucidated. This might be accomplished in different manners, such as the use of mouse models like Eag1 knockout mice.52 It would be very interesting to investigate whether these knockout mice are resistant to the development of liver cirrhosis and HCC.32
Another very important issue to investigate is the mechanism of the oncogenic potential of the channel. Eag1 expression is regulated by the p53-mir-34-E2F1pathway.53 It has been suggested that mutated or inactive p53 increases Eag1 expression; p53 is mutated in most tumors including HCC.54 The increased Eag1 expression could lead to the activation of different proteins such as HIF-1, cyclins D/E, ERK1/2, and FAK complex as well as increase the intracellular calcium concentration and induce vascular endothelial growth factor release. All these changes may lead to angiogenesis, therapy resistance, cell proliferation, migration, and survival, contributing to carcinogenesis.55 However, the precise molecular mechanism of how Eag1 may contribute to HCC development remains to be elucidated.
Liquid biopsies studying HCC circulating cells may provide novel insights into HCC diagnosis and prognosis.56,57 Because Eag1 channels might serve as early markers for different types of cancer, it may be very useful to detect Eag1 mRNA/protein expression in such liquid biopsies. This approach may help to find the urgently needed HCC markers, which are hardly available. However, one of the biggest challenges to overcome would be the sensitivity and specificity of the HCC detection method based on Eag1 expression. In addition, prospective studies should be made to know if cirrhotic patients expressing Eag1 channels would develop HCC.
Conclusion
Because of the poor prognosis of HCC, it is very important to find novel preventive and therapeutic alternatives. Recent studies have suggested that astemizole may be a very promising preventive option for patients at risk of developing HCC as well as a hopeful therapeutic option for HCC patients. It has also been recently proposed that Eag1 may be a potential early biomarker of HCC. Astemizole-based prevention and therapy and early Eag1 detection in the liver should help to reduce mortality from this disease.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286(1):9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 5.Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis. 2012;1(6):180–182. doi: 10.1002/cld.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman M, Llovet JM. Smoking, hepatitis B virus infection, and development of hepatocellular carcinoma. J Natl Cancer Inst. 2011;103(22):1642–1643. doi: 10.1093/jnci/djr430. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Llovet JM. Hepatitis B virus and hepatocellular carcinoma. J Hepatol. 2003;39(Suppl 1):S59–S63. doi: 10.1016/s0168-8278(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Xie L, Yang WS, et al. Risk factors of hepatocellular carcinoma – current status and perspectives. Asian Pac J Cancer Prev. 2012;13(3):743–752. doi: 10.7314/apjcp.2012.13.3.743. [DOI] [PubMed] [Google Scholar]
- 9.Hong M, Li S, Tan HY, Wang N, Tsao SW, Feng Y. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. Int J Mol Sci. 2015;16(12):28705–28745. doi: 10.3390/ijms161226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 11.Imawari M. Liver cancer, prevention and early diagnosis. JMAJ. 2002;5(3):130–133. [Google Scholar]
- 12.Wang CH, Wey KC, Mo LR, Chang KK, Lin RC, Kuo JJ. Current trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16(9):3595–3604. doi: 10.7314/apjcp.2015.16.9.3595. [DOI] [PubMed] [Google Scholar]
- 13.Ingle PV, Samsudin SZ, Chan PQ, et al. Development and novel therapeutics in hepatocellular carcinoma: a review. Ther Clin Risk Manag. 2016;12:445–455. doi: 10.2147/TCRM.S92377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomin A, Cazzagon N, Sergio A, Vanin V, Farinati F. Hepatitis B virus-related hepatocellular carcinoma: primary, secondary, and tertiary prevention. Eur J Cancer Prev. 2011;20(5):381–388. doi: 10.1097/CEJ.0b013e328346399b. [DOI] [PubMed] [Google Scholar]
- 15.Chan CY, Lee SD, Lo KJ. Legend of hepatitis B vaccination: the Taiwan experience. J Gastroenterol Hepatol. 2004;19(2):121–126. doi: 10.1111/j.1440-1746.2004.03153.x. [DOI] [PubMed] [Google Scholar]
- 16.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan HL, Wong GL, Tse CH, Chan HY, Wong VW. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204(3):408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(suppl 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122(6):1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 20.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5(1):145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 21.Parikh NI, Vasan RS. Assessing the clinical utility of biomarkers in medicine. Biomark Med. 2007;1(3):419–436. doi: 10.2217/17520363.1.3.419. [DOI] [PubMed] [Google Scholar]
- 22.Hartwell L, Mankoff D, Paulovich A, Ramsey S, Swisher E. Cancer biomarkers: a systems approach. Nat Biotechnol. 2006;24(8):905–908. doi: 10.1038/nbt0806-905. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez SA. Novel biomarkers for hepatocellular carcinoma surveillance: has the future arrived? Hepatobiliary surgery and nutrition. 2014;3(6):410–414. doi: 10.3978/j.issn.2304-3881.2014.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu K, Dai Z, Zhou J. Biomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapy. Biomark Res. 2013;1(1):1–8. doi: 10.1186/2050-7771-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edhemovic I, Brecelj E, Gasljevic G, et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Ocol. 2014;110(3):320–327. doi: 10.1002/jso.23625. [DOI] [PubMed] [Google Scholar]
- 26.Pardo LA, Stühmer W. Eag1: an emerging oncological target. Cancer Res. 2008;68(6):1611–1613. doi: 10.1158/0008-5472.CAN-07-5710. [DOI] [PubMed] [Google Scholar]
- 27.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardo LA, del Camino D, Sanchez A, et al. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18(20):5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Rasgado JA, Acuña-Macías I, Camacho J. Eag1 channels as potential cancer biomarkers. Sensors (Basel) 2012;12(5):5986–5995. doi: 10.3390/s120505986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmerlein B, Weseloh RM, Mello de Queiroz F, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. doi: 10.1186/1476-4598-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ousingsawat J, Spitzner M, Puntheeranurak S, et al. Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res. 2007;13(3):824–831. doi: 10.1158/1078-0432.CCR-06-1940. [DOI] [PubMed] [Google Scholar]
- 32.de Guadalupe Chávez-López M, Pérez-Carreón JI, Zuñiga-García V, et al. Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumor Biol. 2015;36(8):6149–6158. doi: 10.1007/s13277-015-3299-0. [DOI] [PubMed] [Google Scholar]
- 33.Diaz L, Ceja-Ochoa I, Restrepo-Angulo I, et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009;69(8):3300–3307. doi: 10.1158/0008-5472.CAN-08-2036. [DOI] [PubMed] [Google Scholar]
- 34.Farias LM, Ocana DB, Diaz L, et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64(19):6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 35.Gómez-Varela D, Zwick-Wallasch E, Knötgen H, et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 2007;67(15):7343–7349. doi: 10.1158/0008-5472.CAN-07-0107. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz CS, Montante-Montes D, Saqui-Salces M, et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol Rep. 2011;26(6):1377–1383. doi: 10.3892/or.2011.1441. [DOI] [PubMed] [Google Scholar]
- 37.Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001;7(5):345–356. [PubMed] [Google Scholar]
- 38.Weber C, Mello de Queiroz F, Downie BR, Suckow A, Stuhmer W, Pardo LA. Silencing the activity and proliferative properties of the human EagI potassium channel by RNA Interference. J Biol Chem. 2006;281(19):13030–13037. doi: 10.1074/jbc.M600883200. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Becerra R, Diaz L, Camacho J, et al. Calcitriol inhibits ether-a go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp Cell Res. 2010;316(3):433–442. doi: 10.1016/j.yexcr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Gavrilova-Ruch O, Schonherr K, Gessner G, et al. Effects of imipramine on ion channels and proliferation of IGR1 melanoma cells. J Membr Biol. 2002;188(2):137–149. doi: 10.1007/s00232-001-0181-3. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Quiroz J, Camacho J. Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med Chem. 2011;11(3):307–314. doi: 10.2174/187152011795347513. [DOI] [PubMed] [Google Scholar]
- 42.Downie BR, Sanchez A, Knötgen H, et al. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem. 2008;283(52):36234–36240. doi: 10.1074/jbc.M801830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zúñiga-García V, Chávez-López Mde G, Quintanar-Jurado V, et al. Differential expression of ion channels and transporters during hepatocellular carcinoma development. Dig Dis Sci. 2015;60(8):2373–2383. doi: 10.1007/s10620-015-3633-9. [DOI] [PubMed] [Google Scholar]
- 44.Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int J Mol Sci. 2016;17(3):280. doi: 10.3390/ijms17030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin XJ, Chong Y, Guo ZW, et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16(7):804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser J. Malignant messengers. Science. 2016;352(6282):164–166. doi: 10.1126/science.352.6282.164. [DOI] [PubMed] [Google Scholar]
- 47.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med. 2015;3(1):4. doi: 10.3978/j.issn.2305-5839.2014.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verma M, Kagan J, Sidransky D, Srivastava S. Proteomic analysis of cancer-cell mitochondria. Nat Rev Cancer. 2003;3(10):789–795. doi: 10.1038/nrc1192. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S, Mohan A, Guleria R. Biomarkers in cancer screening, research and detection: present and future: a review. Biomarkers. 2006;11(5):385–405. doi: 10.1080/13547500600775011. [DOI] [PubMed] [Google Scholar]
- 51.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205(3):115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 52.Ufartes R, Schneider T, Mortensen LS, et al. Behavioural and functional characterization of Kv10.1 (Eag1) knockout mice. Hum Mol Genet. 2013;22(11):2247–2262. doi: 10.1093/hmg/ddt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H, Li Z, Chen C, et al. Transcriptional and post-transcriptional mechanisms for oncogenic overexpression of ether a go-go K+ channel. PLoS One. 2011;6(5):e20362. doi: 10.1371/journal.pone.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Benedetti VM, Welsh JA, Yu MC, Bennett WP. p53 mutations in hepatocellular carcinoma related to oral contraceptive use. Carcinogenesis. 1996;17(1):145–149. doi: 10.1093/carcin/17.1.145. [DOI] [PubMed] [Google Scholar]
- 55.Ouadid-Ahidouch H, Ahidouch A, Pardo LA. Kv10.1 K(+) channel: from physiology to cancer. Pflugers Arch. 2016;468(5):751–762. doi: 10.1007/s00424-015-1784-3. [DOI] [PubMed] [Google Scholar]
- 56.Xue R, Li R, Guo H, et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):998–1008. doi: 10.1053/j.gastro.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 57.Zaki MYW, Reeves HL. The genetic heterogeneity of hepatocellular carcinoma and the implications for personalised medicine. Transl Cancer Res. 2016;5(S1):S1–S4. [Google Scholar]
- 58.Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Family Physician. 2011;84(12):1353–1359. [PubMed] [Google Scholar]
- 59.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 60.Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(suppl 1):144–152. doi: 10.1111/j.1440-1746.2010.06546.x. [DOI] [PubMed] [Google Scholar]
- 61.Geng M, Xin X, Bi LQ, Zhou LT, Liu XH. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World J Gastroenterol. 2015;21(38):10732–10738. doi: 10.3748/wjg.v21.i38.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20(33):11630–11640. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol. 2016;27(8):1467–1474. doi: 10.1093/annonc/mdw219. [DOI] [PubMed] [Google Scholar]
- 64.Jahan S, Ashfaq UA, Qasim M, Khaliq S, Saleem MJ, Afzal N. Hepatitis C virus to hepatocellular carcinoma. Infect Agent Cancer. 2012;7(1):2. doi: 10.1186/1750-9378-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013 Sep;22(3):305–310. [PubMed] [Google Scholar]
- 66.Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. 2014;20(43):15943–15954. doi: 10.3748/wjg.v20.i43.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali Kamkar MM, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabet Metab Disord. 2014;13:57. doi: 10.1186/2251-6581-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21(14):4103–4110. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kikuchi L, Oliveira CP, Carrilho FJ. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Biomed Res Int. 2014;2014:6. doi: 10.1155/2014/106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. 2014;3(1):31–40. doi: 10.1159/000343856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purohit V, Rapaka R, Kwon OS, Song BJ. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci. 2013;92(1):3–9. doi: 10.1016/j.lfs.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kew MC. Obesity as a cause of hepatocellular carcinoma. Ann Hepatol. 2015;14(3):299–303. [PubMed] [Google Scholar]
- 73.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernandez-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28. doi: 10.1186/1471-2407-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prieto PA, Cha CH. DKK1 as a serum biomarker for hepatocellular carcinoma. Hepatobiliary Surge Nutr. 2012;2(3):127–128. doi: 10.3978/j.issn.2304-3881.2012.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tung EK, Ng IO. Significance of serum DKK1 as a diagnostic biomarker in hepatocellular carcinoma. Future Oncol. 2012;8(12):1525–1528. doi: 10.2217/fon.12.147. [DOI] [PubMed] [Google Scholar]
- 76.Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13(8):817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 77.Xu Z, Liu L, Pan X, et al. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore) 2015;94(12):e659. doi: 10.1097/MD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res. 2013;4(6):539–546. doi: 10.1016/j.jare.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu R, Ding S, Tan W, et al. Performance of protein induced by vitamin k absence or antagonist-II (PIVKA-II) for hepatocellular carcinoma screening in Chinese population. Hepat Mon. 2015;15(7):e28806. doi: 10.5812/hepatmon.28806v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beneduce L, Castaldi F, Marino M, et al. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer. 2005;103(12):2558–2565. doi: 10.1002/cncr.21106. [DOI] [PubMed] [Google Scholar]
- 81.Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7(2):139–149. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang K, Guo W, Li N, et al. Alpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: a large-scale, long-term study. Br J Cancer. 2014;110(7):1811–1819. doi: 10.1038/bjc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang SY, Lin BD, Li BR. Evaluation of the diagnostic value of alpha-1-fucosidase, alpha-fetoprotein and thymidine kinase 1 with ROC and logistic regression for hepatocellular carcinoma. FEBS Open Bio. 2015;5:240–244. doi: 10.1016/j.fob.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280(10):2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 85.Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132(11):1723–1728. doi: 10.5858/132.11.1723. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Ruan YB, Guan Y, Liu SH. Expression of IGF-II in early experimental hepatocellular carcinomas and its significance in early diagnosis. World J Gastroenterol. 2003;9(2):267–270. doi: 10.3748/wjg.v9.i2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Yao M, Dong Z, Zhang Y, Yao D. Circulating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol. 2014;35(1):9–20. doi: 10.1007/s13277-013-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simão A, Madaleno J, Silva N, et al. Plasma osteopontin is a biomarker for the severity of alcoholic liver cirrhosis, not for hepatocellular carcinoma screening. BMC Gastroenterol. 2015;15:73. doi: 10.1186/s12876-015-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Novellino L, Rossi RL, Bonino F, et al. Circulating hepatitis B surface antigen particles carry hepatocellular microRNAs. PLoS One. 2012;7(3):e31952. doi: 10.1371/journal.pone.0031952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang L, Cheng Q, Zhang BH, Zhang MZ. Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from China. Medicine (Baltimore) 2015;94(10):e603. doi: 10.1097/MD.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu AM, Yao TJ, Wang W, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open. 2012;2(2):e000825. doi: 10.1136/bmjopen-2012-000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Motawi TK, Shaker OG, El-Maraghy SA, Senousy MA. Serum microRNAs as potential biomarkers for early diagnosis of hepatitis C virus-related hepatocellular carcinoma in Egyptian patients. PLoS One. 2015;10(9):e0137706. doi: 10.1371/journal.pone.0137706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wen Y, Han J, Chen J, et al. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int J Cancer. 2015;137(7):1679–1690. doi: 10.1002/ijc.29544. [DOI] [PubMed] [Google Scholar]
- 94.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 95.European Association for Study of Liver. European Organisation for Research and Treatment EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 96.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 97.Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to trans-arterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]