Abstract
Background
Met and HER-2 are proto-oncogenes encoding receptor tyrosine kinase c-Met and HER-2, respectively. Hepatocyte growth factor (HGF) is a ligand of c-Met. The frequency of c-Met, HGF, and HER-2 expressions in gastric cancer and their association with other clinicopathological factors have not been fully understood.
Patients and methods
Patients with stage 1–4 disease were analyzed. Expressions of c-Met, HGF, and HER-2 were examined using immunohistochemistry.
Results
A total of 143 patients, 97 males and 46 females, were included. C-Met scores were 3(+) in 31.5%, 2(+) in 27.3%, and 1(+) in 10.5% of the patients. There was no statistically significant difference in age, sex, tumor location, differentiation, Lauren classification, TNM staging, presence of distant metastasis, depth of tumor invasion (T), lymphovascular invasion, and survival between c-Met subgroups. Overall HGF positivity was 20.6%. HER-2 scores were 3(+) in 9.1%, 2(+) in 9.8%, and 1(+) in 16.1% of the patients. HER-2 overexpression was associated with better differentiation, intestinal subtype, and advanced stage. C-Met overexpressions were 84.6% in the HER-2-overexpression-positive group and 56.2% in the HER-2-overexpression-negative group. There were no statistically significant differences in survival between the high c-Met-expression-positive and -negative stage 3 and stage 4 patients and between the HGF-positive and -negative groups. The mean survival was 11.6±6.3 months in the HER-2-overexpression-positive stage 4 group and 11.9±6.8 months in the HER-2-overexpression-negative stage 4 group. There were no statistically significant differences in survival between the two groups.
Conclusion
c-Met was not associated with any prognostic factors in gastric cancer. HER-2 was associated with better differentiation, intestinal subtype, advanced stage, and c-Met overexpression.
Keywords: gastric cancer, HER-2, c-Met, HGF, clinicopathological features, prognostic factors
Introduction
The incidence and mortality of gastric cancer, which was once the most common cancer worldwide, are decreasing, due to a drop in the rate of distal gastric cancer in the Western world.1 However, despite advances in diagnosis and treatment, gastric cancer has a very poor prognosis, and the 5-year survival rate of stomach cancer is only ~20%. The etiology of gastric cancer is multifactorial and includes both dietary and nondietary factors.2 Gastric cancer is the second leading cause of cancer deaths in men and the third in women. The development of gastric cancer is a complex, multistep process involving multiple genetic and epigenetic alterations of oncogenes, tumor suppressor genes, DNA repair genes, cell cycle regulators, and signaling molecules.2 There is a need to identify new therapeutic targets to be used in the treatment of gastric cancer.
Receptor tyrosine kinases (RTKs) consist of ligand-binding extracellular domains that identify the subfamilies of RTKs, a transmembrane domain and a tyrosine kinase part.3 The RTK c-Met is encoded by MET oncogene. This receptor and its hepatocyte growth factor (HGF) ligand pathway stimulate the proliferation, invasion, angiogenesis, and protection of cancer cells from apoptosis.4 High c-Met expression has been reported in a number of cancers, including lung, colorectal, prostate, pancreatic, head and neck, gastric, hepatocellular, ovarian, and renal cancers and glioma, melanoma, and a number of sarcomas.5 The observed median proportions of high c-Met expression were 59% (range 26%–82%) and 16% (range 8%–29%), respectively, in studies using immunohistochemistry (IHC) and other methods.4 The findings of current literature on c-Met overexpression and its relationship with prognosis and other clinicopathological variables are controversial. Some studies have demonstrated no relationships between c-Met overexpression and other clinicopathological variables.6,7 However, some other studies have reported a strong relationship between c-Met overexpression and tumor invasion depth (T), lymph node metastasis, survival, and intestinal-type tumors.8,9 Increased expression of HGF and c-Met has been linked to poor prognosis and prediction of peritoneal dissemination.10
HER-2 is a member of the human epidermal growth factor receptor family.11 It is the product of the human HER-2 gene weighing 185 kDa with tyrosine kinase activity.12 This RTK protein regulates signal transduction that is important for cell proliferation, differentiation, and survival.11 Overexpression of HER-2 is a frequent molecular event in multiple human cancers, including breast, ovarian, pulmonary, colorectal, and gastric carcinomas.13,14 The rate of HER-2 overexpression varies in different gastric carcinoma studies. The rate of HER-2 overexpression has been reported to be between 5% and 62% in different studies.15 ToGA clinical trial showed that the humanized monoclonal antibody against HER-2, trastuzumab (Herceptin), could effectively prolong overall survival and progression-free survival and increase the response rate in HER-2-positive advanced gastric carcinoma.11
Other molecules have also been investigated for their role in tumor growth and as a target for treatment, such as c-Met. In the present study, we aimed to determine the frequency of c-Met, HGF, and HER-2 overexpression in gastric cancer and their association with prognosis and clinicopathological factors.
Patients and methods
Patients
Gastric cancer patients diagnosed between the years 2006 and 2011 were included. Hospital files and hospital database system were retrospectively analyzed. A total of 150 patients whose pathology slides were available and evaluable in Hacettepe University Hospital were enrolled. Patients were evaluated for age, sex, tumor location, tumor differentiation, Lauren classification, tumor node metastasis (TNM) stage (by seventh Union for International Cancer Control/American Joint Committee on Cancer), distant metastases, depth of tumor invasion (T), lymphovascular invasion, peritoneal carcinomatosis, and survival using medical records. If Lauren classification, differentiation, and lymphovascular invasion information were not reported, the slides of these patients were evaluated again to gather this information. The patients who died just after surgery and did not receive a standard therapy or those patients whose information was missing were not included in the survival analyses. The study protocol was reviewed and approved by the Institutional Ethics Committee of Hacettepe University Faculty of Medicine. This is retrospective translational research and written consent is not required and also not available because most or the patients died at the time of the study.
Tissue samples, IHC, and evaluation of immunohistochemical staining
Ten percentage of formalin-fixed and paraffin-embedded surgical or endoscopic tumor sample blocks were selected. After fixation, tumor samples were embedded in paraffin. Then, tumor sample slides with a 4 μm thickness were prepared from paraffin blocks. Immunohistochemical staining was performed using an automatic immunostainer (Ventana Medical Systems, Inc., AZ, USA). Slides were deparaffinized in xylene for 20 minutes. Antigen retrieval time was 90 minutes for c-Met, 30 minutes for HER-2, and 60 minutes for HGF antibodies. After titrating the primary antibody for 2 hours, amplification was carried out, counterstained with hematoxylin for 8 minutes and then incubated with bluing reagent for 4 minutes. When the slides were taken out of the automatic immunostainer, they were washed in 70% alcohol for 5 minutes, 80% alcohol for 5 minutes, and 100% alcohol for 5 minutes; treated with xylene; left undisturbed for 10 minutes; and then kept at room temperature. After the slides dried, they were covered with ethylene. Lyophilized mouse monoclonal antibodies for HER-2 (novocastra) with an optimal dilution of 1/800, c-Met (novocastra) with an optimal dilution of 1/30, and HGF with an optimal dilution of 1/25 were used. Human breast, prostate, and tonsil tissues were used as positive control for, respectively, HER-2, c-Met, and HGF antibody. Each tissue sample was evaluated and scored by one pathologist who was blind to patients’ data. A pathologist reported c-Met expression as the cytoplasmic and membranous staining intensity from 0 to 3+ (Table 1). HGF expression has been graded as positive or negative according to the presence of cytoplasmic staining intensity at 10× microscopical examination. c-Met overexpression is defined as intensity 2 or 3(+) staining. HER-2 immunohistochemical scoring was done according to the scoring system suggested by Hofmann et al.16 Staining intensity of 3+ score was accepted as overexpression.
Table 1.
c-Met scoring system according to membranous and cytoplasmic c-Met staining patterns
| Staining pattern | Score |
|---|---|
| Membranous 3+ staining, cytoplasmic negative, 1+, 2+, or 3+ staining; membranous 2+ and cytoplasmic 2+, 1+ or negative staining; cytoplasmic 3+ and membranous negative, 1+, 2+ or 3+ staining | 3 |
| Membranous 1+ and cytoplasmic 2+ staining; cytoplasmic 2+ and membranous negative staining | 2 |
| Membranous 1+ and cytoplasmic 1+ or negative staining | 1 |
| Membranous negative and cytoplasmic 1+ or negative staining | 0 |
Statistical analyses
All statistical analyses were performed using the SPSS software Version 18 (SPSS Inc., Chicago, IL, USA). Quantitative data are presented as mean ± SD. The Chi-square, Fisher’s exact test, or Mann–Whitney U-test, where appropriate, was used to compare the proportions in different groups. P-value <0.05 was considered to be significant. Kaplan–Meier method was used for survival analysis, and comparisons between different subgroups were performed using the log-rank test.
Results
Paraffin tumor blocks of 150 gastric cancer patients were achieved but clinical information of seven cases were missing. Finally, 143 cases, 97 males and 46 females, were included. The mean age was 57.3±13.1 (range 28–90 years). C-Met scores were 3(+) in 31.5%, 2(+) in 27.3%, and 1(+) in 10.5% and negative in 30.8% of the patients. C-Met expression (2+ and 3+) rate was 58.7% (84/143). HER-2 scores were 3(+) in 9.1%, 2(+) in 9.8%, 1(+) in 16.1%, and negative in 65.0% of the patients. HGF was positive in 20.6% and negative in 79.4% of the patients (n=126; Table 2). The median overall survival was 21.2±3.8 months in 102 patients who were included in the survival analysis (Figure 1).
Table 2.
General features of the patients and cMET, HER-2, and HGF staining results
| Clinicopathological factors | Patients, n (n=143) | % |
|---|---|---|
| Age (years) | 57.3±13.1 | |
| Sex | ||
| Male | 97 | 67.8 |
| Female | 46 | 32.2 |
| Tumor location | ||
| Proximal | 29 | 20.3 |
| Distal | 100 | 69.9 |
| Diffuse | 14 | 9.8 |
| Tumor differentiation | ||
| Well | 16 | 11.2 |
| Moderate | 40 | 28.0 |
| Poor | 87 | 60.8 |
| Lauren classification | ||
| Diffuse | 45 | 31.5 |
| Intestinal | 71 | 49.7 |
| Mix | 27 | 18.9 |
| Tumor stage (TNM) | ||
| 1 | 9 | 6.3 |
| 2 | 22 | 15.4 |
| 3 | 62 | 43.4 |
| 4 | 50 | 35.0 |
| Distant metastasis | ||
| Positive | 50 | 35.0 |
| Negative | 93 | 65.0 |
| Depth of invasion (T) | n=120 | |
| pT1 | 7 | 5.8 |
| pT2 | 7 | 5.8 |
| pT3 | 26 | 21.7 |
| pT4 | 80 | 66.7 |
| Lymphovascular invasion | n=120 | |
| Positive | 106 | 88.3 |
| Negative | 14 | 11.7 |
| c-Met expression | ||
| Negative | 44 | 30.8 |
| 1+ | 15 | 10.5 |
| 2+ | 39 | 27.3 |
| 3+ | 45 | 31.5 |
| Overexpression (2+ vs 3+) | 84 | 58.7 |
| HER-2 expression | ||
| Negative | 93 | 65.0 |
| 1+ | 23 | 16.1 |
| 2+ | 14 | 9.8 |
| 3+ (overexpression) | 13 | 9.1 |
| HGF expression | n=126 | |
| Negative | 100 | 79.4 |
| Positive | 26 | 20.6 |
| Survival (months) | n=102 | |
| 21.2±3.8 | ||
Abbreviation: HGF, hepatocyte growth factor.
Figure 1.
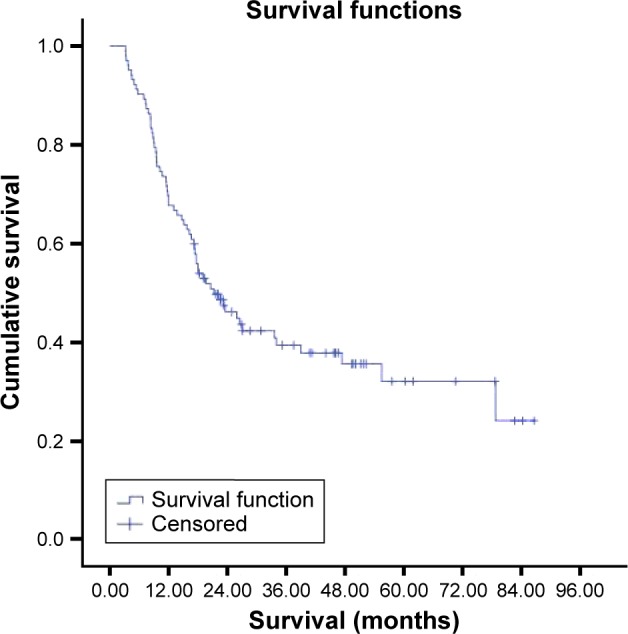
Survival curve of all patients.
Note: The median overall survival was 21.2 months in 102 patients who were included in survival analysis.
C-Met staining report
Of the 143 gastric carcinoma tissue samples, 44 (30.8%) were scored as 0, 15 (10.5%) as 1(+), 39 (27.2%) as 2(+), and 45 (31.5%) as 3(+). The overexpression (2+ and 3+) rate was 58.7% (84/143; Figure 2). The c-Met status was not associated with the sex, tumor location, tumor differentiation, Lauren classification, TNM stage (by seventh UICC/AJCC), presence or absence of distant metastases, depth of tumor invasion (T), and lymphovascular invasion (P=0.462, P=0.595, P=0.636, P=0.387, P=0.823, P=0.823, P=0.820, and P=0.678). HER-2 overexpression was 13.1% in the c-Met-overexpression-positive group and 3.4% in the c-Met-overexpression-negative group. HGF expression was 21.0% in the c-Met-overexpression-positive group. Only one patient had peritoneal carcinomatosis in both HGF/c-Met-positive groups. The median survival was 19.6±7.4 months in the c-Met-overexpression-positive group and 23.3±3.0 months in the c-Met-overexpression-negative group (Tables 2 and 3). There were no statistically significant differences in survival between the two groups (P=0.711).
Figure 2.
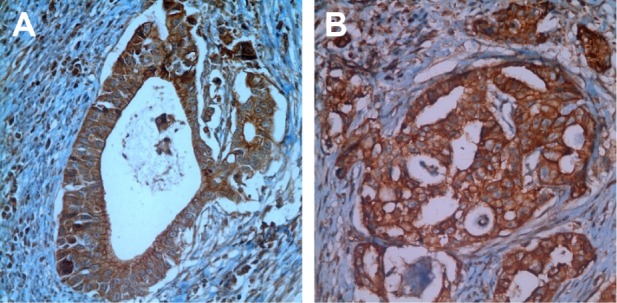
(A and B) Immunohistochemical staining using c-Met antibody showing 3(+) reaction in gastric cancer cells (×40).
Table 3.
Relationship between clinicopathological factors and c-Met overexpression in gastric cancers
| Clinicopathological factors | c-Met overexpression positive, n (%) (n=84) | c-Met overexpression negative, n (%) (n=59) | P-value |
|---|---|---|---|
| Age (years) | 60 | 56 | 0.084 |
| Sex | 0.462 | ||
| Male | 59 (70.2) | 38 (64.4) | |
| Female | 25 (29.8) | 21 (35.6) | |
| Tumor location | 0.595 | ||
| Proximal | 19 (22.6) | 10 (16.9) | |
| Distal | 56 (66.7) | 44 (74.6) | |
| Diffuse | 9 (10.7) | 5 (8.5) | |
| Tumor differentiation | 0.636 | ||
| Well | 10 (11.9) | 6 (10.2) | |
| Moderate | 21 (25.0) | 19 (32.2) | |
| Poor | 53 (63.1) | 34 (57.6) | |
| Lauren classification | 0.387 | ||
| Diffuse | 26 (31.0) | 19 (32.2) | |
| Intestinal | 45 (53.6) | 26 (44.1) | |
| Mix | 13 (15.5) | 14 (23.7) | |
| Tumor stage (TNM) | 0.823a | ||
| 1 | 6 (7.1) | 3 (5.1) | |
| 2 | 14 (16.7) | 8 (13.6) | |
| 3 | 34 (40.5) | 28 (47.5) | |
| 4 | 30 (35.7) | 20 (33.9) | |
| Distant metastasis | 0.823 | ||
| Positive | 30 (35.7) | 20 (33.9) | |
| Negative | 54 (64.3) | 39 (66.1) | |
| Depth of invasion (T) | n=71 | n=49 | 0.820a |
| pT1 | 5 (7.0) | 2 (4.1) | |
| pT2 | 4 (5.6) | 3 (6.1) | |
| pT3 | 15 (21.1) | 11 (22.4) | |
| pT4 | 47 (66.2) | 33 (67.3) | |
| Lymphovascular invasion | n=71 | n=49 | 0.678 |
| Positive | 62 (87.3) | 44 (89.8) | |
| Negative | 9 (12.7) | 5 (10.2) | |
| HER-2 expression | 0.014a | ||
| Negative | 48 (57.1) | 45 (76.3) | |
| 1+ | 16 (19.0) | 7 (11.9) | |
| 2+ | 9 (10.7) | 5 (8.5) | |
| 3+ | 11 (13.1) | 2 (3.4) | |
| HGF expression | n=81 | n=45 | 0.896 |
| Negative | 64 (79.0) | 36 (80.0) | |
| Positive | 17 (21.0) | 9 (20.0) | |
| Survival (month) | n=59 | n=43 | 0.711 |
| 19.6±7.4 | 23.3±3.0 |
Note:
Mann–Whitney U-test was used.
Survival was investigated in stages 1, 2, 3, and 4 c-Met overexpression groups. But all patients in stage 1 were alive and median survival was not reached in stage 2, which could not be evaluated (Figure 3). The median survival was 15.1±3.1 months in the c-Met-overexpression-positive stage 3 group and 17.2±5.8 months in the c-Met-overexpression-negative stage 3 group (Figure 4). There were no statistically significant differences in survival between the two groups (P=0.706). The median survival was 11.6±5.7 months in the c-Met-overexpression-positive stage 4 group and 11.9±7.1 months in the c-Met-overexpression-negative stage 4 group (Figure 5). There were no statistically significant differences in survival between the two groups (P=0.229).
Figure 3.
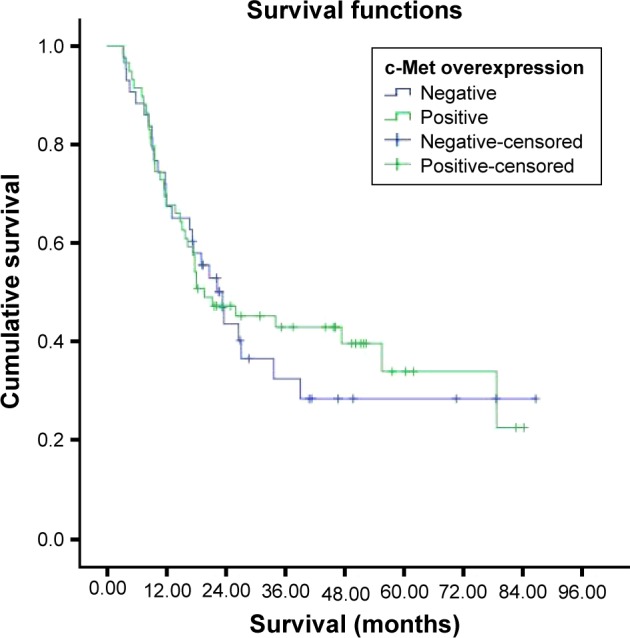
Survival curves of the patients according to c-Met expression.
Figure 4.
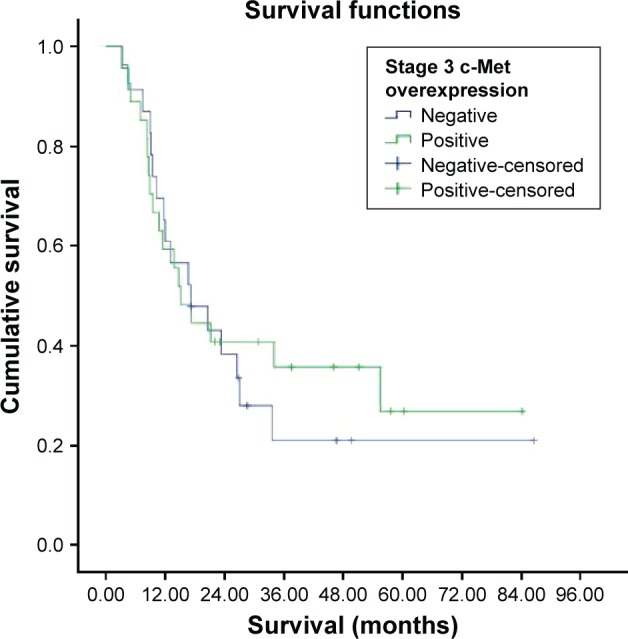
Survival curve for stage 3 c-Met overexpression groups.
Figure 5.
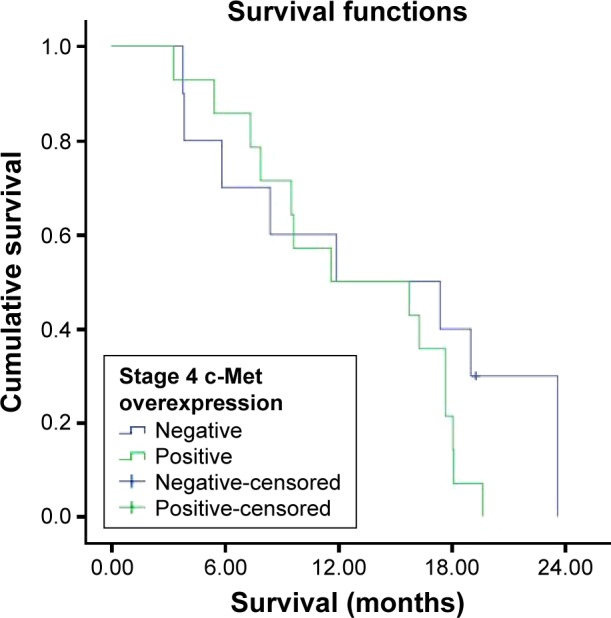
Survival curve for stage 4 c-Met overexpression groups.
Hepatocyte growth factor
The median survival was 20.6±4.0 months in the HGF-expression-positive group (n=20) and 19.0±3.5 months in the HGF-expression-negative group (n=68; Figures 6 and 7). No statistically significant differences in survival were found between the two groups (P=0.863).
Figure 6.
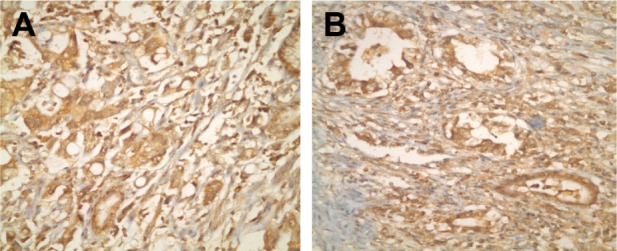
(A and B) Immunohistochemical staining using HGF antibody showing positive reaction in gastric cancer cells (×40).
Abbreviation: HGF, hepatocyte growth factor.
Figure 7.
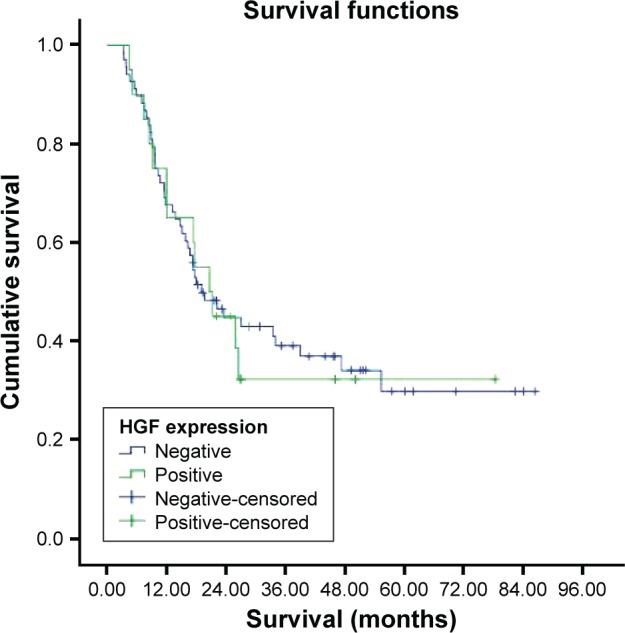
Survival curve for HGF expression groups.
Abbreviation: HGF, hepatocyte growth factor.
Survival was investigated in stages 1, 2, 3, and 4 HGF expression groups. But all patients in stage 1 were alive and median survival was not reached in stage 2, which could not be evaluated. The median survival was 20.6±6.7 months in the HGF-expression-positive stage 3 group and 15.1±2.4 months in the HGF-expression-negative stage 3 group. There were no statistically significant differences in survival between the two groups (P=0.753). The median survival was 17.4±8.2 months in the HGF-expression-positive stage 4 group and 11.6±6.1 months in the HGF-expression-negative stage 4 group (Figures 8 and 9). There were no statistically significant differences in survival between the two groups (P=0.719).
Figure 8.
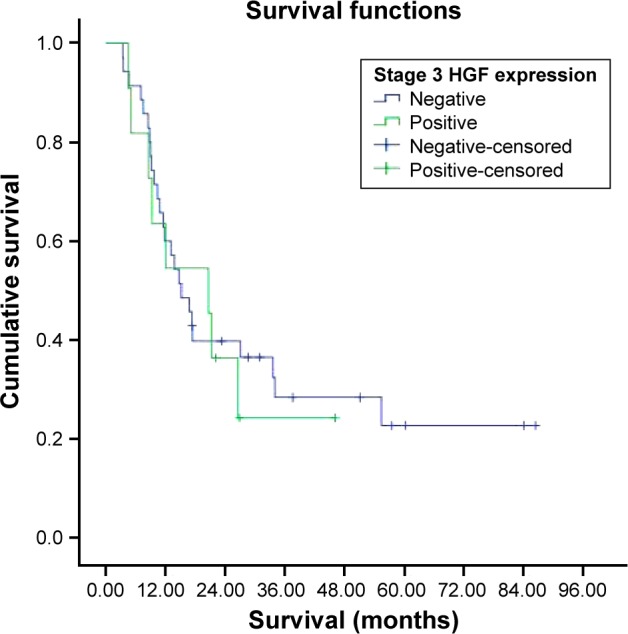
Survival curve for stage 3 HGF expression groups.
Abbreviation: HGF, hepatocyte growth factor.
Figure 9.
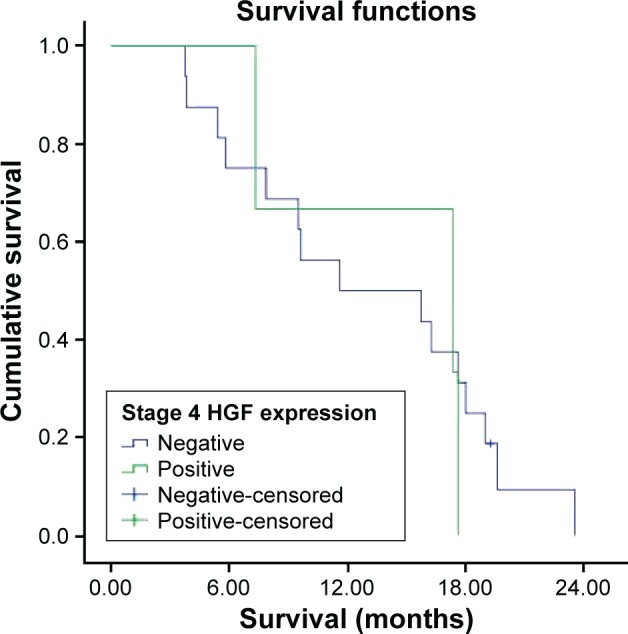
Survival curve for stage 4 HGF expression groups.
Abbreviation: HGF, hepatocyte growth factor.
HER-2 staining report
Of the 143 gastric carcinoma tissue samples, 93 (65.0%) were scored as 0, 23 (16.1%) as 1(+), 14 (9.8%) as 2(+)9, and 13 (9.1%) as 3(+), which indicated overexpression (Figure 10).
Figure 10.
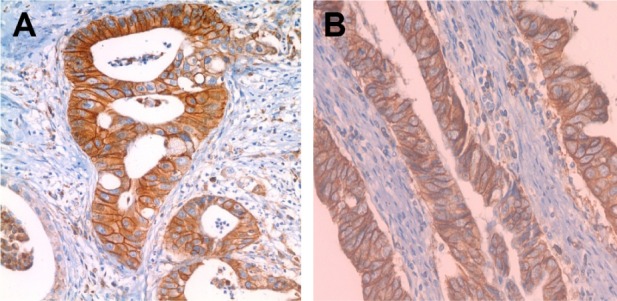
(A and B) Immunohistochemical staining using HER-2 antibody showing 3(+) reaction in gastric cancer cells (×40).
The HER-2 status was not associated with the sex, tumor location, distant metastases, depth of tumor invasion, and lymphovascular invasion (P=0.061, P=0.144, P=0.063, P=0.776, P=1.000). The HER-2 status was associated with tumor differentiation, Lauren classification, and TNM (P=0.003, P=0.008, and P=0.045). C-Met overexpression was 84.6% in the HER-2-overexpression-positive group and 56.2% in the HER-2-overexpression-negative group. The median survival was 18.1±5.2 months in the HER-2-overexpression-positive group and 22.2±4.1 months in the HER-2-overexpression-negative group (Table 4). There were no statistically significant differences in survival between the two groups (P=0.774).
Table 4.
Relationship between clinicopathological factors and HER-2 expression
| Clinicopathological factors | HER-2 overexpression positive, n (%) (n=13) | HER-2 overexpression negative, n (%) (n=130) | P-value |
|---|---|---|---|
| Age (years) | 60.7±14.5 | 57.0±12.9 | 0.330 |
| Sex | 0.061 | ||
| Male | 12 (92.3) | 85 (65.4) | |
| Female | 1 (7.7) | 45 (34.6) | |
| Tumor location | 0.144 | ||
| Proximal | 5 (38.5) | 24 (18.5) | |
| Distal | 8 (61.5) | 92 (70.8) | |
| Diffuse | – | 14 (10.8) | |
| Tumor differentiation | 0.003 | ||
| Well + moderate | 10 (76.9) | 46 (35.4) | |
| Poor | 3 (23.1) | 84 (64.6) | |
| Lauren classification | 0.008 | ||
| Diffuse + mix | 2 (15.4) | 70 (53.8) | |
| Intestinal | 11 (84.6) | 60 (46.2) | |
| Tumor stage (TNM) | 0.045a | ||
| 1 | 1 (7.7) | 8 (6.2) | |
| 2 | – | 22 (16.9) | |
| 3 | 4 (30.8) | 58 (44.6) | |
| 4 | 8 (61.5) | 42 (32.3) | |
| Distant metastasis | 0.063 | ||
| Positive | 8 (61.5) | 42 (32.3) | |
| Negative | 5 (38.5) | 88 (67.7) | |
| Depth of invasion (T) | n=8 | n=112 | 0.776a |
| pT1 | 1 (12.5) | 6 (5.4) | |
| pT2 | – | 7 (6.3) | |
| pT3 | 2 (25.0) | 24 (21.4) | |
| pT4 | 5 (62.5) | 75 (67.0) | |
| Lymphovascular invasion | n=8 | n=112 | 1.000 |
| Positive | 7 (87.5) | 99 (88.4) | |
| Negative | 1 (12.5) | 13 (11.6) | |
| c-Met overexpression | 0.047 | ||
| Positive | 11 (84.6) | 73 (56.2) | |
| Negative | 2 (15.4) | 57 (43.8) | |
| Survival (months) | n=8 | n=94 | 0.774 |
| 18.1±5.2 | 22.2±4.1 |
Note:
Mann–Whitney U-test was used.
Survival was investigated in stages 1, 2, 3, and 4 HER-2 overexpression groups. But all patients in stage 1 survived, and there were not enough patients in stages 2 and 3; hence, survival could not be evaluated. The median survival was 11.6±6.3 months in the HER-2-overexpression-positive stage 4 group and 11.9±6.8 months in the HER-2-overexpression-negative stage 4 group (Figures 11 and 12). There were no statistically significant differences in survival between the two groups (P=0.969).
Figure 11.
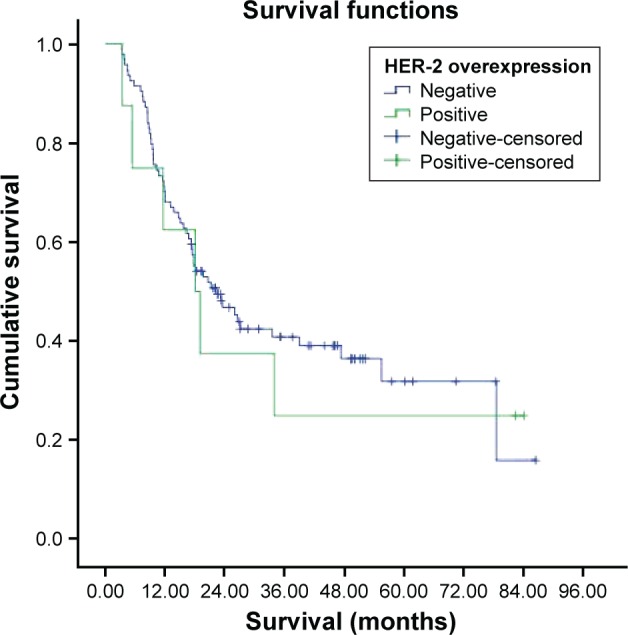
Survival curve for HER-2 overexpression groups.
Figure 12.
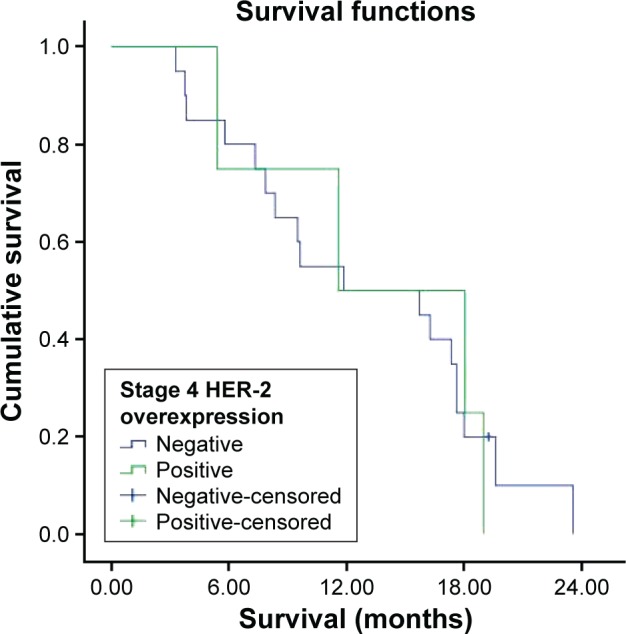
Survival curve for stage 4 HER-2 overexpression groups.
Discussion and conclusion
In this study, we aimed to determine the frequency of c-Met, HGF, and HER-2 overexpression, which play an important role in gastric cancer development, and their association with prognosis in terms of overall survival and other clinicopathological factors. The survival durations of the patients and patient subgroups are given in Table 5.
Table 5.
Median overall survival of the patients and patient subgroups
| Overall survival(months) | 95% confidence interval | |
|---|---|---|
| All patients (n=102) | 21.2 | 13.9–28.6 |
| c-Met | ||
| Positive (n=59) | 19.6 | 5.0–34.2 |
| Negative (n=43) | 23.3 | 17.4–29.2 |
| Stage 3(+) | 15.1 | 9.1–21.1 |
| Stage 3(−) | 17.2 | 5.7–28.6 |
| Stage 4(+) | 11.6 | 0.4–22.8 |
| Stage 4(−) | 11.9 | 0–25.8 |
| HGF | ||
| Positive (n=20) | 20.6 | 12.7–28.5 |
| Negative (n=68) | 19.0 | 12.2–25.8 |
| Stage 3(+) | 20.6 | 7.5–33.6 |
| Stage 3(−) | 15.1 | 10.3–19.9 |
| Stage 4(+) | 17.4 | 1.3–33.4 |
| Stage 4(−) | 11.6 | 0–23.6 |
| HER-2 | ||
| Positive | 18.1 | 7.8–28.3 |
| Negative | 22.2 | 14.2–30.2 |
| Stage 4(+) | 11.6 | 0–24.0 |
| Stage 4(−) | 11.9 | 0–25.2 |
Abbreviation: HGF, hepatocyte growth factor.
c-Met expression was negative in 30.8% of patients and highly expressed in 58.7% of the patients. HER-2 expression was negative in 65% of the patients, whereas it was 2(+) in 9.8% and 3(+) in 9.1% of the patients. HGF was found to be positive only in 20.6% of the patients. In a previous study, Lee et al17 reported c-Met overexpression in 23.7% of 438 gastric cancer patients. In this study, c-Met protein expression was determined by IHC and scored according to membranous staining. In another study by Nakajima et al,8 c-Met overexpression was 46.1% in 128 gastric cancer patients, whereas Retterspitz et al6 reported c-Met overexpression to be 48.9% in 94 gastric cancer patients. In this study, c-Met protein expression was determined by IHC and scored by cytoplasmic staining. In a study by Drebber et al,7 c-Met overexpression was detected to be 73.7% in 114 gastric cancer patients. In this study, c-Met protein expression was determined by IHC and scored by both membranous and cytoplasmic staining. In our study, c-Met overexpression was 58.7% in 125 patients. The literature reports a difference in the frequency of c-Met overexpression in gastric cancer. This difference may be caused by the different methods used for c-Met expression. In our study, we used both membranous and cytoplasmic staining for c-Met expression, and accordingly, we have created a scoring system as given in Table 1. Some studies used only cytoplasmic staining, only membranous staining, or both, showing c-Met expression in the literature. Furthermore, different studies used different criteria for scoring. Therefore, investigation of c-Met expression by IHC methods definitely warrants standard scoring criteria and we believe that our scoring system proposed by Sokmensuer as given in Table 1 seems to be the optimal one. Besides the scoring system, there is also controversy about the correlation with c-Met overexpression and clinicopathological variables in the literature. In the study by Nakajima et al,8 overexpression of c-Met was correlated with depth of tumor invasion and lymph node metastasis. The survival rate of patients with c-Met overexpression (+) gastric cancer was poorer than that of patients with gastric cancers with no overexpression. In the study by Janjigian et al,9 positive c-Met staining with IHC was associated with Lauren intestinal histology. In the study by Retterspitz et al,6 c-Met overexpression was not associated with any clinicopathological factors such as Lauren or WHO classification, depth of invasion, nodal metastasis, TNM stage, and grade. In another study by Drebber et al,7 c-Met overexpression was not associated with any clinicopathological factors such as age, sex, Lauren and WHO classification, differentiation, and TNM stage. In the study by Toiyama et al,10 increased HGF and c-Met had a significant association with poor prognosis and predicted peritoneal dissemination. In our study, there was no statistically significant difference in age, sex, tumor location, differentiation, Lauren classification, TNM staging, presence of distant metastasis, depth of tumor invasion (T), lymphovascular invasion, and survival between c-Met subgroups. Coexpression of HGF/c-Met was observed in 17 patients and only one patient had peritoneal carcinomatosis in our study.
In the study by Cirne-Lima et al,18 HER-2 positivity was 5.4% in 37 gastric cancer patients. In the study by Xu et al,19 HER-2 overexpression was 11.9% in 126 gastric cancer patients. In this study, the IHC score was determined according to the ToGA trial, and HER-2 positivity status was defined as IHC3+ or IHC2+ plus gene-amplified. Geng et al,20 and Ismail et al,21 reported HER-2 positivity to be 19.1% and 25.8%, respectively. In their study, samples with scores 2+ or 3+ were considered to be HER-2 positive. In our study, HER-2 3(+) staining was 9.1% by the IHC method. This result seems to be consistent with the figures reported in the current literature. Unfortunately, in our study HER-2 2+/equivocal score could not be confirmed with fluorescence in situ hybridization (FISH) testing. Therefore, overexpression might have been higher if FISH test was applied.
In the study by Zhou et al,22 HER-2 overexpression was closely correlated with the Lauren type, degree of differentiation, tumor size, and lymph node metastasis. In the study by Xu et al,19 HER-2 was significantly associated with improved disease-free survival. In the study by Geng et al, HER-2 overexpression in primary tumor correlated with lymph node metastasis, distant metastasis, and AJCC stage. Patients with HER-2 positivity had poor survival.20 In our study, the HER-2 status was associated with tumor differentiation, Lauren classification, and TNM stage. The HER-2 status was not associated with the sex, tumor location, distant metastases, depth of tumor invasion, and lymphovascular invasion.
ToGA clinical trial showed that the humanized monoclonal antibody against HER-2, trastuzumab (Herceptin), could effectively prolong overall survival and progression-free survival and increases the response rate in HER-2-positive advanced gastric carcinoma.11 The use of trastuzumab for HER-2-positive patients is routinely recommended for the treatment of advanced gastric cancer.23 Certainly new molecular targets are needed that can be targeted for the treatment of HER-2-negative patients. In our study, c-Met overexpression was 56.2% in HER-2-overexpression-negative group. Unfortunately, targeting c-Met has been disappointing for the treatment of patients with advanced gastric cancer. If new molecular targets are indentified that have a role in tumor growth and survival, then better molecular classification of gastric cancer and new treatments options for this disease may be developed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yalcin S. Gastric cancer in Turkey-a bridge between west and East. Gastrointest Cancer Res. 2009;3(1):29–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4(7):156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morishita A, Gong J, Masaki T. Targeting receptor tyrosine kinases in gastric cancer. World J Gastroenterol. 2014;20(16):4536–4545. doi: 10.3748/wjg.v20.i16.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu S, Yu Y, Zhao N, Cui J, Li W, Liu T. C-Met as a prognostic marker in gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8(11):e79137. doi: 10.1371/journal.pone.0079137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225(1):1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 6.Retterspitz MF, Mönig SP, Schreckenberg S, et al. Expression of {beta}-catenin, MUC1 and c-met in diffuse-type gastric carcinomas: correlations with tumour progression and prognosis. Anticancer Res. 2010;30(11):4635–4641. [PubMed] [Google Scholar]
- 7.Drebber U, Baldus SE, Nolden B, et al. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19(6):1477–1483. [PubMed] [Google Scholar]
- 8.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85(9):1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Janjigian YY, Tang LH, Coit DG, et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1021–1027. doi: 10.1158/1055-9965.EPI-10-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toiyama Y, Yasuda H, Saigusa S, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130(12):2912–2921. doi: 10.1002/ijc.26330. [DOI] [PubMed] [Google Scholar]
- 11.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232(4758):1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 13.Wang SC, Hung MC. HER2 overexpression and cancer targeting. Semin Oncol. 2001;28(5 suppl 16):115–124. doi: 10.1016/s0093-7754(01)90289-1. [DOI] [PubMed] [Google Scholar]
- 14.Koeppen HK, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38(2):96–104. doi: 10.1046/j.1365-2559.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 15.Movagharnejad K, Sharbatdaran M, Sheffaee S, Kashifard M, Sedaghat S. HER-2/neu marker examination using immunohistochemical method in patients suffering from gastric adenocarcinoma. Int J Mol Cell Med. 2013;2(4):199–203. [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee HE, Kim MA, Lee HS, et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107(2):325–333. doi: 10.1038/bjc.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirne-Lima FK, Rosa Ade S, Kulczynski JM, Mattana DS, Corezolla K, Moreira LF. Immunohistochemical expression of HER-2/NEU-CERBB-2 in patients with adenocarcinoma of the stomach. Rev Col Bras Cir. 2009;36(2):131–134. doi: 10.1590/s0100-69912009000200007. [DOI] [PubMed] [Google Scholar]
- 19.Xu CC, Yue L, Wei HJ, et al. Significance of TFF3 protein and Her-2/neu status in patients with gastric adenocarcinoma. Pathol Res Pract. 2013;209(8):479–485. doi: 10.1016/j.prp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Geng Y, Chen X, Qiu J, et al. Human epidermal growth factor receptor-2 expression in primary and metastatic gastric cancer. Int J Clin Oncol. 2014;19(2):303–311. doi: 10.1007/s10147-013-0542-9. [DOI] [PubMed] [Google Scholar]
- 21.Ismail HM, Moneer M, El-Baradie M, Khorshid O, Touny A. Clinicopathologic and prognostic significance of overexpression of her-2/neu and p53 oncoproteins in gastric carcinoma using tissue microarray. J Egypt Natl Canc Inst. 2007;19(2):147–157. [PubMed] [Google Scholar]
- 22.Zhou F, Li N, Jiang W, et al. Prognosis significance of HER-2/neu overexpression/amplification in Chinese patients with curatively resected gastric cancer after the ToGA clinical trial. World J Surg Oncol. 2012;10:274. doi: 10.1186/1477-7819-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Joint Commitee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Stomach. 7th ed. Springer; New York Dordrecht Heidelberg London: 2010. [Google Scholar]


