Abstract
Objective
Parkinson’s disease (PD) is a neurodegenerative disorder resulting in a wide variety of symptoms. The current study examined the influence of apathy, depression and motor symptoms on quality of life (QoL) in PD patients. Information was drawn from an 18-month period.
Method
Participants (N = 397) were assessed for apathy (Apathy Scale; Starkstein et al., 1992), depression (Beck Depression Inventory-II; Beck, Steer, Ball & Ranieri, 1996), motor severity (Unified Parkinson’s Disease Rating Scale, Part III; UPDRS; Fahn, Elton & Committee, 1987), and QoL (Parkinson’s Disease Questionnaire-39; Jenkinson, Fitzpatrick, Peto, Greenhall, & Hyman,1997) at 3 time points: an initial clinical evaluation (baseline), a 6-month follow-up, and an 18-month follow-up. Latent growth-curve models were used to determine the influence of apathy, depression, and motor symptoms on QoL trajectories.
Results
Greater difficulties with QoL at baseline showed the strongest relationship to more severe depression symptoms, followed by more severe motor symptoms, younger age, and less education (all p values < .001). Worsening of QoL over the 18-month period was only predicted by a worsening of depression (p = .003). The relationship between QoL and depression symptoms remained significant in a subsample of nondepressed PD patients.
Conclusion
Overall, findings from the current study suggest that self-reported QoL among PD patients is primarily related to depression. Future efforts to improving clinical care of PD patients may benefit by focusing on improving psychosocial adjustment or treatments targeting depression.
Keywords: Parkinson’s disease, depression, apathy, quality of life
Parkinson’s disease (PD) is a neurodegenerative movement disorder characterized by a loss of dopamine in the substantia nigra and degeneration of multiple motor and nonmotor circuits. Dopaminergic depletion leads to a disruption of multiple cortical-striatal-thalamocortical circuits (Alexander, Delong & Strick, 1986). Disruption of these circuits leads to a wide variety of symptoms including motor, mood, cognitive, and autonomic complaints.
Mood symptoms in PD include apathy (a loss of motivated goald-irected behavior) and depression, which overlap but are dissociable from each other (Kirsch-Darrow, Fernandez, Marsiske, Okun & Bowers, 2006). Although symptoms of depression and apathy may overlap, previous studies have suggested that apathy and depression have separate underlying mechanisms, different trajectories/progressions, and varying contributions to general well-being/quality of life (QoL; Aarsland, Påhlhagen, Ballard, Ehrt&Svenningsson, 2012; Zahodne, Marsiske et al., 2012; Jones et al., 2014) in PD patients. Specifically, when compared with depression, apathy has been shown to be more related to other symptoms that are heavily influenced by dopamine loss in PD, such as frontal-executive cognitive functioning and severity of motor symptoms (Zgaljardic et al., 2007; Butterfield, Cimino, Oelke, Hauser & Sanchez-Ramos, 2010; Zahodne, Marsiske et al., 2012).
Despite the fact that apathy and motor symptoms may be more directly related to striatal dopamine depletion (a hallmark pathological characteristic of PD), depression may be more important in terms of self-reported QoL (Karlsen, Larsen, Tandberg & Mæland, 1999; Kuopio, Marttila, Helenius, Toivonen, & Rinne, 2000). Examining the independent influences of motor symptoms and apathy versus depression on QoL is important because typical treatments of PD may differentially target these symptoms (Zahodne, Bernal-Pacheco et al., 2012).
In the current study, we sought to examine the influence of apathy, depression, and motor symptoms on QoL among PD patients over an 18-month period. Based on past observations of QoL being primarily related to depression, it was predicted that the trajectory of QoL would show the strongest relationship to depressive symptoms (Karlsen et al., 1999; Kuopio et al., 2000; Jones et al., 2014).
Method
Study Design and Participants
A longitudinal design was used and included a convenience sample of PD patients receiving care at the University of Florida (UF) Center for Movement Disorders and Neurorestoration in Gainesville. The study was approved by the Institutional Review Board (IRB) of UF and all participants provided written expression of informed consent and were registered in an IRB-approved database (INFORM).
Data were collected from the INFORM clinical research database, consisting of 7,963 movement-disordered patients and including 2,960 PD participants. A diagnosis of idiopathic PD was based on the UK Brain Bank criteria by a movement-disorder neurologist (Hughes, Daniel, Kilford, & Lees, 1992). A query of the INFORM database was conducted to include PD participants seen between July, 2002 and April, 2014 who met the following criteria: (a) an initial appointment (baseline), (b) a 6-month follow-up, and (c) an 18-month follow-up. Exclusion criteria included prior history of brain surgery (e.g., deep-brain stimulation, pallidotomy), severe psychiatric disturbance (e.g., schizophrenia), or severe sensory defects (e.g., blindness, deafness). Results from the query resulted in a final sample size of 397 participants.
Measures
Motor symptoms were assessed by the Motor subscore (Part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn, Elton & Committee, 1987). Scores on the motor scale of the UPDRS range from 0 to 56, with higher scores representing greater motor-symptom severity. All UPDRS scores were obtained while patients were “on” their normal dopaminergic medication regimen.
Depression symptoms were assessed by the Beck Depression Inventory-II (BDI-II; Beck, Steer, Ball &Ranieri, 1996). The BDI-II has been shown to be a valid measure of depression-symptom severity among individuals with PD (Leentjens, Verhey, Luijckz, &Troost, 2000). Scores range from 0 to 63, with higher scores representing greater depression-symptom severity. A cut-off score of 14 or 15 has been recommended as an indicator of clinically significant depression in individuals with PD, with higher scores representing greater depression severity.
Apathy was assessed by the Apathy Scale (AS; Starkstein et al., 1992). Scores on the AS range from 0 to 42, with higher scores reflecting greater apathy symptoms. The AS has been shown to be a valid measure of apathy in PD, with a recommended cut-off score of 13 or 14; higher scores represent greater apathy (Leentjens et al., 2008).
The Parkinson’s Disease Questionnaire-39 (PDQ-39) was used as a measure of QoL (Jenkinson, Fitzpatrick, Peto, Greenhall, & Hyman,1997). The PDQ-39 measures QoL across eight separate domains: Mobility (10 items), Activities of Daily Living (ADLs; six items), Emotional Well-Being (six items), Stigma (four items), Social Support (three items), Cognition (four items), Communication (three items), and Bodily Discomfort (three items). Scores range from 0 to 100, with higher scores indicating greater difficulties or complaints in QoL.
Statistics
Using SPSS AMOS Graphics 21, latent growth-curve (LGC) models were used to analyze data. Full-information, maximum-likelihood parameter estimation was used to account for missing data. The dependent variable (QoL) was examined for outliers (i.e., values greater or less than 3 SDs outside the mean). Like all outlier criteria, 3 SDs is arbitrary. We selected it to reduce the impact of only the most extremely implausible values (values beyond what we would expect from 99% of the population) so that the distributions could be left as unaltered as possible. Outliers were trimmed and set to closest allowable value (mean ± 3 SDs). The dependent variable was additionally Blom-transformed to normalize the distribution.
Model fit was inspected using χ2, comparative fit index (CFI), normed fit index (NFI), and root mean-square error of approximation (RMSEA). Smaller values of χ2 and RMSEA represent better fit (values below .05 are recognized as good fit, although a .08 cutoff value has been suggested as being reasonable; Jöreskog & Sörbom, 1993). Larger CFI and NFI values (above .90) are indicative of adequate fit (Hu & Bentler, 1999). Fit differences between models were evaluated using the change in χ2 tests.
An initial unconditional univariate model was conducted to examine intercept (between) and slope (within) variance in QoL. The linear growth term was computed from three mean-centered time points, −0.67 (BASELINE), −0.17 (6-month follow-up), and 0.87 (18-month follow-up; Aiken & West, 1991). Univariate models were repeated for time-varying predictors (i.e., apathy, depression, and motor symptoms).
A conditional latent growth model was computed in which the intercept term (i.e., baseline) of the three independent variables (i.e., depression [BD-III], apathy [AS], and motor symptoms [UPDRS Part III]) was allowed to predict the intercept terms of the dependent variable (QoL). In addition, the linear growth terms of the predictors were allowed to predict the linear growth of QoL. A fully recursive model resulted in an inadmissible negative variance estimate; therefore this reduced model was used to account for all unexplained random variance in rate of change. Three static covariates (age, gender, and education) were included in the final model and predicted the QoL intercept and growth terms. All predictors were allowed to correlate with each other, as were the uniquenesses of the latent dependent variables. Presented models allow for heterogeneity of variance over time (i.e., residuals of the measured variables were not constrained to equality over measurement occasions).
Results
The sample included 397 Parkinson patients. The sample characteristics are shown in Table 1.
Table 1.
Sample Characteristics: N = 397 PD patients, no DB, 267 Men (66.5%), 133 Women (33.5%)
| Variable | Baseline mean (SD; range) | 6-month mean (SD; range) | 18-month mean (SD; range) |
|---|---|---|---|
| Age (years) | 66.7 (10) | ||
| Years of education | 15.1 (3) | ||
| Years with symptoms | 6.4 (6) | ||
| UPDRS Motor score, on medication | 27.1 (10; 3–56) | 26.5 (11; 1–56) | 27.9 (12; 1–56) |
| BDI-II | 8.6 (6; 0–31) | 8.2 (6; 0–37) | 9.0 (7; 0–33) |
| Apathy Scale | 11.8 (6; 0–28) | 12.3 (7; 0–33) | 12.8 (7; 0–31) |
| PDQ-39 | 24.2 (16; 1–79) | 21.5 (15; 1–75) | 24.4 (16; 1–70) |
Note. DBS = deep-brain stimulation; UPDRS = Unified Parkinson’s Disease Rating Scale; BDI-II = Beck Depression Inventory-II; PDQ-39 = Parkinson’s Disease Questionnaire-39.
Overall, participants were in their mid-60s with approximately 3 years of college education, and predominantly male (66%). Results for mood symptoms revealed that 13%, 13.5%, and 18.2% of the sample had depression symptoms that exceeded clinical cut-off (raw score of 15) on the BDI-II at baseline, 6 months, and 18 months, respectively. Clinically meaningful apathy was present in 31.3%, 35%, and 37.8% of the sample (using a 14 point cut-off) at baseline, 6 months, and 18 months, respectively.
Unconditional Univariate Growth Models
An initial univariate model was computed to examine the intercept and linear slope of QoL. The model revealed a significant amount of variance in the intercept (p < .001) and slope terms (trend; p = .057) unexplained by the growth model. The linear term was not significant (ns). Models were repeated for depression, apathy, and motor symptoms. Findings are summarized in Table 2.
Table 2.
Summary of Unconditional Univariate Growth Models
| Variable | Fixed intercept | Random intercept | Fixed slope | Random slope | df | χ2 | RMSEA | CFI | NFI |
|---|---|---|---|---|---|---|---|---|---|
| Standardized QoL | 0.02 | .80*** | 0.04 | .13* | 1 | 27.79*** | .26888 | .95 | .95 |
| Motor symptoms | 27.3*** | 84.0*** | .66** | 15.9*** | 1 | 2.82* | .07 | 1.0 | 1.0 |
| Depression | 8.7*** | 27.3*** | .25 | 10.2* | 1 | 3.45* | .08 | .99 | .99 |
| Apathy | 12.3*** | 32.2*** | 1.0*** | 8.7* | 1 | 0.142 | <.01 | 1.0 | 1.0 |
Note. QoL= quality of life. Standardized (Blom-transformed) values of QoL were used in the model. RMSEA = root mean-square error of approximation; CFI = comparative fit index; NFI = normed fit index.
p< .1.
p< .05.
p< .001.
Briefly, there was a significant amount of variance between (intercepts) and within (slope) unexplained by the model for motor symptoms, apathy (trend for random-variance of slope), and depression (trend for random-variance of slope).
Conditional Multivariate Growth Model
The conditional growth model is depicted in Figure 1.
Figure 1.
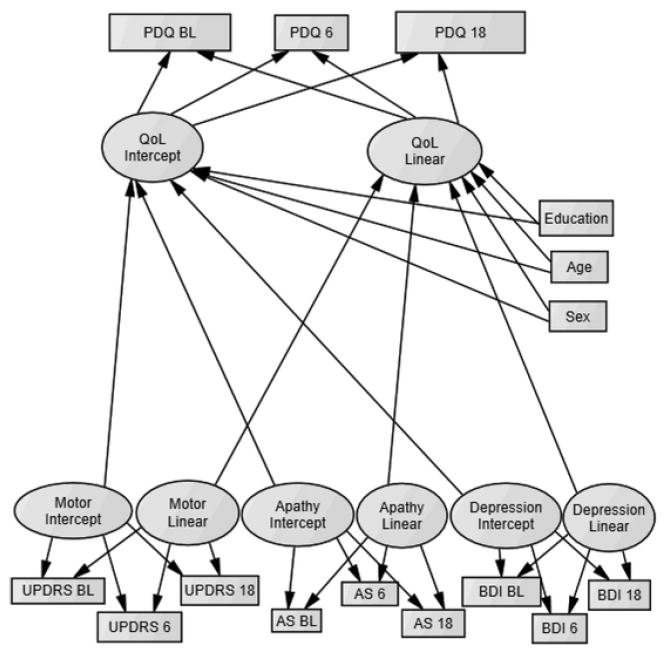
Depiction of final conditional latent grrowth model. PDQ = Parkinson’s Disease Questionnaire-39; AS = Apathy Scale; BDI = Beck Depression Inventory-II; QoL = Quality of Life. Measures were assessed at baseline (BL), a 6-month follow up (6) and an 18-month follow up (18). Latent predictors (motor, apathy, and depression terms) and static predictors (age, education, and gender) were allowed to correlate with each other (correlations not shown in figure).
Briefly, the model included the intercept and linear growth terms of QoL (the dependent variable), which was predicted by motor symptoms, depression, and apathy. Static covariates (age, education, and gender) were additionally entered as predictors of QoL. The overall model demonstrated appropriate fit, χ2(df = 54, N = 397) = 174.01, p < .001; RMSEA = .075, p = .001; CFI = .948; NFI = .928, and explained 80% of the variance between subjects and fully explained all of the variance within subjects. This suggests that the unexplained slope variance in QoL reported from the unconditional univariate model is fully accounted for by the current conditional model.
Correlations of latent predictors (see Table 3) revealed that the motor, depression, and apathy intercept terms were positively correlated with each other; meaning that individuals who, on average, experienced greater amounts of one symptom tended to also experience greater amounts of the other two symptoms. In addition, the linear slope terms of motor symptoms, depression, and apathy were significantly and positively correlated with each other. Individuals who experienced worsening of one symptom tended to experience worsening of the other two symptoms.
Table 3.
Correlations Among Latent Predictors in the Conditional Model
| Predictor | Depression intercept | Motor intercept | Apathy slope | Depression slope | Motor slope |
|---|---|---|---|---|---|
| Apathy intercept | .643** | .382** | 0.215 | 0.132 | 0.168 |
| Depression intercept | .262** | 0.156 | 0.171 | 0.11 | |
| Motor intercept | .418** | 0.13 | .280* | ||
| Apathy slope | .690** | .548** | |||
| Depression slope | .412** |
p< .05.
p< .001.
Williams’ t tests were computed to examine whether motor symptoms (intercept and slope terms) were significantly more correlated with apathy or depression (intercept and slope terms). Results from the Williams’ t tests revealed that the correlation between motor symptoms and apathy was significantly stronger than the correlation of motor symptoms and depression. This was true for both intercept/between-subject terms, Williams’ t(394) = 3.04, p = .002, and slope/within-subject terms, Williams’ t(394) = 4.09, p < .001. Results suggest that motor symptoms have a stronger correlation with apathy than depression, both between and within subjects.
Inspection of the multivariate conditional model revealed that, on average (i.e., between subjects), poorer QoL was related to more severe depression and motor symptoms (but not to apathy). Moreover, poorer QoL was also associated with younger age and female gender. The linear slope of QoL was predicted by the depression slope, such that individuals who experienced a worsening of QoL also experienced a worsening of depression. Figure 2 depicts the influence of depression and motor symptoms on QoL. No other predictors of change in QoL reached statistical significance (see Table 4).
Figure 2.
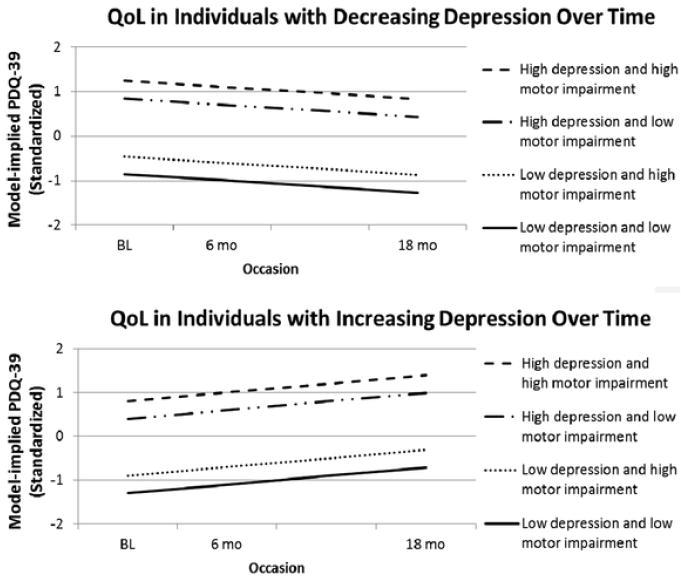
Influence of depression and motor symptoms on quality of life in the conditional multivariate model. Figure depicts the intercept differences in QoL (higher values = greater difficulties in QoL) among individuals with high (≥1 SD above the mean) and low (≥1 SD below the mean) numbers of depressive and motor symptoms. The top figure shows the slope of individuals who experience ≥1 SD reduction/decrease of depressive symptoms, and the bottom figure reflects individuals who experience ≥1 SD worsening/increase of depressive symptoms. Standardized (Blom-transformed) values of the PDQ = Parkinson’s Disease Questionnaire-39 (PDQ-39) are depicted; BL = baseline; 6 mo = 6-month follow-up; 18 mo = 18-month follow-up.
Table 4.
Regression Estimates in the Conditional Multivariate Growth Model
| Independent variable | Standardized β | Significance |
|---|---|---|
| Dependent variable = QoL intercept | ||
| Depression intercept | 0.757 | <.001 |
| Motor intercept | 0.209 | <.001 |
| Apathy intercept | 0.071 | ns |
| Age | -.097 | <.001 |
| Gender | 0.083 | 0.03 |
| Education | -.003 | ns |
| Dependent variable = QoL linear slope | ||
| Depression linear slope | 0.869 | 0.002 |
| Motor slope | 0.244 | ns |
| Apathy slope | -.216 | ns |
| Age | -.003 | ns |
| Gender | -.118 | ns |
| Education | -.187 | ns |
Note. QoL = quality of life
Conditional Multivariate Growth Model in Nondepressed PD Participants
To examine the relationship of motor and mood symptoms to QoL in nondepressed PD patients, the conditional multivariate growth model was repeated in a subsample of PD patients without depression at baseline (BDI-II cut off of 15). The overall model demonstrated appropriate fit, χ2(df = 54, N = 345) = 146.01, p < .001; RMSEA = .069, p = .010; CFI =.952; NFI = .929, and explained 80% of the variance between subjects and fully explained all of the variance within subjects. As mentioned above, this suggests that the unexplained within variance in QoL reported from the unconditional univariate model is fully accounted for by the current conditional model.
Inspection of the multivariate conditional model revealed that findings in the subsample of nondepressed PD patients were generally consistent with that of the total sample. Specifically, poorer QoL between subjects was related to more severe depression (β = .762, p < .001) and motor symptoms (β = .179, p < .001), but not to apathy. Poorer QoL was also associated with younger age (β = −.094, p = .021) and female gender (β = .085, p = .047). The linear slope of QoL was predicted by the depression slope (β = .889, p < .001) and gender (β = .192, p = .040), such that individuals who experienced a worsening of QoL also experienced a worsening of depression and tended to be female.
Discussion
We examined the relationship of apathy, depression, motor symptoms, and QoL over an 18-month period in the current study of 397 PD patients. Our main finding suggests that QoL is largely related to depressive symptoms among PD patients, regardless of whether individuals with PD experience clinical levels of depression. This finding is consistent with past studies of motor and nonmotor predictors of QoL in PD (Antonini et al., 2012; Kuopio et al., 2000; Karlsen et al., 1999; Jones et al., 2014). In addition to depression, QoL had a smaller relationship to motor symptoms, gender, and age. Apathy was not related to QoL.
The strong influence of depression, rather than motor symptoms and apathy, on QoL is important because it points to differences in underlying biological mechanisms. Specifically, apathy and motor symptoms have been shown to be related to striatal-dopamine depletion (a defining characteristic of PD), which disrupts frontal cortical regions involved with motivation, initiation, and motor functioning (Cummings, 1993). Depression, however, has been shown to be related to the disruption of additional neurotransmitter systems (serotonin or norepinephrine) or psychosocial factors (Aarsland et al., 2012). The idea of apathy, motor symptoms, and depression reflecting different underlying mechanisms is consistent with past studies showing the separate symptoms to have dissociable trajectories (Zahodne, Marsiske et al., 2012). The findings from the current study support the view that QoL in PD may be primarily related to depressive symptoms, which may not be specific to striatal-dopamine dysfunction.
In terms of demographic predictors, female gender and younger age were associated with worse QoL over the 18-month period. Compared with men, women have previously reported greater difficulties with functioning, which may contribute to worse QoL (Kuopio et al., 2000).The relationship between younger age and worse QoL among PD patients is consistent with previous findings and may reflect older individuals having greater adaptation to PD (Kuopio et al., 2000).
Despite being highly correlated with depression (which was the strongest predictor of QoL), apathy was not a significant predictor of QoL. This finding is consistent with previous studies showing apathy to have only a minimal influence on QoL, independent of depression (Jones et al., 2014; Leroi et al., 2011). It is possible that apathy may be more detrimental to QoL among caregivers, rather than patients. Preliminary studies have shown that patient apathy is related to caregiver burden among those caring for individuals with PD or other neurodegenerative diseases (Leroi et al., 2012; Merrilees et al., 2013; Ricci et al., 2009).
Correlations among latent predictors (motor symptoms, apathy, and depression) showed mild to moderate correlations within and between subjects. A previous LGC modeling study revealed that Parkinsonian motor symptoms (indicated by UPDRS motor scores) and apathy follow similar trajectories, but that depression followed a divergent trajectory (Zahodne, Marsiske et al., 2012). As mentioned above, the rationale for the divergent trajectories relates to separate underlying neurotransmitter mechanisms for apathy and motor-symptom severity (i.e., dopamine mediated) versus depression (i.e., heterogeneous neurotransmitter systems). Although this study showed UPDRS motor scores to correlate with both depression and apathy, there was a pattern of motor-symptom severity showing a significantly higher correlation with apathy symptoms than depression.
Limitations in the current study include the sample consisting of a convenience recruitment of PD patients seen at an outpatient center over an 18-month period, possibly affecting the generalizability of the findings. We were not able to assess the influence of mood and motor symptoms on caregiver burden, nor did we examine the influence of anxiety or cognitive functioning on QoL. Regarding the latter, past studies have suggested cognitive functioning to have only a mild impact on QoL relative to depression (Karlsen et al., 1999). Also, we did not assess participants’ use of antidepressants. It is unknown whether the use of antidepressants moderates the relationship between depression and QoL; however, results from a previous randomized controlled trial did not include a significant relationship between tricyclic or selective-serotonin reuptake inhibitor (SSRI) antidepressant use on QoL in PD (Menza et al., 2009). Differences in Parkinsonian medications, as well as differences in on–off medication status, were not accounted for in the current study. Different PD medications may have an influence on apathy or depression and should be addressed in future studies. In terms of statistical analyses, we were not able to assess the influence of predictors on quadratic or nonlinear changes in QoL. Future studies could benefit from addressing these limitations, as well as investigating possible biological mechanisms (i.e., neurotransmitter systems) of predictors to increase our knowledge of the trajectories of mood and motor symptoms.
Overall, findings from the current study suggest that self-reported QoL among PD patients is primarily related to depression. Future efforts to improving clinical care of PD patients may benefit by focusing on improving psychosocial adjustment or treatments targeting multiple neurotransmitter systems, in addition to the traditional management of dopamine-mediated symptoms. Such an idea is consistent with a previous Movement Disorder Society task force statement emphasizing the high occurrence of misdiagnosis of nonmotor symptoms and the paucity of efficacious treatments for nonmotor symptoms (Seppi et al., 2011).
Acknowledgments
University of Florida (UF) and the UF National Parkinson Foundation Center of Excellence, Gainesville, Forida provided funding for this study. Dr. Marsiske has received research support from the United States Department of Health and Human Services, National Institutes of Health (NIH) and the McKnight Research Foundation. Dr. Okun has received research grants from the NIH, National Parkinson Foundation (NPF), Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann–Strauss Foundation, Tourette Syndrome Association, and UF Foundation. Dr. Bowers has received research support from the NIH, Michael J. Fox Foundation, NPF, and McKnight Research Foundation. Jacob Jones was responsible for study concept, data analysis, interpretation, and initial paper draft; Michael Marsiske was responsible for review of analysis and revision of draft; Michael Okun was responsible for study concept and revision of draft; Dawn Bowers was responsible for study concept, study supervision and coordination, interpretation, and review of all written drafts. This paper has been run through the iThenticate system and the first author certifies that all of the text is original.
Contributor Information
Jacob D. Jones, Department of Clinical and Health Psychology and Center for Movement Disorders and Neurorestoration, McKnight Brain Institute, University of Florida
Michael Marsiske, Department of Clinical and Health Psychology, University of Florida.
Michael S. Okun, Department of Neurology and Center for Movement Disorders and Neurorestoration, McKnight Brain Institute, University of Florida
Dawn Bowers, Department of Clinical and Health Psychology, Department of Neurology, and Center for Movement Disorders and Neurorestoration, McKnight Brain Institute, University of Florida.
References
- Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease: Epidemiology, mechanisms and management. Nature Reviews Neurology. 2012;8:35–47. doi: 10.1038/nrneurol.2011.189. http://dx.doi.org/10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. http://dx.doi.org/10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Antonini A, Barone P, Marconi R, Morgante L, Zappulla S, Pontieri FE, Colosimo C, et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. Journal of Neurology. 2012;259:2621–2631. doi: 10.1007/s00415-012-6557-8. http://dx.doi.org/10.1007/s00415-0126557-8. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories–IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. http://dx.doi.org/10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Butterfield LC, Cimino CR, Oelke LE, Hauser RA, Sanchez-Ramos J. The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology. 2010;24:721–730. doi: 10.1037/a0019650. http://dx.doi.org/10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R . UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. http://dx.doi.org/10.1080/10705519909540118. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. http://dx.doi.org/10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age and Ageing. 1997;26:353–357. doi: 10.1093/ageing/26.5.353. http://dx.doi.org/10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- Jones JD, Butterfield LC, Song W, Lafo J, Mangal P, Okun MS, Bowers D. Anxiety and depression are better correlates of Parkinson’s disease quality of life than apathy. The Journal of Neuropsychiatry and Clinical Neurosciences Advance online publication. 2014 doi: 10.1176/appi.neuropsych.13120380. http://dx.doi.org/10.1176/appi.neuropsych.13120380. [DOI] [PMC free article] [PubMed]
- Jöreskog KG, Sörbom D. LISREL 8: Structural equation modeling with the SIMPLIS command language. Skokie, IL: Scientific Software International; 1993. [Google Scholar]
- Karlsen KH, Larsen JP, Tandberg E, Mæland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1999;66:431–435. doi: 10.1136/jnnp.66.4.431. http://dx.doi.org/10.1136/jnnp.66.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology. 2006;67:33–38. doi: 10.1212/01.wnl.0000230572.07791.22. http://dx.doi.org/10.1212/01.wnl.0000230572.07791.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuopio A-M, Marttila RJ, Helenius H, Toivonen M, Rinne UK. The quality of life in Parkinson’s disease. Movement Disorders. 2000;15:216–223. doi: 10.1002/1531-8257(200003)15:2<216::aid-mds1003>3.0.co;2-#. http://dx.doi.org/10.1002/1531-8257(200003)15:2<216∷AID-MDS1003>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Leentjens AFG, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, Goetz CG, et al. Apathy and anhedonia rating scales in Parkinson’s disease: Critique and recommendations. Movement Disorders. 2008;23:2004–2014. doi: 10.1002/mds.22229. http://dx.doi.org/10.1002/mds.22229. [DOI] [PubMed] [Google Scholar]
- Leentjens AFG, Verhey FRJ, Luijckx GJ, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson’s disease. Movement Disorders. 2000;15:1221–1224. doi: 10.1002/1531-8257(200011)15:6<1221::aid-mds1024>3.0.co;2-h. http://dx.doi.org/10.1002/1531-8257(200011)15:6<1221∷AID-MDS1024>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leroi I, Harbishettar V, Andrews M, McDonald K, Byrne EJ, Burns A. Carer burden in apathy and impulse control disorders in Parkinson’s disease. International Journal of Geriatric Psychiatry. 2012;27:160–166. doi: 10.1002/gps.2704. http://dx.doi.org/10.1002/gps.2704. [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Buyske S, Dicke A, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72:886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. http://dx.doi.org/10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees J, Dowling GA, Hubbard E, Mastick J, Ketelle R, Miller BL. Characterization of apathy in persons with frontotemporal dementia and the impact on family caregivers. Alzheimer Disease and Associated Disorders. 2013;27:62–67. doi: 10.1097/WAD.0b013e3182471c54. http://dx.doi.org/10.1097/WAD.0b013e3182471c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci M, Guidoni SV, Sepe-Monti M, Bomboi G, Antonini G, Blundo C, Giubilei F. Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD) Archives of Gerontology and Geriatrics. 2009;49:e101–e104. doi: 10.1016/j.archger.2008.10.001. http://dx.doi.org/10.1016/j.archger.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Sampaio C, et al. The Movement Disorder Society evidence-based medicine review update: Treatments for the non-motor symptoms of Parkinson’s disease. Movement Disorders. 2011;26:S42–S80. doi: 10.1002/mds.23884. http://dx.doi.org/10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Bernal-Pacheco O, Bowers D, Ward H, Oyama G, Limotai N, Okun MS, et al. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson’s disease? The Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:326–330. doi: 10.1176/appi.neuropsych.11090210. http://dx.doi.org/10.1176/appi.neuropsych.11090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Marsiske M, Okun MS, Rodriguez RL, Malaty I, Bowers D. Mood and motor trajectories in Parkinson’s disease: Multivariate latent growth curve modeling. Neuropsychology. 2012;26:71–80. doi: 10.1037/a0025119. http://dx.doi.org/10.1037/a0025119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Rocco M, Mattis PJ, Gordon MF, Eidelberg D, et al. Relationship between self-reported apathy and executive dysfunction in nondemented patients with Parkinson disease. Cognitive and Behavioral Neurology. 2007;20:184–192. doi: 10.1097/WNN.0b013e318145a6f6. http://dx.doi.org/10.1097/WNN.0b013e318145a6f6. [DOI] [PMC free article] [PubMed] [Google Scholar]


