Abstract
We review evidence for a high degree of neuroplasticity of the central auditory pathways in early childhood, citing evidence of studies of the P1 and N1 cortical auditory evoked potentials in congenitally deaf children receiving cochlear implants at different ages during childhood, children with auditory neuropathy spectrum disorder and children with hearing loss and comorbid multiple disabilities. We discuss neuroplasticity, including cortico-cortical de-coupling and cross-modal re-organization that occurs in deafness. We provide evidence for the clinical utility of the P1 cortical auditory evoked potential (CAEP) as a non-invasive biomarker that can be used to objectively assess maturation of auditory cortex in clinical cases of cochlear implant patients and candidates. Finally, we present clinical case studies in which the P1 CAEP biomarker proved useful in clinical decision-making regarding intervention in cases of single-sided deafness, auditory neuropathy spectrum disorder, mild hearing loss and hypoplastic auditory nerve.
Keywords: Neuronal Plasticity, Biomarkers, Cochlear implants, Auditory Cortex
Introduction
Cortical auditory evoked potentials (CAEPs) are a non-invasive electroencephalographic (EEG) technique that can be used to objectively assess maturation of the auditory cortex in infants and children. CAEPs can be recorded using most EEG systems routinely used in the clinical setting. The obligatory CAEP response is comprised of three components: the P1, N1, and P2. The P1 component originates from the primary auditory cortex and thalamus [1-4] reflecting the summed synaptic transmission along the ascending auditory pathway. Benefits of CAEP testing include the fact that infants and children can be awake and alert throughout testing, eliminating the need for sleep or sedation in order to obtain the responses. For children of whom behavioral audiometric responses may be unreliable (e.g. infants, children with multiple disabilities), CAEPs allow for objective and reliable responses at higher levels of the brain than the routinely used auditory brainstem response (ABR) can provide [5].
The latency of the P1 component varies as a function of age, occurring around 300 ms in newborns, decreasing rapidly over the first 2-3 years of life, and then gradually decreasing over the second decade of life until reaching a mature adulthood latency around 60 ms [6-12] The P1 CAEP response can therefore serve as a biomarker of cortical maturation such that we can compare responses from children with hearing loss to confidence intervals from normative data to assess the developmental status of the central auditory pathways [10].
A sensitive or critical period refers to a time window in which the potential for neuroplasticity is at its maximum. Neuroplasticity refers to brain’s ability to alter its structure or function in response to intrinsic or extrinsic input. Over the first 4 years of life, neurons begin to form synapses, during a period synaptic overshoot [13, 14]. Cortical plasticity is affected by the amount and quality of stimulation. If one or both are out of order, the brain compensates. If sufficient auditory stimulation is not introduced within the sensitive period, then normal synapse formation and normal development of auditory cortex cannot occur. In addition to synaptogenesis and synaptic pruning, many other factors including neurogenesis, cell death, inhibition, excitation, receptor trafficking, synapse strength, long-term potentiation (LTP), and long-term depression (LTD) underlie the existence and time window of the sensitive period for auditory cortical plasticity.
In order to describe the time limits for a sensitive period for cochlear implantation, Sharma et al., (2002) recorded P1 CAEPs in 245 congenitally deaf children who received cochlear implants at various ages and plotted them against 95% confidence intervals of normal development of the P1 response [10]. P1 CAEP responses were elicited in cochlear implanted children using a /ba/ speech stimulus at least 6 months following cochlear implantation. The vast majority of children who received a cochlear implant before the age of 3.5 years, exhibited a P1 response with a latency falling within normal limits for the child’s age. Only half of children implanted between ages 3.5 and 7 years exhibited normal P1 latencies. For children implanted after the age of 7 years, nearly all children exhibited delayed or abnormal P1 latencies [15]. Taken together, these results provide evidence of a brief sensitive period of approximately 3.5 years during which the central auditory pathways are maximally plastic. Cochlear implantation within this period allows for normal maturation of the auditory cortical pathways as reflected by the latency of the P1 biomarker.
The findings in Sharma et al. are in concordance with other studies demonstrating similar age cutoffs for a sensitive period of the central auditory system during development [15]. For example, Lee et al. [16-19] measured resting glucose metabolism in prelingually deaf children using positron emission tomography (PET) imaging, and correlated the pre-implantation glucose metabolism rates to speech perception scores post-implantation. Glucose hyper-metabolism in auditory cortex was taken to be an indicator of the degree to which auditory cortex had become re-purposed by other modalities, as auditory cortex would otherwise be hypo-metabolic due to deafness. In their study, the early-implanted children (before age 4 years) showed the highest levels of glucose hypometabolism and better speech perception outcomes, while the late-implanted children (after age 6.5 years) showed high levels of glucose hyper-metabolism and poor speech perception abilities. Overall, results of the Lee studies provided complementary evidence of a brief, sensitive period in early childhood when the auditory cortex is maximally receptive to electrical stimulation via a cochlear implant. Evidence of a sensitive period in humans is further supported by evidence of a similar sensitive period in congenitally deaf cats [20-22]. Animal studies have established the existence of a similar, brief sensitive period of approximately 3-6 months in congenitally deaf cats. If implantation occurred beyond this window, late-implanted cats demonstrated auditory local field potentials that are markedly reduced in amplitude and increased in latency when compared to normal hearing and early-implanted cats, similar to findings in human studies [20].
Behavioral outcomes further substantiate the existence and time limits for the sensitive period. For example, early implanted children have been shown to achieve higher levels of speech perception abilities and higher language scores [23-27] compared to late implanted children. Taken together, the behavioral and brain imaging data suggest that consequences of auditory deprivation may hinder normal development of the central auditory pathways, unless cochlear implantation occurs early in life, preferably by one year of age, but best within a sensitive period of 3.5 years [28].
Sensitive periods for cochlear implantation: evidence from the N1 biomarker
The later components of the CAEP response, the N1 and P2 components, reflect higher levels of auditory cortical processing from cortical-cortical circuits, thalamo-cortical pathways, and secondary auditory cortex [28]. The N1/P2 complex emerges as a bifurcation from the broad P1 peak as a child ages. While the P1 component of the CAEP response is present at birth, the N1 component cannot be reliably recorded in normal hearing children until approximately seven years of age using an appropriate stimulation rate [6-9], [11], [29]. Sharma et al (2015) recorded CAEP responses in 41 children with normal hearing and 80 children with cochlear implants in response to a /ba/ speech stimulus at an inter-stimulus interval of 610 ms to determine presence of the N1 response in children who were early, middle, and late-implanted (see Sharma 29 for methodology used). In this study, 71% of normal hearing children exhibited an N1 CAEP response between ages 6 and 9 years. Similarly, 71% of children who were early implanted (before age 3.5 years) showed the emergence of an N1 component between ages 6 and 9 years. Only 40% of children of children who were implanted between ages 3.5 and 7 years exhibited an N1 response, and almost no children who were implanted after age 7 years exhibited an N1 response. Therefore, similar to the P1 response, the N1 CAEP also appears to be abnormal when implantation occurs after the sensitive period. With further research, the N1 component may prove clinically useful in assessing higher-order auditory cortical development in children with hearing loss, in conjunction with the P1 biomarker, which assesses auditory cortical maturation of lower levels in auditory cortex.
Cortical decoupling and cross-modal reorganization after the end of the sensitive period
Why are language outcomes so poor for late-implanted children and why do so few children who are late-implanted ever develop normal cortical auditory evoked potential (CAEP) responses? One hypothesis suggested by Kral et al. is that higher-order cortex may become de-coupled from primary auditory cortex [20-22]. In studies of normal hearing cats, higherorder cortico-cortical auditory pathways and secondary auditory cortex project back to infragranular layers of primary auditory cortex, and then subsequently back to subcortical auditory areas. However, in congenitally deaf cats implanted beyond the sensitive period, studies indicate near absence of activity in infragranular layers of auditory cortex [20-22]. Lack of activity in infragranular layers thus may result in a functional de-coupling between primary and secondary auditory cortices, and between auditory cortex and subcortical auditory regions. Given the role of secondary auditory cortex auditory in processing and integrating auditory stimuli, it is possible that such a potential partial or complete de-coupling may explain poorer speech and language outcomes in late-implanted children.
If auditory stimulation is not delivered in a timely fashion, and a functional de-coupling occurs between primary and secondary auditory cortices, then it is possible that areas of the auditory cortex may become susceptible to recruitment by other sensory modalities. This is a phenomenon known as cross-modal cortical re-organization, and refers to the process when deprivation in one sensory modality (i.e. audition, as in deafness) results in the recruitment of auditory cortex by other non-deprived sensory modalities (i.e. vision or somatosensation) to carry out processing in the recruiting modality. Evidence of cross-modal recruitment by vision has been documented in deaf adults [30-35] and in adults with lesser degrees of hearing loss [36]. Evidence of cross-modal recruitment by the somatosensory system has also been documented in deaf adults [38]. More recently, cross-modal re-organization has also been documented in pediatric populations with hearing loss including children with cochlear implants [29, 39] and single-sided deafness [40]. Along with demographic factors such as age at implantation and duration of deafness which may contribute to variability in behavioral outcomes in patients receiving cochlear implants, cross-modal reorganization may be a factor that may explain some of the variability in speech perception outcomes in children [29] and in adults [30], [31], [36-38], [41]. Further research will help determine whether markers of cross-modal reorganization might help predict cochlear implants success or guide the rehabilitation process.
Clinical utility of the P1 CAEP biomarker in auditory neuropathy spectrum disorder
Recent studies have suggested that there may be a slightly altered sensitive period for cochlear implantation in a sub-set of children with auditory neuropathy spectrum disorder (ANSD) [42], [45]. ANSD is a relatively recently defined disorder characterized by dyssynchronous firing of the auditory nerve and auditory brainstem, characterized by present otoacoustic emissions (OAEs), an absent or abnormal ABR, and a robust cochlear microphonic upon reversal in stimulus polarity [42-49]. Auditory behavioral thresholds in patients with ANSD may fluctuate and are not predictive of speech perception abilities [50-54]. While children who are congenitally deaf receive little to no auditory input, children with ANSD receive inconsistent auditory input [42], [45], [48], [49], [55]. Given that both intrinsic and extrinsic inputs play a large role in refinement of the central auditory system pathways, inconsistent or degraded sensory input, as in ANSD, may result in detrimental effects at the level of the auditory cortex. For example, deficits in cortical phase synchrony which is considered a marker of phase-locking at the auditory cortex, was shown to be significantly reduced in children with ANSD in comparison with normal hearing children and children with sensorineural hearing loss [56].
The P1 CAEP biomarker is a valuable tool in which to assess maturation of the central auditory system in children with ANSD [42], [45], [54], [56]. Research from our laboratory suggests that children with ANSD display three P1 CAEP developmental patterns: 1) present P1 with a latency falling within normal limits for the child’s age; 2) delayed P1 responses of lower amplitude falling outside of normal limits for the child’s age; and 3) children with abnormal or absent P1 responses [43], [49]. Given the strong correlation between markers of cortical development and behavioral outcomes [42], [43], [51], [58-60], normal P1 responses observed in some of the ANSD children may indicate only mild levels of neural dyssynchrony, still allowing for age-appropriate development of the central auditory pathways, whereas absent or abnormal P1 responses may indicate the highest levels of neural dyssynchrony, resulting in absent cortical development. Delayed P1 responses in ANSD may represent moderate levels of neural dyssynchrony [43]. This hypothesis is consistent with recent evidence from our laboratory in which cortical development in ANSD children was significantly correlated with behavioral performance [43], [61]. Children with ANSD have been shown to derive benefit from cochlear implantation in a recent study showing that in a group of 24 children with ANSD, nearly all of the children exhibited a present P1 response (either normal or delayed in latency) post-implantation and there was no evidence of abnormal CAEP morphology post-implantation [42]. Further, studies from our laboratory suggest that the sensitive period for cochlear implantation in children with ANSD is approximately 2 years, with ANSD children implanted before the age of 2 years exhibited age-appropriate P1 latencies, whereas ANSD children implanted after age 2 years tended to show delayed P1 cortical responses [42]. Taken together, measures of auditory cortical development in children with ANSD may prove useful in deciding on optimal ages for implantation, selecting appropriate treatment options for these children, and monitoring cortical auditory maturation following audiological intervention.
Clinical utility of the P1 CAEP biomarker in children with multiple disabilities
An estimated 20-40% of infants and children present with additional physical, sensory, psychiatric, neurological, cognitive, and intellectual disabilities concomitant with hearing loss [62]. Many of these children require long-term, intensive treatment and rehabilitation from medical and health professionals. While not all children with hearing loss have multiple disabilities present with cognitive and developmental delays, more than 30% present with developmental delays which may impact cognitive, motor, and language development [63-66]. Thus, early hearing loss identification and intervention in these children is critical in allowing these children to meet their highest developmental potential.
Major barriers to early intervention among children with hearing loss concomitant with additional disabilities include the large amount of heterogeneity in this population, making it difficult to predict outcomes; lack of appropriate measures to objectively assess hearing aid and cochlear implant candidacy; and paucity of objective measures to monitor progress following audiological intervention. While several studies have demonstrated the efficacy of hearing aids [67] and cochlear implantation in children with additional disabilities [68-75], these children tend to show slower progress and on average poorer outcomes compared to children with hearing loss with no additional disabilities. This makes predicting outcomes following intervention via hearing aids or cochlear implants a particular challenge for medical professionals. Additionally, obtaining reliable behavioral audiometric results in this population can be inconsistent, where auditory thresholds and speech perception results may prove unreliable, or in children who never develop closed-set or open-set word recognition abilities [5], [76]. Using sedation to obtain electrophysiological measures (i.e. ABR testing) to evaluate hearing sensitivity also comes with high risks in children with some types of multiple disabilities, such as infants and children with CHARGE syndrome [77] (also see Wang [78] for review on the effects of general anesthesia on neurodevelopment in children). Further, there exists the need to monitor development in this population of children after they receive clinical intervention with hearing aids or cochlear implants. Due to these factors and the complex nature of medical problems in children multiple disabilities, these children do not always receive intervention within the sensitive period, despite the advent of newborn hearing screening and early identification of their hearing loss [5]. Such factors may contribute to poorer outcomes in children with multiple disabilities receiving audiological intervention beyond the sensitive period [5].
In this realm, the P1 CAEP biomarker represents a non-invasive method to objectively assess the maturational status of the central auditory pathways in candidacy evaluations for hearing aids or cochlear implants, as well as to monitor cortical auditory development following intervention in multiply disabled children [5]. CAEPs are a non-invasive EEG technique that does not require the patient to be asleep or sedated, making it a viable option in cases where there are high risks with anesthesia or in infants and children who have undergone multiple exposures to anesthesia due to numerous life-threatening health complications requiring surgery.
Patterns of deprivation and plasticity in the CAEP waveform
All of this basic research over the past 20 years in our laboratory has allowed us to examine patterns of cortical development to develop clinical biomarkers of central auditory maturation. Children are now being identified with hearing loss very early due to the implementation of newborn hearing screening. However, there are no objective biomarkers to determine whether an infant or young child is benefitting from amplification via hearing aids, or whether the child might benefit from a cochlear implant. The P1 biomarker thus represents an objective, non-invasive way of assessing the maturational status of the central auditory system in this population.
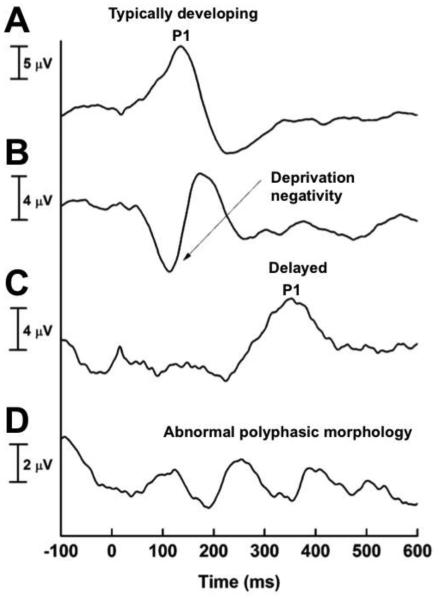
Four patterns in the P1 CAEP waveform become observable in infants and young children based on the developmental status of the central auditory pathways: 1) typical development of the auditory cortical pathways (e.g. in normal hearing children and children with hearing loss receiving sufficient auditory stimulation within the sensitive period), in which a normal P1 CAEP latency is observed; 2) under-stimulated development of the auditory cortical pathways (e.g. in children with severe-profound hearing loss who are not receiving adequate auditory stimulation), in which a large deprivation (called a deprivation negativity) is observed before the P1 CAEP response; 3) partially stimulated development of the central auditory pathways (e.g. children who are receiving some but not sufficient auditory stimulation necessary to drive development of the auditory cortical pathways), in which a present albeit delayed P1 response is observed; and 4) abnormal development of the auditory cortical pathways (e.g. children with significant auditory deprivation), in whom polyphasic morphology indicates abnormal development and re-organization of the central auditory system (Figure 1) [79, 80]. These patterns of deprivation and plasticity in four children are exhibited in Figure 1. Given these distinct morphological differences in the CAEP response, changes in the morphology of the CAEP response can be useful in monitoring the maturational status of the central auditory pathways before and after audiological intervention. For example, in early-implanted children with severe-profound hearing loss, they may initially present with a delayed P1 and deprivation negativity prior to cochlear-implantation, but the latency of the P1 response typically falls within normal values within 3-6 months after cochlear implant activation [15]. Contrastingly, polyphasic morphology may persist in an older child implanted well after the sensitive period of approximately 3.5 years. Using these morphological changes can be useful in distinguishing the effects of different levels of deprivation the development of the auditory cortex.
Figure 1.
Four patterns of central auditory system maturation using the P1 cortical auditory evoked potential (CAEP) biomarker. CAEP response for a typically developing central auditory pathways, marked by a P1 response falling within normal limits for the child’s age (A); CAEP response for an un-stimulated central auditory system marked by a deprivation negativity and delayed P1 response (B); CAEP response for a child with a partially stimulated central auditory system (C); CAEP response for a child with abnormal development of the central auditory system, marked by polyphasic morphology (D). Adapted with permission from Sharma, Nash, & Dorman (2009). Cortical development, plasticity, and re-organization in children with cochlear implants. Journal of Communication Disorders, 42(4), 272-9.
Case studies
In a series of papers we have described the use of the P1 biomarker to establish candidacy for cochlear implantation [12], [29], [49], [54], [81]. In the following section, we describe more complicated cases to highlight the use of the P1 biomarker in cases of single-sided deafness before and after cochlear implantation, mild hearing loss before and after consistent hearing aid use, auditory nerve hypoplasia, and ANSD. CAEPs were recorded in response to a speech syllable /ba/ using a 3-electrode montage for unaided and hearing aid subjects and a 5-channel electrode montage to minimize cochlear implant artifact for cochlear implanted subjects (see Gilley [81] for methodology used to reduce cochlear implant artifact in CAEP recordings). CAEPs were recorded using a 100ms pre-stimulus interval and a 600ms post-stimulus recording window with analog filter set to 0.1 to 1000 Hz using the Compumedics Neuroscan EEG system. The auditory stimulus was delivered via a speaker in the sound field located at 45 degrees azimuth (bilateral condition) or via insert earphones (ear-specific) at a suprathreshold level. Following data collection, CAEPs were baseline corrected to the entire stimulus interval, eye blink and artifact rejection was performed (±100 uV), and remaining sweeps were averaged. At least two sweeps of 250 accepted runs each were then grand averaged, from which P1 latencies were selected. All testing was conducted in an electromagnetically shielded sound boot. Patients watched a video or cartoon of their choice with video audio set on mute throughout the duration of testing.
Case 1: single-sided deafness
The patient is a female child identified with a moderate hearing loss in her right ear at a school hearing screening at age 5 years; her hearing loss in her right ear progressed to severe-profound by age 9 years. The child has normal hearing in her left ear. The etiology of her hearing loss is unknown. She underwent trials with a contralateral routing of signal hearing aid (CROS) and FM system. At the age of 9.86 years, she received a cochlear implant in her right ear. (For in-depth review of this case, see Sharma [40]).
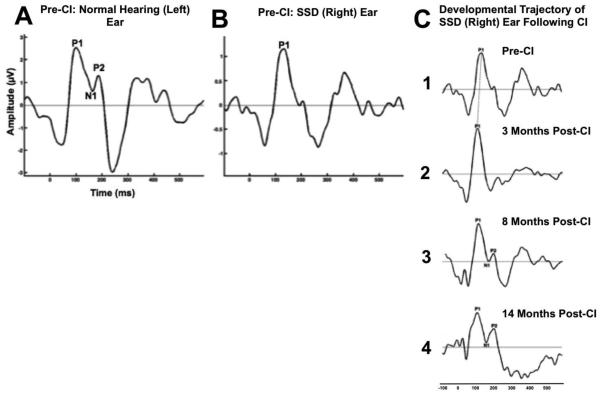
Figure 2 shows the child’s CAEP response in her normal hearing ear (Figure 2A) and single-sided deaf SSD (right) ear (Figure 2B) prior to cochlear implantation. As shown in Figure 2A, the CAEP response in the normal hearing ear shows age-appropriate morphology (presence of all 3 CAEP peaks, the P1, N1, and P2 components), with a P1 response in that ear falling within normal limits for the child’s age. However, responses in her SSD ear pre-CI indicate a developmentally immature response compared to the normal hearing ear, with the presence of just the P1 component occurring at a borderline normal latency. However, following cochlear implantation there is a dramatic change in the developmental morphology of the child’s SSD ear. As shown in Figure 2C, by 3 months there is a decrease in P1 latency, eventually falling within the normal range (Figure 2C2), by 8 months there is the emergence of the later CAEP (N1 and P2) components (Figure 2C3), and by 14 moths post-implantation comes the emergence of an age-appropriate CAEP response (Figure 2C4). Taken together, results indicate a high degree of neuroplasticity in the SSD ear, even after cochlear implantation occurred well after the 3.5-year sensitive period at an age of 9.86. In this case, monitoring via the CAEP recordings provided first evidence of the objective benefit of cochlear implantation in pediatric SSD in terms of auditory-cortical maturation. Electrophysiological findings in this case were consistent with the child’s excellent behavioral outcomes in terms of her speech perception in background noise.
Figure 2.
Cortical auditory evoked potential (CAEP) response for a child diagnosed with normal hearing in her left ear (A) and single-sided deafness in her right ear after age 5 years (B). The child received a cochlear implant (CI) in her right ear age 9.86 years. Pre-CI, the child demonstrates an age-appropriate CAEP response with normal morphology and a P1 response falling within normal limits in the child’s normal hearing ear (A), and a borderline delayed P1 response marked by immature morphology for the child’s age in her deaf ear (B). The developmental trajectory of the child’s SSD ear following CI shows a decrease in P1 latency within 3 months (C2), the development of higher-order N1 and P2 CAEP components within 8 months (C3), and the emergence of an age-appropriate CAEP response with normal P1 latency and morphology within 14 months after CI (C4).
Case 2: benefit from hearing aids
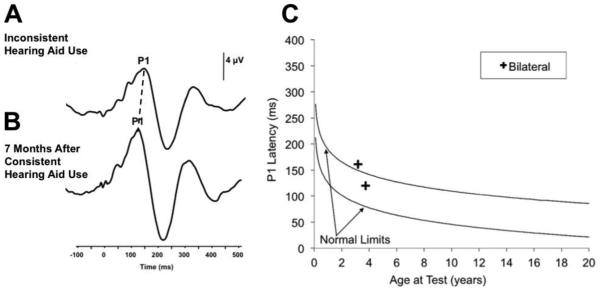
The patient was a female who referred on her newborn hearing screening and diagnosed with a mild sensorineural hearing loss within a year of birth. The child was fit with hearing aids bilaterally at age 2.25 years of age. The child’s audiologist and early intervention specialist reported continued concerns regarding the spoken language development in this child. Additionally, these professionals expressed concerns regarding parental compliance in ensuring the child was wearing her hearing aids during all waking hours. This patient was seen in our laboratory to assess the maturation of her central auditory pathway with the P1 response biomarker at age 3.17 years. Results from this test session indicated a present P1 CAEP response occurring at a latency slightly outside of the 95% confidence intervals for the child’s age (Figure 3C). This result indicated that the auditory input the child was receiving was allowing for some development of the central auditory system, albeit delayed when compared to normal hearing, typically developing children of the same age. Results from this test session were used as a counseling tool by the audiologist to encourage the child’s parents to try to implement more consistent hearing aid use. The patient returned for a second visit 7 months later, following parent reports of the child’s hearing aid use during all waking hours. The P1 response recorded at this visit fell within normal limits for this child’s age, indicating age-appropriate cortical auditory maturation, consistent with parent report of more regular and consistent hearing aid use (Figure 3C). The decrease in P1 responses from the first to second test session can be visualized in Figure 3A, 3B. These results suggest that with continued regular use of her hearing aids, this patient’s P1 responses will remain within normal limits, and that her auditory system will continue to develop normally. Behavioral results suggest that the patient is proceeding with language development.
Figure 3.
Cortical auditory evoked potential (CAEP) response plotted against P1 CAEP norms for a child diagnosed with a mild hearing loss before age 2 years. While a borderline delayed P1 CAEP response was observed during inconsistent hearing aid use (A, C), 11 months after consistent hearing aid use the child’s P1 CAEP response fell within normal limits for her age (B, C).
Case 3: auditory nerve hypoplasia
The patient was a female child who was first diagnosed with a mild to severe unilateral hearing loss at approximately 6.5 years of age in her left ear. There was no previously recorded documentation of medical traumas, illnesses, nor any familial history of significant health problems. At time of initial diagnosis an MRI was collected, revealing no evidence of an enlarged vestibular aqueduct (EVA) or any other cochlear abnormalities. Etiology of the hearing loss in the left ear was confirmed by MRI results, which showed an absence and/or hypoplastic auditory nerve on the left side. The auditory nerve was visible on the right side, consistent with the child’s normal hearing in that ear.
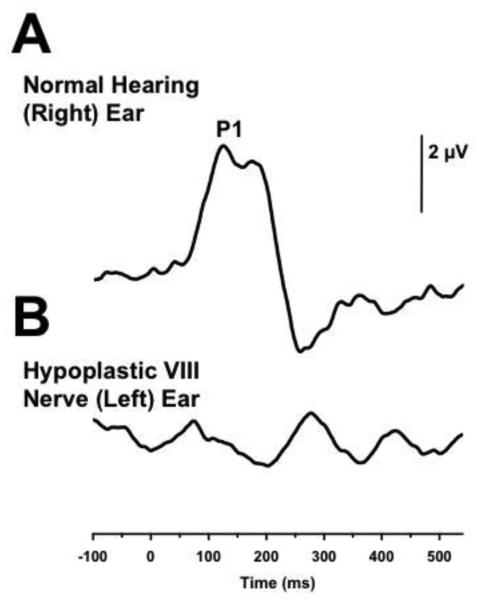
In this particular case of a child with an identified hypoplastic nerve unilaterally, we evaluated the maturation and functionality of the patient’s central auditory pathway using a P1 CAEP biomarker. The results recorded for the child’s right normal hearing ear indicated a replicable CAEP P1 response falling within normal limits for the child’s age (Figure 4A). Wave morphology and peak latencies corresponded to age-matched data, suggestive of normative functionality and development. The CAEP response in the child’s left ear with the hypoplastic nerve was characterized by a replicable, albeit questionable, delayed P1 response. Further, amplitude of CAEP responses in the left ear were significantly reduced in amplitude when compared to the child’s normal hearing right ear (Figure 4B). The morphology of the CAEP response in the left ear is polyphasic in nature, indicating atypical development of the central auditory pathways arising from this ear (Figures 1D, 4B). These results suggest that child is not receiving sufficient auditory stimulation for normal development of the central auditory pathway of her left ear, consistent with the diagnosis of auditory nerve hypoplasia in this ear [80], [81] The child is currently undergoing a trial with a bone conduction hearing aid device (BAHA).
Figure 4.
Cortical auditory evoked potential (CAEP) response plotted against P1 CAEP norms for a 6-year old child with normal hearing in the right ear and a moderate-severe hearing loss due to VIII nerve hypoplasia in the left ear. A normal P1 CAEP response is observed in the child’s normal hearing ear (A). However, a replicable albeit questionable P1 CAEP response for the child’s age marked by polyphasic morphology is observed in the child’s left ear with the hypoplastic auditory nerve (B).
Case 4: auditory neuropathy spectrum disorder
The patient was a male child born full-term with no perinatal complications, and who passed his newborn hearing screening. The child was adopted at birth, so there is limited information available regarding familial history. Postnatal history is significant for reflux and child was diagnosed with bilateral severe to profound hearing loss at 4 months of age. Initial ABR and auditory steady state response (ASSR) results were indicative of an auditory neuropathy (neural dyssynchrony) diagnosis bilaterally. The patient was fit bilaterally with hearing aids at approximately 19 months of age.
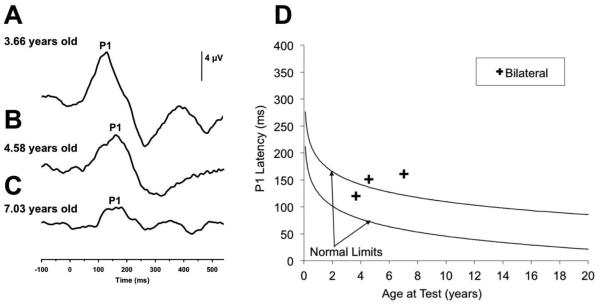
In this case of a child with ANSD, we evaluated the functionality and maturation of the central auditory pathway using the P1 CAEP biomarker. CAEPs were used to assess if adequate acoustic stimulation was being delivered to the patient’s auditory cortices with appropriate hearing aid amplification. P1 biomarkers were evaluated at 3.66 years, 4.58 years, and 7.03 years of age. As visualized in Figure 5A, the P1 response waveform for the child at 3.66 years fell within normal limits for the child’s age, indicating the child was receiving sufficient auditory stimulation allowing for normal development of the central auditory pathways bilaterally. When CAEP responses were assessed at age 4.58 years, a decrease in P1 amplitude and increase in P1 latency were observed bilaterally, with the P1 latency occurring at a borderline normal latency for the child’s age (Figure 5B). Testing at this date indicated a delayed P1 latency suggestive of a regression in age-appropriate maturation of the central auditory pathways when compared to results of testing at age 3.66 years. Further, CAEP responses recorded at 7.03 years of age also showed a delayed P1 latency indicating further regression of typical development of the auditory cortex, marked by a significant reduction in P1 CAEP amplitude (Figure 5C). P1 CAEP latency at age 7.03 years fell outside of the 95% confidence intervals based on the child’s chronological age (Figure 5D). Based on the changes in the child’s P1 latencies from 3.66 to 7.03 years of age, we can infer that the child is no longer receiving adequate acoustic stimulation in the right ear critical for normal development of the auditory cortical pathway. The P1 findings are consistent with a rapid decline in the child’s behavioral speech perception and language development. The CAEP findings are in congruence with the child’s diagnosis of ANSD and continued cortical monitoring was recommended. The child is currently undergoing consideration for cochlear implantation by his audiologic team.
Figure 5.
Cortical auditory evoked potential (CAEP) response plotted against P1 CAEP norms for a child diagnosed with auditory neuropathy (AN). While the child shows a P1 CAEP response with a latency within normal limits for the child’s age when evaluated at age 3.66 (A), by age 4.58 years P1 CAEP latency becomes delayed and reduces in amplitude (B). By age 7.03 years the child’s delayed P1 CAEP response continues to decrease in amplitude, indicating a regression in age-appropriate development of the central auditory pathways.
Conclusions
In this review, we outlined evidence of the existence of a sensitive period in early childhood, during which appropriate auditory stimulation yields optimal developmental outcomes. We then described the clinical utility of the P1 CAEP response as a biomarker of auditory cortical maturation in children with hearing loss, using clinical cases. We have highlighted the clinical power of the P1 CAEP biomarker in assessing the maturational status of the central auditory system in children with congenital deafness, useful in conjunction with other clinical assessments of cochlear implant candidacy. The P1 CAEP biomarker exhibits clinical utility for more complex audiological cases, including infants and children with ANSD and children with multiple disabilities concomitant with hearing loss, both highly heterogeneous populations in whom measures of speech and language development may prove inconsistent, unreliable, and/or limited. Using the P1 biomarker in conjunction with the traditional audiological test battery may provide objective evidence useful in making decisions about audiological intervention, as well as monitoring cortical maturation following intervention.
Acknowledgments
Fundings.—The research was supported by the National Institutes of Health: R01 DC 006257, T32DC012280-01 A1.
Footnotes
Conflicts of interest.—The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Erwin RJ, Buchwald JS. Mid latency auditory evoked responses in the human and the cat model. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:461–467. [PubMed] [Google Scholar]
- 2.McGee T, Kraus N. Auditory development reflected by middle latency response. Ear Hear. 1996;17:419–429. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalograph Clin Neurophys. 1994;92:204–14. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont JJ, Ponton CW, Don M, Waring MD, Kwong B. Maturational delays in cortical evoked potentials in cochlear implant users. Acta Otolaryngol. 1997;117:161–3. doi: 10.3109/00016489709117760. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Glick H, Campbell J, Biever A. Central auditory development in children with hearing loss: Clinical relevance of the P1 CAEP biomarker in hearing-impaired children with multiple disabilities. Hear Bal Comm. 2013:11. doi: 10.3109/21695717.2013.812378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephal Clin Neurophysiol. 1997;104:540–5. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 7.Ceponiene R, Cheour M, Näätänen R. Interstimulus interval and auditory event-related potentials in children:Evidence for multiple generators. Electroencephalogr Clin Neurophysiol. 1998;108:345–54. doi: 10.1016/s0168-5597(97)00081-6. [DOI] [PubMed] [Google Scholar]
- 8.Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity:Evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111:220–36. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham J, Nicol T, Zecker S, Kraus N. Speech-evoked neurophysiologic responses in children with learning in problems:Development and behavioral correlates of perception. Ear Hear. 2000;21:554–68. doi: 10.1097/00003446-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Dorman M, Spahr A. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002a;13:1365–8. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- 11.Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2004;116:648–57. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203:134–43. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Huttenlocher PR, Dabholkar AA. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Kral A. Unimodal and cross-modal plasticity in the ‘deaf’ auditory cortex. Int J Audiol. 2007;46:479–93. doi: 10.1080/14992020701383027. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear. 2002b;23:532–9. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, et al. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–50. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- 17.Kang E, Lee DS, Kang H, Lee JS, Oh SH, Lee MC, et al. Neural changes associated with speech learning in deaf children following cochlear implants. Neuroimage. 2004;22:1173–81. doi: 10.1016/j.neuroimage.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Kang E, Oh SH, Kang H, Lee DS, Lee MC, et al. Preoperative differences of cerebral metabolism rate to the outcome of cochlear implants in congenitally deaf children. Hear Res. 2005;203:2–9. doi: 10.1016/j.heares.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Oh SH, Kim CS, Kang EJ, Lee DS, Lee HJ, Chang SO, et al. Speech perception after cochlear implantation over a 4-year time period. Acta Otolaryngol. 2003;123:148–53. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- 20.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000;10:714–26. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- 21.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness:Central auditory system plasticity and sensory deprivation. Cereb Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- 22.Kral A, Tillein J, Heid S. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15:552–62. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- 23.Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Advantages of cochlear implantation in pre-lingual deaf children before 2 years of age when compared with later implantation. Laryngoscope. 2004;114:1462–9. doi: 10.1097/00005537-200408000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Kirk K, Miyamoto R, Lento C, Ying E, O’Neill T, Fears B. Effects of age at implantation in young children. Ann Otol Rhinol Layrngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- 25.Geers EA. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Otorhinolaryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- 26.Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tajudeen BA, Waltzman SB, Jethanamest D, Svirsky MA. Speech perception in congentially deaf children receiving cochlear implants in the first year of life. Otol Neurotol. 2010;31:1254–60. doi: 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35:111–22. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma A, Campbell J, Cardon G. Developmental and crossmodal plasticity in deafness:Evidence from the P1 and N1 event related potentials in cochlear implanted children. Int J Psychophysiol. 2015;95:135–44. doi: 10.1016/j.ijpsycho.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley KA, Tobey EA. Cross-modal plasticity and speech perception in pre- and post-lingually deaf cochlear implant users. Ear Hear. 2011;32:2–15. doi: 10.1097/AUD.0b013e3181e8534c. [DOI] [PubMed] [Google Scholar]
- 31.Doucet ME, Bergeron F, Lassonde M, Ferron P, Lepore F. Crossmodal reorganization and speech perception in cochlear implant users. Brain. 2006;129:3375–83. doi: 10.1093/brain/awl264. [DOI] [PubMed] [Google Scholar]
- 32.Fine I, Finney EM, Boynton GM, Dobkins KR. Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. J Cogn Neurosci. 2005;17:1621–37. doi: 10.1162/089892905774597173. [DOI] [PubMed] [Google Scholar]
- 33.Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–3. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 34.Finney EM, Clmementz BA, Hickok G, Dobkins KR. Visual stimuli activate auditory cortex in deaf subjects: Evidence from MEG. Neuroreport. 2003;14:1425–7. doi: 10.1097/00001756-200308060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Neville HJ, Schmidt A, Kutas M. Altered visual-evoked potentials in congenitally deaf adults. Brain Res. 1983;266:127–32. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- 36.Campbell J, Sharma A. Cross-modal re-organization in adults with early stage hearing loss. PLoS One. 2014;9:e90594. doi: 10.1371/journal.pone.0090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandmann P, Dillier N, Eichele T, Meyer M, Kegel A, Pascual-Marqui RD, et al. Visual activation of auditory cortex reflects maladaptive plasticity in cochlear implant users. Brain. 2012;135:555–68. doi: 10.1093/brain/awr329. [DOI] [PubMed] [Google Scholar]
- 38.Schierholz I, Finke M, Schulte S, Hauthal N, Kantzke C, Rach S, et al. Enhanced audio-visual interactions in the auditory cortex of elderly cochlear-implant users. Hear Res. 2015;328:133–47. doi: 10.1016/j.heares.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Gilley PM, Sharma A, Dorman F. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma A, Glick H, Campbell J, Torres J, Doman M, Zeitler DM. Cortical plasticity and reorganization in pediatric single-sided deafness pre- and post-cochlear implantation. Otol Neurotol. 2015 doi: 10.1097/MAO.0000000000000904. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci. 2013;7:71. doi: 10.3389/fnsys.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardon G, Sharma A. Central auditory maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder who use cochlear implants. Int J Audiol. 2013;52:577–86. doi: 10.3109/14992027.2013.799786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A, Cardon G. Cortical development and neuroplasticity in auditory neuropathy spectrum disorder. Hear Res. 2015;330:221–32. doi: 10.1016/j.heares.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starr A, Picton TW, Siniger Y, Hood LJ, Berlin CI. Auditory Neuropathy. Brain. 1996;119:741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 45.Berlin C, Bordelon J, John P, Wilenski D, Annette K, Hood L. Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear. 1998;19:37–47. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Berlin C, Li L, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony:Diagnosis and management. Ment Retard Dev Disabil Res Rev. 2003;9:225–31. doi: 10.1002/mrdd.10084. [DOI] [PubMed] [Google Scholar]
- 47.Sininger Y, Oba S. Patients with auditory neuropathy:Who are they and what can they hear? In: Sininger Y, Starr A, editors. Auditory neuropathy:A new perspective on hearing disorders. Singular; San Diego: 2001. pp. 67–82. [Google Scholar]
- 48.Cardon G, Campbell J, Sharma A. Plasticity in the developing auditory cortex: Evidence from children with sensorineural hearing loss and auditory neuropathy spectrum disorder. J Am Acad Audiol. 2012;23:396–411. doi: 10.3766/jaaa.23.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell J, Cardon G, Sharma A. Clinical application of the P1 cortical auditory evoked potential biomarker in children with sensorineural hearing loss and auditory neuropathy spectrum disorder. Semin Hear. 2011;32:117–22. doi: 10.1055/s-0031-1277236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rance G, Beer D, Cone-Wesson B, Shepherd R, Dowell R, King A, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20:238. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23:239–25. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Rapin I, Gravel J. Auditory neuropathy: physiologic and pathologic evidence calls for more diagnostic specificity. Int J Pediatr Otorhinolaryngol. 2003;67:707–28. doi: 10.1016/s0165-5876(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 53.Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–63. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- 54.Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49:367–80. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]
- 55.Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242:1437–41. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- 56.Nash-Kille A, Sharma A. Inter-trial coherence as a marker of cortical phase synchrony in children with sensorineural hearing loss and auditory neuropathy spectrum disorder fitted with hearing aids and cochlear implants. Clin Neurophysiol. 2014;125:1459–70. doi: 10.1016/j.clinph.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardon G, Sharma A. Cortical auditory evoked potentials in auditory neuropathy spectrum disorder: clinical implications. Perspect Hear Hear Disord in Children. 2011;21:31–7. [Google Scholar]
- 58.Michalewski H, Starr A, Nguyen T, Kong Y, Zeng F. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116:669–80. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 59.Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): Effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120:1352–63. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarenga KF, Amorim R, Agostinho-Pesse RS, Costa OA, Nascimento LT, Bevilacqua MC. Speech perception and cortical auditory evoked potentials in cochlear implant users with auditory neuropathy spectrum disorders. Int J Pediatr Otolaryngol. 2012;76:1332–1338. doi: 10.1016/j.ijporl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Ann Otol Rhinol Laryngol Suppl. 2000;185:42–4. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]
- 62.Picard M. Children with permanent hearing loss and associated disabilities: Revisiting current epidemiological data and causes of deafness. Volta Review. 2004;104:221–36. [Google Scholar]
- 63.Meinzen-Derr J, Wiley S, Grether S, Choo DI. Language performance in children with cochlear implants and additional disabilities. Laryngoscope. 2010;120:405–13. doi: 10.1002/lary.20728. [DOI] [PubMed] [Google Scholar]
- 64.Birman CS, Elliott EJ, Gibson WP. Pediatric cochlear implants: additional disabilities prevalence, risk factors, and effect on language outcomes. Otol Neurotol. 2012;33:1347–52. doi: 10.1097/MAO.0b013e31826939cc. [DOI] [PubMed] [Google Scholar]
- 65.Wiley S, Jahnke M, Meinzen-Derr J, Choo D. Perceived qualitative benefits of cochlear implants in children with multi-handicaps. Int J Ped Otorhinol. 2005;69:791–8. doi: 10.1016/j.ijporl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Wiley S, Meinzen-Derr J, Grether S, Choo DI, Hughes ML. Longitudinal functional performance among children with cochlear implants and disabilities:a prospective study using the Pediatric Evaluation of Disability Inventory. Int J Pediatr Otorhinolaryngol. 2012;76:693–7. doi: 10.1016/j.ijporl.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Kaga K, Shindo M, Tamai F, Tanaka Y. Changes in auditory behaviors of multiply handicapped children with deafness after hearing aid fitting. Acta Otolaryngol Suppl. 2007;559:9–12. doi: 10.1080/03655230701596368. [DOI] [PubMed] [Google Scholar]
- 68.Corrales CE, Oghalai JS. Cochlear implant considerations in children with additional disabilities. Curr Otorhinolaryngol. 2013;1:61–8. doi: 10.1007/s40136-013-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berrettini S, Forli F, Genovese E, Santarelli R, Arslan E, Chilosi AM, Cipriani P. Cochlear implantation in deaf children with associated disabilities: challenges and outcomes. Int J Audiol. 2008;47:199–208. doi: 10.1080/14992020701870197. [DOI] [PubMed] [Google Scholar]
- 70.Hamzavi J, Baumgartner WD, Egelierler B, Franz P, Schenk B, Gstoettner W. Follow up of cochlear implanted handicapped children. Int J Pediatr Otorhinolaryngol. 2000;56:169–74. doi: 10.1016/s0165-5876(00)00420-1. [DOI] [PubMed] [Google Scholar]
- 71.Beer J, Harris MS, Kronenberger WG, Holt RF, Pisoni DB. Auditory skills, language development, and adaptive behavior of children with cochlear implants and additional disabilities. Int J Audiol. 2012;51:491–8. doi: 10.3109/14992027.2012.664291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donaldson AI, Heavner KS, Zwolan TA. Measuring progress in children with autism spectrum disorder who have cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130:666–71. doi: 10.1001/archotol.130.5.666. [DOI] [PubMed] [Google Scholar]
- 73.Holt RF, Kirk KI. Speech and language development in cognitively delayed children with cochlear implants. Ear Hear. 2005;26:132–48. doi: 10.1097/00003446-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Pyman B, Blamey P, Lacy P, Clark G, Dowell R. The development of speech perception in children using cochlear implants: effects of etiologic factors and delayed milestones. Am J Otol. 2000;21:57–61. [PubMed] [Google Scholar]
- 75.Waltzman SB, Scalchunes V, Cohen NL. Performance of multiply handicapped children using cochlear implants. Am J Otol. 2000;21:329–35. doi: 10.1016/s0196-0709(00)80040-x. [DOI] [PubMed] [Google Scholar]
- 76.Trimble K, Rosella LC, Propst E, Gordon KA, Papaioannou V, Papsin BC. Speech Perception Outcome in Multiply Disabled Children Following Cochlear Implantation:Investigating a Predictive Score. J Am Acad Audiol. 2008;19:602–11. doi: 10.3766/jaaa.19.8.4. [DOI] [PubMed] [Google Scholar]
- 77.Blake K, MacCuspie J, Hartshorne TS, Roy M, Davenport SL, Corsten G. Postoperative airway events of individuals with CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2009;73:219–26. doi: 10.1016/j.ijporl.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Xu Z, Miao CH. Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: An updated systematic review with meta-regression. PLoS One. 2014;9:e85760. doi: 10.1371/journal.pone.0085760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma A, Dorman MF. Central auditory development in children with cochlear implants: Clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- 80.Sharma A, Nash AA, Dorman M. Cortical development, plasticity, and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272–9. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roland P, Henion K, Booth T, Campbell JD, Sharma A. Assessment of cochlear implant candidacy in patients with cochlear nerve deficiency using the P1 CAEP biomarker. Cochlear Implants Int. 2012;13:16–25. doi: 10.1179/146701011X12962268235869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilley PM, Sharma A, Dorman M, Finley CC, Panch AS, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006;117:1772–82. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]