Abstract
Firemaster® 550 (FM 550) is a commercial mixture of organophosphate and brominated flame retardants currently in use as a replacement for pentaBDE. Its organophosphate components include triphenyl phosphate (TPHP) and a suite of isopropylated triarylphosphate isomers (ITPs); its brominated components include 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEH-TEBP). Taken together, these chemicals have been shown to be endocrine disrupting and potentially toxic, and human exposure to them is widespread. In this study, maternal transfer of FM 550 components, and in some cases their metabolites, was investigated in dosed Wistar rats. Gestational and lactational transfer were examined separately, with dams orally exposed to 300 or 1000 µg of FM 550 for 10 consecutive days during gestation (gestational day [GD] 9–18) or lactation (postnatal day [PND] 3–12). Levels of parent compounds were measured in fetus and whole pup tissue homogenates, and in dam and pup serum, and several metabolites were measured in dam and pup urine. EH-TBB body burdens resulting from lactational transfer were approximately 200- to 300-fold higher than those resulting from placental transfer, whereas low levels of BEH-TEBP were transferred during both lactation and gestation. TPHP and ITPs were rapidly metabolized by the dams and were not detected in whole tissue homogenates. However, diphenyl phosphate (DPHP) and mono-isopropylphenyl phenyl phosphate (ip-PPP) were detected in urine from the dosed animals. This study is the first to confirm ip-PPP as a urinary metabolite of ITPs and establish a pharmacokinetic profile of FM 550 in a mammalian model.
Key words: Firemaster 550; lactational transfer; gestational transfer; metabolites; rodent.
Flame retardants are a group of chemical compounds that are applied to textiles, electronics, and other consumer products in order to suppress or delay their combustion, and to meet federal and state flammability standards (Van Esch et al., 1997). Adopted in 1975, California Technical Bulletin 117 (CA TB117) required the filling material used in upholstered furniture to withstand a 12-second open flame test (Stapleton et al., 2012a,b). Although CA TB117 was recently replaced by CA TB117-2013, which requires smolder resistance of materials used in upholstered furniture, the use of additive flame retardants remains a cheap and convenient method for North American foam manufacturers to maintain compliance with existing flammability standards. Until recently when concerns about its persistence, bioaccumulation, and toxic properties arose, foam manufacturers primarily used pentaBDE, a commercial polybrominated diphenyl ether (PBDE) mixture, to satisfy CA TB117. Following the voluntary phase-out of pentaBDE in the US in 2005, foam manufacturers turned to alternative flame retardants to treat their foam. In a survey of 102 foam samples collected from residential couches in the United States in 2011, Firemaster® 550 (FM 550) was the second most commonly detected flame retardant (Stapleton et al., 2012a,b). FM 550 is a novel, commercial flame retardant mixture made up of 4 components: 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEH-TEBP), triphenyl phosphate (TPHP), and a suite of isopropylated triarylphosphate isomers (ITPs) (Fig. 1). Because flame retardants are not chemically bound to the foam filling in furniture, they can migrate out of the treated material over time and accumulate in indoor house dust, leading to exposure. Ingestion of house dust via hand-to-mouth activity is a major exposure pathway for flame retardants, and FM 550 components have been frequently detected in house dust from around the world at levels ranging from 3.0 ng/g to 15 030 ng/g (Ali et al., 2011, 2012; Al-Omran and Harrad, 2015; Dodson et al., 2014; Stapleton et al., 2008). In addition, EH-TBB and BEH-TEBP have been detected in atmospheric particles from the European Arctic and in marine mammals, underscoring the potential for long-term transport of these compounds and illustrating their current far-reaching global distribution (Lam et al., 2009; Salamova et al., 2014; Zhu et al., 2014). Furthermore, EH-TBB and BEH-TEBP have been measured in human hair and nails and the urinary metabolite of EH-TBB, 2,3,4,5-tetrabromobenzoic acid (TBBA), has been frequently detected in recent analyses of human urine, suggesting that human exposure to FM 550 is widespread (Butt et al., 2014; Hoffman et al., 2014; Liu et al., 2015). Other metabolites of FM 550 measured previously in urine include diphenyl phosphate (DPHP), isopropylphenyl phenyl phosphate (ip-PPP), and mono-(2-ethylhexyl) tetrabromophthalate (TBMEHP) (Van den Eede et al., 2013b, 2014; Silva et al., 2015) (Fig. 1).
FIG. 1.
Parent compounds and corresponding urinary metabolites of the 4 FM 550 components. *Not measured in this study.
Despite its use and known exposure, there were no studies on the health effects of FM 550 in rodents or mammals prior to 2012. Since that time, toxicological evidence suggests that FM 550 may be obesogenic and endocrine disrupting (Fang et al., 2015; Patisaul et al., 2013; Pillai et al., 2014). Exposure to FM 550 has been associated with weight gain, anxiety-like behavior, early-onset of puberty in females, and perturbed thyroid hormone levels in perinatally exposed rats (Patisaul et al., 2013). TPHP exposure has also been correlated with increases in both T3 and T4 concentrations in zebrafish larvae and an increase in the expression of genes involved in thyroid hormone synthesis in rat pituitary cell lines (Kim et al., 2015). In addition, a recent study on a range of brominated and organophosphate flame retardants flagged mono-ITP as a priority chemical warranting additional testing, as it was active in 10 out of 11 tested developmental and neurotoxicity assays at concentrations <30 μM (Behl et al., 2015). Furthermore, the 2 brominated components of FM 550, EH-TBB and BEH-TEBP, have been shown to induce DNA damage in the hepatic tissue of fathead minnows and increase the production of E2 in mammalian H295R cells (Bearr et al., 2010; Saunders et al., 2013). While there have been a number of studies demonstrating the potential toxicity of FM 550 components, there has been little research investigating the toxicokinetic properties of FM 550 in mammalian systems. Such information is critical to informing future toxicological research and will facilitate the risk management of this recently introduced chemical, which is prioritized for risk assessment by the EPA (U.S. Environmental Protection Agency, 2014).
This study seeks to fill the aforementioned data gap by investigating the toxicokinetic properties of FM 550, with a specific focus on gestational and lactational transfer of its components. Since lipophilic chemicals are known to partition into both breast milk and the placenta, it is possible that FM 550 is capable of both gestational and lactational transfer, yet no studies to date have examined this end point (Balakrishnan et al., 2010; Gómara et al., 2007; Shen et al., 2007). Because FM 550 is a mixture of organophosphate and brominated compounds with varying physicochemical properties, it is likely that the components exhibit differential pharmacokinetic behaviors. In order to establish a pharmacokinetic profile of FM 550 transfer during pregnancy and nursing, we sought to determine the accumulation of parent and metabolite components of FM 550 in rat fetuses and in rat pups separately, following 10 days of daily exposure to the dam.
MATERIALS AND METHODS
Materials
Animal care, maintenance, and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals” and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee (IACUC). A supervising veterinarian approved and monitored all procedures throughout the duration of the project. For each experiment, all Wistar rats were obtained from Charles River (Raleigh, North Carolina) and/or bred in house as indicated in humidity-and-temperature controlled rooms, each with a 12 h:12 h light, dark cycle at 25 °C and 45–60% average humidity at the Biological Resource Facility at NCSU. As in our prior studies (Patisaul et al., 2012, 2009), and in accordance with recommended practices for endocrine-disrupting chemicals (EDC) research (Hunt et al., 2009; Li et al., 2008; Richter et al., 2007), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles, soy-free diet, woodchip bedding, and thoroughly washed polysulfone caging.
Individual authentic standards of EH-TBB, 13C6-EH-TBB, BEH-TEBP, 13C6-BEH-TEBP, 13C18-TPHP, d15TPHP, TBBA, 13C6-TBBA, and 13C12-2,2’,3,4,5,5’-hexachlorodiphenyl ether (13C12-CDE141) were purchased from Wellington Laboratories (Guelph, Ontario, Canada). The recovery standard used for EH-TBB and BEH-TEBP in pup tissue extracts, 4’-fluoro-2,3’,4,6-tetrabromodiphenyl ether (FBDE-69), was purchased from Chiron (Trondheim, Norway). Isopropylphenyl phenyl phosphate (ip-PPP) and 13C2-DPHP were synthesized at Duke University by the Small Molecule Synthesis Facility (Durham, North Carolina) and d10-DPHP was synthesized by the Max Planck Institute for Biophysical Chemistry (Goettingen, Germany). Pyrrolidine, TPHP (99% pure), DPHP (99% pure), and 2,3,5-triiodobenzoic acid (TIBA) were purchased from Sigma-Aldrich (St. Louis, Missouri). All solvents used (methanol, ethyl acetate, hexane, dicholoromethane, and acetonitrile) were HPLC grade (EMD Millipore Corporation, Bellerica, Massachusetts).
Dosing Preparation
All animals were orally dosed using a concentrated solution prepared in the Stapleton lab that was coded before transfer to the Patisaul lab to ensure blinded dosing. A commercial mixture of FM 550 was obtained from Great Lakes Chemical (West Lafayette, Indiana) and each dosing solution was prepared by diluting the appropriate amount of FM 550 in 100% ethanol and stirring for 6 hours. Dosing solutions were stored in amber bottles at 4 °C until use. At the time of dosing, 20 µl of each dosing solution was pipetted onto ¼ of a soy-free food treat pellet (chocolate flavored AIN-76A Rodent Diet Test Tabs, Test Diet, Richmond, Indiana) as we have done previously (Patisaul, et al., 2013).
Animal Husbandry and Exposure
Both gestational and lactational exposure windows were selected to be 10 days in duration to facilitate a repeated dosing regimen and to allow for the appreciable accumulation of bioaccumulative FM 550 components.
Prenatal exposure and placental transfer
Adult Wistar rats (n = 18 females and 26 males) were obtained from Charles River and maintained on Teklad 2020 (phytoestrogen-free) diet, then paired and monitored for the presence of a sperm plug, which was designated gestational day (GD) 0. The males were then removed and the dams were housed individually. All paired females were successfully impregnated. There were 3 exposure groups: Vehicle, 300 µg (approx. 1 mg/kg) FM 550, and 1000 µg (approx. 3.3 mg/kg) FM 550 (the animals were not dosed by individual weight; rather, the average weight at the time of dosing was 300 g). Oral exposure to the pregnant dam was initiated on GD 9 and terminated on GD 18. Fetal sacrifice was chosen to occur relatively late in gestation (GD 18) but confidently before birth (∼GD 21) in order to have a reasonable amount of fetal tissue to extract (∼1 g).
Postnatal exposure and lactational transfer
Animals (n = 18 females and the same 26 males from the placental transfer experiment) were obtained, bred and reared under the same conditions as described above. Of the 18 females, 1 failed to conceive and therefore was not used in this study. On PND 0, litters were culled to 8 pups (4 males, 4 females) to ensure sufficient lactational access for each pup. Oral exposure to the dam occurred daily from PND 3–12 via food treat as described above: Vehicle, 300 µg (approx. 1 mg/kg) FM 550, and 1000 µg (approx. 3.3 mg/kg) FM 550. Because there is evidence suggesting that exposure to certain EDCs during pregnancy may cause impaired lactation in the dam, FM 550 exposure was delayed until PND 3 (Lew et al., 2009; Rudel et al., 2011; Vorderstrasse et al., 2004). To ensure consistent lactational transfer among litters and equal exposure windows, PND 12 was chosen as the time of sacrifice for the pups. In addition, PND 12 is confidently prior to natural weaning in rats and before rats start to scavenge for other food sources around PND 14. Supplementary Figure 1 provides schematic representation of dosing and sample collection.
Tissue and Urine Collection
Prenatal exposure and placental transfer
On GD 18, 4 h after final dosing, all dams and fetuses were weighed and sacrificed by CO2 asphyxiation and rapid decapitation. Dam urine and blood were collected. Trunk blood from the dams was collected on ice and spun at 4°C for 10 min at 13,000 rpm. Serum was extracted with disposable glass pipets and stored at −80°C. The head of all the fetuses was removed for a separate study, and the remaining carcass was weighed and frozen on dry ice.
Postnatal exposure and lactational transfer
On PND 12, 4 h after final dosing, dams were sacrificed as described above and pups were sacrificed by rapid decapitation without CO2. Animals were weighed prior to tissue collection. Dam and pup urine and blood were collected. From each dam, 2 whole pup carcasses of each sex, male and female, were reserved and frozen. Pup sex was verified at necropsy and only 3 of the 20 litters were found to have uneven male:female ratios (7:1, 1:7, and 5:3). All tissues were wet weighed and frozen on dry ice. Blood was collected on ice and serum was extracted as described above.
Chemical Analyses
Parent compound extraction and clean up
TPHP, mono-ITP, EH-TBB, and BEH-TEBP were analyzed in decapitated (but otherwise whole) fetuses collected from dams on GD 18 and in decapitated (but otherwise whole) rat pups on PND 12. Whole fetus or approximately 1 g of homogenized pup tissue was ground with sodium sulfate, spiked with 13C-TPHP, 13C-EH-TBB, and 13C-BEH-TEBP, and extracted using either sonication in dichloromethane (DCM) or 12 h Soxhlet treatment in DCM. Sonication and Soxhlet extractions were compared and results acquired from the 2 methods did not significantly differ. Total lipid content of the tissue was measured using gravimetric analysis of an aliquot of the extract. Purification of the fetus extracts was performed following a slightly modified version of the solid phase extraction (SPE) method described in Van den Eede et al. (2012). Briefly, 8 mL of hexane was used to elute EH-TBB and BEH-TEBP, and 10 mL of ethyl acetate was used to elute TPHP and ITPs from Supelclean™ ENVI-Florisil® SPE cartridges (6 mL, 1.0 g; Supelco, Bellefonte, Pennsylvania). Purification of the pup tissue extracts was achieved using 8 g of deactivated (2.5%) Florisil® and sequential elution with hexane and ethyl acetate. Extracts were cleaned using 2 consecutive chromatography columns. Fractions collected from purifications were concentrated under a gentle nitrogen stream.
Urinary metabolites extraction and clean up
DPHP, ip-PPP, and TBBA were analyzed in urine collected from dams at the time of sacrifice (either GD 18 or PND 12) and in urine collected from pups at the time of sacrifice (PND 12). Due to low volumes, pup urine was pooled within litters. TBBA extraction was performed according to a previously described method (Hoffman et al., 2014). Briefly, samples were spiked with 13C-TBBA and the sample pH was reduced to <2 using acetic acid. Samples were purified using SampliQ OPT SPE columns (6 mL; 150 mg; Agilent, Santa Clara, California) and TBBA was eluted with 4 mL MeOH. DPHP and ip-PPP extraction was performed according the method described in Cooper et al. (2011). Briefly, samples were spiked with dDPHP and acidified using formic acid. Samples were purified using StrataX-AW SPE columns (3 mL; 60 mg; Phenomenex, Torrance, California) and DPHP and ip-PPP were eluted with 2 mL of 5% pyrrolidine in acetonitrile. Purified urine extracts were blown down to near dryness under nitrogen, reconstituted with 0.5 mL MeOH:H2O (1:1 v/v), and transferred to a Mini-Uniprep Syringeless Filter (PTFE, 0.2 μm; Whatman/GE Healthcare, Piscataway, New Jersey) prior to analysis.
Serum extraction and clean up
TPHP, EH-TBB, and BEH-TEBP were analyzed in serum collected from dams at the time of sacrifice (either GD 18 or PND 12) and in serum collected from pups at the time of sacrifice (PND 12). Due to low volumes, pup serum was pooled within litters. Analyte extraction was performed as outlined in a previously published method (Butt et al., 2016). Briefly, samples were spiked with 13C-TPHP, 13C-EH-TBB, and 13C-BEH-TEBP and acidified with formic acid. Samples were extracted by ultrasonification, isolated using Oasis HLB columns (500 mg; 6 ml; Waters, Milford, Massachusetts) and cleaned with Sep-Pak silica columns (1 g; 6 ml; Waters, Milford, Massachusetss). Analytes were eluted from the Waters Oasis HLB columns with 50:50 dichloromethane:ethyl acetate and eluted from the silica columns using dichloromethane. Purified serum extracts were blown down to near dryness under nitrogen and reconstituted with 100 μl of hexane prior to analysis.
Instrumental analysis
Analyte quantification was performed using the following internal standards: 13C-EH-TBB for EH-TBB, 13C-BEH-TEBP for BEH-TEBP, 13C-TPHP for TPHP, 13C-TBBA for TBBA, and dDPHP for DPHP and ip-PPP. Recovery standards were used as follows: 13C-CDE-141 for EH-TBB and BEH-TEBP in whole fetuses, FBDE-69 for EH-TBB and BEH-TEBP in pup tissue, dTPHP for TPHP, TIBA for TBBA, and 13C-DPHP for DPHP and ip-PPP. EH-TBB, BEH-TEBP, TPHP, and ITPs were quantified using our previously published GC/MS methods (Stapleton et al., 2008, 2009). TBBA, DPHP, and ip-PPP were quantified using previously published LC/MS/MS methods (Butt et al., 2014; Cooper et al., 2011; Roberts et al., 2012) (See Supplementary Figs. 2–4 for representative chromatograms). Recovery of 13C-EH-TBB averaged in 96 ± 8% in fetus extracts, 87 ± 27% in pup tissue extracts and 83 ± 7% in serum extracts; recovery of 13C-BEH-TEBP averaged 92 ± 17% in fetus extracts, 94 ± 54% in pup tissue extracts, and 87 ± 11% in serum extracts; recovery of 13C-TPHP averaged 100 ± 6% in fetus extracts, 99 ± 8% in pup tissue extracts, and 90 ± 10% in serum extracts. Average recovery of dDPHP and 13C-TBBA in urine extracts was 85 ± 7% and 79 ± 24%, respectively. All sample values were blank corrected using the average blank level, and method detection limits (MDLs) were determined using 3 times the standard deviation of the average lab blanks normalized to average tissue mass or urine/serum volume extracted (Supplementary Table 1).
Statistical Analyses
Statistical analyses were performed using StatView® software (version 5.0.1; SAS Institute Inc., Cary, North Carolina) and statistical significance was set at α = 0.05. No outliers were removed from any of the data sets and data were not log-transformed for any of the analyses. Measurements that were <MDL were replaced with values below the appropriate MDL using a random number generator, as recommended by Antweiler and Taylor (2008). For analyses of parent compound accumulation in whole tissue, 6 dams (n = 6) were included in control and high dose groups for both gestational and lactational experiments, and 2 fetuses (1 male and 1 female) were analyzed from each dam. One fetus was lost during processing in the low dose gestational group, and 1 dam failed to conceive in the low dose lactational group; as a consequence, the sample size was n = 5 at the low dose for both lactational and gestational experiments. A 2-factor repeated measures ANOVA was performed with sex being considered a repeated measure and the litter as the definition of n =1. Dose (control, low, or high) and transfer route (gestational or lactational) were the 2 factors. For the serum and urine analyses, pup serum and urine was pooled among litters, and a 2-factor ANOVA was performed with dose (control, low, or high) and rat category (gestational dam, lactational dam, or lactational pup) as the 2 factors. For each rat category at low and high doses n = 4–6; n ranged from 2 to 6 depending on rat category in the control dose group due to low urine and serum volumes (see Supplementary Fig. 1 for indication of sampling deviation from study design). Upon determining significant interactions (ANOVA < 0.05), post hoc analysis was performed using Fisher’s protected least significant difference (PLSD) test.
RESULTS
Growth Parameters
At all doses tested, and for both gestational and lactational transfer, no sex differences in either accumulation of FM 550 components or their metabolites were observed. For this reason, male and females have been combined to calculate mean analyte concentrations in Table 1. In addition, the number of fetuses/pups per litter and distribution of sexes in each litter were not significantly altered as a result of FM 550 exposure. There were also no overt signs of toxicity or changes in growth for either dams or pups across dose and transfer route groups. Lipid content of the extracts did not differ between either dose group or sex and averaged 12 ± 3% for pups and 1.3 ± 0.6% for fetuses. Due to the significant difference in lipid content between pups and fetuses and because lipid content may affect the mechanism by which transfer might occur, analyte concentration results are presented as ng/g wet weight (ng/g ww) rather than on a lipid normalized basis. Body burdens (total amount in each animal in ng) are also reported to account for the difference in the volumes of distribution between pups and fetuses. Method detection limits (MDLs) for each analyte per sample type are shown in Supplementary Table 1.
TABLE 1.
Summary of Parent Compound and Metabolite Findings In Fetus/Whole Pup Tissue and Dam/Pup Urine and Serum
| Vehicle control |
Low dose |
High dose |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | ||
| Concentrations in whole fetus (GD 18) or whole pup (PND 12) homogenates (ng/g ww)* | |||||||
| TPHP | Gestational* | <MDL | – | <MDL | – | <MDL | – |
| Lactational* | <MDL | – | <MDL | – | <MDL | – | |
| ITP | Gestational* | <MDL | – | <MDL | – | <MDL | – |
| Lactational* | <MDL | – | <MDL | – | <MDL | – | |
| EH-TBB | Gestational* | <MDL | – | 1.3 | ±0.4 | 4.6 | ±0.4 |
| Lactational* | <MDL | – | 14.3 | ±1.0 | 65.1 | ±3.6 | |
| BEH-TEBP | Gestational* | <MDL | – | 0.5 | ±0.04 | 1.9 | ±0.4 |
| Lactational* | <MDL | – | <MDL | – | 0.9 | ±0.2 | |
| Concentration in urine (ng/mL) | |||||||
| DPHP | Gestational dam | <MDL | – | 2972 | ±948 | 3381 | ±694 |
| Lactational dam | <MDL | – | 2599 | ±929 | 5412 | ±1507 | |
| Pup* | <MDL | – | <MDL | – | <MDL | – | |
| ip-PPP | Gestational dam | <MDL | – | 37 | ±9 | 90 | ±18 |
| Lactational dam | <MDL | – | 34 | ±10 | 67 | ±15 | |
| Pup | <MDL | – | <MDL | – | <MDL | – | |
| TBBA | Gestational dam | <MDL | – | 385 | ±110 | 1245 | ±526 |
| Lactational dam | <MDL | – | 52 | ±23 | 223 | ±55 | |
| Pup* | <MDL | – | <MDL | – | 8.7 | ±4.9 | |
| Concentration in dam serum (ng/mL) | |||||||
| TPHP | Gestational | <MDL | – | <MDL | – | <MDL | – |
| Lactational | <MDL | – | <MDL | – | <MDL | – | |
| ITP | Gestational | <MDL | – | <MDL | – | <MDL | – |
| Lactational | <MDL | – | <MDL | – | <MDL | – | |
| EH-TBB | Gestational | <MDL | – | <MDL | – | 6.8 | ±1.7 |
| Lactational | <MDL | – | <MDL | – | 5.8 | ±1.9 | |
| BEH-TEBP | Gestational | <MDL | – | <MDL | – | 3.2 | ±0.4 |
| Lactational | <MDL | – | <MDL | – | 3.6 | ±0.7 | |
*Male and female samples were combined to calculate mean concentrations and standard errors.
Gestational Transfer
Tissue levels
To evaluate gestational transfer of FM 550, decapitated (but otherwise whole) rat fetuses collected at GD 18 were analyzed for individual components. Both EH-TBB and BEH-TEBP body burdens showed a dose-dependent increase in fetuses, demonstrating placental transfer of the 2 brominated components of FM 550. Average EH-TBB concentrations were 1.3 ± 0.1 ng/g ww and 4.6 ± 0.4 ng/g ww and average EH-TBB body burdens were 1.0 ± 0.4 ng and 5.3 ± 3.5 ng in the low and high dose groups, respectively, corresponding to 0.0013% and 0.0018% of the total dose administered to the dams (Fig. 2). Average BEH-TEBP concentrations and body burdens were 0.5 ± 0.04 ng/g ww and 0.5 ± 0.1 ng in the low dose group and 1.9 ± 0.4 ng/g ww and 1.9 ± 0.4 ng in the high dose group, corresponding to 0.0019% and 0.0024% of the total administered dose, respectively (Fig. 3). Average TPHP and ITP body burdens in the 2 dose groups did not differ significantly from controls and background levels (<3.1 ng/g ww), suggesting no accumulation (Table 1).
FIG. 2.
Lactational transfer results in greater EH-TBB concentrations and body burdens in fetus and pup tissue compared with gestational transfer. EH-TBB (ng/g wet weight) was measured in fetus tissue following daily FM 550 exposure to the dam from gestational day 9 to 18 and in pup tissue following daily FM 550 exposure to the dam from postnatal day 3 to 12 (shown in panel A). EH-TBB body burdens (ng) were calculated based on total body mass of each decapitated pup or fetus (shown in panel B). Doses are as follows: control = ethanol vehicle, low = 300 µg/day, high = 1000 µg/day. Gestational transfer is shown in solid gray and lactational transfer is shown in dashed gray. For each dose, n = 4–6 and *p <.005. The minimum detection limit for gestational transfer was 0.3 ng/g wet weight and the minimum detection limit for lactational transfer was 0.2 ng/g wet weight. No significant differences were found between males and females.
FIG. 3.
Gestational transfer results in greater BEH-TEBP concentrations but lower body burdens in fetus/pup tissue compared with lactational transfer. BEH-TEBP (ng/g wet weight) was measured in fetus tissue following daily FM 550 exposure to the dam from gestational day 9 to 18 and in pup tissue following daily FM 550 exposure to the dam from postnatal day 3 to 12 (shown in panel A). BEH-TEBP body burdens (ng) were calculated based on total body mass of each decapitated pup or fetus (shown in panel B). Doses are as follows: control = ethanol vehicle, low = 300 µg/day, high = 1000 µg/day. Gestational transfer is shown in solid gray and lactational transfer is shown in dashed gray. For each dose, n = 4–6 and *p <.005. The minimum detection limit for gestational transfer was 0.1 ng/g wet weight and the minimum detection limit for lactational transfer was 0.6 ng/g wet weight. No significant differences were found between males and females.
Urinary levels
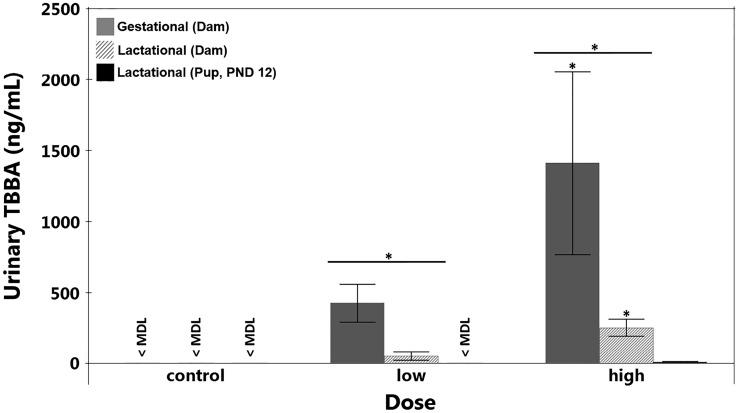
Urinary metabolites were also measured in urine collected from the dams. Average concentrations of urinary TBBA, the primary metabolite of EH-TBB, in dams dosed from GD 9 to 18 were 385 ± 110 ng/mL and 1245 ± 526 ng/mL in the low and high dose groups, respectively (Fig. 4). DPHP and ip-PPP were detected in the urine of gestationally exposed dams and displayed a dose-dependent response (Fig. 5). Average DPHP concentrations were 2972 ± 948 ng/mL in the low dose group and 3381 ± 694 ng/mL in the high dose group, whereas average ip-PPP concentrations were 37 ± 9 ng/mL in the low dose group and 90 ± 18 ng/mL in the high dose group (Table 1).
FIG. 4.
Dams dosed with FM 550 during gestation show higher levels of urinary TBBA compared with dams dosed during lactation. TBBA (ng/mL) was measured in dam urine following daily FM 550 exposure from gestational day 9 to 18 and in dam and pup urine following daily FM 550 exposure to the dam from postnatal day 3 to 12. Doses are as follows: control = ethanol vehicle, low = 300 µg/day, high = 1000 µg/day. Dams dosed during gestation are shown in solid gray, dams dosed during lactation are shown in dashed gray, and pups at PND 12 are shown in black. For each dose, n = 2–6 and *p <.05. The minimum detection limits for dams dosed during gestation or lactation were 9.6 ng/mL, and minimum 2.4 ng/mL for pups at PND 12. TBBA was measured in pup urine at levels higher than the MDL only at the high dose.
FIG. 5.
Organophosphate metabolites of FM 550 components measured in dam urine show a dose response. DPHP and ip-PPP (ng/mL) were measured in dam urine following daily FM 550 exposure from gestational day 9 to 18 and in dam and pup urine following daily FM 550 exposure to the dam from postnatal day 3 to 12. Doses are as follows: control = ethanol vehicle, low = 300 µg/day, high = 1000 µg/day. For each dose, n = 3–6 and *p <.05. For DPHP, the minimum detection limit was 25.6 ng/mL in dam urine and 6.4 ng/mL for pup urine. For ip-PPP, the minimum detection limit was 17.1 ng/mL in dam urine and 4.3 ng/mL in pup urine. Neither DPHP nor ip-PPP were detected above MDL in pup urine.
Serum levels
TPHP and ITPs were not detected above MDL levels in the serum of gestating dams (<5.4 ng/mL) in any of the dose groups. EH-TBB was not detected in serum of gestating dams in the low dose group (<1.8 ng/mL), but was detected in serum of gestating dams in the high dose group at an average concentration of 6.8 ± 1.7 ng/mL. Similarly, BEH-TEBP was not detected in the serum of low dose gestating dams (<0.9 ng/mL), but was detected at average concentrations of 3.2 ± 0.4 ng/mL in the serum of high dose gestating dams (Fig. 6).
FIG. 6.
EH-TBB and BEH-TEBP detected in dam serum at the high dose. TPHP, ITPs, EH-TBB, and BEH-TEBP (ng/mL) were measured in dam serum following daily FM 550 exposure from gestational day 9 to 18, and in dam and pup serum following daily FM 550 exposure to the dam from postnatal day 3 to 12. Doses are as follows: control = ethanol vehicle, low = 300 µg/day, high = 1000 µg/day. TBB is shown in solid gray and BEH-TEBP is shown in dashed gray. None of the compounds were detected above MDL levels in pup serum, and TPHP and ITPs were not detected above MDL levels in any of the tested serum (data not shown). Measured serum levels did not differ statistically between gestating and lactating dams, so the 2 groups have been combined in this plot. For each dose, n = 2–6 and *p <.05. The minimum detection limits are as follows: 5.4 ng/mL for TPHP and ITPs, 1.8 ng/mL for EH- TBB, and 0.9 ng/mL for BEH-TEBP.
Lactational Transfer
Tissue levels
To evaluate lactational transfer of FM 550, decapitated (but otherwise whole) rat pups collected at PND 12 were analyzed for individual components. Both EH-TBB and BEH-TEBP accumulated in whole pup tissue, displaying lactational transfer. Average EH-TBB concentrations and body burdens in whole pup tissues showed a dose-dependent increase in tissues, and were 14.3 ± 1.0 ng/g ww and 343.2 ± 84.9 ng in the low dose group and 65.1 ± 3.6 ng/g ww and 1587.4 ± 334.9 ng in the high dose group respectively (Fig. 2), corresponding to 0.39% and 0.54% of the total administered dose. BEH-TEBP was not detected in whole tissue of pups in the low dose group (<0.6 ng/g ww), but was detected in whole pup homogenates in the high dose group at an average concentration of 0.9 ± 0.2 ng/g ww and an average body burden of 27.9 ± 8.0 ng (Fig. 3) corresponding to 0.026% of the total administered dose.
Urinary levels
Average TBBA concentrations in the urine of dams dosed during lactation were 52 ± 23 ng/mL and 223 ± 55 ng/mL in the low and high dose groups, respectively (Fig. 4). TBBA levels in pooled pup urine from the low dose group were <2.4 ng/mL (Table 1), but were detected and measured at 8.7 ± 4.9 ng/mL in the high dose group, corresponding to 0.0050% of the total administered dose (Fig. 4). While there was no TPHP or ITP accumulation in whole pup tissues in any of the treatments (<2.2 ng/g ww), DPHP and ip-PPP, their respective metabolites, were detected in the dam urine and displayed a dose-dependent concentration (Fig. 5). Average DPHP concentrations were 2599 ± 929 ng/mL in the low dose group and 5412 ± 1507 ng/mL in the high dose group, whereas average ip-PPP concentrations were 34 ± 10 ng/mL in the low dose group and 67 ± 15 ng/mL in the high dose group (Table 1). DPHP and ip-PPP were not detected in pup urine (<6.4 and 4.3 ng/mL, respectively).
Serum levels
TPHP and ITPs were not detected above MDL levels in the serum of lactating dams (<5.4 ng/mL) across all dose groups. EH-TBB was not detected in serum of gestating dams in the low dose group (<1.8 ng/mL), but was detected in serum of gestating dams in the high dose group at an average concentration of 5.8 ± 1.9 ng/mL. Similarly, BEH-TEBP was not detected in the serum of low dose gestating dams (<0.9 ng/mL), but was detected at average concentrations of 3.6 ± 0.7 ng/mL in the serum of high dose gestating dams (Fig. 6).
DISCUSSION
As FM 550 is a current and common-use flame retardant mixture found in residential furniture, with ubiquitous exposure in most US home environments, it is important to evaluate the potential accumulation and metabolism of these chemicals, particularly during a vulnerable developmental period. This study sought to provide important pharmacokinetic data on this flame retardant mixture. Our data demonstrate that the components of FM 550 can be transferred to the developing fetus both throughout gestation and during lactation postnatally. The differences in the transfer routes and metabolism observed highlight the importance of understanding the effects of these chemicals on development.
Organophosphate Components
TPHP and ITPs were not detected in either whole fetus or pup tissue, indicating they did not undergo either gestational or lactational transfer (Table 1). This is not surprising as TPHP was shown to be readily metabolized by rat and human liver microsomes (Van den Eede et al., 2013a ; Sasaki et al., 1984). These compounds were also not detected in any of the serum samples tested above MDL levels (<5.4 ng/mL), although serum analysis was limited by low volumes (∼250 μl). Supporting rapid metabolism of both TPHP and ITPs by the dam, DPHP, and ip-PPP were detected and quantified in a dose-dependent manner in urine from both gestationally and lactationally dosed dams. Although our study indicates that TPHP does not undergo gestational or lactational transfer in rats, a study involving paired herring gulls and their eggs demonstrated that TPHP can undergo in ovo transfer (Greaves and Letcher, 2014). In addition, other studies have detected TPHP in human breast milk at low levels (median TPHP concentration = 0.28 ng/g ww, which is well below the MDL for TPHP in this study) (Kim et al., 2014; Sundkvist et al., 2010). While measured in other biological samples (Butt et al., 2014; Hoffman et al., 2015), this was the first study to the authors’ knowledge to confirm ip-PPP as a urinary metabolite of ITPs in a controlled dosing experiment. This establishes ip-PPP as a useful biomarker for exposure to ITPs in human populations. It is interesting to note that the formulation for FM 550 is approximately 18% TPHP and 32% mono-ITP, and yet a disproportionately small amount of ip-PPP was measured in the rat urine compared with DPHP (Fig. 5) (Klosterhaus et al., 2009). This discrepancy may suggest that some of the ITP isomers may be metabolized to both ip-PPP and DPHP, or that the metabolism rates for TPHP and ITPs differ. In addition, it is possible that the major elimination pathway for ip-PPP is feces rather than urine. Metabolites of tricresyl phosphate were found to be mostly excreted in the feces in a number of different animal studies (WHO, 1990, EHC 110). Similarly, TPP, ITPs and their metabolites may also be excreted in the feces, although this was not measured in the present study.
Brominated Components
EH-TBB
Our study showed that EH-TBB accumulates in tissue to a greater extent via lactational transfer than during gestational transfer (Fig. 2). EH-TBB concentrations were 11–14 times greater and EH-TBB body burdens were 200–300 times greater in whole pup tissue compared with whole fetuses in both low and high dose groups. It is generally accepted that most xenobiotics enter breast milk through passive diffusion from the plasma, and their concentration in the milk depends on their lipophilicity, molecular size, maternal plasma level, protein binding in maternal circulation, half-life, and degree of ionization (Agatonovic-Kustrin et al., 2002). Like EH-TBB (logKOW = 7.73), 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), p,p′-dichlorodiphenyldichloroethylene (p,p’-DDE), and hexachlorobenzene (HCB) have logKOW values near 7 and have been shown to be transferred to offspring almost exclusively through milk, rather than by placental transfer (Lyche et al., 2004; Nakashima et al., 1997; You et al., 1999). This can be accounted for by the mobilization of the maternal depots of lipophilic compounds during milk production. Gallenberg and Vodicnik (1987) showed that very low density lipoproteins (VLDLs) provide molecular binding sites for lipophilic compounds. VLDL are composed of cholesterol, triglycerides, and proteins, and enable movement of lipids in the bloodstream (Iverson et al., 1995). Shortly before parturition and throughout lactation, VLDLs are directed to the mammary gland for milk production. Also during this stage of pregnancy the activity of lipoprotein lipase, and enzyme that hydrolyzes VLDL to supply free fatty acids necessary for milk production, is drastically reduced in adipose and greatly enhanced in mammary tissue (Hamosh et al., 1970). Importantly, there is evidence to suggest the involvement of VLDLs in the redistribution kinetics of 2,4,5,2’,4’,5’-hexachlorobiphenyl (PCB-153) (Mühlebach et al., 1991). Low density lipoproteins have been found to bind PCB-153 at 30 non-interactive binding sites, with a binding constant K = 2.7 × 105 and a dissociation constant Kd = 7.2 × 10−6 M −1 (Becker and Gamble, 1982). The partition coefficient of PCB-153 in a phosphate buffer solution designed to mimic serum for human VLDL was measured to be 1.2 × 106 M−1 (Maliwal and Guthrie, 1982). It has been shown in rats that serum VLDL concentrations change over the course of pregnancy, increasing during the third trimester and decreasing following lactation (Spindler-Vomachka and Vodicnik, 1984). Spindler-Vomachka and Vodicnik (1984) also demonstrated that PCB1-53 is preferentially associated with VLDL during pregnancy and lactation in comparison to virgin females (PCB-153 levels found in the VLDL of dams at GD 18 were 3-fold higher compared with the levels found in virgin rats.) By passive diffusion and by binding VLDL, lipophilic chemicals including EH-TBB can partition into milk and later be transferred from dam to pup during nursing. Interestingly, milk composition varies over the course of lactation, and uptake of lipophilic chemicals is proportional to the fat content of the milk (Clewell and Gearhart, 2002). As such, lactational transfer of EH-TBB is likely to vary at different times during lactation corresponding to milk fat content fluctuations. The relatively low levels of gestational transfer of EH-TBB might also be explained by the presence of a placental lipid gradient during pregnancy. The total lipid concentration in human maternal venous plasma has been measured as about 4 times that of umbilical plasma at term (Parker et al., 1983). This lipid gradient may inhibit the distribution of EH-TBB across the placenta during pregnancy. Whereas EH-TBB levels differed between whole fetus and pup homogenates, EH-TBB serum concentrations in gestating and lactating dams 4 h following the final dosing did not differ significantly (Figs. 2 and 6).
BEH-TEBP
BEH-TEBP concentrations were slightly higher in fetuses, but BEH-TEBP body burdens were higher in pups (Fig. 3). Whereas BEH-TEBP body burdens were approximately 13–14 times greater in pups compared with fetuses in the high dose group, they were comparatively lower than EH-TBB body burdens observed in pups. Schecter et al. (2010) and Wolff (1983) showed that higher molecular weight compounds exhibit lesser blood to milk partitioning than lower molecular weight compounds of the same class. Lesser blood to milk partitioning of BEH-TEBP, which has a higher molecular weight and higher estimated logKOW compared with EH-TBB (706.14 Da and 11.95 compared to 549.92 Da and 7.73), could account for lower observed levels of BEH-TEBP tissue accumulation resulting from lactational transfer (U.S. Environmental Protection Agency, 2012). Similarly, Zhou et al. (2014) observed lesser serum to breast milk partitioning of BEH-TEBP compared with EH-TBB in humans. BEH-TEBP was only detected in serum above MDL levels in dams at the high dose, also supporting a low absorption potential (Fig. 6).
Brominated Metabolites
Urinary TBBA concentrations were 5–7 times higher in dams dosed during gestation compared with dams dosed during lactation in both low and high dose groups (Fig. 4). This suggests that greater amounts of EH-TBB are cleared from dams via urine during pregnancy than during lactation, owing perhaps to fewer VLDL binding sites in gestational stages of pregnancy or to the partitioning of TBBA from plasma to milk. Breast milk has a slightly lower pH (7.08) compared to maternal plasma (7.42), so this pH gradient facilitates the partitioning of weak bases from plasma to milk (Clewell and Gearhart, 2002). Because the pKa of TBBA has been estimated to be 2.3, TBBA would be almost entirely deprotonated in both milk and plasma (Fang and Stapleton, 2014). Since TBBA is mostly in its charged form, partitioning from plasma to milk is not a likely explanation for the lower levels observed in the urine of lactating dams. Alternatively, lower levels of TBBA found in the urine of lactating dams could be a result of simple dilution, as both gestating and lactating dams were allowed water ad libitum. In fact, there is evidence that lactating Sprague Dawley rats drink, on average, more water than their gestating counterparts (Cao et al., 2015; Slone et al., 2012). This is also supported by the high variability in urinary TBBA levels, indicating differential water intake (Fig. 4). While this “dilution effect” was not observed for DPHP and ip-PPP, it is possible these compounds are passively excreted with urinary concentrations independent of urine flow, whereas TBBA could be actively excreted with its urinary concentration dependent on urine flow (Boeniger et al., 1993). In this way, we might see differences in urinary levels of TBBA between gestating and lactating rats, but not in urinary levels of DPHP and ip-PPP as reflected in our results. Multidrug resistance-associated protein (MRP) transporters and p-glycoprotein have been found to facilitate active xenobiotic elimination (Hodgson, 2012). Specifically, endosulfan has been shown to exhibit p-glycoprotein-mediated efflux in cell culture experiments (Bain and LeBlanc, 1996). Bain and LeBlanc (1996) also showed in their experiments that lipophilicity and molecular mass were major determinants of p-glycoprotein binding, with optimum binding occurring with compounds that had a log Kow between 3.6 and 4.5 and a molecular weight between 391 and 490 Da. Incidentally, TBBA has a log Kow of approximately 4.28 (predicted ACD/Labs) and a molecular weight of 437.7 Da and could potentially bind p-glycoprotein for active efflux.
Although we did not evaluate TBMEHP in this study, it has been measured at much lower levels (∼90× lower) than TBBA in the urine of rats dosed with Uniplex FPR-45, a commercial flame retardant containing >95% BEH-TEBP and <5% EH-TBB (Silva et al., 2015). Because FM 550 is approximately 30% EH-TBB and 9% BEH-TEBP by formula, we predict that TBMEHP would be present at increasingly lower levels than TBBA in rat urine, potentially below method detection limits. It is also likely that a majority of BEH-TEBP is excreted in the feces. Knudsen et al. (2016) found that after orally administering 0.071 or 7.1 mg/kg of radioactively labeled BEH-TEBP to female Sprague Dawley rats, 92–98% was detected unchanged in the feces.
Study Limitations
While this study provided some novel insights into the gestational and lactational transfer of FM 550 components, it is not without its limitations. As mentioned previously, some FM 550 components and their metabolites may be excreted predominantly in the feces; however, our study did not investigate fecal elimination. Another limit of the current study is that urine was not collected at periodic time intervals, prohibiting calculations of average excretion rates for each FM 550 component. In addition, limited mobilization from adipose and other tissues in the dam might contribute to the observed differences in transfer of FM 550 components to the pups, although our study did not analyze specific dam tissues. For instance, Patisaul et al. (2013) observed TBB accumulation in adipose tissue, liver, and muscle of dams following perinatal dosing.
Implications
The current study provides important information about FM 550 body burdens in fetuses and pups following 10 days of exposure to the dam at different developmental windows. Our dosing method mimics the predicted main exposure route for brominated flame retardants—dietary ingestion, which likely occurs through either chronic hand-to-mouth contact or inadvertent ingestion of household dust (Johnson et al., 2010; Stapleton et al., 2012a,b). Prior to this study, experiments concerning FM 550 have focused primarily on human exposure and toxic endpoints. This study is one of the first to assess the pharmacokinetic properties of the components of FM 550 and contributes novel and important data regarding this commercial mixture, which can help support human exposure studies and risk assessments. These data can also inform physiologically based pharmacokinetic (PBPK) models for semi-volatile organic compounds (SVOCs). Future work should focus on more detailed pharmacokinetic endpoints such as the tissue distribution of FM 550 components and metabolites, mechanisms involved in metabolism and excretion, and the formation of ip-PPP from ITP metabolism in real time.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors are grateful to Sheryl Arambula and Meghan Rebuli for assisting with tissue collection, and the staff of the NCSU Biological Resource Facility for overseeing animal care and husbandry. FM 550 was provided by Chemtura.
FUNDING
National Institutes of Health (IR56ES022957 to H.B.P. and H.M.S); National Institute of Environmental Health Sciences (T32-ES021432, T32-ES007046); North Carolina State University Center for Human Health and the Environment (P30ES025128 to H.B.P.).
REFERENCES
- Agatonovic-Kustrin S., et al. (2002). Molecular descriptors that influence the amount of drugs transfer into human breast milk. J. Pharm. Biomed. Anal. 29, 103–119. [DOI] [PubMed] [Google Scholar]
- Ali N., et al. (2011). “Novel” brominated flame retardants in Belgian and UK indoor dust: Implications for human exposure. Chemosphere 83, 1360–1365. [DOI] [PubMed] [Google Scholar]
- Ali N., et al. (2012). Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere 88, 1276–1282. [DOI] [PubMed] [Google Scholar]
- Al-Omran L. S., Harrad S. (2015). Polybrominated diphenyl ethers and “novel” brominated flame retardants in floor and elevated surface house dust from Iraq: Implications for human exposure assessment. Emerg. Contam. 2, 7–13. [Google Scholar]
- Antweiler R. C., Taylor H. E. (2008). Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environ. Sci. Technol. 42, 3732–3738. [DOI] [PubMed] [Google Scholar]
- Bain L. J., LeBlanc G. A. (1996). Interaction of structurally diverse pesticides with thehuman MDR1 gene product P-glycoprotein. Toxicology and Applied Pharmacology. 141(1), 288–298. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B., et al. (2010). Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 202, 393.e1–e7. [DOI] [PubMed] [Google Scholar]
- Bearr J. S., et al. (2010). Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environ. Toxicol. Chem. 29, 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. M., Gamble W. (1982). Determination of the binding of 2,4,5,2’,4',5'-hexachlorobiphenyl by low density lipoprotein and bovine serum albumin. J. Toxicol. Environ. Health 9, 225–234. [DOI] [PubMed] [Google Scholar]
- Behl M., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Boeniger M. F., Lowry L. K., Rosenberg J. (1993). Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association Journal. 54, 615–627. [DOI] [PubMed] [Google Scholar]
- Butt C. M., et al. (2014). Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Butt C. M., et al. (2016). Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal. Bioanal. Chem. 408, 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., et al. (2015). Soy but not bisphenol A (BPA) or the phytoestrogen genistin alters developmental weight gain and food intake in pregnant rats and their offspring. Reprod. Toxicol. 58, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell R. a., Gearhart J. M. (2002). Pharmacokinetics of toxic chemicals in breast milk: Use of PBPK models to predict infant exposure. Environ. Health Perspect. 110, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E. M., et al. (2011). Analysis of the flame retardant metabolites bis (1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E., et al. (2014). Urinary biomonitoring of phosphate flame retardants: Levels in california adults and recommendations for future studies. Environ. Sci. Technol. 48, 13625–13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., et al. (2015). Characterizing the peroxisome proliferator-activated receptor (PPARγ) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ. Health Perspect. 123, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Stapleton H. M. (2014). Evaluating the bioaccessibility of flame retardants in house dust using an in vitro tenax bead-assisted sorptive physiologically based method. Environ. Sci. Technol. 48, 13323–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenberg L. A., Vodicnik M. J. (1987). Potential mechanisms for redistribution of polychlorinated biphenyls during pregnancy and lactation. Xenobiotica 1717, 299–310. [DOI] [PubMed] [Google Scholar]
- Gómara B., et al. (2007). Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ. Sci. Technol. 41, 6961–6968. [DOI] [PubMed] [Google Scholar]
- Greaves A. K., Letcher R. J. (2014). Comparative body compartment composition and in ovo transfer of organophosphate flame retardants in North American Great Lakes herring gulls. Environ. Sci. Technol. 48, 7942–7950. [DOI] [PubMed] [Google Scholar]
- Hodgson E. (2012). Introduction to Pesticide Biotransformation and Disposition. Pesticide Biotransformation and Disposition. 1–3. [Google Scholar]
- Hamosh M., et al. (1970). Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride pregnant and lactating rats. Biochim. Biophys. Acta 210, 473–482. [DOI] [PubMed] [Google Scholar]
- Hoffman K., et al. (2014). Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ. Health Perspect. 122, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., et al. (2015). High exposure to organophosphate flame retardants in infants: Associations with baby products. Environ. Sci. Technol. 49, 14554–14559. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., et al. (2009). The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 81, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson S. J., et al. (1995). Lipoprotein lipase activity and its relationship to high milk fat transfer during lactation in grey seals. J. Comp. Physiol. B. 165, 384–395. [DOI] [PubMed] [Google Scholar]
- Johnson P. I., et al. (2010). Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ. Sci. Technol. 44, 5627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., et al. (2014). Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116, 91–97. [DOI] [PubMed] [Google Scholar]
- Kim S., et al. (2015). Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat. Toxicol. 160, 188–196. [DOI] [PubMed] [Google Scholar]
- Klosterhaus S., et al. (2009) Characterization of organophosphorous chemicals in a PentaBDE replacement mixture and their detection in biosolids. Poster presentation, 11th Annual Workshop on Brominated Flame Retardants, Ottawa, Ontario, Canada.
- Knudsen G. A., Sanders J. M., Birnbaum L. S. (2016). Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: Effects of dose, route and repeated administration. Xenobiotica 1–10. doi:10.1080/00498254.2016.1174793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. C. W., et al. (2009). Temporal trends of hexabromocyclododecanes (HBCDs) and polybrominated diphenyl ethers (PBDEs) and detection of two novel flame retardants in marine mammals from Hong Kong, South China. Environ. Sci. Technol. 43, 6944–6949. [DOI] [PubMed] [Google Scholar]
- Lew B. J., et al. (2009). Activation of the aryl hydrocarbon receptor during different critical windows in pregnancy alters mammary epithelial cell proliferation and differentiation. Toxicol. Sci. 111, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. A., et al. (2008). Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology 29, 504–519. [DOI] [PubMed] [Google Scholar]
- Liu L. Y., et al. (2015). Analysis of polybrominated diphenyl ethers and emerging halogenated and organophosphate flame retardants in human hair and nails. J. Chromatogr. A 1406, 251–257. [DOI] [PubMed] [Google Scholar]
- Lyche J. L., et al. (2004). Levels of PCB 126 and PCB 153 in plasma and tissues in goats exposed during gestation and lactation. Chemosphere 55, 621–629. [DOI] [PubMed] [Google Scholar]
- Maliwal B. P., Guthrie F. E. (1982). In vitro uptake and transfer of chlorinated hydrocarbons among human lipoproteins. J. Lipid Res. 23, 474–479. [PubMed] [Google Scholar]
- Mühlebach S., et al. (1991). The use of 2,4,5,2’,4',5'-hexachlorobiphenyl (6-CB) as an unmetabolizable lipophilic model compound. Pharmacol. Toxicol. 69, 410–415. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., et al. (1997). Hexachlorobenzene accumulated by dams during pregnancy is transferred to suckling rats during early lactation. J. Nutr. 127, 648–654. [DOI] [PubMed] [Google Scholar]
- Parker C. R., et al. (1983). Analysis of the potential for transfer of lipoprotein-cholesterol across the human placenta. Early Hum. Dev. 8, 289–295. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., et al. (2013). Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J. Biochem. Mol. Toxicol. 27, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., et al. (2012). Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One 7, e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., et al. (2009). Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai H. K., et al. (2014). Ligand binding and activation of PPARγ by Firemaster 550: Effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 122, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., et al. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. C., et al. (2012). In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem. Res. Toxicol. 25, 1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A., et al. (2011). Environmental exposures and mammary gland development: State of the science, public health implications, and research recommendations. Environ. Health Perspect. 119, 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamova A., et al. (2014). Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site. Environ. Sci. Technol. 48, 6133–6140. [DOI] [PubMed] [Google Scholar]
- Sasaki K., et al. (1984). Metabolism of phosphoric acid triesters by rat liver homogenate. Bull. Environ. Contam. Toxicol. 33, 281–288. [DOI] [PubMed] [Google Scholar]
- Saunders D. M. V., et al. (2013). In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol. Lett. 223, 252–259. [DOI] [PubMed] [Google Scholar]
- Schecter A., Colacino J., Sjödin A., Needham L., Birnbaum L. (2010). Partitioning of polybrominated diphenyl ethers (PBDEs) in serum and milk from the same mothers. Chemosphere 78, 1279–1284. [DOI] [PubMed] [Google Scholar]
- Shen H., et al. (2007). From mother to child: Investigation of prenatal and postnatal exposure to persistent bioaccumulating toxicants using breast milk and placenta biomonitoring. Chemosphere 67, S256–S262. [DOI] [PubMed] [Google Scholar]
- Silva M. J., et al. (2015). Quantification of tetrabromo benzoic acid and tetrabromo phthalic acid in rats exposed to the flame retardant Uniplex FPR-45. Arch. Toxicol. 90, 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone E. J., et al. (2012). Estimation of individual rodent water consumption from group consumption data for gestation, lactation, and postweaning life stages using linear regression models. Ilar J. 53, E99–112. [DOI] [PubMed] [Google Scholar]
- Spindler-Vomachka M., Vodicnik M. J. (1984). Distribution of 2,4,5,2’,4',5'-hexachlorobiphenyl among lipoproteins during pregnancy and lactation in the rat. J. Pharmacol. Exp. Ther. 230, 263–268. [PubMed] [Google Scholar]
- Stapleton H. M., et al. (2008). Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 42, 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., et al. (2009). Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., et al. (2012a). Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., et al. (2012b). Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 120, 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist A. M., et al. (2010). Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 12, 943–951. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Estimation Program Interface (EPI) Suite. Ver. 4.1. Nov, 2012. Available at http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm. Accessed November 18, 2014.
- U.S. Environmental Protection Agency. TSCA Work Plan for Chemical Assessments: 2014 Update. Available at http://www.epa.gov/sites/production/files/2015-01/documents/tsca_work_plan_chemicals_2014_update-final.pdf. Accessed February 8, 2016.
- Van den Eede N., et al. (2014). Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 74, 1–8. [DOI] [PubMed] [Google Scholar]
- Van den Eede N., Dirtu A. C., Ali N., Neels H., Covaci A. (2012). Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 89, 292–300. [DOI] [PubMed] [Google Scholar]
- Van den Eede N., Maho W., et al. (2013a). First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 223, 9–15. [DOI] [PubMed] [Google Scholar]
- Van den Eede N., Neels H., et al. (2013b). Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 1303, 48–53. [DOI] [PubMed] [Google Scholar]
- Van Esch G. J., et al. (1997). Flame retardants: A general introduction. Environmental Health Criteria, Vol. 192. IPCS, WHO, Geneva.
- Vorderstrasse B. A., et al. (2004). A novel effect of dioxin: Exposure during pregnancy severely impairs mammary gland differentiation. Toxicol. Sci. 78, 248–257. [DOI] [PubMed] [Google Scholar]
- Wolff M. S. (1983). Occupationally derived chemicals in breast milk. Am. J. Ind. Med. 4, 259–281. [PubMed] [Google Scholar]
- World Health Organization. (1990). United Nations Environment Programme., International Labour Organisation., World Health Organization., & International Program on Chemical Safety. Tricresyl phosphate, p. 122.
- You L., et al. (1999). Transplacental and lactational transfer of p,p′-DDE in Sprague–Dawley rats. Toxicol. Appl. Pharmacol. 157, 134–144. [DOI] [PubMed] [Google Scholar]
- Zhu B., et al. (2014). Changes of accumulation profiles from PBDEs to brominated and chlorinated alternatives in marine mammals from the South China Sea. Environ. Int. 66, 65–70. [DOI] [PubMed] [Google Scholar]
- Zhou S. N., et al. (2014). Measurements of selected brominated flame retardants in nursing women: Implications for human exposure. Environ. Sci. Technol. 48, 8873–8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.