Abstract
Kinesin is a motor protein that processively moves step by step along a microtubule. To investigate the effects of temperature on run length, i.e., processivity of kinesin motility, we performed a single-molecular bead assay at temperature range of 20–40°C. An increase in the walking velocity of kinesin corresponded to the Arrhenius activation enthalpy of 48 kJ/mol, being consistent with the previous reports. Here, we found that the run length increased, that is, the kinesin processivity enhanced with increasing temperature. Then, we estimated the probability of detachment of kinesin from a microtubule per one 8-nm stepping event, and found that it diminishes from 0.014 to 0.006/step with increasing temperature from 20 to 40°C. And we noticed that prolonged incubation at 30, 35 and 40°C significantly slowed down the walking velocity, but further increased the run length and duration. Those results are interpreted according to the effect of temperature on the rate constants of some key kinetic steps in the ATPase cycle.
Keywords: TIRF, molecular motors, microtubule, hand-over-hand mechanism, run length
Conventional kinesin is a microtubule-based motor protein that transports vesicles and organelles within nerve cells. An important property of kinesin is processivity, that is, individual motor molecules can move continuously along a microtubule with hundreds of 8-nm steps1,2 for about 1 μm without dissociating3–5. Processivity is attributable to the synchronization of attachment to and detachment from a microtubule of two heads of kinesin coupled with ATPase cycle6. Recent studies have focused on examining the coupling between each step of movement and the ATPase cycle6–11. Despite motility has been extensively studied from various aspects by single-molecule methods11–13, the molecular mechanism of processivity has not yet been fully explored, although a consensus on the hand-over-hand model has been reached2.
The interaction between kinesin and microtubule is regulated not only by the nucleotide state of kinesin, but also by solvent conditions and environment. For example, an increase in salt concentration (ionic strength) lowers the processivity14,15. An increase in temperature increases the walking velocity16,18, although the maximum tension (stall force) is independent of temperature16. Here, to investigate the effect of temperature on the processivity, we performed a single-molecular bead assay at temperature range of 20–40°C. In the previous report16, we briefly described qualitative results obtained at 15–35°C showing the enhancement of processivity at higher temperature.
Here, a single native kinesin molecule purified from bovine brain was attached to a polystyrene bead, and the bead movement on microtubules was examined with total internal reflection fluorescence microscope. We measured the distance over which kinesin molecules continue to move along a microtubule without detachment (run length), the period of time for each run (duration) and the average velocity obtained from the slope of the time course of bead displacement (walking velocity). The quantitative analysis of the results showed that the probability of detachment of kinesin from a microtubule decreases, implying the enhancement of processivity, with increasing temperature. We also noticed that the processivity of kinesin is enhanced as the incubation time becomes longer, especially at higher temperatures.
Materials and methods
Proteins
Conventional kinesin was prepared from bovine brain according to the method of Kojima et al19. Tubulin was purified from porcine brain and labeled with tetramethylrhodamine succinimidyl ester (C-1171, Molecular Probes, USA) according to Hyman20. Fresh bovine and porcine brains were purchased from a local slaughterhouse. Microtubules were stabilized with 20 μM taxol.
Preparation of kinesin-bound beads
Kinesin-bound beads were prepared according to Kojima et al.19 with slight modifications. We used fluorescent poly-styrene beads (0.2 μm in diameter, carboxylate-modified, orange, F-8809; Molecular Probes). For the preparation of kinesin-bound beads, kinesin was mixed with beads at a 1:1 molar ratio, assuming the molecular weight of kinesin is 380 kDa. By using optical tweezers, we confirmed that even when mixed at a 1:5 (beads:kinesin) ratio, more than 90% of the beads that interact with a microtubule have single kinesin molecules bound7,8,21–23.
Flow cell for beads assay
The fluorescent microtubules were introduced into a flow cell and incubated for 2 min to allow binding of the micro-tubule to glass surface. The cell was washed with a solution containing 0.8 mM MgCl2, 64 mM PIPES (piperazine-1,4-bis(2-ethanesulfonic acid), pH 6.8), 0.8 mM EGTA, 1 mg/ml filtered casein and 20 μM taxol to remove unattached microtubules and left for 2 min to coat the glass surface with casein. The cell was then filled with an assay solution containing the kinesin-bound beads and an oxygen scavenging enzyme system [approximately 0.1 nM kinesin-bound beads, 1.4 mM MgCl2, 56 mM PIPES (pH 6.8), 0.7 mM EGTA, 1.0 mg/ml filtered casein, 1 mM ATP, 40 μM taxol, 10 mM dithiothreitol (DTT), 4.5 mg/ml glucose, 50 units/ml glucose oxidase, 50 units/ml catalase] and sealed with enamel. The above procedure was performed at room temperature regardless of the temperature examined. All the chemicals were of reagent grade.
Microscope
Tetramethylrhodamine-labeled microtubules and kinesin-bound fluorescent beads were visualized by total internal reflection fluorescence microscopy (IX70, Olympus, Japan) with a green laser (μGreen #4301-050, Uniphase, USA), which illuminates only ∼150 nm in depth above the cover-slip24. Fluorescence images were collected using an SIT camera (C2400, Hamamatsu Photonics, Japan) equipped with an image intensifier (VS4-1815, Video Scope, USA) and recorded on a Digital Videocassette Recorder (DSR-20, Sony, Japan).
Temperature control
For experiments at 20 and 25°C, the room temperature was adjusted by an air conditioner. For experiments at 30, 35, and 40°C, the stage of the inverted microscope with a flow cell mounted on it was covered by a thermal insulation chamber (Olympus), and the temperature inside it was adjusted. The temperature was measured by a thermometer mounted on the microscope stage near the flow cell and kept within ±1°C of the required temperature.
Analysis of the processive movement of a kinesin-bound bead
The method of movement analysis employed in this article was different from the previous one16, where a kinesin-bound bead had been manipulated and placed in contact with a microtubule by optical tweezers, which allowed to determining exactly the share of beads with bound kinesin molecules. Here we examined the kinesin-bound beads that spontaneously attached to and moved along a microtubule and then dissociated after a while (Supplementary Movies). As evident from the movies, the proportion of beads that interacted with a microtubule was small. It is still possible the beads with several kinesin molecules bound might have been included in our analysis. However, considering the bead-kinesin geometry, the probability of multiple kinesin molecules simultaneously interacting with a microtubule is sufficiently low under our experimental conditions.
Fluorescence images of moving beads were digitally recorded at a video rate (30 frames/s), and the center of the fluorescence intensity distribution of the bead image was determined using a custom-written software program supplied with the Halcon image processor (MVTec Software GmbH, Germany). From the analysis of a bead movement along a microtubule we obtained the three parameters characterizing kinesin motility at each temperature: the run length (distance travelled by a bead after attaching to and before detaching from a microtubule; in μm), the duration (time interval of the continuous movement of the bead along a microtubule; in seconds), and the velocity (the average slope of the time course of bead movement, which was determined by the least squares method; in μm/s). The recording of video started after incubating the flow cell at each temperature for 1 min, and continued for 10 min. Note that the flow cell was prepared at room temperature just before each experiment.
Results and Discussion
Effect of temperature on the time course of movement of a kinesin-bound bead along a microtubule
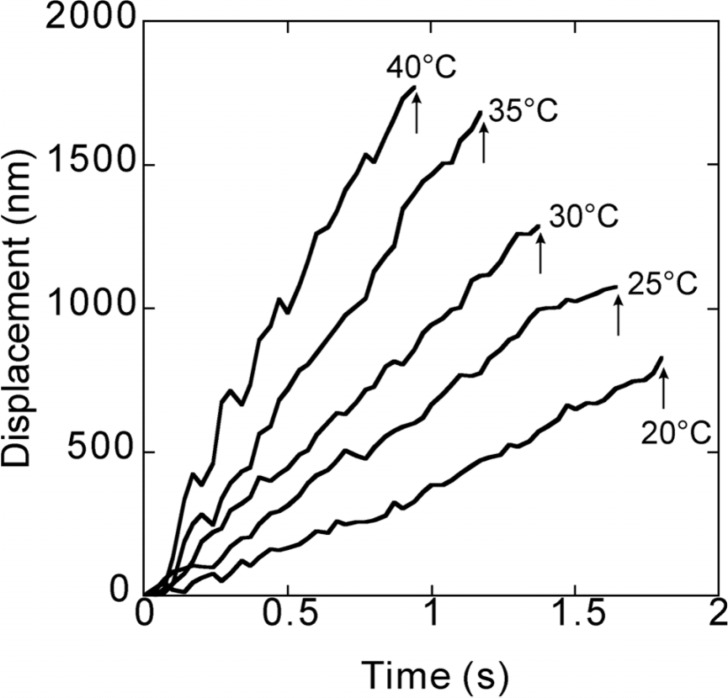
Figure 1 shows typical traces of movement of a kinesin-bound bead at various temperatures we examined, i.e., 20, 25, 30, 35 and 40°C. We collected the data between 1 and 11 min after the beginning of incubation at each temperature. From these data, we obtained the values of run length, duration and velocity (parameters characterizing the processivity of molecular motors) of single kinesin molecules moving along a microtubule. Here, the run length is defined as the distance, which a bead travelled continuously before detachment. The duration is the period of time during which the bead continuously moved (the abscissa of the final position of raw data as shown in Fig. 1). The velocity was obtained from the average slope of the time course of bead displacement. As these data show, with increasing temperature both run length and velocity increased, whereas duration decreased. Note that only two parameters of these three are theoretically independent : the value of one parameter can be calculated from the others according to the relationship (run length) = (velocity) × (duration). However, this relationship is not exactly kept in practice, because the velocity is obtained as the average slope of the time course of bead movement, whereas both run length and duration are determined from the coordinates of the end point of the time course of the bead movement (see Fig. 1). In spite of the difference between the values obtained theoretically and experimentally, all the experimental data exhibit good agreement with the above relationship throughout the analysis. These results show therefore that the temperature dependence of the run length is weaker than that of the velocity, because the temperature dependence of duration is opposite.
Figure 1.
Typical traces showing the time course of bead movement along a microtubule at various temperatures (Supplementary Movies 1–5. Note that those movies do not necessarily correspond to the data shown here). These traces were taken between 1 and 6 min of the incubation at each temperature. Attachment of the bead to a micro-tubule occurred at the zero point. Arrows indicate the time at which the kinesin-bound bead detached from the microtubule, detected by the disappearance of the fluorescent image of the bead. Run length was defined as the ordinate of the detachment point. Duration is the period of time between attachment and detachment of the bead. Walking velocity was estimated from the average slope of the time course of the bead movement obtained by the least squares method.
Effect of temperature on run length
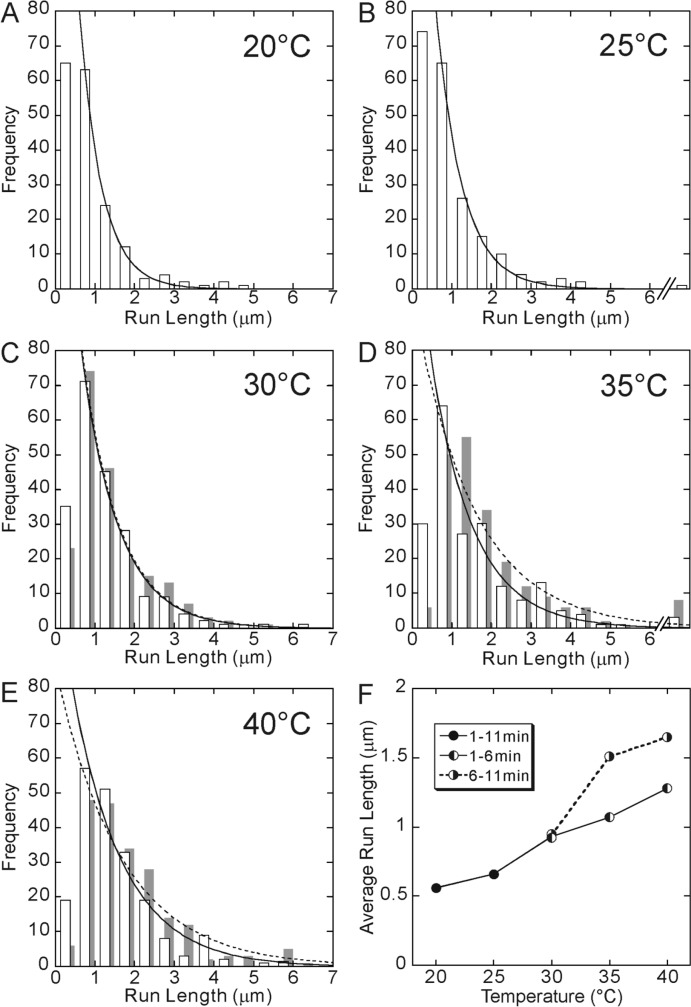
Figure 2 shows the distribution of run length at each temperature, which could be fitted by a single-exponential function, being consistent with the previous results15,25. As the temperature increased, the average run length became longer, and eventually increased more than twice from 0.6 μm to 1.3 μm with increasing temperature from 20 to 40°C. The results are summarized in Fig. 2F (points connected by a solid line). The fact that the run length increases at higher temperature was briefly mentioned in our previous paper16. Similar results were also reported for ncd and Eg5 molecular motors4.
Figure 2.
Temperature dependence of run length. The data at 20°C (A) and 25°C (B) were obtained between 1 and 11 min after the incubation at each temperature. On the other hand, for 30°C (C), 35°C (D) and 40°C (E), the data obtained between 1 and 6 min of incubation are shown by open bars, whereas those obtained between 6 and 11 min of incubation are shown by gray bars, because they showed a different set of values although the shape of the distribution was the same, i.e., approximated by a single exponential. Distribution of run lengths was fitted by an exponential function (a solid or a dashed curve). Here, the run lengths shorter than 0.5 μm were excluded from the analysis. The average run length, which was defined as the characteristic run length of the exponential function, obtained from each distribution shown here, was 0.56, 0.66, 0.93 (0.94), 1.07 (1.51) and 1.28 (1.65) μm at 20, 25, 30, 35 and 40°C, respectively (the values in the parentheses, the data for 6–11 min). In Fig. 2F, these values are shown by closed circles for 20 and 25°C and left-half filled circles for 30, 35 and 40°C connected by a solid line. Right-half filled circles (connected by a dashed line) show the average run length obtained from the data taken between 6 and 11 min of incubation at 30, 35 and 40°C.
As we reported previously8,16, the activity of kinesin motility appeared to change on lengthening the incubation time at higher temperatures. To quantitatively examine this for the data obtained at 30, 35 and 40°C, the data were divided into two groups, corresponding to the two periods, i.e., the one between 1 and 6 min and the other between 6 and 11 min after the beginning of incubation, because the two sets of data significantly differed at these temperatures; the average run length increased on lengthening the incubation time, although the shape of distribution remained the same. The results are shown by gray bars in Figs. 2C–E and right-half filled circles in Fig. 2F (points connected by a dashed line). For the data obtained at 20 and 25°C, the data is presented for both groups combined, because no difference was observed between them. This result suggests that the alteration of the motility of kinesin occurs during long incubation at the elevated temperature. We will discuss it in more detail below.
Effect of temperature on duration
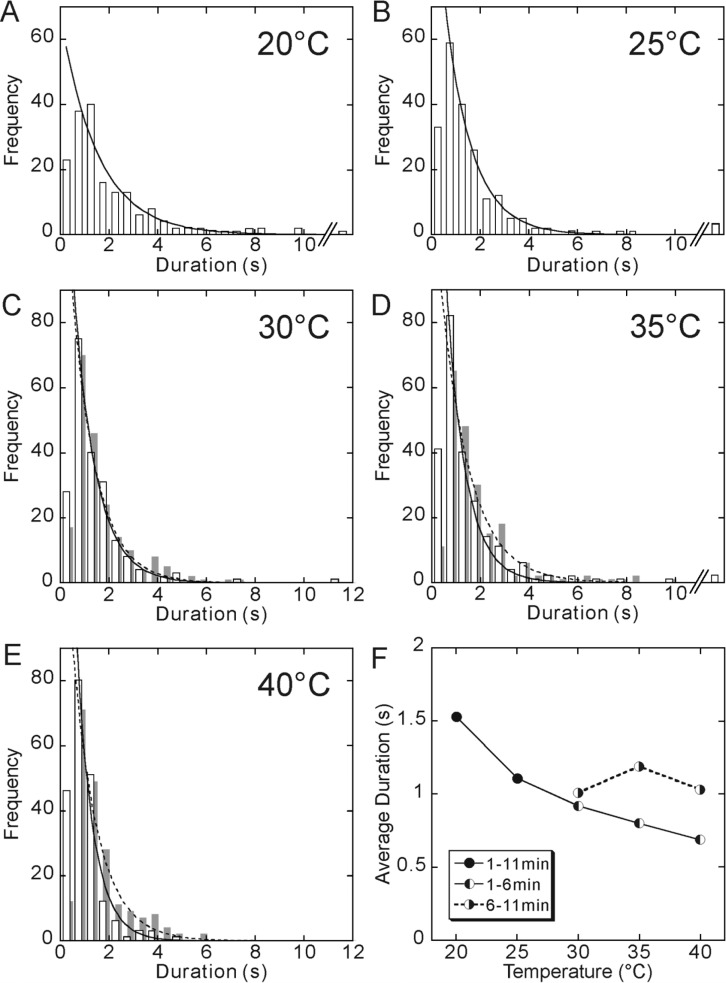
The distribution of duration at each temperature was also fitted by a single exponential function (Fig. 3), implying that both the run length and duration vary stochastically, and the detachment of kinesin from a microtubule occurs at one kinetic step. As the temperature increased, the average value of duration decreased, by about a half, from 1.5 s at 20°C to 0.7 s at 40°C. Thus, the temperature dependence of duration is opposite to that of the run length.
Figure 3.
Temperature dependence of duration. The data at 20°C (A) and 25°C (B) were obtained between 1 and 11 min of incubation at each temperature. On the other hand, for 30°C (C), 35°C (D) and 40°C (E), the data obtained between 1 and 6 min of incubation are shown by open bars, whereas the data obtained between 6 and 11 min of incubation are shown by gray bars, because they showed a different set of values, although the shape of the distribution was the same, i.e., approximated by a single exponential. Every distribution of duration was fitted by an exponential function (a solid or a dashed curve). Only runs longer than 0.5 s were included in the analysis. The average duration, which was defined as the characteristic time of the exponential function, was 1.53, 1.11, 0.92 (1.01), 0.80 (1.19) and 0.69 (1.03) s, at 20, 25, 30, 35 and 40°C, respectively (the values in the parentheses, the data for 6–11 min). In Fig. 3F, these values are shown by closed circles for 20 and 25°C and left-half filled circles for 30, 35 and 40°C connected by a solid line. Right-half filled circles (connected by a dashed line) show the average duration obtained from the data taken between 6 and 11 min of incubation at 30, 35 and 40°C.
Similarly, we analyzed the data for two different incubation periods. The results are summarized in Fig. 3F, showing that the elongation of the incubation time at higher temperatures prolonged the average duration. This tendency was similar to that of the run length. But note that with lengthening the incubation time, the temperature dependence of duration became weaker (Fig. 3), whereas it became stronger in case of the run length (Fig. 2F).
Effect of temperature on walking velocity
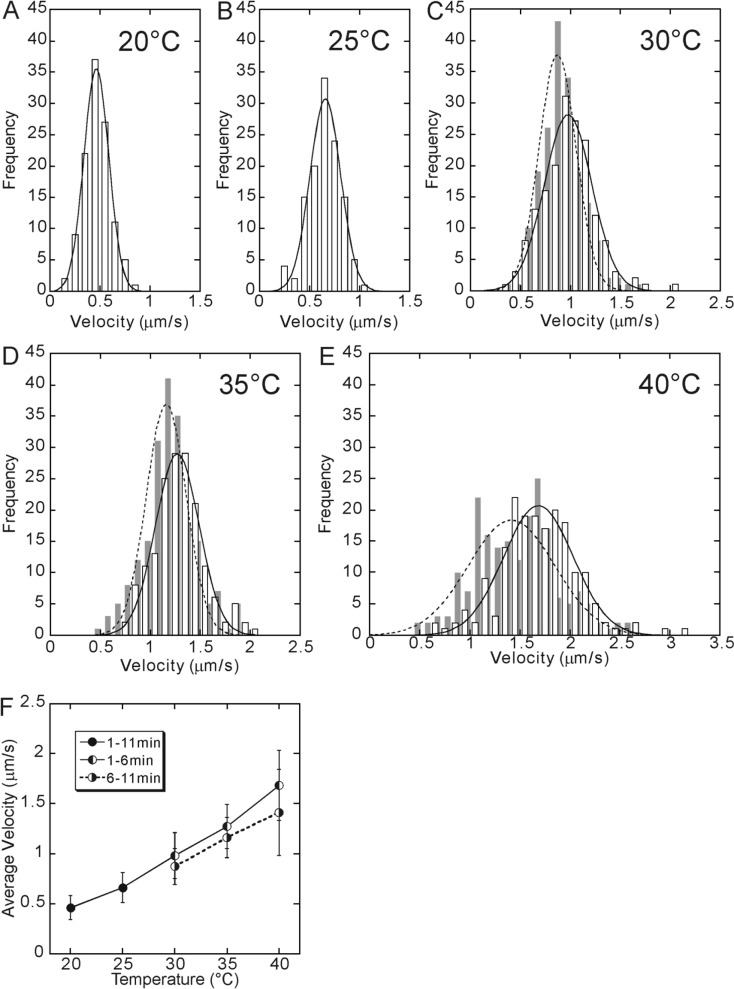
Next, we examined the effect of temperature on the velocity of a kinesin molecule moving along a microtubule. As Fig. 4 shows, the distribution of velocity exhibited a sharp peak and could be simulated by a Gaussian because there is a positive correlation between the run length and duration. The average value of velocity, i.e., the peak value of the Gaussian distribution, increased with increasing temperature as previously reported16,18.
Figure 4.
Temperature dependence of walking velocity. The data at 20°C (A) and 25°C (B) were obtained between 1 and 11 min of incubation at each temperature. On the other hand, for 30°C (C), 35°C (D) and 40°C (E), the data obtained between 1 and 6 min of incubation are shown by open bars, whereas the data obtained between 6 and 11 min after the incubation are shown by gray bars, because they showed a different set of values although the shape of the distribution was the same, i.e., approximated by a single Gaussian. The average walking velocity, which was defined as a peak of the Gaussian distribution, was 0.46, 0.66, 0.98 (0.87), 1.27 (1.16) and 1.68 (1.41) μm/s at 20, 25, 30, 35 and 40°C, respectively (the values in the parentheses, the data for 6–11 min). In Fig. 4F, these values are shown by closed circles for 20 and 25°C and left-half filled circles for 30, 35 and 40°C connected by a solid line. Right-half filled circles (connected by a dashed line) show the average velocity obtained from the data taken between 6 and 11 min of incubation at 30, 35 and 40°C.
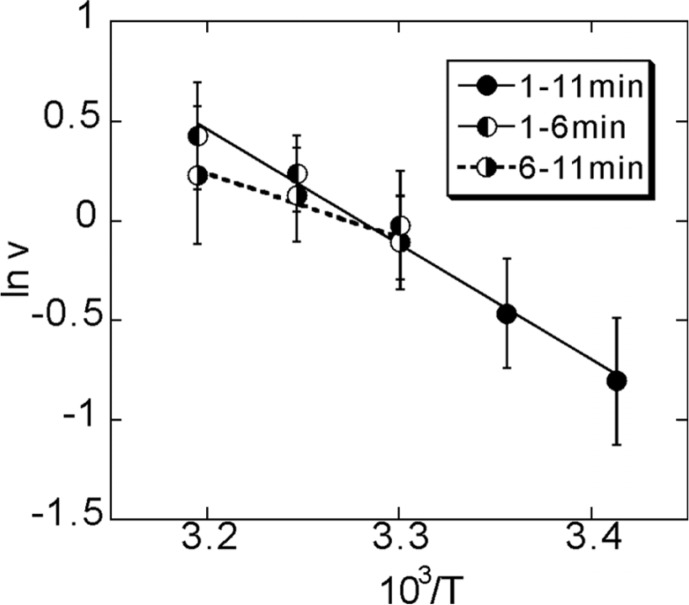
The Arrhenius plot of the velocity obtained between 1 and 6 min of incubation was linear within the temperature range we examined (Fig. 5). The activation enthalpy was estimated to be 48 kJ/mol from the slope of the Arrhenius plot, being consistent with the previous data16,17. Here we found that, for the data obtained between 6 and 11 min of incubation, the linear relationship deviated from the straight line above around 30°C. These results imply that the protein functions are generally deteriorated by incubation at the elevated temperatures. The thermally deteriorated kinesin molecules may exhibit a slower rate of ATPase hydrolysis, and the walking velocity is therefore decreased. However, the detachment rate decreases to a larger extent, so that not only the duration but the run length as well is prolonged.
Figure 5.
Arrhenius plot of walking velocity (ν) vs. absolute temperature (T). The activation enthalpy was estimated as 48 kJ/mol and 27 kJ/mol from the slope of the solid line and the dashed lines, respectively. Symbols are the same as in Figs. 2–4.
Effect of temperature on the probability of detachment during one cycle of stepping
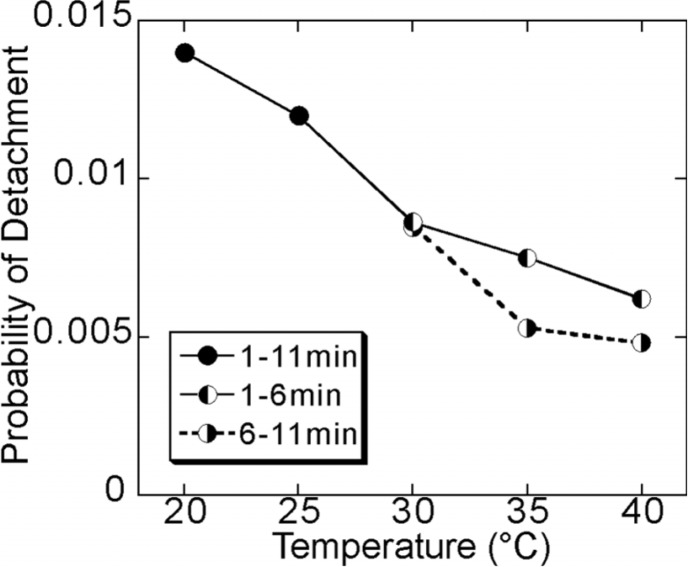
Based on the above data, we estimated the probability, p, of detachment of kinesin from a microtubule during one cycle of stepping. Now, we assume that the number of cycles (steps) in each processive movement, n, is determined by n = (run length)/(step size). Because the probability that a kinesin molecule detaches after the n-th step is equal to (1−p)n−1p, the average number of steps, <n>, is expressed as <n>=∑n=1∞n(1−p)n–1p=1/p. Thus, the value of p can be determined from the inverse of <n>, that is, the value of p is proportional to the inverse of the average run length. The results of this analysis show that the probability of detachment during one stepping cycle is around 0.01 and decreases with an increase in temperature, meaning that the processivity is enhanced at elevated temperatures (Fig. 6).
Figure 6.
Temperature dependence of the probability of detachment per each 8 nm step. The probability of detachment was determined to be 0.0143, 0.0121, 0.0086 (0.0085), 0.0075 (0.0053) and 0.0062 (0.0048) at 20, 25, 30, 35 and 40°C, respectively (the values in the parentheses, the data for 6–11 min). Symbols are the same as in Figs. 2–5. For more details, see the text.
What is the cause for the larger processivity at higher temperature? If the two heads work independently from each other, a plausible cause would be that the duty ratio increases as temperature increases. Here, the duty ratio is defined as the share of strongly bound states during each cycle of stepping. Note that the strong binding occurs in the nucleotide-free and AMPPNP (an ATP analogue)-bound states, as demonstrated by a large unbinding force as compared to a small unbinding force in the ADP-bound state7,17,22. The duty ratio is expected to increase if the proportion of the lifetime of such strongly bound states during one ATPase cycle increases. If the hydrophobic interaction is involved in the strong binding, it is understandable that the duty ratio increases with increasing temperature. In practice, such endothermic properties of protein-protein interaction have been observed for the strong binding (a rigor bond) formed between actin and myosin II in the absence of ATP26,27. Whether the hydrophobic interaction is involved in the strongly bound state of the kinesin-microtubule complex should be examined in future.
The detachment of kinesin from a microtubule will probably occur when both heads are in a weakly bound (ADP-bound) state. So, if the duty ratio increases, the probability that both heads are in a weakly bound state decreases. This simple interpretation would be reasonable if the two heads of kinesin would have worked independently. However, this is not the case because, in practice, the two heads of kinesin must work cooperatively to ensure highly-efficient processive movement.
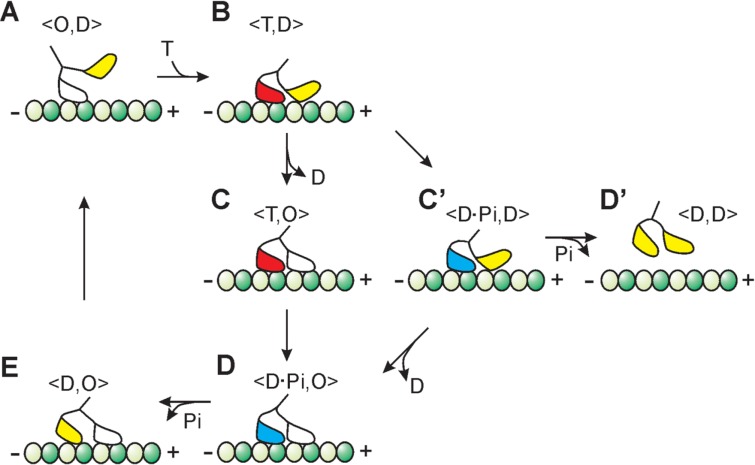
Thus, another possible and more plausible reason for the higher processivity at the elevated temperatures will be as follows. Let us consider the kinetic scheme of kinesin motility based on a simple hand-over-hand model as schematically shown in Fig. 78,11,23,25,28. During processive run of 8 nm steps, kinesin successively adopts a single-headed strongly bound <O, D> state (A in Fig. 7), the <T, D> state at which the trailing head is strongly bound and the leading head is weakly bound or detached (B in Fig. 7), double-headed strongly bound <T, O> and <D·Pi, O> states (C and D in Fig. 7, respectively) and the <D, O> state at which the leading head is strongly bound and the trailing head is weakly bound (E in Fig. 7). In this scheme, the spontaneous detachment of kinesin is assumed to occur in the <D, D> state (D′ in Fig. 7), which is occasionally formed after passing through the <D·Pi, D> state (C′ in Fig. 7). It is to be noted that the D·Pi state may be a weakly bound state as well11,25,29,30. Because every step occurs stochastically, the detachment of kinesin is plausible even if the rate constants of the B→C and C′→D steps are much higher than those of the B→C′ and C′→D′ steps, respectively.
Figure 7.
Simple kinetic pathway of the processive movement of kinesin. Each 8 nm step occurs stochastically and one ATP is hydrolysed in one cycle. T, D, Pi and O represent ATP, ADP, inorganic phosphate and nucleotide-free state, respectively. Here, we assume that there is a turning point, the <T, D> state (B), in which the pathway diverges; that is, one pathway proceeds to the next stepping event (from C to D, E, A and B), whereas the other proceeds to the state of detachment of kinesin from a microtubule (from C′ to D′). Dark- and light-green circles represent β- and α-subunits of tubulin, respectively. The plus and the minus ends of microtubule are represented by + and −, respectively. Manner of binding of each kinesin’s head with α- and β-subunits of tubulin is not realistic and is only schematically drawn in all nucleotide states.
Now, based on this scheme, the interpretation of an increase in the processivity with increasing temperature is as follows. First, a kinetic constant at every kinetic step (especially, a rate-limiting step) becomes larger with increasing temperature, which results in an increase of the walking velocity (ATPase activity). However, the temperature dependence of the kinetic constants will be different for every step. Therefore, the most plausible interpretation is that the degree of an increase of the kinetic constant at the B→C step is higher than that of the B→C′ step. Besides, this may also be the case for the temperature dependence of the kinetic constants for the C′ →D and C′ →D′ transitions. From this consideration, it is interesting to infer that the rate of detachment of ADP from the bound leading head is accelerated much higher than the other transitions with increasing temperature (both B→C and C′ →D steps).
Deterioration of motility at elevated temperatures
It has been reported that the melting transition of a part of the α-helix in the stalk region of kinesin occurs in a stepwise fashion at 25–30°C and 45–50°C31. Thus, there is a possibility that the deterioration observed here after 6–11 min incubation at 30–40°C may be attributable to a partial melting of the α-helix observed at 25–30°C. The denaturation of kinesin that forces a detachment from a microtubule may occur due to the melting transition of the α-helix at 45–50°C.
The interpretation of the effect of thermal deterioration, i.e., a decrease in the walking velocity and an increase in the processivity with longer incubation at elevated temperatures up to 40°C, based on the scheme shown in Fig. 7, is as follows. Due to the thermal deterioration, the kinetic constants decrease, resulting in a decrease in the walking velocity (ATPase activity). However, the rate of ADP release from the leading head (the B→C and C′→D steps) decreases to a smaller degree compared to the rates of the competing events in the trailing head (i.e., the B→C′ and C′→D′ transitions, respectively). Thus, all effects of temperature on the processivity and the walking velocity of kinesin observed in the present study are attributable explicitly to the temperature dependence of the kinetic constants, especially the rate of ADP release from the leading head in the hand-over-hand model shown in Fig. 7.
We previously reported that much longer incubation (20–30 min) at higher temperature (50°C) denatured kinesin that tends to detach from a microtubule in the presence of ATP16. This may be attributable to the melting of the α-helix observed at 45–50°C31. The partly deteriorated kinesin molecules obtained by incubation for 6–11 min at 30–40°C may be an intermediate between the native and the denatured ones. One possible intermediate state may be that either one of two heads of kinesin is thermally deteriorated. If it is the case, it would be interesting to examine how this thermally half-deteriorated kinesin walks along a microtubule as compared to the alternate stepwise motion of kinesin with heads genetically engineered12,13.
We should mention here the possibility that our measurements may have been effected to some extent by the contribution of beads to which more than one kinesin molecules are bound and are able to interact with a microtubule at the same time (see Materials and Methods). Therefore, the enhancement of the processivity in the partially deteriorated state may be the property of a heterogeneous ensemble of more than one kinesin molecules (rather than the heterogeneity of the two heads of the same molecule as discussed above). This situation may be similar to that in the gliding assay in which multiple kinesin molecules interact with a sliding microtubule.
Acknowledgments
We thank Drs. S. Uemura and I. Fujiwara of Waseda University for their technical assistance at the early stage of this research. We also thank Dr. S. V. Mikhailenko for his critical reading of the manuscript. This research was partly supported by Grants-in-Aid for Specially Promoted Research and for The 21st Century COE Program (Physics of Self-Organization Systems) at Waseda University from the Ministry of Education, Sports, Culture, Science and Technology of Japan (S. I.).
References
- 1.Svoboda K, Schmidt CF, Schnapp BJ, Block SM. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993;365:721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 3.Block SM, Goldstein LS, Schnapp BJ. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 4.Crevel IM, Lockhart A, Cross RA. Kinetic evidence for low chemical processivity in ncd and Eg5. J. Mol. Biol. 1997;273:160–170. doi: 10.1006/jmbi.1997.1319. [DOI] [PubMed] [Google Scholar]
- 5.Hancock WO, Howard J. Kinesin’s processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA. 1999;96:13147–13152. doi: 10.1073/pnas.96.23.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackney DD. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl. Acad. Sci. USA. 1994;91:6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi K, Uemura S, Ishiwata S. Equilibrium and transition between single- and double-headed binding of kinesin as revealed by single-molecule mechanics. Biophys. J. 2003;84:1103–1113. doi: 10.1016/S0006-3495(03)74926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi K, Ishiwata S. Nucleotide-dependent single-to double-headed binding of kinesin. Science. 2001;291:667–669. doi: 10.1126/science.291.5504.667. [DOI] [PubMed] [Google Scholar]
- 9.Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama M, Higuchi H, Yanagida T. Chemomechani-cal coupling of the forward and backward steps of single kinesin molecules. Nat. Cell Biol. 2002;4:790–797. doi: 10.1038/ncb857. [DOI] [PubMed] [Google Scholar]
- 11.Cross RA. The kinetic mechanism of kinesin. Trends Biochem. Sci. 2004;29:301–309. doi: 10.1016/j.tibs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Kaseda K, Higuchi H, Hirose K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat. Cell Biol. 2003;5:1079–1082. doi: 10.1038/ncb1067. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi H, Bronner CE, Park HW, Endow SA. Rapid double 8-nm steps by a kinesin mutant. EMBO J. 2004;23:2993–2999. doi: 10.1038/sj.emboj.7600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert SP, Webb MR, Brune M, Johnson KA. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995;373:671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vale RD, Funatsu T, Pierce DW, Romberg L, Harada Y, Yanagida T. Direct observation of single kinesin molecules moving along microtubules. Nature. 1996;380:451–453. doi: 10.1038/380451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi K, Ishiwata S. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 2000;272:895–899. doi: 10.1006/bbrc.2000.2856. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi K, Ishiwata S. Thermal activation of single kinesin molecules with temperature pulse microscopy. Cell Motil. Cytoskeleton. 2001;49:41–47. doi: 10.1002/cm.1019. [DOI] [PubMed] [Google Scholar]
- 18.Böhm KJ, Stracke R, Baum M, Zieren M, Unger E. Effect of temperature on kinesin-driven microtubule gliding and kinesin ATPase activity. FEBS Lett. 2000;466:59–62. doi: 10.1016/s0014-5793(99)01757-3. [DOI] [PubMed] [Google Scholar]
- 19.Kojima H, Muto E, Higuchi H, Yanagida T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 1997;73:2012–2022. doi: 10.1016/S0006-3495(97)78231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman A. A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence. J. Cell Sci. Suppl. 1991;14:125–127. doi: 10.1242/jcs.1991.supplement_14.25. [DOI] [PubMed] [Google Scholar]
- 21.Svoboda K, Block SM. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Uemura S, Kawaguchi K, Yajima J, Edamatsu M, Toyoshima YY, Ishiwata S. Kinesin-microtubule binding depends on both nucleotide state and loading direction. Proc. Natl. Acad. Sci. USA. 2002;99:5977–5981. doi: 10.1073/pnas.092546199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura S, Ishiwata S. Loading direction regulates the affinity of ADP for kinesin. Nat. Struct. Biol. 2003;10:308–311. doi: 10.1038/nsb911. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara I, Takahashi S, Tadakuma H, Funatsu H, Ishiwata S. Microscopic analysis of polymerization dynamics with individual actin filaments. Nature Cell Biol. 2002;4:666–673. doi: 10.1038/ncb841. [DOI] [PubMed] [Google Scholar]
- 25.Yajima J, Alonso MC, Cross RA, Toyoshima YY. Direct long-term observation of kinesin processivity at low load. Curr. Biol. 2002;12:301–306. doi: 10.1016/s0960-9822(01)00683-2. [DOI] [PubMed] [Google Scholar]
- 26.Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch. Biochem. Biophys. 1977;180:404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- 27.Ishiwata S, Manuck BA, Seidel JC, Gergely J. Saturation transfer electron paramagnetic resonance study of the mobility of myosin heads in myofibrils under conditions of partial dissociation. Biophys. J. 1986;49:821–828. doi: 10.1016/S0006-3495(86)83711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vale RD, Milligan RA. The way things move: Looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 29.Crevel IM, Lockhart A, Cross RA. Weak and strong states of kinesin and ncd. J. Mol. Biol. 1996;257:66–76. doi: 10.1006/jmbi.1996.0147. [DOI] [PubMed] [Google Scholar]
- 30.Sosa H, Peterman EJ, Moerner WE, Goldstein LS. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nat. Struct. Biol. 2001;8:540–544. doi: 10.1038/88611. [DOI] [PubMed] [Google Scholar]
- 31.de Cuevas M, Tao T, Goldstein LS. Evidence that the stalk of Drosophila kinesin heavy chain is an α-helical coiled coil. J. Cell Biol. 1992;116:957–965. doi: 10.1083/jcb.116.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.