ABSTRACT
Evofosfamide (TH-302) is a hypoxia-activated prodrug of the cytotoxin bromo-isophosphoramide. In hypoxic conditions Br-IPM is released and alkylates DNA. Ifosfamide is a chloro-isophosphoramide prodrug activated by hepatic Cytochrome P450 enzymes. Both compounds are used for the treatment of cancer. Ifosfamide has been approved by the FDA while evofosfamide is currently in the late stage of clinical development. The purpose of this study is to compare efficacy and safety profile of evofosfamide and ifosfamide in preclinical non-small cell lung cancer H460 xenograft models. Immunocompetent CD-1 mice and H460 tumor-bearing immunocompromised nude mice were used to investigate the safety profile. The efficacy of evofosfamide or ifosfamide, alone, and in combination with docetaxel or sunitinib was compared in ectopic and intrapleural othortopic H460 xenograft models in animals exposed to ambient air or different oxygen concentration breathing conditions. At an equal body weight loss level, evofosfamide showed greater or comparable efficacy in both ectopic and orthotopic H460 xenograft models. Evofosfamide, but not ifosfamide, exhibited controlled oxygen concentration breathing condition-dependent antitumor activity. However, at an equal body weight loss level, ifosfamide yielded severe hematologic toxicity when compared to evofosfamide, both in monotherapy and in combination with docetaxel. At an equal hematoxicity level, evofosfamide showed superior antitumor activity. These results indicate that evofosfamide shows superior or comparable efficacy and a favorable safety profile when compared to ifosfamide in preclinical human lung carcinoma models. This finding is consistent with multiple clinical trials of evofosfamide as a single agent, or in combination therapy, which demonstrated both anti-tumor activity and safety profile without severe myelosuppression.
KEYWORDS: Evofosfamide, hypoxia-activated prodrug, hypoxia, ifosfamide, non-small cell lung cancer, xenograft
Abbreviations
- BW
body weight
- Br-IPM
a brominated analog of isophosphoramide mustard
- CAA
chloroacetaldehyde
- Doc
docetaxel
- DCE
dechloroethylifosfamide
- EMT
epithelial to mesenchymal transition
- Evo
evofosfamide
- ILS
increased life span
- Ifo
ifosfamide
- MTD
maximum tolerated dose
- MST
median survival time
- NSCLC
non-small cell lung cancer
- TGI
tumor growth inhibition
- Sun, sunitinib; V
vehicle
Introduction
Prodrugs are derivatives of drug molecules that undergo an enzymatic and/or chemical transformation in vivo to release the active parent drug, which can then exert the desired pharmacological effect.1,2 In general, the rationale behind the use of prodrugs is to optimize the absorption, distribution, metabolism, excretion, and unwanted toxicity (so called ADMET properties) of the parent drugs.3 It is estimated that currently about 10% of worldwide marketed drugs can be classified as prodrugs.3,4
Ifosfamide (3-(2-chloroethyl)-2-[(2-chloroethyl)-amino] tetrahydro-2H-1,3,2-oxazaphosphorin-2-oxide) is a prodrug which is metabolized in the liver by hepatic cytochrome P450 (CYP)-catalyzed 4-hydroxylation to produce the active DNA-alkylating agent isophosphoramide mustard (IPM).5 It is approved for the treatment of testicular cancer 6 and also used as a treatment for a variety of other cancers, including breast cancer, lymphoma, soft tissue sarcoma, osteosarcoma, bone tumor, lung cancer, cervical cancer, and ovarian cancer.5 The main acute side effects of ifosfamide include those commonly seen with other antineoplastic agents such as neutropenia, thrombocytopenia, nausea and vomiting, alopecia, and hypersensitivity reactions. Ifosfamide is also associated with more specific toxicities due to its metabolism byproducts, including hemorrhagic cystitis, neurotoxicity (encephalopathy), and nephrotoxicity (Fig. 1A).5,7 With the co-administration of mesna uroprotection, the primary dose-limiting toxicity of ifosfamide is myelosuppression.8
Figure 1.
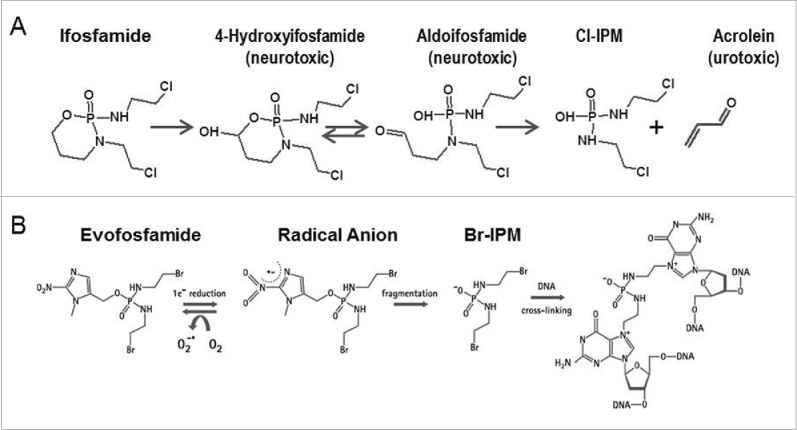
(A) Metabolism of byproducts of ifosfamide induced toxicity (B) Mechanism of action of evofosfamide.
To improve the selectivity of tumor cell killing and the sparing of normal tissue, prodrug forms that can be selectively activated in tumor tissue have been widely investigated. There are several mechanisms potentially exploitable for selective prodrug activation in tumors including utilizing unique aspects of tumor physiology such as selective enzyme expression, hypoxia, and low extracellular pH.9
Hypoxia activated prodrugs (HAPs) are designed to be selectively activated in hypoxic regions of tumors and release cytostatic or cytotoxic effectors. Evofosfamide (previously known as TH-302, (1-methyl-2-nitro-1H-imidazole-5-yl) methyl N,N'-bis (2-bromoethyl) diamidophosphate) is a nitroimidazole-linked prodrug of a brominated version of isophosphoramide mustard (Br-IPM). The 2-nitroimidazole moiety of evofosfamide acts as an oxygen concentration sensor. Evofosfamide is reduced at the nitroimidazole site of the prodrug by intracellular reductases when exposed to hypoxic conditions, and releases the DNA-alkylating Br-IPM10 (Fig. 1B). Evofosfamide exhibits hypoxia-selective in vitro cytotoxicity across a wide variety of human cancer cell lines11 and in vivo anti-tumor efficacy in a panel of preclinical xenograft models.12-14 Evofosfamide is currently being tested in multiple clinical trials including Phase III trials for the treatment of sarcoma (NCT01440088) and pancreatic adenocarcinoma (NCT01746979), based on encouraging Phase II results.15,16
Lung cancer has become the number one killer among cancers worldwide, and non-small cell lung cancer accounts for approximately 85% of all cases of lung cancer.17,18 Ifosfamide has been evaluated extensively for the treatment of NSCLC.19 In combination therapy with other active agents, ifosfamide has contributed to high response rates in NSCLC.20 Evofosfamide in combination with pemetrexed was evaluated in the patients with solid tumors including NSCLC. A previous Phase I/II study showed encouraging activity21 and a Phase II study of the pemetrexed and evofosfamide combination compared to pemetrexed and placebo is currently underway (NCT02093962).
As prodrugs, evofosfamide and ifosfamide produce a similar DNA cross-linking moiety via different mechanisms of activation. In the present study, we compared the efficacy and safety profile of evofosfamide and ifosfamide in preclinical non-small cell lung cancer H460 xenograft models.
Results
Evofosfamide prolonged survival time longer than ifosfamide in the H460 intrapleural orthotopic model
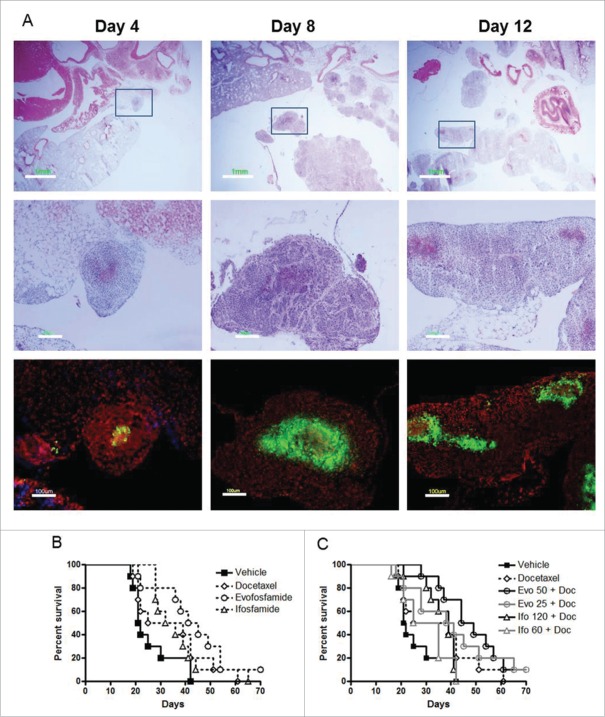
Four days after H460 cells inoculation into the pleural space of nude mice, tumor cells or tumor cell clusters were found attached to the surface and the edge of the lung as well as other mediastinal organs. Over the next 4 d the tumor nodules spread within the entire lung, and invaded the whole thoracic cavity by 12 d (Fig. 2A). Hypoxia was detected as early as 4 d after inoculation. Based on the characterization of disease progression and tumor hypoxia, the randomization and drug treatment was initiated 7 d after inoculation (Day 1).
Figure 2.
Antitumor activity of evofosfamide or ifosfamide in combination with docetaxel in the metastatic H460 intrapleural model. A, metastasis progression and tumor hypoxia characterization on 4 days, 8 days, and 12 d after H460 cells inoculation. Top panel, H & E staining; middle panel, enlarged images of inserts; and bottom panel, immunofluorescence staining of pimonidazole, a marker of hypoxia, on the consecutive sections of middle panel; green, hypoxia; blue, Hoechst 33342; and red, propidium iodide; B, Kaplan-Meier plot analysis of evofosfamide or ifosfamide as monotherapy. C, Kaplan-Meier plot analysis of evofosfamide or ifosfamide in combination with docetaxel.
To investigate the antitumor activity of evofosfamide in comparison to ifosfamide, 10 animals per group were treated for QD × 5/wk × 2 wks with evofosfamide 50 mg/kg, ip, or ifosfamide 120 mg/kg, ip alone or in combination with docetaxel, 5 mg/kg, iv, Q7D × 2. In the combination therapy groups with docetaxel, evofosfamide and ifosfamide dosed at 25 and 60 mg/kg, respectively, were tested as well. Vehicle treated animals started to show body weight loss more than 20% from Day 18. In a 150-day observation, all dead or euthanized mice showed pleural metastases at necropsy. At the end of the study, 10% animals survived in evofosfamide 50 mg/kg monotherapy, and the evofosfamide 50 and 25 mg/kg combination therapy groups. All other mice were dead or euthanized by Day 65.
Comparing to the Median Survival Time (MST) of 22 d in the vehicle-treated group, all drug-treated animals showed an increased MST by Kaplan-Meier curve analysis. However, a statistical significant difference from the vehicle treatment group was only found in the evofosfamide-treated groups, including evofosfamide 50 mg/kg monotherapy, evofosfamide 25 and 50 mg/kg combination therapy groups, with the increase in life span of 100%, 84%, and 116%, respectively. Ifosfamide monotherapy, docetaxel monotherapy, or ifosfamide and docetaxel combination groups did not significantly increase life span compared to vehicle treatment. Evofosfamide 50 mg/kg in combination with docetaxel significantly prolonged the survival time compared with ifosfamide 120 mg/kg in combination with docetaxel (p < 0.001) (Figs. 2B and C, Table 1).
Table 1.
Comparison of antitumor activity of evofosfamide and ifosfamide alone, or in combination with docetaxel in the metastatic H460 intrapleural model.
| MST (Day) | T/C% | ILS% | |
|---|---|---|---|
| Vehicle | 22 | ||
| Evofosfamide 50 mg/kg | 43* | 200* | 100 |
| Ifosfamide 120 mg/kg | 34 | 158 | 58 |
| Docetaxel 5 mg/kg | 32 | 149 | 49 |
| Evo 50 mg/kg + Doc 5 mg/kg | 47*,# | 216*,# | 116 |
| Evo 25 mg/kg + Doc 5 mg/kg | 40* | 184* | 84 |
| Ifo 120 mg/kg + Doc 5 mg/kg | 39 | 181 | 81 |
| Ifo 60 mg/kg + Doc 5 mg/kg | 30 | 140 | 40 |
MST: Median Survival Time
T/C%: MST of treated group/MST of Vehicle Group × 100
ILS: Increase in life span, ILS=T/C%-1
Evo: evofosfamide
Ifo: ifosfamide
Doc: docetaxel
*, p < 0.05 vs. vehicle
#, p < 0.05 vs. same dose of monotherapy
Evofosfamide showed comparable activity to ifosfamide in the H460 ectopic xenograft model at Maximum Tolerated Dose (MTD) level
In the ectopic H460 xenograft model, animals were randomized and treated when tumor size was 100–150 mm3. Ten animals per group were treated with QD × 5/wk × 2 wks with evofosfamide 50 mg/kg, ip or ifosfamide 120 mg/kg, ip alone or in combination with docetaxel, 10 mg/kg, iv, Q7D × 2. In the combination therapy groups, evofosfamide and ifosfamide, 25 mg/kg and 60 mg/kg, respectively, were tested as well. As showed in Fig. 3, evofosfamide 50 mg/kg monotherapy yielded similar efficacy as ifosfamide, with Tumor Growth Inhibition (TGI) of 74% in evofosfamide vs. 68% in ifosfamide. However, evofosfamide alone did not induce any body weight loss during the study but ifosfamide alone caused 4% body weight loss on average. When in combination with docetaxel, evofosfamide showed less body weight loss compared with ifosfamide (6% vs. 9%, respectively). Given docetaxel alone yielded 6% body weight loss, the addition of evofosfamide in the combination did not induce more body weight loss. Notably, evofosfamide 25 mg/kg in combination with docetaxel produced similar antitumor activity as the evofosfamide 50 mg/kg combination group or ifosfamide 120 mg/kg combination therapy treatment.
Figure 3.
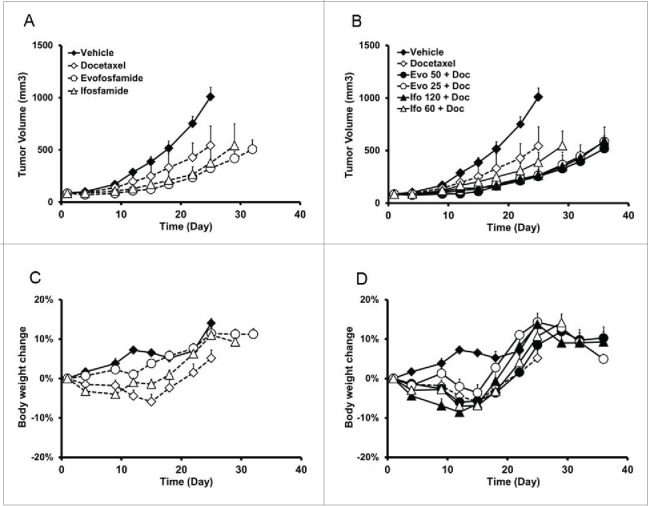
Antitumor efficacy and safety profile of evofosfamide or ifosfamide in combination with docetaxel in the ectopic H460 xenograft model. A and B, tumor growth of evofosfamide or ifosfamide alone (A), or in combination with docetaxel (B). C and D, body weight change induced by evofosfamide or ifosfamide alone (C), or in combination with docetaxel (D). Animals were monitored daily and tumor growth was quantified twice a week. Data are expressed as Mean ± SEM of 10 animals per group. Evo, evofosfamide; Ifo, ifosfamide; Doc, docetaxel.
Antitumor activity of evofosfamide but not ifosfamide was breathing oxygen concentration dependent in the H460 ectopic xenograft model
To further compare the difference of mechanism of action between evofosfamide and ifosfamide, tumor hypoxia was modified by exposing H460 tumor-bearing animals to different oxygen levels in controlled atmospheric breathing chambers gassed with 95%, 21%, or 10% O2. When tumor size reached 100 mm3, animals were treated with vehicle, evofosfamide 50 mg/kg, or ifosfamide 120 mg/kg, ip, QD × 5/wk × 2 wks. On treatment days, all mice were exposed to the different levels of oxygen in the controlled atmosphere chamber for 30 min before and 2 h after each dose. As shown in Figs. 4A and B, tumor growth rates in the vehicle or ifosfamide-treated groups were not affected by the different oxygen breathing conditions. However, with evofosfamide treatment, TGI was dependent on breathing oxygen concentration with the lower breathing oxygen concentration group achieving the superior efficacy profile. TGIs in 95%, 21%, and 10% O2 breathing condition groups after dosing evofosfamide were 50%, 80% and 90%, respectively. Consistent results were obtained in two separate experiments. Body weight changes were similar across most of groups, however, animals treated with ifosfamide showed more body weight loss when breathing 95% O2 (Figs. 4C and D).
Figure 4.
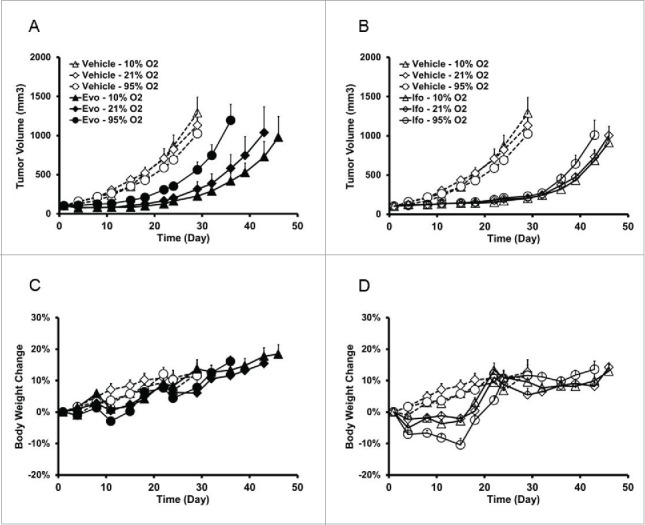
Evofosfamide, but not ifosfamide, exhibits controlled oxygen concentration breathing condition-dependent antitumor activity in the ectopic H460 xenograft model. A and C, antitumor activity; B and D, body weight change as a readout of toxicity. Data are expressed as Mean ± SEM of 10 animals per group. Evo, evofosfamide; Ifo, ifosfamide; Doc, docetaxel. (A and C are from Reference 12).
Evofosfamide showed favorable hematological profile compared to ifosfamide
Evofosfamide and ifosfamide monotherapy induced hematologic change was investigated in CD-1 immunocompetent mice following a regimen of QD × 5/wk × 2 wks. Four hours after the last treatment, animals were sacrificed with CO2, blood was collected via heart puncture, and hematological analysis was performed using a Hemavet 950 blood analyzer. 25, 50 and 75 mg/kg of evofosfamide, and 30, 60, 90 and 120 mg/kg of ifosfamide were employed. As shown in Fig. 5A, after two weeks treatment, ifosfamide 120 mg/kg significantly reduced white blood cells (WBCs) count, including neutrophils, lymphocytes, and monocytes, compared with vehicle-treated animals. Evofosfamide 50 mg/kg did not significantly reduce the same blood cell counts compared with the vehicle-treated animals.
Figure 5.
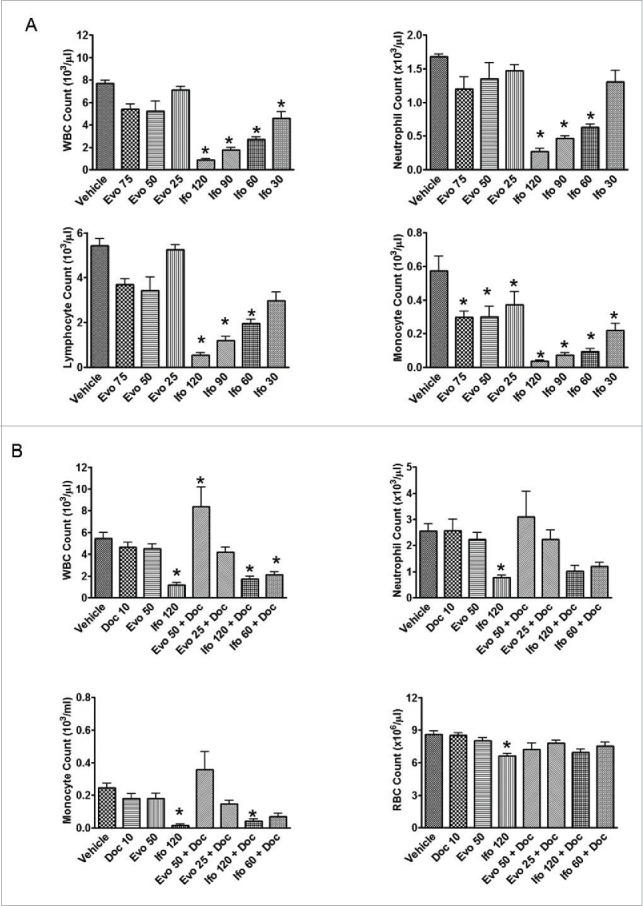
Effect of evofosfamide or ifosfamide on hematologic change in CD1 and H460 tumor bearing nude mice. The means and standard errors from the 5–6 mice per group are presented. A, blood samples were collected 4 hrs after the last treatment from non-tumor bearing CD-1 mice. B, blood samples were collected 3 d after the last treatment from H460 bearing nude mice; *, p < 0.05 as compared to Vehicle.
In the xenograft models, evofosfamide 50 mg/kg showed similar body weight loss as ifosfamide dosed at 90 to 120 mg/kg with the regimen of QD × 5/wk × 2 wks. However, evofosfamide exhibited superior safety profile with the hematology end points profiled the last treatment. Ifosfamide at 120 mg/kg, but not evofosfamide at 50 mg/kg, significantly reduced WBC and red blood cells (RBC). Consistent results were obtained with both the CD-1 and immunocompromised nude mice models. When in combination with the conventional chemotherapeutic agent docetaxel, evofosfamide did not add hematoxicity, but ifosfamide at both 120 and 60 mg/kg in combination with docetaxel induced a significant reduction of WBC compared with vehicle treatment (p < 0.05). (Fig. 5B).
Evofosfamide showed superior antitumor activity as compared to ifosfamide in the H460 ectopic xenograft model at equal hematoxicity level
Following the QD × 5/wk × 2 wks regimen, evofosfamide 50 mg/kg and ifosfamide 30 mg/kg showed similar levels of hematological changes. Therefore, we used the H460 ectopic model to test the efficacy of evofosfamide and ifosfamide at an equal hematoxicity level for aligning the doses employed. Ifosfamide at 30 mg/kg did not exhibit any antitumor activity; on the other hand, evofosfamide at 50 mg/kg yielded 56% TGI, which was consistent with previous data. Interestingly, the combination treatment of evofosfamide and ifosfamide enhanced antitumor activity in this model, with a TGI of 75% (Fig. 6A). In another study, evofosfamide or ifosfamide in combination with sunitinib was investigated in the H460 ectopic model. Sunitinib was administered at 80 mg/kg, QDx19, PO; evofosfamide at 50 mg/kg, QD × 5/wk × 2 wks, ip and ifosfamide at 30, 60 and 90 mg/kg, QD × 5/wk × 2 wks, ip. Evofosfamide and ifosfamide started 7 d after sunitinib treatment initiation. Evofosfamide alone yielded 76% TGI comparing to 44% in the ifosfamide 30 mg/kg group. In the combination treatment group, compared with TGI of 82% by sunitinib alone, evofosfamide and sunitinib combination group yielded a TGI of 92%, but did not reach significant difference (Fig. 6B). No enhanced antitumor activity was observed in ifosfamide and sunitinib combination group that yielded TGI of 84% only.
Figure 6.
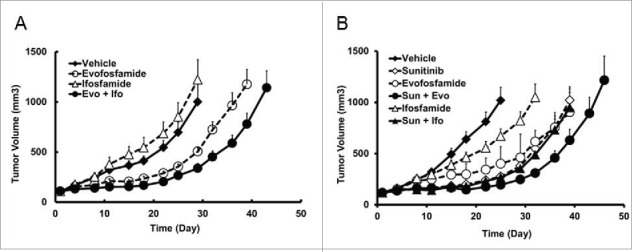
At an equivalent hematoxicity level, antitumor activity of evofosfamide and ifosfamide in the H460 ectopic xenograft models. Evo, was given at 50 mg/kg, ip, and Ifo was given at 30 mg/kg ip at a regimen of QDx5/wk × 2 wks; A, antitumor activity as monotherapy. B, sunitinib was given at 80 mg/kg, QDx19, oral. Evo or Ifo was given 7 d after the initiation of sunitinib treatment. Data are expressed as Mean ± SEM of 10 animals per group.
Discussion
The antitumor activity and safety profile of evofosfamide, a hypoxia-activated prodrug, was compared with ifosfamide, a CYP-activated prodrug, in the preclinical models. We demonstrated increased antitumor activity and favorable safety profile of evofosfamide versus ifosfamide.
Animal weight loss is a main index of drug-induced toxicity in preclinical cancer drug discovery. MTD is usually defined as the maximum dose that causes no drug-related lethality and produced <20% loss of initial animal weight.22 With such definition, MTDs of evofosfamide and ifosfamide in mice were 50 and 120 mg/kg, respectively. At an equal body weight loss level, evofosfamide showed superior antitumor activity to ifosfamide in the metastatic intrapleural model and comparable efficacy in the ectopic H460 xenograft model. We set up an orthotopic model by intrapleural inoculation of H460 cells.23 The implantation of tumor in the organ specific orthotopic site leads to an increased tumorigenicity and metastatic potential as compared to the ectopic models and thus could be more relevant as a model of clinical situation.24,25 As a single agent, evofosfamide at 50 mg/kg but not ifosfamide at 120 mg/kg, significantly prolonged survival compared to vehicle in the orthotopic model. In the combination with docetaxel treatment groups, evofosfamide 50 mg/kg significantly increased survival time compared with ifosfamide 120 mg/kg group. More importantly, in a 150-day observation time, all surviving animals were from evofosfamide-treated groups. In addition, in both orthotopic and ectopic H460 xenograft models, in combination with docetaxel, evofosfamide at the one-half MTD dose of 25 mg/kg exhibited similar antitumor activity as ifosfamide at MTD dose of 120 mg/kg.
Many published reports reveal that the results of preclinical xenograft models were not retrospectively predictive of clinical activity.26 However, Nomura and colleagues reasoned this could be due to inappropriate drug dosing.26-31 The MTD of most chemotherapeutic drugs given to mice is higher than the corresponding allometrically scaled ‘equivalent’ human dose.32 Therefore, it is possible that MTD-based dosing in xenograft models could lead to a high rate of false positives. When the clinically equivalent dose was used, the pattern of response in mice was similar to the activity of the drug in the respective human cancer setting.27-31 It is well-known that hematoxicity represents one of the major limitations of chemotherapy treatment.32 Therefore, we investigated the doses of evofosfamide and ifosfamide that induced equivalent hematoxicity by conducting a series of studies in both immunocompromised and immunocompetent mice. Neutropenia is defined as <500 neutrophils/µl blood as reported by Walsh et al.33,34 In the present study, and 2 weeks' treatment of ifosfamide at 120 mg/kg induced neutropenia. MTD of evofosfamide, 50 mg/kg, also reduced neutrophil count after 5 day's treatment, but did not reach the degree of neutropenia. Evofosfamide and ifosfamide, 50 mg/kg and 30 mg/kg, respectively, induced equivalent hematoxicity in the preclinical xenograft studies. We employed these doses in the H460 ectopic model and found that ifosfamide alone at 30 mg/kg did not show any antitumor activity while evofosfamide significantly inhibited tumor growth.
While no direct clinical studies comparing evofosfamide with ifosfamide have been conducted, the safety and efficacy of the 2 compounds can be indirectly compared based on historical studies. The main adverse events from ifosfamide administered as a single agent are myelosuppression and urotoxicity. Myelosuppression following ifosfamide is dose-dependent and primarily manifests as leukopenia including neutropenia or thrombocytopenia.5 This contrasted to main side effect of evofosfamide as skin and mucosa toxicity.35 When in combination with doxorubicin in the clinical sarcoma trials, the indirect comparison reports less hematological toxicity with doxorubicin plus evofosfamide.15 The ongoing Phase 3 study investigating doxorubicin vs. evofosfamide plus doxorubicin (NCT01440088) will contribute to a better understanding of the differences between ifosfamide and evofosfamide when combined with doxorubicin, albeit a historical, but not a direct comparison.
Evofosfamide and ifosfamide are both prodrugs which require metabolic activation to exert their cytotoxic activity. Evofosfamide's bioactivation to its DNA cross-linking metabolite, BrIPM, is mediated by CYP450 reductase and other one-electron reductases. 11,36 In contrast, the metabolism of ifosfamide is mainly catalyzed by CYP3A4 to 4-hydroxy-ifosfamide and further to 2- and 3-dechloroethylifosfamide (DCE) and chloroacetaldehyde (CAA), which is presumed to be neuro- and nephrotoxic. As 4-hydroxy-ifosfamide is unstable and exists in equilibrium with its tautomeric form aldophosphamide, the latter decomposes spontaneously to the DNA cross-linking metabolite, IPM, and acrolein, which is also known to be nephrotoxic.37
Evofosfamide is a second-generation HAP designed to address and potentially overcome some of the recognized limitations of earlier HAPs. These included shifting the oxygen selectivity to more extreme hypoxia (<0.5%), designing the prodrug to be insensitive to 2-electron reductases and not be metabolized by cytochrome P450s.35 Selective hypoxic region targeting by evofosfamide has been reported in both in vitro and in vivo studies.11,12 In the current study, lower oxygen level breathing concentrations yielded greater efficacy for evofosfamide. The controlled oxygen breathing condition-dependent antitumor activity was not observed in ifosfamide treated animals.
In summary, our results indicate that evofosfamide exhibits greater antitumor activity and favorable safety profile compared to ifosfamide. These observations provide a translational rationale support for the on-going clinical trial to evaluate the efficacy and safety profile of evofosfamide in the treatment of non-small cell lung cancer.
Materials and methods
Compounds
Evofosfamide was manufactured at Syngene (Bangalore, India). Ifosfamide and docetaxel were purchased from Sigma Aldrich (St. Louis, USA). Sunitinib was purchased from Ontario Chemical (Guelph, Canada). Evofosfamide and ifosfamide were solubilized and diluted in 0.9% saline; docetaxel was dissolved in 5% ethanol, 5% cremophor and 90% Water for Injection; and sunitinib was formulated in 10mM sodium citrate (pH 3.5).
Cell line and experimental animals
H460, a human NSCLC cell line, was purchased from the American Type Culture Collection (Manassas, Virginia, USA). Cells were passaged in RPMI 1640 medium complemented with 10% fetal bovine serum and maintained at 37°C in 5% CO2/ 95% air.
Homozygous female nude mice (Nu-Foxn 1nu NU/NU, Charles River Laboratories) were used for the xenograft models. Mice were given food and water ad libitum and housed in microisolator cages. Four- to 6-week-old animals were tagged with microchips (Locus Technology) for identification.
Five-week old female CD-1 mice (Charles River Laboratories) were used for maximum tolerated dose (MTD) determination and safety profiling investigation. All animal studies were approved by the Institutional Animal Care and Use Committee of Threshold Pharmaceuticals.
Drug treatments
The maximum tolerated dose (MTD) was determined by dose escalations in a small number of CD-1 immunocompetent mice or non-tumor bearing nu/nu mice, with 4–5 mice per group. The MTD was defined as the highest dose resulting in less than 20% weight loss for any one animal in an experimental group, no significant changes in general clinical signs, and no abnormal gross anatomical findings after necropsy, and no animal deaths. General clinical signs included: respiratory rate, behavior, and response to normal stimuli.38 At a regimen of QD × 5/wk × 2wks, ip, the MTD of evofosfamide and ifosfamide determined to be was 50 mg/kg and 120 mg/kg, respectively. Docetaxel was given at 10 mg/kg, Q7Dx2, iv in the ectopic study and the dose was reduced to 5 mg/kg in the intrapleural orthotopic model because aggressive disease progression itself induced body weight loss as well. When the combination therapy was scheduled, evofosfamide or ifosfamide was given 4 hrs prior to docetaxel. Sunitinib was given at 80 mg/kg, QD × 19, oral. Evofosfamide or ifosfomide was given 7 d after the initiation of sunitinib treatment in the combination setting. The doses of evofosfamide, ifosfamide and docetaxel used in all studies were no higher than MTD.
In vivo xenograft models
For the intrapleural orthotopic model, 1 × 106 H460 cells were implanted through the chest wall into the left pleural space of nude mice (i.pl.) in a volume of 100 µl PBS using a 26 gauge needle. The depth of needle penetration through the intercostal muscles was controlled to avoid lung injury and hemorrhage into the pleural space.23
For the ectopic model, 1 × 106 H460 cells were prepared in 30% Matrigel (BD Biosciences, Franklin Lakes, NJ) mixed with 70% RPMI 1640 medium. A total volume of 0.2 ml was implanted in the subcutaneous space of the right flank in mice.
For xenograft experiments employing controlled oxygen concentration breathing chambers, groups of mice were placed in a controlled atmospheric chamber and exposed to 95% O2 (carbogen with 5% CO2), 21% O2 (air) or 10% O2, for 30 min. followed by drug or vehicle control administration, and the animals remained in the controlled breathing chambers for 2 additional hours. The chambers were flushed continuously with gases at a rate of 5 L/min.
In vivo antitumor activity
With the intrapleural orthotopic model, treatment was initiated when the tumor had begun to invade the surrounding tissues, 7 d after the injection of the H460 NSCLC cells. Each treated group consisted of 10–12 mice. Animal mortality was checked daily, and the antitumor activity was evaluated as follows: T/C % = median survival time (MST) of the treated group/MST of the control group × 100. Results were also expressed as the percentage of Increased Lifespan (ILS, T/C of treated group - 100). Kaplan-Meier plots were constructed to show the percentage animals remaining in the study as a function of time following treatments. A comparison of the survival curves between all the treated and control groups was performed with a log-rank test, which takes censored values into account. A p level < 0.05 was considered statistically significant.
With the ectopic model, tumor growth and body weight were measured twice a week after cell implantation. Tumor volume was calculated as (length × width2)/2. When the mean value of tumor volume was approximately 100–150 mm3, mice were randomized into 10 mice per group and the treatment started (Day 1). Antitumor activity was assessed by tumor growth kinetics and Tumor Growth Inhibition (TGI) and Tumor Growth Delay (TGD). TGI was defined as (1-ΔT/ΔC) × 100, where ΔT/ΔC is the ratio of the change in mean tumor volume of the treated group (ΔT) and of the control group (ΔC). Animals were culled when individual tumor size was over 2000 mm3 or mean tumor volume exceeded 1000 mm3 in the group. TGI was determined on the last measurement when all the animals in the vehicle group were survived. Data are expressed as the mean ± SEM. One-way analysis of variance with Dunnett's test (GraphPad PRISM 4, La Jolla, CA) was used for analysis. A p level < 0.05 was considered statistically significant.
Safety profile
CD-1 female mice or H460 tumor-bearing nude mice (5 mice per group) were used. Four hours or 3 d after the last treatment, animals were euthanized, and blood from each animal was immediately withdrawn by cardiac puncture into EDTA-containing tubes. The blood samples were immediately analyzed for hematological parameters with a Hemavet 950 (Drew Scientific, Miami Lakes, FL) and also centrifuged at 5000 r.p.m. for 5 min to collect plasma fraction for liver and kidney function tests. One kidney from each animal was collected and fixed in 10% neutral buffered formalin, and embedded in paraffin for 5-µm thick tissue sections. Periodic acid–Schiff (PAS) staining was performed to evaluate morphology of kidney.
Histology and immunofluorescence
To characterize tumor hypoxia in the intrapleural orthotopic model, 4, 8 and 12 d after inoculation of 106 H460 cells into intrapleural space, 3–4 animals in each group were euthanized. The hypoxia biomarker pimonidazole hydrochloride (Hypoxyprobe, Natural Pharmacia International, Burlington, MA) was intraperitoneally (ip) injected one hour before animal sacrifice at 60 mg/kg. 10 mg/kg of Hoechst 33342 was iv injected via tail vein 1 min before animal sacrifice to label the tumor blood perfusion. Lungs and mediastinal organs were collected, and embedded in OCT. 8-µm thick tissue sections were cut and adhered to poly-L-lysine-coated glass microscope slides. Frozen sections were stored at −80°C until use. Part of the samples were fixed in 10% neutral buffered formalin and embedded in paraffin for 5-µm thick tissue sections. Hematoxylin & Eosin (H & E) staining was performed to evaluate the morphology of the tumors and progression. To detect blood perfusion, Hoechst 33342 was observed under UV light with blue filter. For immunofluorescence staining, FITC-conjugated anti-pimonidazole monoclonal antibody (HP2-1000, Natural Pharmacia International, 1:50) using green filter under fluorescence was examined, and propidium iodide (PI) was used as counterstain. All images were captured under consistent illumination and exposure for their respective stains. No image post-processing was performed.
Disclosure of potential conflicts of interest
This research was funded by Threshold Pharmaceuticals, Inc., and Merck KGaA. J Sun, D Ahluwalia, Q Liu, D Ferraro, D Jung, M Matteucci, and C Hart are employees of Threshold Pharmaceuticals, Inc., and hold either stock or stock options in the company. Y Wang is a former employee of Threshold Pharmaceuticals, Inc., and held either stock or stock options in the company.
References
- 1.Smith Williams, Introduction to the Principles of Drug Design and Action, 4th Ed, USA: Taylor and Francis Group, 216-30. [Google Scholar]
- 2.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jaervinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov 2008; 7:255-70; PMID:18219308; http://dx.doi.org/ 10.1038/nrd2468 [DOI] [PubMed] [Google Scholar]
- 3.Huttunen KM, Raunio H, Rautio J: Prodrugs – from serendipity to rational design. Pharmacol Rev 2011; 63:750-71; PMID:21737530; http://dx.doi.org/ 10.1124/pr.110.003459 [DOI] [PubMed] [Google Scholar]
- 4.Stella VJ. Prodrugs: Some thoughts and current issues. J Pharm Sci 2010; 99:4755-65; PMID:20821387; http://dx.doi.org/ 10.1002/jps.22205 [DOI] [PubMed] [Google Scholar]
- 5.Brade WP, Herdrich K, Varini M. Ifosfamide- pharmacology, safety and therapeutic potential. Cancer Treat Rev 1985; 12:1-47; PMID:3896483; http://dx.doi.org/ 10.1016/0305-7372(85)90011-8 [DOI] [PubMed] [Google Scholar]
- 6.Dechant KL, Brogden RN, Pilkington T, Faulds D.. Ifosfamide/Mesna: A review of its antineoplastic activity, pharmacokinetic properties and therapeutic efficacy in Cancer. Drugs 1991; 42: 428-67; PMID: 1720382; http://dx.doi.org/ 10.2165/00003495-199142030-00006 [DOI] [PubMed] [Google Scholar]
- 7.Brade W, Seeber S, Herdrich K. Comparative activity of ifosfamide and cyclophosphamide. Cancer Chemother Pharmacol 1986; 18 (suppl 2): S1-S9; PMID:3545522; http://dx.doi.org/ 10.1007/BF00647438 [DOI] [PubMed] [Google Scholar]
- 8.Boni C, Zanelli F. Ifosfamide in non-small cell lung cancer. Oncology 2003; 65 Suppl 2:50-4; PMID:14586148; http://dx.doi.org/ 10.1159/000073359 [DOI] [PubMed] [Google Scholar]
- 9.Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem 2001; 36:577-95; PMID:11600229; http://dx.doi.org/ 10.1016/S0223-5234(01)01253-3 [DOI] [PubMed] [Google Scholar]
- 10.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman RM, Ammons WS, Hart CP, Matteucci M. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem 2008; 51:2412-20; PMID:18257544; http://dx.doi.org/ 10.1021/jm701028q [DOI] [PubMed] [Google Scholar]
- 11.Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, et al.. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther 2012; 11:740-51; PMID:22147748; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0634 [DOI] [PubMed] [Google Scholar]
- 12.Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD, Hart CP. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res 2012; 18:758-70; PMID:22184053; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1980 [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD, Hart CP. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol 2012; 69:1487-98; PMID:22382881; http://dx.doi.org/ 10.1007/s00280-012-1852-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, Liu Q, Sun JD, Van Camp B, Hart CP, et al.. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood 2010; 116:1524-27; PMID:20530289; http://dx.doi.org/ 10.1182/blood-2010-02-269126 [DOI] [PubMed] [Google Scholar]
- 15.Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S, Ganjoo KN. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol 2014; 32:3299-306; PMID:25185097; http://dx.doi.org/ 10.1200/JCO.2013.54.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J Jr, Chiorean EG, Rosen PJ, et al.. Randomized Phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2015; 33:1475-81; PMID:25512461; http://dx.doi.org/ 10.1200/JCO.2014.55.7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navada S, Lai P, Schwartz AG, Kalemkerian GP. Temporal trends in small cell lung cancer: analysis of the national Surveillance Epidemiology and End-Results (SEER) database [abstract 7082]. J Clin Oncol 2006; 24 (18S) suppl:384S [Google Scholar]
- 18.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008; 83:355-67; PMID:18316005; http://dx.doi.org/ 10.4065/83.3.355 [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Martin C, Balaña C. Ifosfamide in non-small-cell lung cancer. Ann Oncol 1999; 10 Suppl 5: S25-8; PMID:10582135; http://dx.doi.org/ 10.1093/annonc/10.suppl_5.S26 [DOI] [PubMed] [Google Scholar]
- 20.Johnson DH. Overview of ifosfamide in small cell and non-small cell lung cancer. Semin Oncol 1990; 17:24-30; PMID:2159187; http://dx.doi.org/ 10.1016/0169-5002(91)90216-S [DOI] [PubMed] [Google Scholar]
- 21.Vlahovic G, Infante JR, Mita AC, Traynor AM, Molina JR, Lacouture ME, Langmuir VK, Eng C, Kroll S, Borad MJ. Phase I/II study of TH-302 in combination with pemetrexed in patients with solid tumors including NSCLC [abstract]. J Clin Oncol 2010; 28 (15S) suppl: e13535 [Google Scholar]
- 22.Azrak RG, Cao S, Slocum HK, Tóth K, Durrani FA, Yin MB, Pendyala L, Zhang W, McLeod HL, Rustum YM. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res 2004; 10:1121-9; PMID:14871992; http://dx.doi.org/ 10.1158/1078-0432.CCR-0913-3 [DOI] [PubMed] [Google Scholar]
- 23.Nagamachi Y, Tani M, Shimizu K, Tsuda H, Niitsu Y, Yokota J. Orthotopic growth and metastasis of human non-small cell lung carcinoma cell injected into the pleural cavity of nude mice. Cancer Lett 1998; 127:203-09; PMID:9619878 http://dx.doi.org/ 10.1016/s0304-3835(98)00039-1 [DOI] [PubMed] [Google Scholar]
- 24.Talmadge JE, Singh RK, Fidler IJ, Raz A.. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007; 170:793-804; PMID:17322365; http://dx.doi.org/ 10.2353/ajpath.2007.060929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manzotti C, Audisio RA, Patresi G. Importance of orthotopic implantation for human tumors as model systems: relevance to metastasis and invasion. Clin Exp Metastasis 1993; 11:5-11; PMID:8422706; http://dx.doi.org/ 10.1007/BF00880061 [DOI] [PubMed] [Google Scholar]
- 26.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther 2003; 2: S134-9; PMID:14508091; http://dx.doi.org/ 10.4161/cbt.213 [DOI] [PubMed] [Google Scholar]
- 27.Inaba M, Tashiro T, Kobayashi T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T.. Responsiveness of human gastric tumors implanted in nude mice to clinically equivalent doses of various antitumor agents. Jpn J Cancer Res. 1988; 79:517-22; PMID: 3133340 http://dx.doi.org/ 10.1111/j.1349-7006.1988.tb01621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba M, Tashiro T, Kobayashi T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T. Responsiveness of human gastric tumors implanted in nude mice to clinically equivalent doses of various antitumor agents. Jpn J Cancer Res 1988; 79:17-22; PMID:2833480; http://dx.doi.org/ 10.1111/j.1349-7006.1988.tb00005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba M, Kobayashi T, Tashiro T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T. Evaluation of antitumor activity in a human breast tumor/nude mouse model with a special emphasis on treatment dose. Cancer 1989; 64:1577-82; PMID:2507122; http://dx.doi.org/ 10.1002/1097-0142(19891015)64:8%3c1577::AID-CNCR2820640803%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 30.Tashiro T, Inaba M, Kobayashi T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T. Responsiveness of human lung cancer/nude mouse to antitumor agents in a model using clinically equivalent doses. Cancer Chemother Pharmacol 1989; 24:187-92; PMID:2544308; http://dx.doi.org/ 10.1007/BF00300241 [DOI] [PubMed] [Google Scholar]
- 31.Inaba M, Kobayashi T, Tashiro T, Sakurai Y. Pharmacokinetic approach to rational therapeutic doses for human tumor-bearing nude mice. Jpn J Cancer Res 1988; 79:509-16; PMID:3133339; http://dx.doi.org/ 10.1111/j.1349-7006.1988.tb01620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segura C, Bandrés E, Trocóniz IF, García-Foncillas J, Sayar O, Dios-Vieítez C, Renedo MJ, Garrido MJ. Hematological response of topotecan in tumor-bearing rats: modeling of the time course of different cellular populations. Pharm Res 2004; 21:567-73; PMID:15139512; http://dx.doi.org/ 10.1023/B:PHAM.0000022402.00699.5c [DOI] [PubMed] [Google Scholar]
- 33.Walsh TJ, Aoki S, Mechinaud F, Bacher J, Lee J, Rubin M, Pizzo PA. Effects of preventive, early, and late antifungal chemotherapy with fluconazole in different granulocytopenic models of experimental disseminated candidiasis. J Infect Dis 1990; 161:755-60; PMID:2138654; http://dx.doi.org/ 10.1093/infdis/161.4.755 [DOI] [PubMed] [Google Scholar]
- 34.Dale DC, Cottle TE, Fier CJ, Bolyard AA, Bonilla MA, Boxer LA, Cham B, Freedman MH, Kannourakis G, Kinsey SE, et al.. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol 2003; 72:82-93; PMID:12555210; http://dx.doi.org/ 10.1002/ajh.10255 [DOI] [PubMed] [Google Scholar]
- 35.Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, Tibes R, Ramanathan RK, Lewandowski K, Jones SF, et al.. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res 2011; 17:2997-300437; PMID:21415214; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3425 [DOI] [PubMed] [Google Scholar]
- 36.Hunter FW, Hsu HL, Su J, Pullen SM, Wilson WR, Wang J. Dual targeting of hypoxia and homologous recombination repair dysfunction in triple-negative breast cancer. Mol Cancer Ther 2014; 13:2501-1438; PMID:25193512; http://dx.doi.org/ 10.1158/1535-7163.MCT-14-0476 [DOI] [PubMed] [Google Scholar]
- 37.Kurowski V, Wagner T. Comparative pharmacokinetics of ifosfamide, 4-hydroxyifosfamide, chloroacetaldehyde, and 2- and 3-dechloroethylifosfamide in patients on fractionated intravenous ifosfamide therapy. Cancer Chemother Pharmacol 1993; 33:36-42; PMID:8269587; http://dx.doi.org/ 10.1007/BF00686020 [DOI] [PubMed] [Google Scholar]
- 38.Hureaux J, Lagarce F, Gagnadoux F, Rousselet MC, Moal V, Urban T, Benoit JP. Toxicological study and efficacy of blank and paclitaxel-loaded lipid nanocapsules after i.v. administration in mice. Pharm Res 2010; 27:421-30; PMID:20054705; http://dx.doi.org/ 10.1007/s11095-009-0024-y [DOI] [PubMed] [Google Scholar]