Abstract
Purpose
Smoking has a negative impact on disease activity in Crohn’s disease (CD). Smoking may also affect the quality of life, but this has not been evaluated using validated measures over time. We assessed the relationship between smoking and disease-specific quality of life over time in a tertiary referral inflammatory bowel disease cohort.
Patients and methods
Retrospective cohort study from July 2004 to July 2009 in patients with CD identified from the University of Maryland, Baltimore, Institutional Review Board-approved University of Maryland School of Medicine Inflammatory Bowel Disease Program database. Smoking status was classified as current, former, and never. Age was categorized as <40 years, 40–59 years, and ≥60 years. Index visit disease activity and quality of life was measured with the Harvey–Bradshaw index, and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ). Repeated measures linear regression was used to assess the association between smoking and quality of life over time after adjustment for confounding variables.
Results
A total of 608 patients were included, of whom 42% were male; 80% were Caucasian; 22% were current smokers; 24% were former smokers; and 54% were never smokers. Over time, adjusted Harvey–Bradshaw index scores declined in all patients, but current smokers had consistently higher scores. After adjustment for sex, age, and disease duration, never smokers had higher mean SIBDQ scores at index visit compared to former and current smokers (P<0.0001); all increased over time but SIBDQ scores for never smokers remained consistently highest.
Conclusion
Smoking has a negative impact on disease activity and quality of life in patients with CD. Prospects of improved disease activity and quality of life should be proposed as an additional incentive to encourage smoking cessation in patients with CD.
Keywords: inflammatory bowel disease, SIBDQ, Harvey–Bradshaw index
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a multifactorial inflammatory disorder of the gastrointestinal tract that affects over 1 million people in the US.1 While the pathogenesis is unclear, cigarette smoking is well associated with IBD outcomes, resulting in increased risk and exacerbation of CD, and a protective effect in UC.2–5
The Center for Disease Control estimates that there are ~42 million smokers in the US.6 Smoking has been shown to adversely impact outcomes in several disease processes, including cardiovascular disease and human immunodeficiency virus infection.7,8 While smoking seems to ameliorate the effect of UC, it has been well described as a negative predictor in CD, resulting in increased risk of surgery, perianal disease, and an overall complicated disease course.9 Despite its deleterious effect, and its modifiable nature among environmental exposures, the prevalence of smoking has been estimated to range from 20% to 33% in cohort studies.10,11
Decrease in the quality of life has been described among smokers without IBD.12 In CD, smoking has also been shown to have a negative effect on the quality of life in cross-sectional studies.9 To our knowledge, the effect of smoking on the quality of life over time has not been described in IBD using disease-specific validated measures. We sought to describe longitudinal measures of disease-specific quality of life by smoking status in a tertiary center IBD cohort.
Materials and methods
We conducted a retrospective cohort study from July 2004 to July 2009 in patients who were identified from an institutional review board-approved clinical data repository, with a confirmed diagnosis of CD by clinical, endoscopic, radiologic, and histologic criteria. Demographics and clinical variables were extracted from the data repository, including sex, race, age at index visit (categorized as age <40 years, 40–59 years, and ≥60 years), and behavioral phenotype by Montreal classification.13 Perianal disease behavior and upper tract location information were included as modifiers. Behavior and location disease modifiers occur concurrently with other behavior and location categories.
Smoking status was classified as current, former, and never, as determined by the patient at the intake visit. CD activity was measured at each clinical visit with the Harvey–Bradshaw index (HBI),14 and disease-specific quality of life was also measured at each clinical visit using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ).15 Demographics and disease characteristics were compared using the X2 test for categorical variables, and analysis of variance testing was used for continuous variables. Repeated measures linear regression was used to assess the association between smoking status and disease-specific quality of life over time after adjustment for confounding variables described in means and standard deviation. This study was approved by the University of Maryland, Baltimore Institutional Review Board (IRB). A waiver for consent was granted as this was a retrospective analysis of de-identified data from the Universities IRB-approved database.
Results
Demographic characteristics
Six hundred and nine patients with CD were included. Forty-two percent were male, and 80% were Caucasian (Table 1). Twenty-two percent of patients were identified as current smokers, 24% were former smokers, and 54% were never smokers at the initial visit. There were no statistically significant differences in sex, race, or disease location across groups by smoking status.
Table 1.
Demographics of participants with CD from the University of Maryland Baltimore IBD Program by smoking status 2004–2012
| Total N=608 n (100%) |
Never smokers N=326 n (53.6%) |
Former smokers N=147 n (24.2%) |
Current smokers N=135 n (22.2%) |
P-value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 254 (41.9) | 137 (42) | 60 (41) | 57 (42) | 0.96 |
| Race | |||||
| Caucasian | 340 (80) | 176 (78) | 83 (84) | 81 (82) | 0.69 |
| CD behavior | |||||
| Infammatory | 211 (35) | 125 (41) | 53 (39) | 33 (27) | 0.02 |
| Stricturing | 175 (29) | 80 (26) | 48 (35) | 47 (38) | |
| Penetrating | 179 (29) | 101 (33) | 35 (26) | 43 (35) | |
| Perianal disease* | 184 (25) | 92 (28) | 41 (28) | 51 (38) | |
| CD location | |||||
| Ileal | 186 (31) | 96 (34) | 45 (35) | 45 (40) | 0.52 |
| Ileocolonic | 216 (36) | 118 (42) | 51 (39) | 47 (42) | |
| Colonic | 122 (23) | 69 (24) | 33 (25) | 20 (18) | |
| Upper tract** | 79 (13) | 49 (19) | 11 (7) | 19 (14) | |
| Age at diagnosis (years) | |||||
| <40 | 350 (58) | 214 (66) | 66 (45) | 70 (52) | <0.01 |
| 40–59 | 195 (32) | 88 (27) | 54 (37) | 53 (39) | |
| ≥60 | 63 (10) | 24 (7) | 27 (18) | 12 (9) |
Notes:
This is a disease modifer and may coexist with other behavioral phenotypes.
This is a disease modifer and may coexist with other disease locations.
Abbreviations: CD, Crohn’s disease; IBD, infammatory bowel disease.
Disease characteristics
As demonstrated in Table 1, the mean disease duration was 10.3 (±10.3) years at the index visit. The maximum follow-up time was 5 years and the mean follow-up time was 1.6 years. Two hundred and eleven (35%) patients had inflammatory disease phenotype, 175 (29%) had stricturing phenotype, 179 (29%) had perforating behavior phenotype, and 184 (25%) had perianal disease. One hundred and eighty-six (31%) had isolated ileal disease location, 216 (36%) had ileocolonic location, 122 (23%) had isolated colonic disease, and 79 (13%) had upper tract involvement.
Smoking status characteristics
As demonstrated in Table 1, patients diagnosed at age <40 years were more likely to be never smokers compared to patients diagnosed at age 40–59 and ≥60 years (P<0.01). Never smokers and former smokers were more likely to have inflammatory behavior phenotype, whereas current smokers were more likely to have complicated disease behavior (stric-turing or penetrating disease) (P=0.02).
Mean HBI scores were higher at index visit among current smokers (4.6±5.0) than former smokers (4.3±5.3), and never smokers (4.3±5.3) (P<0.0001) (Figure 1). Over time, after adjustment for sex, age at index visit, and disease duration, HBI scores declined significantly over time in all patients, but current smokers had consistently higher scores (Figure 1). In terms of disease-specific quality of life, never smokers had higher mean SIBDQ scores at index visit (48.3±13.6), compared to former smokers (46.6±14.1) and current smokers (39.0±14.1) (P<0.0001) (Figure 2). After adjustment for sex, age at index visit, and disease duration, disease-specific quality of life increased significantly over time in all patients, but quality of life scores for never smokers remained consistently higher than their former and current smoker counterparts.
Figure 1.
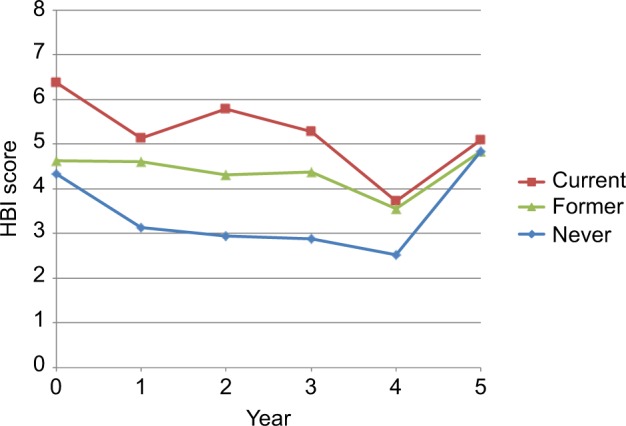
HBI score by smoking status, per year.
Abbreviation: HBI, Harvey–Bradshaw index.
Figure 2.
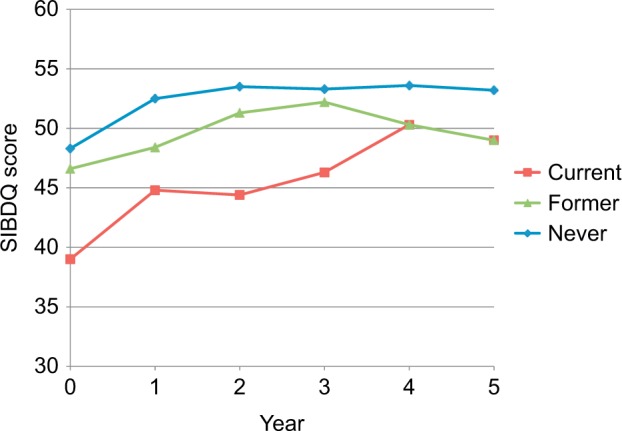
SIBDQ score by smoking status, per year.
Abbreviation: SIBDQ, Short Inflammatory Bowel Disease Questionnaire.
Discussion
To our knowledge, this is the first study examining the longitudinal course of disease activity and quality of life based on smoking status in an IBD cohort using validated measures. In this study, we examined the relationship between smoking and disease activity as well as disease-specific quality of life over time in 608 patients with CD. After adjustment for age, sex, and disease duration, current smokers had significantly increased disease activity and decreased quality of life at the initial visit compared to former and never smokers. The differences in disease activity and quality of life among the three smoking groups persisted over time, although all groups experienced a decrease in disease activity and an increase in quality of life over time.
These findings describe an inverse correlation between smoking status and quality of life in CD, and are consistent with the existing literature.16 The largest study examining this question was a cross-sectional study from the Netherlands, in which smoking status was associated with decrease in the quality of life, as measured by the SIBDQ in over 1,100 patients with CD. This difference was attributed to increased bowel symptoms, as well as worsened emotional factors.9 Interestingly, this difference was more pronounced among young females, though no sex differences were noted in our study. In a more recent cohort study conducted in Australia, the longitudinal effect of smoking on disease progression and risk of surgery was assessed using the Sydney IBD Cohort database, including 1,203 patients with IBD, of whom 626 had CD.10 In this study, no difference in disease location or behavior was detected by smoking status; however, after a median follow-up period of 9 years, smokers with CD exhibited an increased risk of surgery, more frequent hos-pitalizations, and increased risk of peripheral arthritis. In a prospective cohort study of 622 patients with CD, smoking was associated with more frequent disease flares, and this risk was notably increased with smoking >15 cigarettes per day.17 Quality of life measures were not included in these studies.
The observed decrease in the quality of life of CD patients may be due to the negative effect of smoking on disease activity; however, other factors may also be at play. Literature in the field of psychiatry suggests there may be an association between smoking and depression,12 which may also adversely impact disease-activity scores by outside factors that do not necessarily correlate with active inflammation. Our study was limited by the classification of smoking status at the index visit only, without additional longitudinal data on smoking status. We also were not able to adjust for medication use in this cohort, a potential confounder of disease activity and quality of life.
Importantly, patients are often not aware of the negative impact of smoking on CD. In a study of current and former smokers with IBD, only eleven out of 41 current smokers were aware that smoking could exacerbate their disease, and none of the 19 former smokers surveyed were aware of this association.18 In addition, 27 of the 41 smokers had never been asked to stop smoking by their primary doctor. This stresses the role of the provider to educate patients regarding the deleterious effects of smoking in CD. Long-term improvement in quality of life should be proposed as an additional incentive to encourage smoking cessation in patients with CD.
Acknowledgments
This work was supported by the T32 DK067872 Research Training in Gastroenterology NIDDK grant. This study abstract was presented in poster format at the Digestive Diseases Week (DDW) conference in May 2013.19
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12(38):6102–6118. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989;34(12):1841–1854. doi: 10.1007/BF01536701. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101(7):1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 6.Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults – United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 7.Stafford L, Berk M, Jackson HJ. Tobacco smoking predicts depression and poorer quality of life in heart disease. BMC Cardiovasc Disord. 2013;13:35. doi: 10.1186/1471-2261-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crothers K, Griffith TA, McGinnis KA. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russel MG, Nieman FH, Bergers JM, Stockbrügger RW. Cigarette smoking and quality of life in patients with inflammatory bowel disease. South Limburg IBD Study Group. Eur J Gastroenterol Hepatol. 1996;8(11):1075–1081. doi: 10.1097/00042737-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lunney PC, Kariyawasam VC, Wang RR, et al. Smoking prevalence and its influence on disease course and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2015;42(1):61–70. doi: 10.1111/apt.13239. [DOI] [PubMed] [Google Scholar]
- 11.Regueiro M, Kip KE, Cheung O, Hegazi RA, Plevy S. Cigarette smoking and age at diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(1):42–47. doi: 10.1097/00054725-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg MI, Danovitch I, IsHak WW. Quality of life and smoking. Am J Addict. 2014;23(6):540–562. doi: 10.1111/j.1521-0391.2014.12148.x. [DOI] [PubMed] [Google Scholar]
- 13.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 15.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91(8):1571–1578. [PubMed] [Google Scholar]
- 16.Rubin DT, Hanauer SB. Smoking and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12(8):855–862. doi: 10.1097/00042737-200012080-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cosnes J, Carbonnel F, Carrat F, Beaugerie L, Cattan S, Gendre J. Effects of current and former cigarette smoking on the clinical course of Crohn’s disease. Aliment Pharmacol Ther. 1999;13(11):1403–1411. doi: 10.1046/j.1365-2036.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 18.Shields PL, Low-Beer TS. Patients’ awareness of adverse relation between Crohn’s disease and their smoking: questionnaire survey. BMJ. 1996;313(7052):265–266. doi: 10.1136/bmj.313.7052.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quezada S, Langenberg P, Cross R. Cigarette smoking adversely affects disease activity and disease-specific quality of life in patients with Crohn’s disease (CD) evaluated at tertiary referral center. Gastroen-terology. 2013;144(5):S–762. doi: 10.2147/CEG.S104652. [DOI] [PMC free article] [PubMed] [Google Scholar]


