Abstract
Takotsubo cardiomyopathy (TC) is a rare condition, characterized by acute left ventricular (LV) dysfunction in the absence of flow-limiting coronary artery disease, usually provoked by a physical or emotional stressor. The condition is far more common in women. The commonest presenting symptoms in patients with TC are chest pain and shortness of breath, often mimicking an acute coronary syndrome. A number of complications of TC are recognized, and very rarely patients experience cardioembolic phenomena secondary to LV thrombus formation in TC. We present the case of a 48-year-old lady presenting with peripheral limb ischaemia, subsequently found to have an LV thrombus secondary to TC. Diagnosis of TC was made challenging by the absence of chest pain. She required urgent arterial embolectomy and was treated with 6-month oral anticoagulation therapy. She was also commenced on beta-blocker and angiotensin-converting enzyme inhibitor treatment for the management of LV dysfunction.
INTRODUCTION
Takotsubo cardiomyopathy (TC) is a rare condition, characterized by acute left ventricular (LV) dysfunction in the absence of flow-limiting coronary artery disease, usually provoked by a physical or emotional stressor [1]. The condition is far more common in women (>95% of cases are female), particularly those over the age of 50 years [2, 3]. Other risk factors include smoking, alcohol misuse, anxiety disorders and hyperlipidaemia [2].
The commonest presenting symptoms in patients with TC are chest pain and shortness of breath, often mimicking an acute coronary syndrome. Less commonly, patients may present with cardiogenic shock, syncope, or even cardiac arrest [4]. Approximately two-thirds of cases are preceded by a stressful physical or emotional event, such as the death of a loved one, financial crisis or trauma. In one-third, however, no trigger is identified [3]. Electrocardiographic abnormalities in TC include ST-segment changes, predominantly in the precordial leads. Occasionally, QT prolongation or conduction abnormalities are seen [4]. Serum biomarkers of myocardial necrosis, such as cardiac troponins, are usually elevated. These features make distinguishing TC from an acute coronary syndrome a diagnostic challenge, and typically patients will undergo coronary angiography to investigate the possibility of epicardial coronary artery disease [5]. Angiography allows distinction of TC from an acute coronary syndrome by excluding significant (>50%) coronary artery stenosis [5].
Echocardiography and LV angiography demonstrate marked LV dysfunction in TC. Classically, there is apical ballooning of the LV with a hyperdynamic base. There is moderate to severe dysfunction of the mid- and apical segments of the LV, not in keeping with any regional coronary artery territory [6].
No guidelines exist for the treatment of TC. Initially, patients should be managed as for an acute coronary syndrome. Once this is excluded, supportive treatments for LV dysfunction, such as beta-blockade and renin–angiotensin–aldosterone inhibition, should be employed [5]. A number of complications are recognized, including heart failure, LV thrombus formation, ventricular tachyarrhythmia, shock, stroke and LV/ventricular septum rupture [5]. Very rarely, patients experience cardioembolic complications secondary to LV thrombus formation [7]. When complications do arise, management is according to the relevant guidelines.
CASE REPORT
A 48-year-old lady presented with a 2-day history of an exquisitely painful and tender right leg. She also reported the occurrence of progressive dyspnoea over several days but denied chest discomfort. Her past medical history was significant for chronic obstructive pulmonary disease and degenerative spinal disc disease.
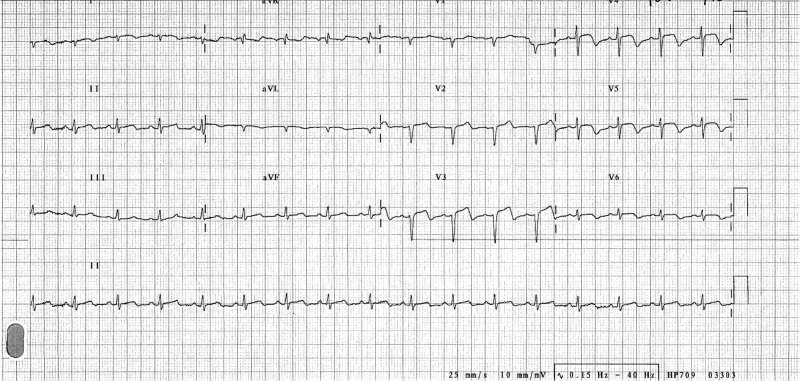
She was hypotensive (blood pressure 79/43 mmHg), tachycardic (heart rate 130 beats per minute) and tachypnoeic (respiratory rate 30 breaths per minute) on admission. There was no fever or hypoxia. She was visibly uncomfortable at rest, due to marked right leg pain and dyspnoea. Examination of her right leg revealed a swollen, cold, pale and tender extremity with no palpable popliteal, posterior tibial or dorsalis pedis pulses. Bilateral basal inspiratory crackles were audible on chest auscultation. Her electrocardiogram (ECG) on admission showed a sinus tachycardia with anterior ST-segment elevation (Fig. 1), and bilateral interstitial oedema was evident on her chest X-ray.
Figure 1:
Twelve-lead ECG. This demonstrates a sinus tachycardia, with ST-segment elevation in leads V3–V5. There is T-wave inversion in leads V3–V6.
In the absence of initial chest pain, it was elected that she would undergo emergency right femoral embolectomy and a right calf fasciotomy for critical right lower limb ischaemia complicated by an acute compartment syndrome. Postoperatively, she required admission to the intensive care unit for progressive pulmonary oedema with increasing dyspnoea.
Blood testing revealed elevated cardiac troponin I values (initial troponin I 1443 ng/ml, repeat troponin I 1456 ng/ml). Transthoracic echocardiography then showed an LV with extensive regional wall motion abnormalities. There was akinesis/hypokinesis of the apical and mid-LV, with preservation of the basal segments (Supplementary Video 1). Overall, the LV function was moderate to severely impaired.
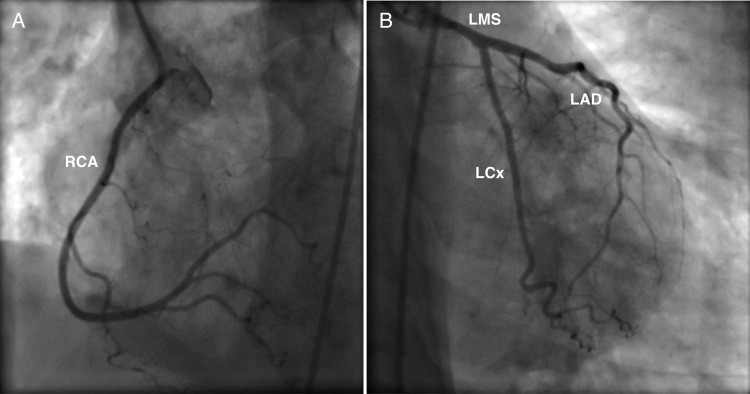
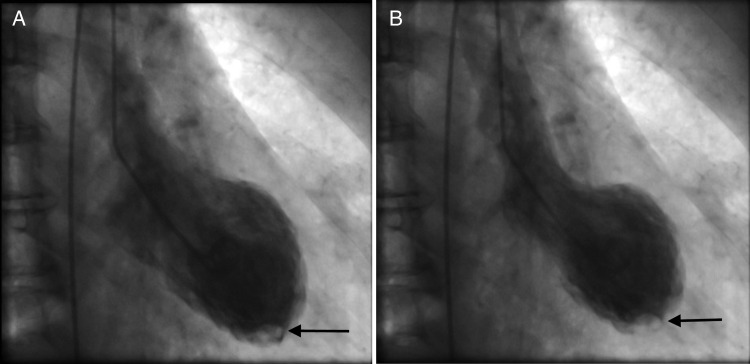
Urgent coronary angiography was performed, which did not demonstrate any flow-limiting coronary artery stenosis (Fig. 2). LV angiography, however, revealed apical ballooning of the LV with an apical filling defect (Fig. 3). These appearances were in keeping TC together with an apical LV thrombus.
Figure 2:
Coronary artery angiogram images demonstrating the patient's unobstructed coronary arteries: (A) the right coronary artery (RCA) and (B) the left coronary system (LMS, left main stem; LAD, left anterior descending; LCx, left circumflex).
Figure 3:
LV angiogram images in diastole (A) and systole (B) showing apical ballooning and an apical filling defect (arrow) highlighting the LV thrombus.
The patient's critical limb ischaemia resolved following surgery. Her pulmonary oedema was treated with intravenous diuretic therapy and her symptoms of dyspnoea resolved. Repeat ECGs showed resolution of the initial ST-segment changes. She was commenced on a 6-month course of oral anticoagulation for management of her LV thrombus complicated by peripheral limb embolism. Her clinical condition improved dramatically. A repeat echocardiogram, performed 3 months after her initial presentation, showed a dramatic improvement in LV systolic function with resolution of the previous wall motion abnormalities and apical ballooning (Supplementary Video 2). This confirmed our diagnosis of TC.
DISCUSSION
We have presented a case of TC with the extremely rare complications of LV thrombus with peripheral embolism. One systematic review showed that only 2.5% of TC cases are complicated by LV thrombus and fewer than 1% by cardioembolic complications [7]. Diagnosis of TC was made challenging by the absence of chest pain and disproportionate right leg pain. There was also no clear stressful precipitant for TC. The patient's pulmonary oedema, ECG changes, elevated cardiac enzymes and echocardiographic findings, however, could not be explained in the absence of significant coronary artery disease. The diagnosis of TC was, therefore, confirmed by LV angiogram.
TC can often represent a diagnostic challenge for clinicians. For this reason, a series of diagnostic criteria for TC have been devised by the Mayo Clinic (Box 1) [5]. This case clearly fulfils three of the four Mayo Clinic Criteria, and the diagnosis of TC was made with confidence. The European Society of Cardiology (ESC) recommends 3 months anticoagulation for TC complicated by LV thrombus [5], and the British Committee for Standards in Haematology recommends ‘long-term’ anticoagulation for patients who suffer from arterial embolism, with an international normalized ratio target of 2.5 [8]. Given that there was peripheral embolism from the LV thrombus in this case, 6 months oral anticoagulation therapy was deemed appropriate. No specific guidelines, however, exist for arterial embolism in the context of LV thrombus, so the duration of anticoagulation was chosen somewhat arbitrarily. Guidelines to address anticoagulation therapy for embolic phenomena secondary to LV thrombus would be of use in clinical practice. She was also commenced on beta-blocker and angiotensin-converting enzyme inhibitor treatment, for management of LV dysfunction. Outpatient echocardiography and follow-up were organized for 3 months post-discharge to allow demonstration of improvement in LV function and symptoms.
Box 1: Mayo Clinic diagnostic criteria for TC [5].
1. Transient hypokinesis, akinesis or dyskinesis of the LV mid-segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial coronary vascular distribution; a stressful trigger is often, but not always present.
2. Absence of obstructive coronary disease or angiographic evidence of acute coronary plaque rupture.
3. New electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin enzymes.
4. Absence of phaeochromocytoma or myocarditis. In both of the above circumstances, the diagnosis of TC should be made with caution, and a clear stressful precipitating trigger must be sought.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Oxford Journal online.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ETHICAL APPROVAL
None required.
CONSENT
The patient presented in this report gave her full written informed consent for this work to be published.
GUARANTOR
G.G. is the guarantor of this work.
REFERENCES
- 1.Sharkey SW, Lesser JR, Maron BJ. Cardiology patient page. Takotsubo (stress) cardiomyopathy. Circulation 2011;124:e460–2. [DOI] [PubMed] [Google Scholar]
- 2.Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of takotsubo cardiomyopathy in the United States. Am Heart J 2012;164:66–71 e1. [DOI] [PubMed] [Google Scholar]
- 3.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. [DOI] [PubMed] [Google Scholar]
- 4.Antonopoulos A, Kyriacou C. Apical ballooning syndrome or takotsubo cardiomyopathy: a new challenge in acute cardiac care. Cardiol J 2008;15:572–7. [PubMed] [Google Scholar]
- 5.Kyriacou C. Identifying takotsubo cardiomyopathy. e-J ESC Counc Cardiol Pract 2012;10. [Google Scholar]
- 6.Movahed MR. Important echocardiographic features of takotsubo or stress-induced cardiomyopathy that can aid early diagnosis. JACC Cardiovasc Imaging 2010;3:1200–1; author reply 1201. [DOI] [PubMed] [Google Scholar]
- 7.de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with takotsubo-like syndrome: a systematic review. Int J Cardiol 2008;131:18–24. [DOI] [PubMed] [Google Scholar]
- 8.Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin – fourth edition. Br J Haematol 2011;154:311–24. [DOI] [PubMed] [Google Scholar]