Abstract
Neural changes related to the learning of the pronunciation of Chinese characters in English speakers were examined using fMRI. We examined the item-specific learning effects for trained characters and the generalization of phonetic knowledge to novel transfer characters that shared a phonetic radical (part of a character that gives a clue to the whole character’s pronunciation) with trained characters. Behavioral results showed that shared phonetic information improved performance for transfer characters. Neuroimaging results for trained characters over learning found increased activation in the right lingual gyrus, and greater activation enhancement in the left inferior frontal gyrus (Brodmann’s area 44) was correlated with higher accuracy improvement. Moreover, greater activation for transfer characters in these two regions at the late stage of training was correlated with better knowledge of the phonetic radical in a delayed recall test. The current study suggests that the right lingual gyrus and the left inferior frontal gyrus are crucial for the learning of Chinese characters and the generalization of that knowledge to novel characters. Left inferior frontal gyrus is likely involved in phonological segmentation, whereas right lingual gyrus may subserve processing visual–orthographic information.
INTRODUCTION
Reading acquisition involves the development of phonological and orthographic representations and the establishment of associations between these two types of representation. Although writing systems vary in the nature of speech sounds and word forms and in how word forms represent units of spoken sounds, increasing evidence from imaging studies has revealed that there are both common and distinct neural mechanisms across different writing systems (Perfetti et al., 2007; Bolger, Perfetti, & Schneider, 2005; Chen, Fu, Iversen, Smith, & Matthews, 2002; Chee, Tan, & Thiel, 1999). On the one hand, as suggested by both bilingual and monolingual studies, reading both English (alphabetic) and Chinese (nonalphabetic) recruits the left inferior occipito-temporal (OT) cortex (covering lingual gyrus, fusiform gyrus, and inferior occipital gyrus) for visual analysis of word forms (Nelson, Liu, Fiez, & Perfetti, 2009; Wong, Jobard, James, James, & Gauthier, 2009; Tan, Liu, et al., 2001) and the left inferior frontal gyrus (IFG; pars opercularis and pars triangularis) for phonological processing (Liu et al., 2009; Booth et al., 2006; Tan, Laird, Li, & Fox, 2005; Li et al., 2004). On the other hand, activations in the left temporo-parietal (TP) cortex (including inferior parietal lobule, angular gyrus, and supramarginal gyrus) have been mainly found in phonological tasks for English but rarely for Chinese (Tan et al., 2005), indicating its role in the mapping from graphemes to phonemes (Booth et al., 2002a, 2002b). Activations in the right OT have been extensively found in orthographic processing for Chinese but not for English (Lee, 2004; Li et al., 2004; Tan et al., 2003; Tan, Liu, et al., 2001), suggesting that the right OT is associated with processing the logographic nature of Chinese characters.
These patterns are also supported by studies of orthographic–phonological learning in adults and by studies of language development. After phonological learning of novel visual word forms, learning-induced increases have been consistently found in the left IFG and OT region exclusively for trained stimuli for alphabetic and nonalphabetic scripts (Xue, Chen, Jin, & Dong, 2006; Bitan, Manor, Morocz, & Karni, 2005; Callan, Callan, & Masaki, 2005; Gronholm, Rinne, Vorobyev, & Laine, 2005; Hashimoto & Sakai, 2004; Sandak et al., 2004). Activation increases in the TP region have been mainly found for alphabetic systems in phonological training studies in typical adults (Breitenstein et al., 2005; Raboyeau et al., 2004; Sandak et al., 2004) or for phonological remediation in dyslexics (Eden et al., 2004; Temple et al., 2003; Small, Flores, & Noll, 1998). In contrast, the recruitment of the right OT has been consistently found for learning Chinese in English speakers (Nelson et al., 2009; Liu, Dunlap, Fiez, & Perfetti, 2007), reflecting accommodative responses to a new writing system that requires distinct orthographic processing (Perfetti et al., 2007; Bolger et al., 2005). Evidence from developmental studies is consistent with these training studies and has also shown age increases in the IFG, OT, and TP regions in the left hemisphere in English (Church, Coalson, Lugar, Petersen, & Schlaggar, 2008; Cone, Burman, Bitan, Bolger, & Booth, 2008; Bitan et al., 2007; Shaywitz et al., 2002, 2007; Booth et al., 2004; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003) and increases in the left IFG, left OT, and right OT regions in Chinese (Cao et al., 2009, 2010).
The generalization of phonological knowledge has been explored within alphabetic systems at both behavioral and neural levels, suggesting that acquired phonological knowledge can facilitate novel word recognition (Ferman, Olshtain, Schechtman, & Karni, 2009; Finley & Badecker, 2009; Bitan & Karni, 2003) and that the left IFG subserves phonological coding in learning (Bitan et al., 2005). However, little is known about the mechanism of phonological generalization in nonalphabetic systems. Although there is no sublexical grapheme–phoneme correspondence rules in Chinese, which uses characters as the basic units of writing to map onto a spoken monosyllable, the orthography–phonology mapping in Chinese is not arbitrary, and the subcomponent “phonetic radical” plays an important role in Chinese reading and learning. In modern Chinese, approximately 72% of Chinese phonograms’ pronunciation can be predicted by their phonetic radical (according to Li & Kang, 1990). The phonetic radical has been found to facilitate the reading of Chinese characters (Seidenberg, 1985). Developmental studies have also shown that the awareness of this orthographic– phonological principle increases over the school years, helps young readers to learn new exemplars, and characterizes good readers (Shu, Anderson, & Wu, 2000; Shu & Anderson, 1997). However, there are no studies examining how the acquisition or application of phonetic knowledge influences the neural mechanisms in Chinese learning and reading. The current study aimed to examine the neural mechanism involved in the generalization of phonetic knowledge in learning Chinese characters.
In this study, English speakers with no previous exposure to nonalphabetic languages learned the pronunciation of Chinese phonograms without explicit instruction on the role of their phonetic radicals. The key purposes of this study were to examine the neural basis of (1) learning the phonology of Chinese characters and (2) the generalization of phonetic knowledge to processing novel words. To examine the generalization of phonetic knowledge, we manipulated whether a phonetic radical indicated the whole word pronunciation or not. When several phonologically related words have the same phonetic radical, the phonetic radical gives a clue to whole word pronunciation. For example, the three words [Image] (hai4), [Image] (hai2), and [Image] (gai1) all have the same phonetic radical [Image] on their right part, which indicates the pronunciation of “ai” (numbers indicate tones). Because this subcomponent gives a clue to word pronunciation, we could examine transfer of this knowledge to novel characters that shared the same phonetic radical with trained characters, such as the new character [Image] (gai1) with the phonetic radical [Image]. Behaviorally, acquiring this knowledge was expected to facilitate the character recognition. At the neural level, we expected that learning and transfer would be associated with changes in the left IFG and OT regions because they have been implicated in phonological analysis. According to the “assimilation and accommodation” hypothesis (Perfetti et al., 2007), if there was an assimilation to processing mechanisms in English, we expected to see activation increases in the left TP region; if there was an accommodation to Chinese processing, we expected learning-related increases in the right OT region.
METHODS
Participants
Twelve adults (average age = 22.8 years, range = 18–26 years, 7 women) participated in this study. All participants were undergraduate or graduate students at the Northwestern University. Participants were native English speakers with no prior Chinese language and other nonalphabetic language experience. Participants were right-handed and had normal hearing and normal or corrected-to-normal vision. None of the participants had a history of learning disability, speech articulation problems, attention deficit hyperactivity disorder, psychiatric disorder, or neurological disease. The institutional review board at the Northwestern University and at the Evanston Northwestern Healthcare Research Institute approved the informed consent procedures. All participants received monetary reimbursement for their participation.
Materials
One hundred forty-four Chinese phonograms evenly consisted of two character types: the phonetically implied (PI) type versus the phonetically unrelated (PU) type. For the PI type, 12 groups of six exemplars shared the same phonetic radical, which indicates the pronunciation of the whole character. Within these groups, the semantic radical of these characters varied from each other (Figure 1A). For the PU type, 12 groups contained six exemplars in which the phonetic radicals were all different, so it was impossible to extract the phonetic radical as a clue to the pronunciation. However, to balance the visual repetition of radicals as compared with the PI type, the six exemplars in each group of the PU type shared the same semantic radical (Figure 1B). All characters were evenly divided into two stimulus sets. Each set included three exemplars of each radical of both character types; as a result, there were a total of 72 characters in each stimulus set. These two stimulus sets were counterbalanced for either training or transfer across participants; that is, for one half of the participants, one stimulus set was used for training and the other for transfer; the reverse arrangement was applied to the other half of the participants. Characters used for training were broken up into six lists of 12 characters. Each list included two types of phonetic radicals (six characters) in the PI type and another two types of semantic radicals (six characters) in the PU type.
Figure 1.
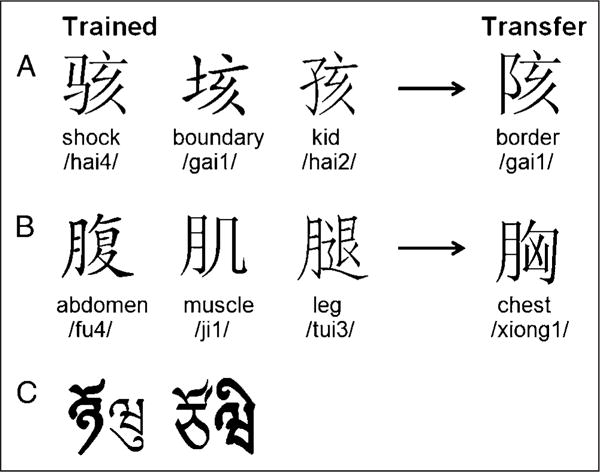
Examples of stimuli with their English translation and their pronunciation in pinyin. Numbers for the pinyin indicate one of four tones. Participants learned only the pronunciations. (A) PI-type characters, the right part of each character (phonetic radical) indicates the “ai” sound; (B) PU-type characters, the right part of each character indicates a different pronunciation, whereas the left part (semantic radical) of each character is the same; (C) two examples of visual stimulus for the baseline task in the fMRI scan with the left part or the right part of the symbol boldfaced.
All characters were approximately 200 × 200 pixel size. The characters with the same radical were presented in three different fonts (SimSun, KaiTi, and Fangsong) to encourage participants to perform the task on the basis of the extraction of orthographic information rather than on the recognition of low-level visual similarity. The stroke count of characters, an indication of visual complexity, did not show significant differences between the two stimulus sets, six lists, or two character types. The corresponding Chinese pronunciation of each Chinese character was given in an auditory format. All pronunciations were recorded in a soundproof booth using a digital recorder and a high-quality stereo microphone. A native Chinese woman read aloud each pronunciation in isolation so that there would be no contextual effects. Sound waves were normalized to 800 msec and equal amplitude (loudness) (WaveLab 2002).
Procedure
Behavioral Training
Figure 2A presents the overall training and testing procedures, which were the same as that in our previous lexical training study (Deng, Booth, Chou, Ding, & Peng, 2008), except that our participants learned Chinese pronunciation of each character rather than its English translation. All training and testing procedures were computerized using an in-house computer program.
Figure 2.
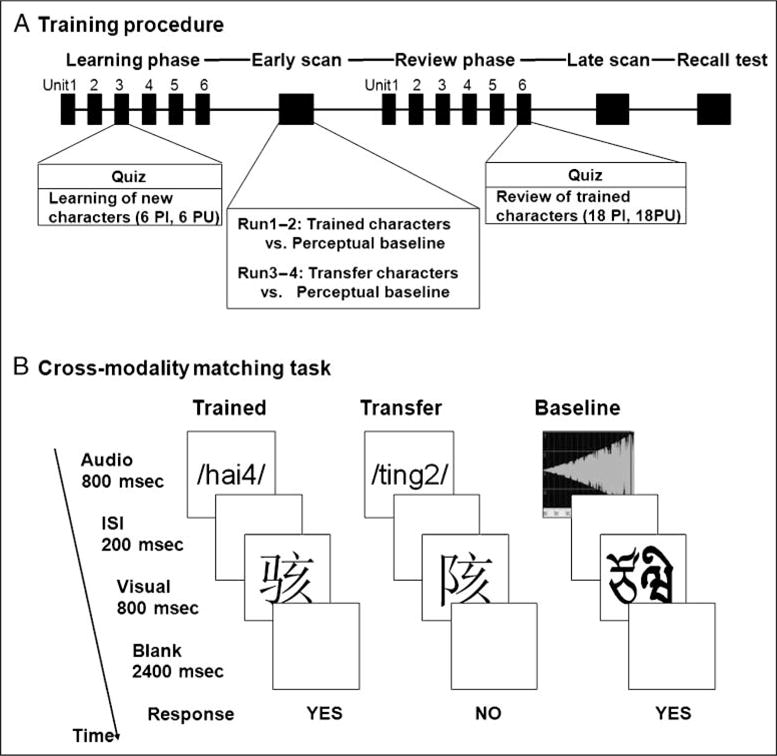
(A) Illustration of overall training procedure; (B) illustration of experimental tasks in the fMRI session.
In the learning phase, participants learned a new list (12 characters) about every other day and finished all six lists (72 characters) within 2 weeks. The learning sequence of the six lists was counterbalanced between participants by a Latin square arrangement. Within each learning unit (i.e., day), participants saw a Chinese character on the center of a screen for 30 sec and simultaneously heard its Chinese pronunciation repeated six times. Each character was presented four times so that the repetition of a specific radical was 12 times. The sequence in which the characters were presented was fixed in each learning unit, ensuring that the three exemplars of a certain radical were not contiguously presented and a certain character was not contiguously repeated. Each learning unit took 24 minutes.
After finishing the six learning units, participants reviewed all trained characters in six review units of three lists each. The arrangement of review lists was designed ensuring that each list was reviewed three times on different days and a particular list would not be reviewed more than twice on consecutive days. Because each participant started with a different learning list, the reviewing sequence also varied across participants. Within each review unit, participants saw a Chinese character on the center of a screen for 10 sec and simultaneously heard its Chinese pronunciation repeated three times. Each character type was repeated four times, resulting in the repetition of a certain radical 12 times. Characters were presented randomly with the constraint that a particular character did not appear contiguously more than twice. Each review unit lasted 24 minutes.
The quiz was taken before learning or reviewing the list(s) on the participant’s next visit to deal with recency effects. Each quiz only covered trained characters from the latest learning or review unit; that is, no novel stimuli were introduced into quizzes. Participants were asked to judge whether the pair of auditory pronunciation and visual character matched or not. Feedback was given after each response. All pairings were presented randomly.
fMRI Sessions
Participants were trained to keep their head still using an infrared tracking device, and then they practiced the baseline task (for details, see next section) in a simulator scanner for 15 minutes. The practice session was administered at least 3 days (ranging from 3 to 7 days) before the first fMRI scan.
Participants were scanned at the end of the learning phase (early) and review phase (late). Within each scanning session, there were two runs for trained characters and another two runs for transfer characters. Runs on trained characters included 90 PI-type pairings, 90 PU-type pairings, 60 baseline trials, and 74 null events (blank screen). The duration of each run was 11.84 minutes. Runs on transfer characters involved 60 PI-type pairings, 60 PU-type pairings, 60 baseline trials, and 56 null events. Each run lasted 8.95 minutes. Both early and late scanning runs used the same protocol. During the fMRI scan, the presentation of trials was optimized for event-related design using Optseq tool (http://surfer.nmr.mgh.harvard.edu/optseq, written by D. Greve, Charlestown, MA).
Figure 2B presents the format and the timing of each trial. Within each trial, an auditory pronunciation was presented for 800 msec, followed by a 200-msec ISI, then a visual character for 800 msec. After a 2400-msec blank screen, the next pair appeared. For runs on trained characters, participants were asked to judge whether the pair matched or not by pressing the yes or no button with their right hand. For runs on transfer characters, participants were instructed to guess whether the pair matched on the basis of their learned knowledge. RT was recorded and defined as the latency from the point that a visual character appeared at the time that a participant pressed the button. The same set of trained and transfer materials was used in the early and late scans, in which each character was always paired with the same pronunciation.
A visual–auditory matching task served as a baseline for all runs. In this task, artificial visual symbols were paired with two kinds of complex tones. To minimize the perceptual differences between lexical and baseline tasks, these visual symbols were created by spatially combining two to four Tibetan characters into a left–right structural character, as seen in Figure 1C. The size of these symbols was the same as that of Chinese characters. Each visual symbol was boldfaced on the left or right. A volume-increasing tone indicated that the boldfaced part should be on the right, whereas a volume-decreasing tone indicated that the boldfaced part should be on the left. Participants were asked to judge whether the position of the boldfaced part of the symbol matched the part indicated by the complex tone. The presentation format of the baseline task was the same as the lexical task.
Delayed Test on Recall: Radical Knowledge Test
To examine the retention of phonetic knowledge over the long term, 3 to 6 months after the training, participants were asked back to the laboratory for a recall test. After going through a regular review unit on the six trained lists, participants were shown a list of trained characters, in which the phonetic radical of each character was circled. They were asked to try to read aloud each character and also to guess the pronunciation of the circled part. The rate for correctly pronouncing the phonetic radical was treated as the score for phonetic knowledge, indicating the extent to which participants had extracted the underlying lexical rule. Only 10 of the 12 subjects were available for delayed testing.
Image Acquisition
Participants lay in the scanner with their head position secured with a specially designed vacuum pillow (Bionix, Toledo, OH). An optical response box (Current Designs, Philadelphia, PA) was placed in the participants’ right hand. Participants viewed visual stimuli that were projected onto a screen via a mirror attached to the inside of the head coil. Participants wore headphones to hear auditory stimuli (Resonance Technology, Northridge, CA).
All images were acquired using a 1.5-T GE scanner. For the functional imaging studies, a susceptibility-weighted single-shot EPI method with BOLD was used. Functional images were interleaved from bottom to top. The following scan parameters were used: echo time = 35 msec, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24, repetition time = 2000 msec. The first two functional runs on trained characters had 349 image volumes each, and the last two runs on transfer characters had 236 image volumes each. In addition, a high-resolution, T1-weighted three-dimensional image was acquired (SPGR, repetition time = 21 msec, echo time = 8 msec, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124). The axial orientation of the three-dimensional image was identical to the functional slices.
fMRI Data Analysis
Data analysis was performed using Statistical Parametric Mapping (SPM2). The functional images were corrected for differences in slice-acquisition time to the middle volume and were realigned to the first volume in the scanning session using affine transformations. No participant had more than 3.0 mm of movement in any plane. Coregistered images were normalized to the Montreal Neurological Institute average template (12 linear affine parameters for brain size and position, 8 nonlinear iterations, and 2 × 2 × 2 nonlinear basis functions). Statistical analyses were calculated on the smoothed data (10-mm isotropic Gaussian kernel), with a high-pass filter (128-sec cutoff period). We used global normalization to scale the mean of each scan to a common value.
Data from each participant were entered into a general linear model using an event-related analysis procedure. Word pairs were treated as individual events for analysis and modeled using a canonical hemodynamic response function. Parameter estimates from contrasts of the canonical hemodynamic response function in single participant models were entered into random-effects analysis across all participants to determine whether activation during a contrast was significant. We first examined activation when processing each type (PI and PU) of trained and transfer characters, relative to null events, separately for early and late sessions (p < .05 FDR-corrected, with a cluster size of 10 or greater). For the trained characters, we examined learning effects (late minus early) for each type of characters. We also examined the learning effects for the baseline condition (baseline minus null) to confirm that the learning effects for the characters were specific to linguistic processing. In addition, we examined the correlation between behavioral and activation changes by entering the difference score in accuracy (late minus early) as a covariate of interest in the contrast of greater activation late versus early in learning. Together, these two methods determined the region(s) that plays a critical role in phonological learning of Chinese.
On the basis of the region(s) that had shown a learning effect on trained characters, we chose the ROI(s) to examine the generalization of knowledge to transfer characters. We did these analyses only for the PI type because only these characters could benefit from the generalization of knowledge extracted from the trained phonetic radicals. There were two key comparisons in this analysis: (1) a comparison of late with early in learning to determine overall the learning effects and (2) the correlation between delayed recall of phonetic radical pronunciations and activation at the late stage. For each comparison, a 6-mm radius sphere ROI was drawn by using the VOI toolbar in SPM2 centered on the peak activation voxel of the regions that showed a learning effect for the trained characters. The coordinates of the peak activation voxel for each ROI were slightly different from those for trained characters, and the variances were within 9 mm in each direction. Only voxels whose activation surpassed the threshold p < .05 uncorrected were used to compute the average beta value for the ROI(s).
RESULTS
Behavioral Results
Figure 3 shows the behavioral performance (accuracy) on quizzes and in the fMRI sessions. Because each subject started the training with a different list because of our counterbalancing, the list(s) in each unit was different among subjects. Therefore, the quiz scores were collapsed into three stages, in which all materials were learned once (Stage 1), reviewed once (Stage 2), and reviewed twice (Stage 3), respectively. In an ANOVA, both main effects of time (Stages 1, 2, and 3) and character type (PI and PU) were significant, Ftime(2, 10) = 10.429, p < .01, Ftype(1, 11) = 13.847, p < .01, with no interaction, indicating that performance on quizzes significantly increased in accuracy over training and PI characters demonstrated significantly higher accuracy than PU characters (Figure 3A). In the scanning sessions, there was an interaction between time and character type on accuracy to trained characters, F(1, 11) = 5.146, p < .05, indicating that the PU type improved over time compared with the PI type (Figure 3B). For responses to transfer characters, a sensitivity index (d′) was calculated to adjust for response bias. The value for d′ is the Z value of the hit rate minus that of the false alarm rate. This value indicates how sensitive an individual is to the experimental manipulation. As shown in Figure 3C, an interaction between time and character type was found, F(1, 11) = 18.875, p < .01. The PI type showed significantly higher d′ late compared with early in learning, F(1, 11) = 60.85, p < .001, whereas d′ for the PU type did not differ over time (p > .08).
Figure 3.
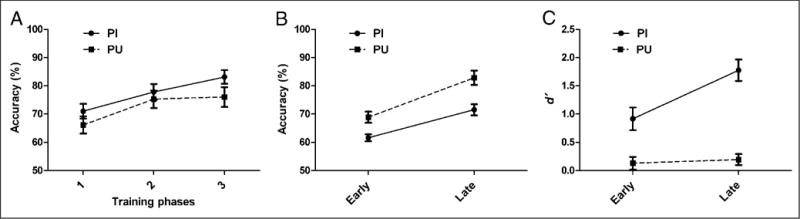
Behavioral results. (A) Mean accuracy (bars indicate one standard error) for the quizzes for PI and PU character types: 1 = collapsed data from all quizzes in the learning phase; 2 = collapsed data from the first two quizzes in the review phase; and 3 = collapsed data from the third and the fourth quizzes in the review phase. (B) Mean accuracy (bars indicate one standard error) for trained characters in the early and late fMRI sessions. (C) Mean d′ (bars indicate one standard error) for transfer characters in the early and late fMRI sessions.
Brain Activations
Neural Responses to Trained Characters
Figure 4 and Table 1 show significantly activated areas for trained Chinese characters for early and late in learning and for the PI and PU types. Activation was similar across character types and learning sessions with activation in all major components of the reading/language network, such as IFG, inferior occipo-temporal cortex including fusiform gyrus, superior temporal gyrus, and posterior parietal cortex including inferior parietal lobule. Activation was bilateral but clearly left lateralized.
Figure 4.
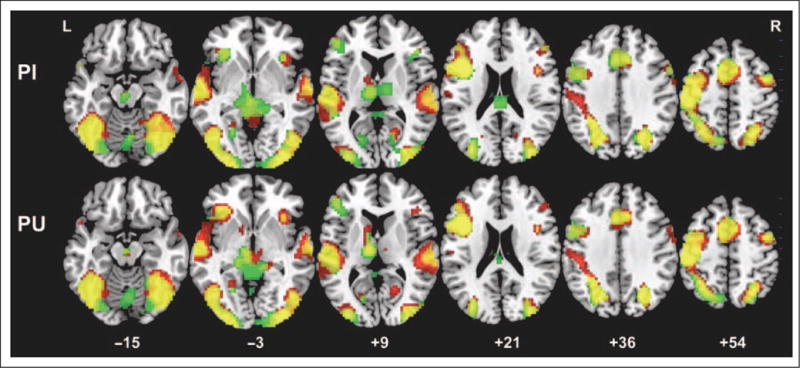
Brain activation maps for both trained PI and PU characters in the early and late scan sessions. Green indicates activation for the early scan; red indicates activation for the late scan; yellow indicates overlapping activation for both scan sessions. p < .05, FDR corrected, greater than 10 voxels.
Table 1.
Overlapping Activations for PI and PU Trained Characters in Early and Late Scan
| Regions | H | BA | Voxels | Z Score | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Early Scan: Both PI and PU Characters | |||||||
| IPL/MOG/IFG | L | 40/19/45 | 4403 | 6.14 | −33 | −51 | 39 |
| MOG/precuneus/IPL | R | 19/7/40 | 1838 | 5.49 | 48 | −69 | −15 |
| Medial frontal gyrus/MFG | L/R | 8/6 | 839 | 5.44 | 0 | 9 | 57 |
| STG | R | 22/21 | 508 | 4.98 | 60 | −12 | 3 |
| IFG | R | 45 | 55 | 4.25 | 45 | 9 | 24 |
| IFG | R | 46/45 | 172 | 4.06 | 48 | 33 | 15 |
| MFG/IFG | R | 6/9 | 160 | 3.95 | 42 | −3 | 57 |
| Posterior cingulate | R | 31 | 62 | 3.91 | 18 | −63 | 12 |
| Thalamus | L | – | 339 | 3.32 | −3 | −27 | −6 |
| Declive | R | – | 17 | 3.11 | 9 | −72 | −21 |
| Thalamus | R | – | 22 | 2.78 | 9 | −12 | 6 |
| Late Scan: Both PI and PU Characters | |||||||
| Fusiform gyrus/IOG/precuneus | L | 37/19/7 | 5502 | 5.73 | −42 | −60 | −21 |
| STG/transverse temporal gyrus | L | 22/41 | 371 | 4.79 | −57 | −21 | 9 |
| Medial frontal gyrus/cingulate gyrus | L/R | 6/32 | 580 | 4.75 | −6 | 9 | 51 |
| MFG | R | 10 | 57 | 3.91 | 45 | 0 | 57 |
| Thalamus | R | – | 17 | 3.81 | 9 | −12 | 6 |
| STG | R | 22 | 178 | 3.73 | 60 | −15 | 3 |
| Corpus callosum | R | – | 52 | 3.53 | 3 | −27 | 24 |
| IFG | R | 9 | 32 | 3.37 | 45 | 9 | 27 |
| IFG | R | 45 | 24 | 3.31 | 33 | 24 | −3 |
| Cuneus | L | 18 | 54 | 3.22 | −24 | −60 | 3 |
H = left (L) or right (R) hemisphere; BA = Brodmann’s area; Voxels = number of voxels in cluster, only cluster 10 or greater are presented; IPL = inferior parietal lobule; MOG = middle occipital gyrus; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; STG = superior temporal gyrus; IOG = inferior occipital gyrus. Areas in boldface indicate the peaks of activation in the clusters. p < .05, FDR corrected.
Figure 5 and Table 2 present the learning-related changes in activation. As shown in Figure 5A, significant learning effects were found in bilateral lingual gyri for both PI and PU characters (p < .001 uncorrected at the voxel level and p < .05 corrected at the cluster level). At a whole-brain level, there was no significant two-way interaction (i.e., character type vs. early/late learning) for these regions (p < .001, uncorrected; 10 or greater voxels). As shown in Figure 5B, greater increases in accuracy were correlated with greater activation increases from early to late in training for the PU characters in the left IFG (Brodmann’s area [BA] 44), middle frontal gyrus (BA 10), and cingulate gyrus (p < .001 uncorrected at the voxel level). To decrease the chances of a false-positive finding, an anatomical mask of left IFG was applied to the correlation analysis for PU characters on the basis of a priori hypotheses regarding the critical role of left IFG in phonological processing (Liu et al., 2009; Booth et al., 2006; Poldrack et al., 1999) and in phonological training (Xue et al., 2006; Bitan et al., 2005; Callan et al., 2005; Sandak et al., 2004). The result showed that activation in left IFG (BA 44) was significant at the voxel level (p < .05 corrected).
Figure 5.
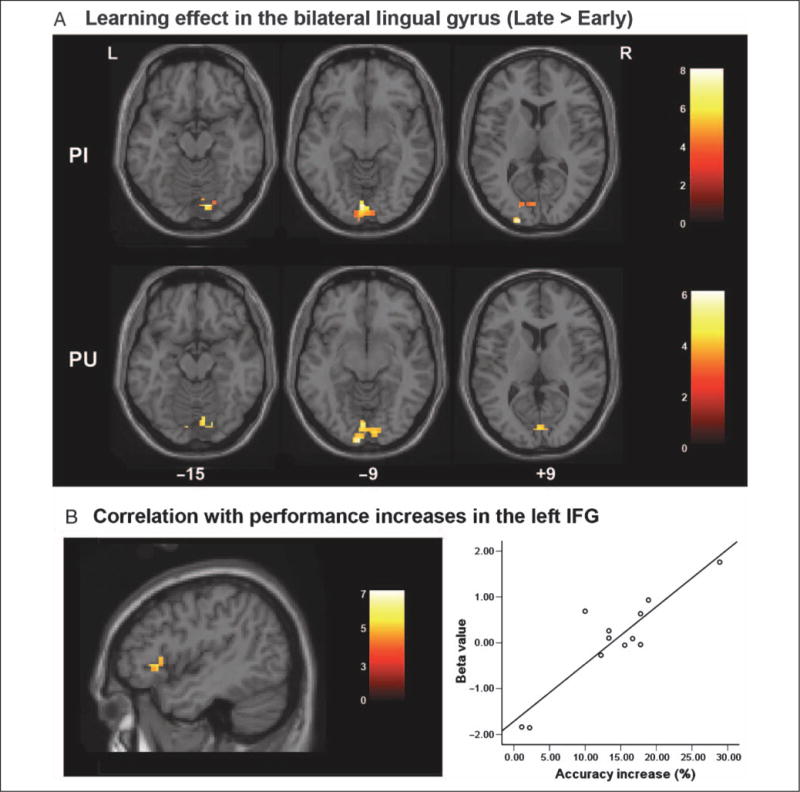
(A) Brain regions that showed greater activation for trained PI and PU characters in late compared with early in learning, mainly in the bilateral lingual gyrus. (B) The left IFG shows greater increases in accuracy correlated with greater activation for trained PU characters in late compared with early in learning.
Table 2.
Learning Effects (Late–Early) for PI and PU Trained Characters and Correlations between Behavioral Improvement and Activation Changes
| Regions | H | BA | Voxels | Z Score | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Learning Effects (Late Minus Early for PI Characters) | |||||||
| Lingual gyrus/MOG | L/R | 18 | 164 | 4.57 | −3 | −84 | −9* |
| Learning Effects (Late Minus Early for PU Characters) | |||||||
| Lingual gyrus | R/L | 18 | 172 | 6.63 | 15 | −78 | −18* |
| Larger Accuracy Increases over Learning Correlated with Greater Activation Increases over Learning (for PI) | |||||||
| Lingual gyrus | L | 18 | 27 | 4.13 | −24 | −72 | −3 |
| MTG | L | 21 | 25 | 4.02 | −66 | −15 | −15 |
| Cingulate gyrus | L | 24 | 27 | 3.84 | −12 | −6 | 33 |
| OFC | L | 11 | 21 | 3.73 | −21 | 45 | −9 |
| SFG/MFG | R | 10 | 15 | 3.71 | 30 | 57 | −3 |
| Anterior cingulate | L | 32 | 22 | 3.60 | −6 | 30 | 12 |
| Larger Accuracy Increases over Learning Correlated with Greater Activation Increases over Learning (for PU) | |||||||
| MFG | L | 10 | 18 | 4.19 | −33 | 51 | 15 |
| IFG | L | 44 | 17 | 3.95 | −48 | 27 | 0** |
| Cingulate gyrus | L | 31 | 19 | 3.57 | −12 | −36 | 36 |
H = left (L) or right (R) hemisphere; BA = Brodmann’s areas; Voxels = number of voxels in cluster, only clusters of 15 or greater are presented; MOG = middle occipital gyrus; MTG = middle temporal gyrus; SFG = superior frontal gyrus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus. Areas in boldface indicate the peaks of activation in the clusters.
Activations were significant at voxel-wise p < .001 uncorrected and at cluster-wise p < .05 corrected.
This activation was significant at voxel-wise p < .05 corrected after applying an anatomical mask based on priori hypotheses.
To support the conclusion that these learning-related changes in activations were linguistically specific, we also conducted the same analysis on the perceptual condition. The results showed no significant differences in brain activations in the perceptual condition for the comparison of late versus early in learning or for the correlation of this difference with behavioral improvements on the perceptual condition.
Neural Responses to Transfer Characters
Figure 6 and Table 3 show significantly activated areas for transfer Chinese characters for early and late in learning and for the PI and the PU types. Activation was similar across character types and learning sessions with activation in all major components of the reading/language network, similar to the effects shown for the trained characters (see previous section).
Figure 6.
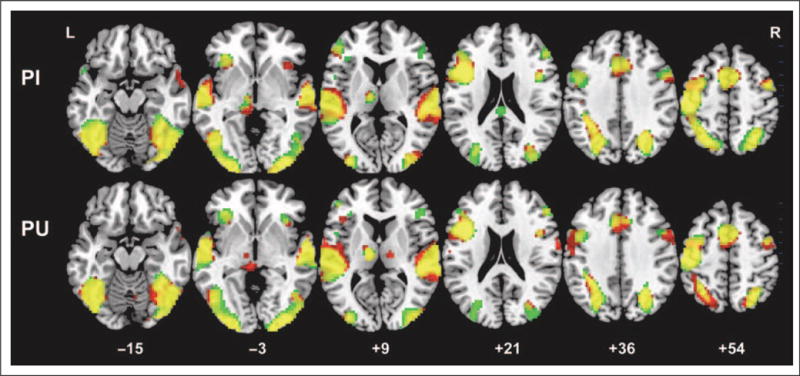
Brain activation maps for both transfer PI and PU characters in the early and late scan sessions. Green indicates activation for the early scan; red indicates activation for the late scan; yellow indicates overlapping activation for both scan sessions. p < .05, FDR corrected, greater than 10 voxels.
Table 3.
Overlapping Activations for PI and PU Transfer Characters in Early and Late Scan
| Regions | H | BA | Voxels | Z Score | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Early Scan: Both PI and PU Characters | |||||||
| STG/transverse temporal gyrus | R | 22/41 | 687 | 6.13 | 60 | −12 | 0 |
| STG | L | 22 | 778 | 5.68 | −66 | −15 | 0 |
| Precuneus | R | 7 | 1594 | 5.60 | 27 | −72 | 30 |
| Fusiform gyrus/MOG | L | 37/19/18 | 799 | 5.59 | −42 | −72 | −18 |
| Precentral gyrus/IPL/IFG | L | 4/40/45 | 1948 | 5.42 | −45 | −15 | 60 |
| Cingulate gyrus/SFG | R/L | 32/6 | 600 | 4.89 | 9 | 18 | 42 |
| Thalamus | L | – | 121 | 4.18 | −9 | −30 | −6 |
| MFG/IFG | R | 6/46 | 243 | 3.84 | 57 | 6 | 42 |
| MFG | R | 46 | 32 | 3.31 | 48 | 33 | 18 |
| IFG | R | 47 | 16 | 3.04 | 36 | 21 | −3 |
| Thalamus | R | – | 21 | 2.58 | 12 | −15 | 6 |
| Late Scan: Both PI and PU Characters | |||||||
| Precuneus/fusiform gyrus | R | 7/37 | 1817 | 5.87 | 27 | −75 | 48 |
| Precentral gyrus/MOG/IPL | L | 4/19/40 | 2684 | 5.80 | −45 | −15 | 48 |
| Transverse temporal gyrus/STG | L | 42/22 | 608 | 4.99 | −60 | −21 | 9 |
| STG/transverse temporal gyrus | R | 22/41 | 522 | 4.94 | 60 | −12 | 0 |
| SFG | R | 6 | 453 | 4.55 | 3 | 9 | 57 |
| IFG | R | 9 | 102 | 4.24 | 45 | 6 | 24 |
| MFG | R | 46 | 91 | 3.79 | 51 | 36 | 18 |
| IFG | L | 45 | 128 | 3.79 | −33 | 30 | 0 |
| Precentral gyrus | R | 6 | 39 | 3.55 | 45 | −3 | 51 |
| Thalamus | L | – | 76 | 3.44 | −12 | −15 | 6 |
| Declive | R | – | 11 | 3.25 | 6 | −72 | −21 |
| IFG | R | 45 | 28 | 2.21 | 36 | 24 | −3 |
H = left (L) or right (R) hemisphere; BA = Brodmann’s areas; Voxels = number of voxels in cluster, only cluster 10 or greater are presented; STG = superior temporal gyrus; MOG = middle occipital gyrus; IPL = inferior parietal lobule; IFG = inferior frontal gyrus; SFG = superior frontal gyrus; MFG = middle frontal gyrus. Areas in boldface indicate the peaks of activation in the clusters. p < .05, FDR corrected.
The analysis of the trained characters showed training-related effects in several regions including bilateral lingual gyri and left IFG, suggesting that these regions were critical for learning Chinese characters. To determine the role that these regions played in the generalization of knowledge to processing the transfer characters, we extracted beta values from these regions separately for the early and late sessions. The t tests revealed no significant differences between the early and the late sessions. Moreover, the difference in activation between the early and the late sessions was not significantly correlated with behavioral improvement across these sessions. However, our delayed recall test is a better measure of generalized knowledge of the phonetic radicals, and better performance on delayed recall was significantly correlated with greater activation in the late session in the right lingual gyrus (r = .69, p < .05) and the left IFG (r = .63, p = .05) (Figure 7).
Figure 7.
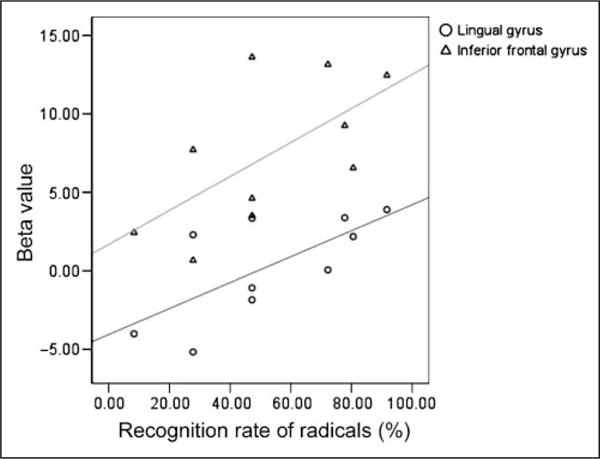
Greater activity in the late scan for the transfer PI characters was correlated with higher recognition rate of radicals in a delayed test given 3 to 6 months after training. Triangles: left IFG (r = .63, p = .05); circles: right lingual gyrus (r = .69, p < .05).
DISCUSSION
Neural changes resulting from English speakers learning the pronunciation of Chinese characters were examined in a cross-modality matching task using fMRI. The novelty of this design is that it allowed participants to implicitly learn sublexical phonetic knowledge during training, and transfer of phonetic knowledge was measured by examining the processing of novel characters that shared a phonetic radical with trained characters (PI). In this way, the current study aimed to explore which brain regions would play crucial role in the acquisition of Chinese phonetic knowledge. In terms of performance, learning-related increases in accuracy were evident on trained items. Generalization of phonetic knowledge was shown by higher performance on PI transfer characters as compared with PU transfer characters. In terms of neural activation, there were learning-related increases for the trained items in the bilateral lingual gyrus, and better learning was correlated with increasing activation in left IFG. These regions were also critical for generalization to novel characters because greater activation in the left IFG and right lingual gyrus was correlated with better performance on delayed recall of phonetic radicals.
Bilateral Lingual Gyri for Chinese Orthographic Processing
Consistent with language training studies in adults (Xue et al., 2006; Callan et al., 2005; Hashimoto & Sakai, 2004; Sandak et al., 2004), the current study found learning-related increases in the left lingual gyrus. As one component in the ventral stream of visual processing, the lingual gyrus is broadly found to be involved in visual object and word processing. It has been suggested that the left lingual region is one of the key components within the brain circuit for skilled visual word recognition in alphabetic languages (Price, 2000; Fiez & Petersen, 1998). Studies in English have shown hypoactivation in this region in dyslexic children (Hoeft et al., 2007; Shaywitz et al., 2002) and an increased activity after remediation (Temple et al., 2003; Small et al., 1998). The left lingual gyrus has also been consistently found to be activated in Chinese character processing across different conditions/tasks, such as greater activation for low-frequency compared with high-frequency characters (Lee et al., 2004; Kuo et al., 2003), for homophone judgments (Kuo et al., 2004; Tan, Feng, Fox, & Gao, 2001), and for pseudowords compared with real words (Xiao et al., 2005). Therefore, the learning effect in the left lingual gyrus in this study may reflect a language-general pattern across languages.
We also showed learning-related increases in the right lingual gyrus and that the magnitude of activation in this region was correlated with delayed recognition of phonetic radicals. These findings are broadly consistent with studies in Chinese adults and developmental studies of Chinese. Some argue that the right lingual gyrus is a critical component of Chinese character processing that is involved in the processing of the logographic nature of characters (Kuo et al., 2004; Tan, Feng, et al., 2001; Tan, Liu, et al., 2001). When learning Chinese characters as a second language, Nelson et al. (2009) and Liu et al. (2007) reported that English speakers recruited the right ventral OT cortex, including the fusiform gyrus and the lingual gyrus as an accommodation for supporting the specific graphic requirements of character learning. Our finding also suggested an accommodation effect in the right lingual gyrus for learning Chinese characters in English speakers. Two recent developmental studies in Chinese have consistently found that adults showed greater activation than children in the right inferior occipital cortex across reading tasks (Cao et al., 2009, 2010), suggesting a greater engagement of this region for the visual–spatial processing of Chinese characters over age.
Perceptual processing studies suggest that the visual system in the left hemisphere may be more involved in local and analytic processes, whereas the right may be more involved in global and holistic processes (Fink et al., 1997; Delis, Robertson, & Efron, 1986). On the basis of this, some have argued that the right visual cortex may be more involved in Chinese character processing because of greater demands on holistic processing (Nelson et al., 2009; Tan, Feng, et al., 2001). However, we found that greater activation in the right lingual gyrus was correlated with better recognition of phonetic radical after the training. Although recognition of radicals within characters may place demands on local processing, it is also possible that the spatial layout of the radicals within the characters places demands on holistic processing. Indeed, in our task, better learners probably more effectively attended to the spatial layout to process the critical phonetic radical information on the right of the character in the presence of other radicals on the left of the character that did not give a clue to the pronunciation. Studies have suggested that attention to letters/words enhanced activation over the right lingual gyrus (Fink et al., 1996; Petersen, Fox, Posner, Mintun, & Raichle, 1989), and learning novel visual patterns increased regional CBF in the right lingual gyrus (Roland & Gulyas, 1995). Therefore, it is possible that the increasing activation in right lingual gyrus may be driven by the attention to processing the learning-specific graphic information, that is, phonetic radical.
The Left IFG for Phonological Processing
Another key region that showed learning effects was the left IFG. This finding is broadly consistent with previous language training studies and developmental studies in both English and Chinese, in which left IFG showed greater involvement as a result of learning or over development (Cone et al., 2008; Bitan et al., 2005, 2007; Xue et al., 2006; Gronholm et al., 2005; Callan et al., 2003; Turkeltaub et al., 2003). Studies in alphabetic languages have suggested that the dorsal aspect of posterior IFG region (mainly BA 44/45) is involved in many aspects of phonological processing (Poldrack et al., 1999), such as phonetic discrimination (Zatorre, Meyer, Gjedde, & Evans, 1996), phonetic segmentation (Burton, Small, & Blumstein, 2001), and controlled phonological retrieval (Gold & Buckner, 2002). Although the Chinese writing system differs dramatically from alphabetic ones in terms of the nature of orthography–phonology mapping, several studies have found regularity/consistency effects in low-frequency conditions in the left IFG in both Chinese (Lee et al., 2004; Peng et al., 2004; Tan, Liu, et al., 2001) and English (Fiebach, Friederici, Muller, & von Cramon, 2002; Fiez, Balota, Raichle, & Petersen, 1999). Moreover, Chen et al. (2002) reported comparable activation for Chinese characters and pinyin reading (an alphabetic system for representing spoken language in Chinese) in the IFG (BA 44/45). These findings suggest that this region may be involved in general phonological processing across writing systems. In this study, we found that greater IFG activation was correlated with better performance of learning on the trained items and also better maintenance of radical knowledge in the long term. According to a post-training questionnaire, most of our learners noticed that characters containing the same phonetic radical rhymed. It is likely that our subjects segmented the auditory syllable into an onset and a rhyme and related this to the character spelling, so greater IFG activation may indicate the role of phonemic segmentation.
Lack of Learning Effect in the Left TP Region
There were no learning effects found in TP region, neither in the left superior temporal gyrus nor in the left inferior parietal lobule. The TP region is thought to be involved in the mapping between orthographic and phonological representations in alphabetic languages (Booth et al., 2002a, 2002b), and there have been studies showing learning effects in this region as a result of training to link novel visual word forms to sounds in alphabetic systems (Breitenstein et al., 2005; Eden et al., 2004; Sandak et al., 2004; Temple et al., 2003). The lack of learning effects in this region in this study suggests little assimilation to the native language in English speakers when learning Chinese. However, evidence from second language acquisition studies has suggested that proficiency may modulate brain activation patterns (Xue, Dong, Jin, & Chen, 2004; Perani et al., 2003; Chee, Hon, Lee, & Soon, 2001). The lack of learning effect in this region in this study may be due to the proficiency levels of our participants.
Performance on Phonological Learning of Chinese Characters
In terms of behavioral performance, our participants showed learning-related increases of accuracy for only trained PU characters (not for transfer ones) and for both trained and transfer PI characters, showing clear evidence of generalization of knowledge of the phonetic radical. There was, however, a surprising drop in behavioral performance for the trained PI characters between the quizzes administered during the training and the tests administered during the scanning. This decline in performance may be due to an acoustic confusion effect (i.e., phonological similarity effect) that has been reported when words with similar phonological features are mistakenly confused (Conrad, 1964). Behavioral verbal learning studies have shown that phonological similarity reduces performance in serial recall (Watkins, Watkins, & Crowder, 1974) and that interference is enhanced as a function of increasing phonological overlap (Li, Schweickert, & Gandour, 2000). Moreover, phonologically similar words have been shown to cause more confusion than visually similar words, irrespective of writing systems (Saito, Logie, Morita, & Law, 2008; Flaherty & Moran, 1999). In this study, trained PI characters that shared phonetic radicals also shared phonological components (i.e., they rhymed); therefore, it is possible that remembering trained PI characters suffers from confusability compared with remembering trained PU characters and is more likely to be forgotten from the training to scanning.
It is interesting to note that the overall accuracy for phonological learning (ranging from 65% to 80%) was slightly lower than that reported for semantic learning (80–90%) in a previous study with the same amount of time in training (Deng et al., 2008). In a recent computer simulation study, Yang, McCandliss, Shu, and Zevin (2008) used connectionist models of Chinese lexical reading to examine the associative learning mechanisms among lexical representations. The training results showed that mapping from orthography to semantics was learned more rapidly with higher accuracy than mappings from orthography to phonology. Therefore, the difference in learning efficiency in the phonological compared with semantic learning of Chinese characters may result from the statistical properties of the writing system.
Semantic versus Phonological Learning
Semantics is a crucial component of learning a new script, and learning semantics may interact with phonological learning. Other studies have separately and jointly examined semantic and phonological learning of scripts (Deng et al., 2008; Liu et al., 2007; Xue et al., 2006; Sandak et al., 2004). In Sandak et al. (2004), English speakers learned either the phonology or the semantics of new alphabetic words. They reported many differences between these training conditions, with phonological training showing greater activation in bilateral angular gyrus and semantic training showing greater activation in left middle temporal gyrus. Xue et al. (2006) examined Chinese speakers learning an artificial script with phonological and then semantic training. They reported that, compared with visual word form training, phonological training induced more activation in the dorsal region of IFG, whereas semantic training caused increased activation in the ventral portion of IFG. Liu et al. (2007) directly compared English speakers learning Chinese characters in different training methods, including pronunciation (P), meaning (M), and pronunciation and meaning (P + M). They found that P + M showed greater activation than either of the other conditions in left middle frontal gyrus and the P + M showed greater activation than M in right superior/middle temporal cortex and bilateral precuneus. In a previous semantic training study (Deng et al., 2008) with a similar experimental design to the current study, we investigated English speakers learning the word meanings of Chinese characters. We found that higher accuracy and better long-term retention were correlated with greater activation in superior parietal cortex, whereas lower accuracy during learning was correlated with greater activation in the ventral portion of left IFG.
In summary, according to the studies that have examined the influence of training phonology and/or semantics, including the current study, it appears that semantic training results in changes in ventral IFG, whereas phonological training results in changes in dorsal IFG (Deng et al., 2008; Xue et al., 2006). These findings coincide with a common agreed upon notion that the more anterior and inferior part of the IFG (i.e., orbitalis) is involved in semantic processing and the more posterior parts of the IFG (i.e., triangular and opercular) are involved in phonological processing (Poldrack et al., 2001; Fiez et al., 1999). More generally, these findings suggest that these two training approaches may rely on different underlying mechanisms, but systematic studies are still needed to examine interactive versus independent effects of semantic versus phonological training across languages.
Conclusion
In this study, we showed that learning the pronunciation of Chinese characters produced learning-related activation increases in the right lingual gyrus and that behavioral improvement was correlated with activation increases in the left IFG (BA 44). We also examined the role of these two regions in the generalization of phonetic knowledge about the radical for processing novel characters and found that greater activation in these regions was correlated with better knowledge of the phonetic radical in a delayed recall test. The current study suggests that right lingual gyrus and the left IFG are critically involved in the learning Chinese characters and in generalizing and maintaining that information over the long term. The left IFG is likely involved in phonological segmentation, whereas the right lingual gyrus may subserve processing visual–orthographic information.
Acknowledgments
This work was supported by grants from the Chinese Natural Science Foundation (Project No. 30270462 to Dan-ling Peng, Project No. 30400133 to Guo-sheng Ding, and Project No. 30700232 to Yuan Deng) and from the program for Changjiang Scholars and Innovative Research Team in University (IRT0710) to Dan-ling Peng. This work was also supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to J. R. B. This study was conducted at the Northwestern University, Evanston, IL 60208, USA.
References
- Bitan T, Burman DD, Chou TL, Lu D, Cone NE, Cao F, et al. The interaction between orthographic and phonological information in children: An fMRI study. Human Brain Mapping. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Karni A. Alphabetical knowledge from whole words training: Effects of explicit instruction and implicit experience on learning script segmentation. Cognitive Brain Research. 2003;16:323–337. doi: 10.1016/s0926-6410(02)00301-4. [DOI] [PubMed] [Google Scholar]
- Bitan T, Manor D, Morocz IA, Karni A. Effects of alphabeticality, practice and type of instruction on reading an artificial script: An fMRI study. Cognitive Brain Research. 2005;25:90–106. doi: 10.1016/j.cogbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, et al. Specialization of phonological and semantic processing in Chinese word reading. Brain Research. 2006;1071:197–207. doi: 10.1016/j.brainres.2005.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster AF, Sommer J, Wolbers T, et al. Hippocampus activity differentiates good from poor learners of a novel lexicon. Neuroimage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL, Blumstein SE. The role of segmentation in phonological processing: An fMRI investigation. Journal of Cognitive Neuroscience. 2001;12:679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- Callan AM, Callan DE, Masaki S. When meaningless symbols become letters: Neural activity change in learning new phonograms. Neuroimage. 2005;28:553–562. doi: 10.1016/j.neuroimage.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Yamada RA. Learning-induced neural plasticity associated with improved identification performance after training of a difficult second-language phonetic contrast. Neuroimage. 2003;19:113–124. doi: 10.1016/s1053-8119(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Cao F, Lee R, Shu H, Yang YH, Xu GQ, Li KC, et al. Cultural constraints on brain development: Evidence from a developmental study of visual word processing in Mandarin Chinese. Cerebral Cortex. 2010;20:1223–1233. doi: 10.1093/cercor/bhp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, et al. Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Human Brain Mapping. 2009;30:797–809. doi: 10.1002/hbm.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Hon N, Lee HL, Soon CS. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. Neuroimage. 2001;13:1155–1163. doi: 10.1006/nimg.2001.0781. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan EW, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Fu S, Iversen SD, Smith SM, Matthews PM. Testing for dual brain processing route in readings: A direct contrast of Chinese character and Pinyin reading using fMRI. Journal of Cognitive Neuroscience. 2002;14:1088–1098. doi: 10.1162/089892902320474535. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone NE, Burman DD, Bitan T, Bolger DJ, Booth JR. Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. Neuroimage. 2008;41:623–635. doi: 10.1016/j.neuroimage.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R. Acoustic confusions in immediate memory. British Journal of Psychology. 1964;55:75–84. [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Deng Y, Booth JR, Chou TL, Ding GS, Peng DL. Item-specific and generalization effects on brain activation when learning Chinese characters. Neuropsychologia. 2008;46:1864–1876. doi: 10.1016/j.neuropsychologia.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, et al. Clinical neural changes following study remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Ferman S, Olshtain E, Schechtman E, Karni A. The acquisition of a linguistic skill by adults: Procedural and declarative memory interact in the learning of an artificial morphological rule. Journal of Neurolinguistics. 2009;22:384–412. [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Finley S, Badecker W. Artificial language learning and feature-based generalization. Journal of Memory and Language. 2009;61:423–437. [Google Scholar]
- Flaherty M, Moran A. Acoustic and visual confusions in immediate memory in Japanese and English speakers. Psychologia. 1999;42:80–88. [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gronholm P, Rinne JO, Vorobyev V, Laine M. Naming of newly learned objects: A PET activation study. Cognitive Brain Research. 2005;25:359–371. doi: 10.1016/j.cogbrainres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Learning letters in adulthood: Direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42:311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences, USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu YT, Chou CC, Ho LT, et al. Frequency effects of Chinese character processing in the brain: An event-related fMRI study. Neuroimage. 2003;18:720–730. doi: 10.1016/s1053-8119(03)00015-6. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, et al. Orthographic and phonological processing of Chinese characters: An fMRI study. Neuroimage. 2004;21:1721–1731. doi: 10.1016/j.neuroimage.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Lee CY, Tsai JL, Kuo WJ, Yeh TC, Wu YT, Ho LT, et al. Neuronal correlates of consistency and frequency effects on Chinese character naming: An event-related fMRI study. Neuroimage. 2004;23:1235–1245. doi: 10.1016/j.neuroimage.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Lee KM. Functional MRI comparison between reading ideographic and phonographic scripts of one language. Brain and Language. 2004;91:245–251. doi: 10.1016/j.bandl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Li XJ, Schweickert R, Gandour J. The phonological similarity effect in immediate recall: Positions of shared phonemes. Memory & Cognition. 2000;28:1116–1125. doi: 10.3758/bf03211813. [DOI] [PubMed] [Google Scholar]
- Li XJ, Wong D, Gandour J, Dzemidzic M, Tong YX, Talavage T, et al. Neural network for encoding immediate memory in phonological processing. NeuroReport. 2004;15:2459–2462. doi: 10.1097/00001756-200411150-00005. [DOI] [PubMed] [Google Scholar]
- Li Y, Kang JS. Research on phonetic radicals of the modern Chinese characters. In: Yuan C, editor. Information analysis of the modern Chinese characters. Shanghai: Shanghai Education Publication; 1990. pp. 29–36. [Google Scholar]
- Liu L, Deng XX, Peng DL, Cao F, Ding GS, Jin Z, et al. Modality- and task-specific brain regions involved in Chinese lexical processing. Journal of Cognitive Neuroscience. 2009;21:1473–1487. doi: 10.1162/jocn.2009.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dunlap S, Fiez J, Perfetti CA. Evidence for neural accommodation to a writing system following learning. Human Brain Mapping. 2007;28:1223–1234. doi: 10.1002/hbm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. 2009;30:810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng DL, Ding GS, Perry C, Xu D, Jin Z, Luo Q, et al. fMRI evidence for the automatic phonological activation of briefly presented words. Cognitive Brain Research. 2004;20:156–164. doi: 10.1016/j.cogbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, et al. The role of age of acquisition and language usage in early, high-proficient bilinguals: An fMRI study during verbal fluency. Human Brain Mapping. 2003;19:170–182. doi: 10.1002/hbm.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Fiez J, Nelson J, Bolger DJ, Tan LH. Reading in two writing systems: Accommodation and assimilation in the brain’s reading network. Bilingualism: Language and Cognition. 2007;10:131–146. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of singe words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, et al. Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. Journal of Cognitive Neuroscience. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–357. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboyeau G, Marie N, Balduyck S, Gros H, Demonet JF, Cardebat D. Lexical learning of the English language: A PET study in healthy French subjects. Neuroimage. 2004;22:1808–1818. doi: 10.1016/j.neuroimage.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Roland PE, Gulyas B. Visual memory, visual-imagery, and visual recognition of larger field patterns by the human brain: Functional anatomy by positron emission tomography. Cerebral Cortex. 1995;5:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- Saito S, Logie RH, Morita A, Law A. Visual and phonological similarity effects in verbal immediate serial recall: A test with kanji materials. Journal of Memory and Language. 2008;59:1–17. [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, et al. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cognitive, Affective & Behavioral Neuroscience. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Seidenberg M. The time course of phonological code activation in two writing systems. Cognition. 1985;19:1–30. doi: 10.1016/0010-0277(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, et al. Age-related changes in reading systems of dyslexic children. Annals of Neurology. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Shu H, Anderson RC. Role of radical awareness in the character and word acquisition of Chinese children. Reading Research Quarterly. 1997;32:78–89. [Google Scholar]
- Shu H, Anderson RC, Wu NN. Phonetic awareness: Knowledge of orthography–phonology relationship in character acquisition of Chinese children. Journal of Educational Psychology. 2000;92:56–62. [Google Scholar]
- Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain and Language. 1998;62:298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- Tan LH, Feng CM, Fox PT, Gao JH. An fMRI study with written Chinese. NeuroReport. 2001;12:83–88. doi: 10.1097/00001756-200101220-00024. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Human Brain Mapping. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong JH, et al. Neural systems of second language reading are shaped by native language. Human Brain Mapping. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences, USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Watkins MJ, Watkins OC, Crowder RG. The modality effect in free and serial recall as a function of phonological similarity. Journal of Verbal Learning and Verbal Behavior. 1974;13:430–447. [Google Scholar]
- Wong ACN, Jobard G, James KH, James TW, Gauthier I. Expertise with characters in alphabetic and non-alphabetic writing systems engage overlapping occipito-temporal areas. Cognitive Neuropsychology. 2009;26:111–127. doi: 10.1080/02643290802340972. [DOI] [PubMed] [Google Scholar]
- Xiao ZW, Zhang JX, Wang XY, Wu RH, Hu XP, Weng XC, et al. Differential activity in left inferior frontal gyrus for pseudowords and real words: An event-related fMRI study on auditory lexical decision. Human Brain Mapping. 2005;25:212–221. doi: 10.1002/hbm.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Chen CS, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: An fMRI training study. Neuroimage. 2006;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Chen CS. Mapping of verbal working memory in nonfluent Chinese-English bilinguals with functional MRI. Neuroimage. 2004;22:1–10. doi: 10.1016/j.neuroimage.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Yang JF, McCandliss BD, Shu H, Zevin JD. Proceedings of the Thirtieth Annual Conference of the Cognitive Science Society. Mahwah, NJ: Lawrence Erlbaum; 2008. Division of labor between semantics and phonology in normal and disordered reading development across languages. [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cerebral Cortex. 1996;6:21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]


