Abstract
Anti-glomerular basement membrane (GBM) disease is commonly a monophasic illness. We present the case of multiple recurrences of anti-GBM disease with varying serum anti-GBM antibody findings. A 33-year-old female tobacco user presenting with hematuria was diagnosed with anti-GBM disease by renal biopsy. Five years later, she presented with alveolar hemorrhage and positive anti-GBM antibody. She presented a third time with alveolar hemorrhage but undetectable anti-GBM antibody. With each occurrence, symptoms resolved with plasmapheresis, intravenous methylprednisone and oral cyclophosphamide. The relationship between anti-GBM antibody findings and disease presentation is complex. Clinicians should be aware of the possibility of seronegative anti-GBM disease.
Keywords: autoantibodies, crescentic glomerulonephritis, glomerulonephritis, plasmapheresis, renal biopsy
Background
Anti-glomerular basement membrane (anti-GBM) disease is a rare condition characterized by the production of auto-antibodies against the alpha-3 chain of type IV collagen. Incidence and prevalence of anti-GBM disease is estimated to be one case per 1 million population [1]. Goodpasture's (pulmonary-renal) syndrome is the most common presentation of anti-GBM disease, characterized by rapidly progressive glomerulonephritis and pulmonary hemorrhage [2]. Anti-GBM antibodies normally can be detected in the circulation by enzyme-linked immunosorbent assay (ELISA) [3]. Typically, anti-GBM disease is a monophasic illness [4, 5]. The disease has good prognosis after a typical treatment regimen consisting of plasma exchange, cyclophosphamide and prednisone: one study reports 100% patient survival and 95% renal survival at 1 year [6]. We present the case of a woman who presented with three episodes of presumed anti-GBM disease and varying presence of anti-GBM antibodies on laboratory assays throughout her disease course.
Case report
A 33-year-old woman with a history of tobacco use presented with gross hematuria in 2001. As part of her outpatient workup for hematuria she underwent a renal biopsy, which showed crescentic glomerulonephritis with 25% crescents on light microscopy and linear deposition of IgG along the GBM on immunofluorescence microscopy. At the time of diagnosis, serum anti-GBM, anti-nuclear antibody (ANA) and anti-neutrophilic cytoplasmic antibody (ANCA) levels were undetectable and there was no evidence of pulmonary involvement. She was treated with plasmapheresis three times per week for seven occurrences, oral prednisone 60 mg daily for 6 months and cyclophosphamide 2 mg/kg/day for 6 months.
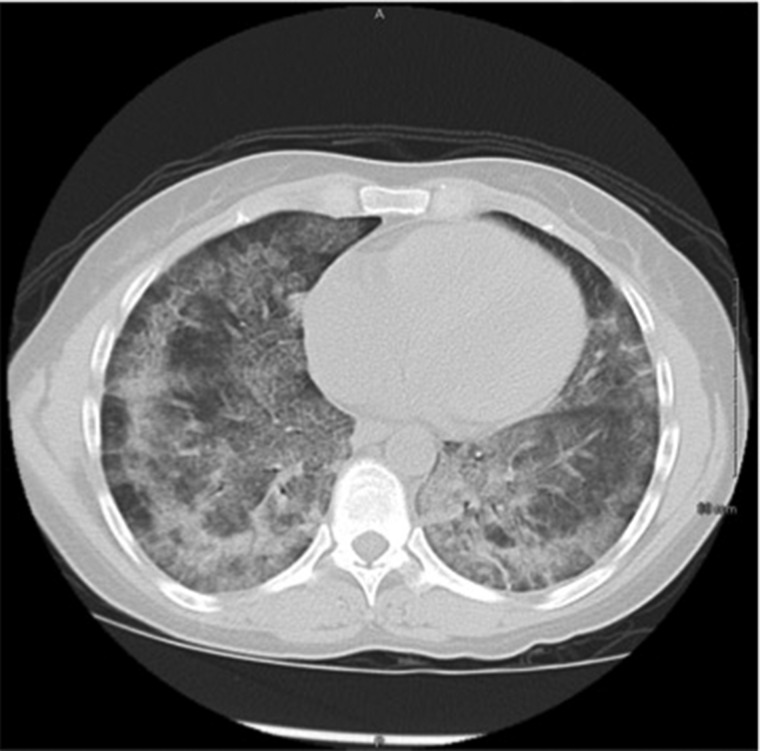
In 2006, 5 years after initial diagnosis, she presented with dyspnea, hemoptysis and gross hematuria. Hemoglobin was 8.5 g/dL and serum creatinine was 0.8 mg/dL. A chest x-ray and computed tomography (CT) were obtained and showed evidence of diffuse alveolar filling consistent with alveolar hemorrhage (Figure 1). Urine analysis was significant for moderate hemoglobin. Serum anti-GBM antibody was 112 AU/mL and serum ANCA was negative. Renal biopsy was not performed but she was treated for a presumed relapse of anti-GBM disease with plasmapheresis for 7 days, methylprednisone 1 g intravenously (IV) daily for three doses followed by 40 mg oral prednisone for 2 months and 2 mg/kg/day cyclophosphamide. She remained on the 2 mg/kg/day cyclophosphamide for 32 months prior to being tapered and discontinued due to loss of follow-up to care. At the end of a 7-day plasmapheresis treatment, anti-GBM antibody levels had returned to 0 UA/mL. During this relapse, she was hospitalized for 5 days and required intensive care unit (ICU) monitoring for 2 days due to hypotension and need for plasmapheresis. The hypotension resolved with two units of packed red blood cells and volume resuscitation. She required 4 L of supplemental oxygen on admission, but prior to discharge she showed no significant desaturations during a 6-min walk test.
Fig. 1.
Chest CT without IV contrast during admission in 2006 revealed diffuse patchy bilateral ground glass opacities, which along with lab evidence of anemia, is consistent with alveolar hemorrhage.
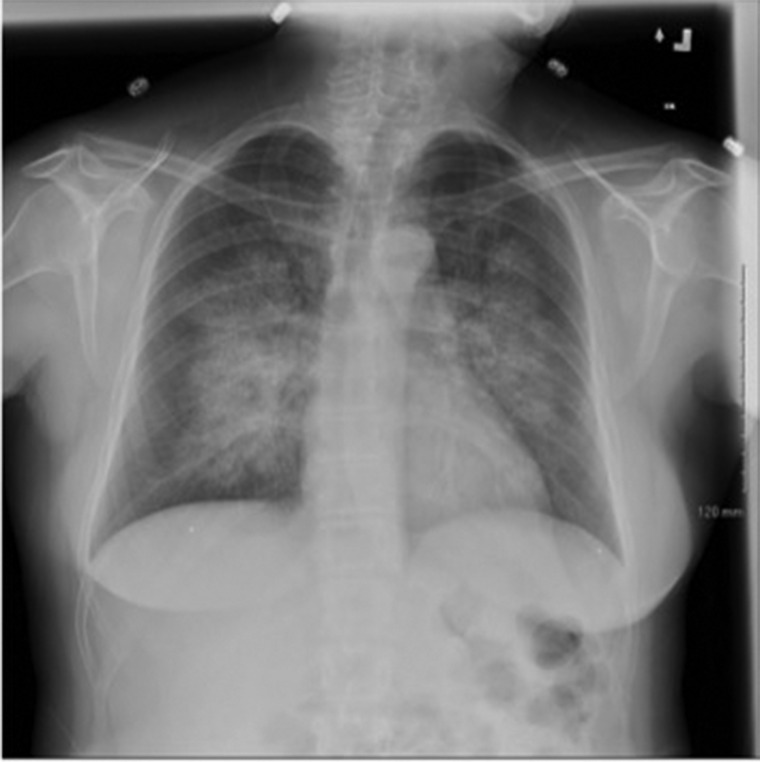
In 2014, 8 years after her relapse, she presented with epistaxis and hemoptysis. On presentation, hemoglobin was 12.0 g/dL and serum creatinine was 0.57 mg/dL. Urine analysis revealed microhematuria with 1+ hemoglobin. Chest x-ray showed bilateral perihilar opacities consistent with diffuse alveolar hemorrhage (Figure 2). During this admission, her serum-anti-GBM antibody was negative. She was again treated as a presumed anti-GBM relapse with plasmapheresis for 7 days, cyclophosphamide 2 mg/kg/day for 3 months and methylprednisone 1 g IV daily for three doses followed by oral prednisone 60 mg/day for 2 months. During this most recent hospitalization, she was initially admitted to the ICU for plasmapheresis then transferred to the general medicine service after 1 day due to stability. She required no supplemental oxygen during the course of her 8-day hospitalization.
Fig. 2.
Chest plain film x-ray during admission in 2014 revealed extensive perihilar opacities.
In 2015, she was seen in the outpatient nephrology clinic for follow-up. Urine analysis showed no evidence of hematuria or proteinuria and labs revealed normal kidney function with a creatinine of 0.7 mg/dL and estimated glomerular filtration rate >90 mL/min/1.73 m2. The patient states that she has been smoking one to one and a half packs per day since 1984 but is now down to 12 cigarettes daily. Despite her two nearly fatal relapses, repeated counseling for smoking cessation and discussions regarding future risk of relapse due to continued cigarette use, the patient continues to smoke cigarettes. In multiple provider notes between 2006 and 2015, she states that she is not ready to quit due to stress at home and family responsibilities. Other significant social history includes consumption of one to two alcoholic beverages daily and she denies illicit drug use. She has been a bartender for over 10 years and has no history of exposure to solvents or hydrocarbons.
Discussion
Relapses are infrequent in anti-GBM disease but have been reported [7–12]. The present report is unique because the patient had three episodes of presumed anti-GBM disease with varying presence of anti-GBM antibody and negative ANCA and ANA. Our patient was only biopsied during her initial presentation. After each of the three episodes she recovered with a similar treatment regimen (Table 1).
Table 1.
Summary of clinical presentations, anti-GBM antibodies, renal biopsy and treatment
| Year | Clinical presentation | Anti-GBM antibodies | Renal biopsy | Treatment |
|---|---|---|---|---|
| 2001 | Gross hematuria; no pulmonary symptoms | Undetectable | Crescentic glomerulonephritis with 25% crescents on light microscopy; Linear deposition of IgG on immunofluorescence microscopy |
|
| 2006 | Dyspnea, hemoptysis, gross hematuria; Diffuse alveolar hemorrhage on chest plain film and CT | 112 AU/mL | Not obtained |
|
| 2014 | Epistaxis, hemoptysis; diffuse alveolar hemorrhage on chest plain film and microhematuria on urine analysis | Undetectable | Not obtained |
|
It is interesting that our patient had variable findings of circulating anti-GBM antibodies with each recurrence. Salama et al. have estimated that seronegative anti-GBM disease may occur in 2–3% of patients in the UK [13]. One possible explanation is that the inability to detect anti-GBM antibodies may reflect technical limitations of the routinely available assay (indirect immunofluorescence, immunoblot and ELISA) rather than antibody negativity. Another possible explanation is that smoking produces autoantibodies that react with a different antigen or epitope compared with typical anti-GBM antibodies, which react with two well-defined epitope regions of the NC1-domain of type IV collagen alpha-3-chains [1, 14]. Ohlsson et al. reported four young women with severe alveolar hemorrhage and favorable renal outcome who were found to have a predominance of IgG4 autoantibodies that were not detected by routine anti-GBM antibody laboratory assays. They concluded that these antibodies might constitute a distinct subgroup of anti-GBM disease [12].
Another recent case series by Serisier et al. has suggested that alveolar hemorrhage in anti-GBM disease that is seronegative by routinely available assays may be more common than previously appreciated [11]. Subjects in the case series were all smokers who presented with alveolar hemorrhage and minimal evidence of active renal involvement. It may be that subjects with very low levels of anti-GBM antibodies develop isolated pulmonary hemorrhage when exposed to the right precipitant such as volatile hydrocarbons or cigarette smoke. Such subjects may represent a subgroup with ‘early’ anti-GBM disease and relatively low circulating antibodies who manifest pulmonary disease due to the direct effects of cigarette smoke on the alveolar walls.
Smoking has been linked to recurrent disease and could have been the trigger in this case, as she has continued to use tobacco since her initial diagnosis [15, 16]. It is possible that smoking may even stimulate production of anti-GBM antibodies by allowing direct contact between the alveolar basement membrane and the immune system [1, 8]. Other environmental exposures that have been implicated in relapse include organic solvents [17], hydrocarbons [18, 19], chlorine gas [20] and hard metal exposure [21]. Some case reports have also suggested cocaine [22] and urinary tract infection [23] as triggers for relapse.
This case emphasizes the need for awareness of the possibility of seronegative anti-GBM disease. Several previous cases and case series have reported seronegative disease particularly in patients with recurrences, tobacco users and those with predominantly pulmonary symptoms. Renal biopsy should be considered in a patient with diffuse alveolar hemorrhage with even minimal renal involvement [11] since early diagnosis of anti-GBM disease allows for earlier initiation of life-saving treatment. Lastly, diagnosis of anti-GBM disease in such patients warrants greater emphasis on the importance of smoking cessation and close monitoring for disease recurrence.
Conflict of interest statement
None declared.
References
- 1.Pusey C. Anti-glomerular basement membrane disease. Kidney Int 2003; 64: 1535–1550 [DOI] [PubMed] [Google Scholar]
- 2.Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int 1973; 3: 74–89 [DOI] [PubMed] [Google Scholar]
- 3.Sinico RA, Radice A, Corace C et al. . Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant 2006; 21: 397–401 [DOI] [PubMed] [Google Scholar]
- 4.Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol 1999; 10: 2446–2453 [DOI] [PubMed] [Google Scholar]
- 5.Jayne DR, Marshall PD, Jones SJ et al. . Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int 1990; 37: 965–970 [DOI] [PubMed] [Google Scholar]
- 6.Levy JB, Turner AN, Rees AJ et al. . Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 2001; 134: 1033–1042 [DOI] [PubMed] [Google Scholar]
- 7.Mehler PS, Brunvand MW, Hutt MP et al. . Chronic recurrent Goodpasture's syndrome. Am J Med 1987; 82: 833–835 [DOI] [PubMed] [Google Scholar]
- 8.Borza DB, Neilson EG, Hudson BG. Pathogenesis of Goodpasture syndrome: a molecular perspective. Semin Nephrol 2003; 23: 522–531 [DOI] [PubMed] [Google Scholar]
- 9.Fonck C, Loute G, Cosyns JP et al. . Recurrent fulminant anti-glomerular basement membrane nephritis at a 7-year interval. Am J Kidney Dis 1998; 32: 323–327 [DOI] [PubMed] [Google Scholar]
- 10.Levy JB, Lachmann RH, Pusey CD. Recurrent Goodpasture's disease. Am J Kidney Dis 1996; 27: 573–578 [DOI] [PubMed] [Google Scholar]
- 11.Serisier DJ, Wong RCW, Armstrong JG. Alveolar haemorrhage in anti-glomerular basement membrane disease without detectable antibodies by conventional assays. Thorax 2006; 61: 636–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohlsson S, Herlitz H, Lundberg S et al. . Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis 2014; 63: 289–293 [DOI] [PubMed] [Google Scholar]
- 13.Salama AD, Dougan T, Levy JB et al. . Goodpasture's disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis 2002; 39: 1162–1167 [DOI] [PubMed] [Google Scholar]
- 14.Segelmark M, Hellmark T, Wieslander J. The prognostic significance in Goodpasture's disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract 2003; 94: 59–68 [DOI] [PubMed] [Google Scholar]
- 15.Lazor R, Bigay-Gamé L, Cottin V et al. . Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine (Baltimore) 2007; 86: 181–193 [DOI] [PubMed] [Google Scholar]
- 16.Donaghy M, Rees AJ. Cigarette smoking and lung haemorrhage in glomerulonephritis caused by autoantibodies to glomerular basement membrane. Lancet 1983; 2: 1390–1393 [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Gattone VH II, Noelken ME et al. . The alpha 3 chain of type IV collagen induces autoimmune Goodpasture syndrome. Proc Natl Acad Sci USA 1994; 91: 6201–6205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beirne GJ, Brennan JT. Glomerulonephritis associated with hydrocarbon solvents: mediated by antiglomerular basement membrane antibody. Arch Environ Health 1972; 25: 365–369 [DOI] [PubMed] [Google Scholar]
- 19.Keller F, Nekarda H. Fatal relapse in Goodpasture's syndrome 3 years after plasma exchange. Respiration 1985; 48: 62–66 [DOI] [PubMed] [Google Scholar]
- 20.Siebels M, Andrassy K, Ritz E. Provocation of pulmonary haemorrhage in Goodpasture syndrome by chlorine gas. Nephrol Dial Transplant 1993; 8: 189. [PubMed] [Google Scholar]
- 21.Lechleitner P, Defregger M, Lhotta K et al. . Goodpasture's syndrome. Unusual presentation after exposure to hard metal dust. Chest 1993; 103: 956–957 [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Rostan y Perez GM, Garcia Bragado F, Puras Gil AM. Pulmonary hemorrhage and antiglomerular basement membrane antibody-mediated glomerulonephritis after exposure to smoked cocaine (crack): a case report and review of the literature. Pathol Int 1997; 47: 692–697 [DOI] [PubMed] [Google Scholar]
- 23.Wu MJ, Moorthy AV, Beirne GJ. Relapse in anti glomerular basement membrane antibody mediated crescentic glomerulonephritis. Clin Nephrol 1980; 13: 97–102 [PubMed] [Google Scholar]