Abstract
Anti-glomerular basement membrane (GBM) antibody disease is a typically monophasic autoimmune disease with severe pulmonary and renal involvement. We report an atypical case of frequently relapsing anti-GBM antibody disease with both anti-GBM antibody–positive flares with pulmonary and renal involvement, and anti-GBM antibody–negative flares that were pulmonary limited with no histologic renal disease. This is the first report of alternating disease phenotype and anti-GBM antibody status over time. Disease severity paralleled the detection of anti-GBM antibodies but was independent of IgG subtype staining along the GBM. This case suggests a role for changing subpopulations of pathogenic antibodies as an explanation for variation in disease phenotype and anti-GBM antibody results.
Keywords: anti-GBM antibody disease, ELISA, glomerulonephritis, Goodpasture's disease, pulmonary hemorrhage
Background
Anti-glomerular basement membrane (GBM) antibody disease is a prototypical autoimmune disease characterized by pulmonary hemorrhage and crescentic necrotizing glomerular disease as a result of antibodies targeting the non-collagenous 1 domain of the α3 subunit of type 4 collagen [α3(IV)NC1] [1]. The disease course is usually monophasic, with initially severe pulmonary and renal involvement, but subsequent relapses are not commonly seen. In >95% of cases, anti-GBM antibodies can be detected in the serum using commercially available ELISAs that use various forms of α3(IV) collagen as the target antigen [2, 3]. However, there have been increasing reports of atypical anti-GBM antibody disease characterized by isolated pulmonary disease with minimal renal involvement or without detectable anti-GBM antibodies [4–9]. Proposed mechanisms to explain negative anti-GBM antibody ELISA testing include low levels of pathogenic antibodies below the detectable limit of the assay, different IgG subtypes (such as IgG4) or non-IgG antibodies that are less detectable by ELISA, low antigen binding affinity or target antigens other than the usual epitopes in α3(IV)NC1 [6, 10–13]. These atypical characteristics have also been suggested to result in fewer pathogenic antibodies and hence might explain cases of isolated pulmonary disease in the context of superimposed lung injury from hydrocarbons or smoking [1, 12–14].
We report a case of anti-GBM antibody disease with a highly unique frequently relapsing disease course that varied between anti-GBM antibody–positive flares with both pulmonary and renal involvement and anti-GBM antibody–negative flares that were pulmonary limited. To our knowledge, this clinical pattern of anti-GBM antibody disease has not been previously described. By comparing ELISA results with IgG subtypes detected along the GBM in serial kidney biopsies, this case provides unique insight into the role of longitudinal changes in antibody characteristics associated with atypical variation in the clinical phenotype of anti-GBM antibody disease.
Case report
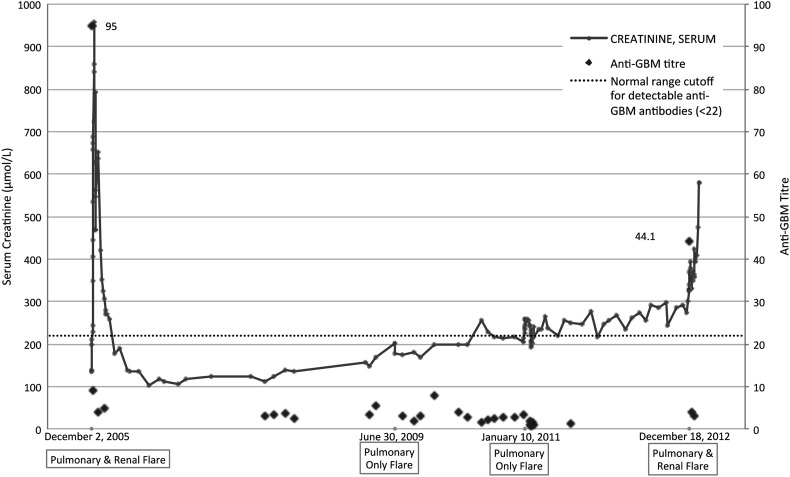
A 41-year-old woman with normal baseline kidney function presented in December 2005 with pulmonary hemorrhage confirmed on CT scan and bronchoscopy, an elevated creatinine of 957 µmol/L (eGFR 5 mL/min/1.73 m2) requiring dialysis, >50 red blood cells (RBCs)/high power field (HPF) on urinalysis, negative anti-neutrophil cytoplasmic antibodies (ANCAs), and positive anti-GBM antibodies (see Figure 1). A renal biopsy showed crescentic glomerulonephritis in which 60% of the 24 glomeruli contained active cellular or fibrocellular crescents, with strong linear capillary glomerular staining on direct immunofluorescence (IF) that was IgG2 dominant (see Figure 2 and Table 1). None of the glomeruli had segmental or global sclerosis. She was treated with daily plasmapheresis, steroids and monthly intravenous (IV) cyclophosphamide for 6 months. Her hemoptysis resolved, and she recovered renal function but had residual chronic kidney disease with baseline creatinine of 170 µmol/L.
Fig. 1.
Serum creatinine, anti-GBM titers and disease presentations over time.
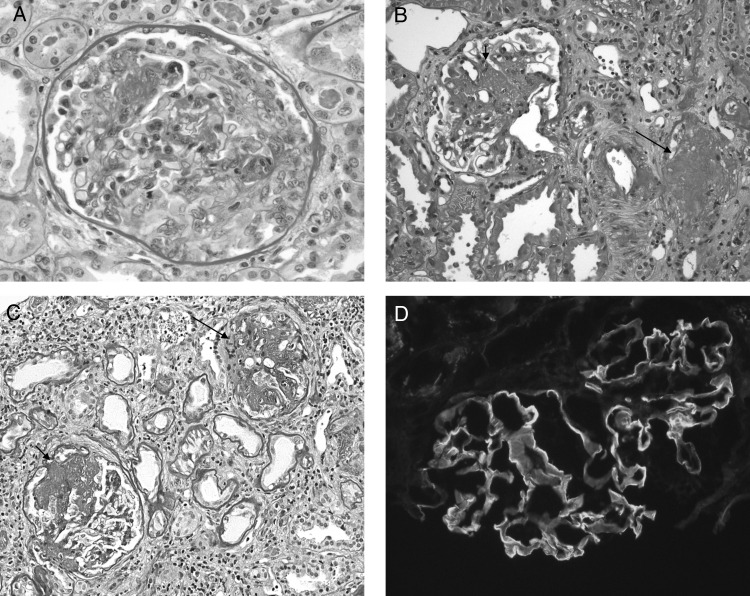
Fig. 2.
Renal biopsy findings. (A) First renal biopsy (2005). A glomerulus with a large cellular crescent characteristic of active crescentic glomerulonephritis [Periodic acid–Schiff (PAS) stain, original magnification ×400]. (B and C) Second (2009) and third (2011) biopsies. Both panels show glomeruli with segmental scarring (short arrows) or global sclerosis (long arrows) (PAS stain, original magnification ×200). (D) Direct immunofluorescence. The glomerulus shows strong linear capillary staining for IgG (original magnification ×200).
Table 1.
Results of linear IgG subtype staining along the GBM on serial kidney biopsies, graded on a scale of 0 to 4+, performed using indirect immunofluorescence
| First biopsy (2005) | Second biopsy (2009) | Third biopsy (2011) | |
|---|---|---|---|
| IgG1 | 1+/2+ | 1+ | 1+/2+ |
| IgG2 | 3+ | 3+ | 1+/2+ |
| IgG3 | 0 | 0 | 0 |
| IgG4 | 1+/2+ | 1+ | 1+ |
In June 2009 she presented with diffuse alveolar hemorrhage confirmed on CT scan and bronchoscopy, creatinine near baseline at 201 µmol/L with an eGFR of 25 mL/min/1.73 m2 and negative anti-GBM antibody and ANCA testing. Urinalysis was negative for RBC or protein. A kidney biopsy showed strong linear capillary IgG staining on IF that was IgG2 dominant, but no active disease (24 glomeruli—17 were globally sclerotic, 2 had segmental scarring and 5 were normal; see Figure 2 and Table 1). She was treated with plasmapheresis, steroids and monthly IV cyclophosphamide followed by azathioprine maintenance therapy, with improvement in her hemoptysis. In 2011, while on low-dose azathioprine, she presented again with pulmonary hemorrhage confirmed on CT scan, creatinine near her baseline at 215 µmol/L with an eGFR of 21 mL/min/1.73 m2 and negative anti-GBM antibody testing. Urinalysis was again negative for RBC or protein. A third renal biopsy again showed linear IgG staining that was IgG1/2 codominant but with no active disease (48 glomeruli—33 were globally sclerotic and the rest were normal or had segmental scarring; see Figure 2 and Table 1). She was treated with plasmapheresis, steroids and oral cyclophosphamide, followed by azathioprine maintenance therapy, with resolution of her pulmonary disease. In December 2012, she presented with a final disease flare while on low-dose azathioprine. She had diffuse alveolar hemorrhage confirmed on CT scan and bronchoscopy, >20 RBCs/HPF on urinalysis, creatinine increased to 677 µmol/L requiring dialysis and positive anti-GBM antibodies (see Figure 1). A renal biopsy was not performed. She was treated with plasmapheresis, steroids and monthly IV cyclophosphamide followed by azathioprine. Her hemoptysis resolved but she remained dialysis dependent. Throughout her disease course she had no evidence of a lymphoproliferative disorder, but she was a lifelong smoker and had recurrent occupational exposures to paints and solvents.
All testing for the presence of anti-GBM antibodies was by the QUANTA Lite GBM ELISA (INOVA Diagnostics, San Diego, CA, USA), which uses purified bovine α3(IV) as substrate and was performed according to the manufacturer's protocol. Banked serum samples were not available for more detailed anti-GBM antibody testing.
Discussion
We describe a unique case of anti-GBM antibody disease that is the first report of an atypical frequently relapsing disease course alternating between combined pulmonary and renal involvement and isolated pulmonary hemorrhage. Renal involvement paralleled the presence or absence of detectable anti-GBM antibodies by conventional ELISAs but was independent of IgG subtype or staining intensity on serial kidney biopsies.
Anti-GBM antibody disease most commonly presents as a monophasic illness that results in crescentic glomerular disease alone or in combination with pulmonary hemorrhage [1]. While the risk of relapse was originally thought to be quite rare, there have been recent reports of classic pulmonary-renal disease with positive anti-GBM antibodies that were followed by a single pulmonary limited relapse without detectable anti-GBM antibodies [5, 7, 8]. These relapses were often triggered by inhalation injury from smoking or hydrocarbons, which has been proposed to cause local alveolar injury precipitating pulmonary disease but without sufficient systemic involvement to result in crescentic glomerulonephritis. This is similar to the current case, in which multiple frequently relapsing pulmonary limited flares without detectable anti-GBM antibodies were likely precipitated by ongoing exposure to cigarettes, paint fumes or solvents. However, to our knowledge this is the first report of pulmonary limited antibody-negative relapses followed by a second occurrence of combined pulmonary and renal disease with reappearance of detectable anti-GBM antibodies. This emphasizes that in rare instances anti-GBM antibody disease can result in multiple relapses associated with inhalational irritants, that organ involvement in the lungs and kidney can fluctuate over time and that anti-GBM antibodies can be variably detectable and parallel more severe renal involvement.
This case offers novel insight into anti-GBM antibody characteristics that have been previously proposed to explain variation in disease severity and false-negative conventional ELISAs. Although IgG4 is the least common subtype seen on kidney biopsy, it has been associated with more mild renal involvement and is less able to bind anti-IgG antisera during ELISA preparation, resulting in false-negative results [13–15]. Low levels of pathogenic antibodies below the detectable limit of ELISAs have also been proposed to explain pulmonary limited disease [6, 8]. However, neither of these explain the variation in clinical disease or anti-GBM assay results seen in this case, given that IgG1 and 2 were the dominant subtypes on serial kidney biopsies, and the stable intensity of linear IgG staining across disease flares suggests sufficient antibody production to result in the same degree of GBM deposition irrespective of the presence or absence of renal involvement. Because commercial ELISAs use various forms of α3(IV)NC1 as a substrate, they are less able to detect antibodies that either target atypical antigens or have lower binding affinity and are removed during the preparation process [9, 10]. More severe disease is known to be associated with increasing binding affinity to target antigens and with epitope spreading to atypical NC1 domains in non-α3 chains, such that the majority of patients with severe disease have antibodies against two or more antigens in different α chains [1, 14, 16, 17]. As such, we propose that this patient may have had multiple subpopulations of antibodies with different characteristics, such as antigen binding affinity or epitope specificity, whose pathogenicity varied over time and resulted in different clinical presentations. This is consistent with the initial and final severe disease flares that had both pulmonary and renal involvement and had detectable antibodies against α3(IV)NC1 of sufficient binding affinity to result in positive ELISAs. The intervening more mild pulmonary limited flares occurred in the context of IgG anti-GBM antibodies, as demonstrated by linear GBM staining on kidney biopsies, but these had sufficiently different properties to result in negative ELISAs. These findings suggest that the population of pathogenic antibodies in anti-GBM antibody disease may not be static and instead may change over time, resulting in disease flares of different severity and organ involvement and inconsistent detection of circulating anti-GBM antibodies by conventional ELISAs.
In summary, we demonstrated a case of highly atypical anti-GBM antibody disease with a frequently relapsing course in which disease flares alternated between combined lung and renal involvement and isolated pulmonary hemorrhage. The variable detection of anti-GBM antibodies by ELISA paralleled disease severity and was independent of persistent linear IgG staining along the GBM, suggesting a role for changing subpopulations of pathogenic autoantibodies as an explanation for variation in disease phenotype.
Conflict of interest statement
None declared. This paper has not been published previously.
Acknowledgements
B.G. is funded by the University of British Columbia Faculty of Medicine Summer Student Research Program. S.J.B. is funded by the Michael Smith Foundation for Health Research.
References
- 1.Cui Z, Zhao MH. Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol 2011; 7: 697–705 [DOI] [PubMed] [Google Scholar]
- 2.Jaskowski TD, Martins TB, Litwin CM et al. . Comparison of four enzyme immunoassays for the detection of immunoglobulin G antibody against glomerular basement membrane. J Clin Lab Anal 2002; 16: 143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinico RA, Radice A, Corace C et al. . Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture's disease: a comparison of different assays. Nephrol Dial Transplant 2006; 21: 397–401 [DOI] [PubMed] [Google Scholar]
- 4.Harrity P, Gilbert-Barness E, Cabalka A et al. . Isolated pulmonary Goodpasture's disease. Pediatr Pathol 1991; 11: 635–646 [DOI] [PubMed] [Google Scholar]
- 5.Lazor R, Bigay-Gamé L, Cottin V et al. . Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine (Baltimore) 2007; 86: 181–193 [DOI] [PubMed] [Google Scholar]
- 6.Salama AD, Dougan T, Levy JB et al. . Goodpasture's disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis 2002; 39: 1162–1167 [DOI] [PubMed] [Google Scholar]
- 7.Benz K, Amann K, Dittrich K et al. . Patient with antibody-negative relapse of Goodpasture's disease. Clin Nephrol 2007; 67: 240–244 [DOI] [PubMed] [Google Scholar]
- 8.Serisier DJ, Wong RC, Armstrong JG. Alveolar haemorrhage in anti-glomerular basement membrane disease without detectable antibodies by conventional assays. Thorax 2006; 61: 636–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolk M, Carl D, Massey HD. Antibody-negative Goodpasture's disease. NDT Plus 2010; 3: 253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia XY, Qu Z, Cui Z et al. . Circulating anti-glomerular basement membrane autoantibodies against α3(IV)NC1 undetectable by commercially available enzyme-linked immunosorbent assays. Nephrology (Carlton) 2012; 17: 160–166 [DOI] [PubMed] [Google Scholar]
- 11.Fervenza FC, Terreros D, Boutaud A et al. . Recurrent Goodpasture's disease due to a monoclonal IgA-kappa circulating antibody. Am J Kidney Dis 1999; 34: 549–555 [DOI] [PubMed] [Google Scholar]
- 12.Ho J, Gibson IW, Zacharias J et al. . Antigenic heterogeneity of IgA anti-GBM disease: new renal targets of IgA autoantibodies. Am J Kidney Dis 2008; 52: 761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson S, Herlitz H, Lundberg S et al. . Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis 2014; 63: 289–293 [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Yan Y, Cui Z et al. . The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHα3(IV)NC1 is associated with disease severity. Hum Immunol 2009; 70: 425–429 [DOI] [PubMed] [Google Scholar]
- 15.Qu Z, Cui Z, Liu G et al. . The distribution of IgG subclass deposition on renal tissues from patients with anti-glomerular basement membrane disease. BMC Immunol 2013; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedchenko V, Vanacore R, Hudson B. Goodpasture's disease: molecular architecture of the autoantigen provides clues to etiology and pathogenesis. Curr Opin Nephrol Hypertens 2011; 20: 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedchenko V, Bondar O, Fogo AB et al. . Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 2010; 363: 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]