Abstract
During the last decade, a new view into the molecular mechanisms of chronic kidney disease-mineral bone disorder (CKD-MBD) has been proposed, with fibroblast growth factor 23 (FGF23) as a novel player in the field. Enhanced serum FGF23 levels cause a reduction in serum phosphate, together with calcitriol suppression and consequent hyperparathyroidism (HPT). In contrast, reduced serum FGF23 levels are associated with hyperphosphatemia, higher calcitriol levels and parathyroid hormone (PTH) suppression. In addition, serum FGF23 levels are greatly increased and positively correlated with serum phosphate levels in CKD patients. In this population, high serum FGF23 concentration seems to predict the occurrence of refractory secondary HPT and to be associated with higher mortality risk in incident haemodialysis patients. In living-donor kidney transplant recipients, a faster normalization of FGF23 and phosphate levels with a lower prevalence of HPT, may be considered a major pathway to investigate.
Keywords: CKD-MBD, FGF23, PTH, secondary hyperparathyroidism
Kidney transplantation is the best renal therapy for eligible end-stage renal disease (ESRD) patients. Kidney transplant recipients have better survival rates than dialysis patients, with lower dialysis-related morbidity, reduced cardiovascular risk, improved quality of life and reduced health economic costs [1, 2].
Due to an organ shortage, however, most patients have to wait while on dialysis for a considerable period of time prior to transplantation, with unfavourable consequences such as compromised graft and patient survival. Living-donor kidney transplantation and pre-emptive kidney transplant, defined as transplant before dialysis, are valid options to expand the organ pool and reduce the waiting time of patients on the waiting list, and the short- and long-term outcomes seem to be favourable compared with deceased-donor kidney transplantation [3]. Abnormalities in serum calcium, phosphorus, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and vitamin D levels occur early in the course of chronic kidney disease (CKD) to become a widespread complication in patients with advanced renal disease [4]. Mineral and bone disorders are common in patients who have undergone kidney transplantation [5]. Although for a long time it has been supposed that successful kidney transplantation to a large extent solves the problem of CKD–mineral and bone disorder (CKD-MBD), mineral and bone disorders are common in kidney transplant recipients, changing only its phenotype. These conditions are caused, to a large extent, by previous bone damage and CKD-MBD persisting after transplantation, de novo CKD-MBD and immunosuppressive therapy. The contribution of each component to the overall scenario changes over time.
In long-term kidney transplant recipients with a well-functioning graft (eGFR >30–45 mL/min), high PTH levels can still be observed in 30–60% at 1 year after transplantation [6]. Post-transplant hyperparathyroidism can be differentiated into a maladaptive response (‘persistent’ hyperparathyroidism) versus a compensatory–adaptive response (de novo hyperparathyroidism). ‘Persistent’ hyperparathyroidism results from pre-existing CKD-MBD with secondary hyperparathyroidism, likely complicating post-transplant follow-up with hypercalcaemia, hypophosphataemia, fracture risk [7], vascular calcification [8] and loss of graft function [9]. Conversely, de novo hyperparathyroidism results in elevated PTH levels, along with deterioration of graft function, to maintain normophosphataemia and normocalcaemia.
Serum levels of the bone-derived phosphaturic hormone FGF23 are extremely high in dialysis patients, reaching levels that can be 1000-fold above the normal range [4, 10], in an attempt to counteract hyperphosphataemia, producing a decrease of 1,25-dihydroxyvitamin D. Additional triggers for FGF23 in late CKD are secondary hyperparathyroidism and Klotho deficiency.
FGF23 levels decline 3 months after transplantation but remain higher than in CKD patients matched for eGFR. Further reductions in FGF23 levels were repeatedly observed over longer follow-up, approximating normal levels 1–3 years after transplantation [6, 11].
In contrast, in a cross-sectional observational study of 279 maintenance kidney recipients with CKD (stages 1–4), Sanchez Fructuoso et al. [12] found that FGF23 levels increased in long-term kidney graft recipients, even in the early stages of CKD, maybe as a result of previous chronic phosphate retention stimulating the secretion of FGF23. These findings support the notion of a persistent (or tertiary) hyperphosphatoninism that in the early post-transplant period mainly reflects previous mineral and bone disorders while in the long term mirrors renal function that is the major determinant of FGF23 serum levels, similar to what is observed in CKD patients. The interactions between FGF23 and PTH are very complex and cause a decrease in vitamin D metabolism. FGF23 and PTH mutually regulate each other in a negative feedback loop where PTH stimulates FGF23 production and FGF23, in turn, suppresses PTH synthesis acting via the Klotho–FGF receptor 1 (FGFR1) complex in the parathyroid gland and in the absence of Klotho via a phosphoinositide-specific phospholipase C gamma (PLCγ)-dependent activation of the calcineurin–nuclear factor of activated T cells (NFAT) pathway [13]. The mitogen-activated protein kinase (MAPK) pathway is likely the dominant pathway in physiology, although its relative contribution is unknown.
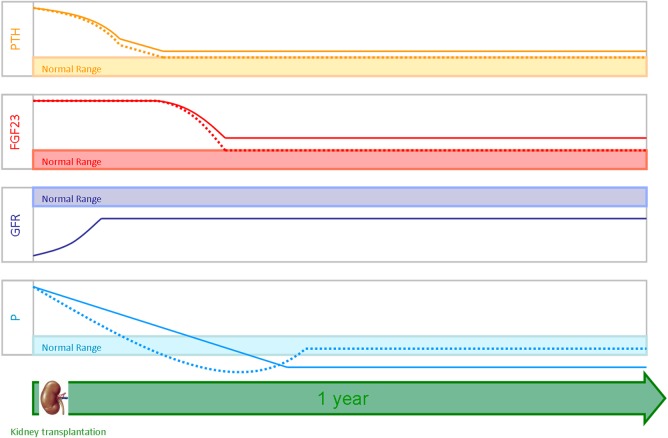
Hypophosphataemia is present in up to 90% of transplant recipients [14], with the majority (70%) of the cases being mild to moderate (serum phosphate level >1.5 to <2.3 mg/dL); phosphate levels remain low for longer than in patients with CKD matched for the GFR [15]. Acute or chronic post-transplant hypophosphataemia may cause detrimental effects. In the early post-transplant period, when serum phosphate levels are lower, muscle weakness may occur [16], whereas effects of chronic hypophosphataemia are less clear. There are few studies on the parallel changes in PTH, FGF23 and phosphate levels and mostly detailed in deceased-donor kidney transplantation with a follow-up of <1 year. Based on the data available, it is estimated that the effect of these phosphaturic hormones on hypophosphataemia changes over time after transplantation. FGF23 has emerged as an important mediator of early hypophosphataemia, and its phosphaturic effect is enforced by persistent hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency. In the long term, hypophosphataemia and renal phosphate loss are mainly related to persistent hyperparathyroidism [17, 18] (Figure 1).
Fig. 1.
Trends of PTH, FGF23 and phosphate (P) levels at 1 year after renal transplantation in a well-functioning graft. Deceased kidney donors are shown with a solid line while living kidney donors are shown with a dashed line.
The role of different strategies in the treatment of CKD-MBD before transplantation is an underestimated issue in the field of mineral and bone disorders in kidney transplant recipients, which expalins the wide variability reported in the literature. In particular, kidney transplant recipients undergoing treatment with cinacalcet before transplantation had high rates of persistent hyperparathyroidism, hypophosphataemia and hypercalcaemia after transplantation [18].
In the first year after transplantation, the faster normalization of FGF23 levels, compared with PTH levels, suggests that FGF23-producing cells may experience a quick reset of their activities in response to the recovery of renal function, all despite persistently elevated PTH levels, which stimulates FGF23 production. The long-lasting PTH-related renal phosphate wasting in kidney transplant recipients induces a negative phosphate balance that is appropriately sensed by osteocytes, thus decreasing FGF23 production in an effort to conserve phosphate, despite the stimulatory effects of high PTH levels.
A few and heterogeneous studies, and with a small number of patients, have analysed the changes in phosphate, PTH and FGF23 levels in living-donor kidney transplants. In a study of 39 living-donor kidney transplant recipients [19], FGF23 levels at 12 months after kidney transplantation were relatively low [20] and intact PTH (iPTH) levels were comparably high relative to the GFR; the pathogenesis of hypophosphataemia at 12 months may be due largely to persistent hyperparathyroidism rather than high FGF23 levels. It could also be argued that FGF23 levels remain inappropriately elevated, especially in light of low serum phosphorus levels and decreased urinary phosphate reabsorption, which therefore suggests that a dysregulation of the FGF23, renal phosphate and PTH axis may be present. An interesting finding in this study is that the pre-transplant FGF23 level was the best predictor of hypophosphataemia at 12 months.
These results are in agreement with those observed in a prospective observational cohort study carried out on 72 kidney transplant recipients, of which 23 received a living-donor transplant (58 on dialysis before transplantation and 14 pre-emptive transplant recipients), until 6 months after transplantation. The pre-transplantation FGF23 level was the main predictor of urinary phosphate excretion and serum phosphate levels in the early post-transplantation period, whereas serum phosphate levels in the sixth month were mainly influenced by PTH at that time. In this study, FGF23 levels decreased within the reference range in 50% of patients in the first month after transplantation and in 77% of patients with pre-emptive transplants. Kidney function improvement was associated with a reduction in FGF23 levels after transplantation.
Prasad et al. [21] addressed this issue by analysing at 1, 3 and 12 months the post-transplantation changes in FGF23, iPTH and phosphate levels in 63 ESRD patients who underwent living-donor transplantation. FGF23 and phosphate levels remained above the normal range in 36.5 and 27% of patients, respectively, at 1 month, in 15.9 and 8%, respectively, at 3 months and in none of the patients at 12 months post-transplantation, while only 11% of patients had persisting hyperparathyroidism 12 months post-transplantation. The authors postulated that two factors were responsible for these results, i.e. the shorter dialysis vintage prior to transplantation and the inclusion of only living-donor transplantation in the study, as renal function and mineral metabolism normalize relatively faster than in deceased-donor transplantation [22]. In kidney transplant recipients, the pathogenesis underlying the imbalance between PTH and FGF23 levels is probably multifactorial, involving persisting bone abnormalities and the effects of immunosuppressive therapy. Except for mycophenolate, immunosuppressive drugs stimulate FGF23 production, impair vitamin D metabolism and consequently increase PTH production [23, 24]. Speculatively the clinical use of calcineurin inhibitors (CNIs) that block calcineurin signalling may increase the susceptibility to develop or may aggravate pre-existing hyperparathyroidism in patients with reduced Klotho expression, such as in kidney transplant recipients [13]. Nevertheless, Prasad et al. demonstrated that FGF23 levels normalized and the prevalence of hyperparathyroidism was lower (when compared with deceased-donor kidney recipients) at 12 months after transplantation, even though all living-donor kidney transplant recipients underwent immunosuppressive schedules, including steroids and CNIs. This is an important finding of this study, but unfortunately there are only a few experimental studies on this specific issue, and the feasibility of clinical evaluations is hampered by the inability to assess the effects of the individual drugs, as they are always administered in combination.
Furthermore, the authors showed that the percentage decrease in FGF23 and iPTH levels was significantly associated during the entire follow-up. This interesting finding confirms the close interplay existing between FGF23 and PTH and suggests a substantial integrity/recovery of the receptor and signalling pathways in this cohort of living-donor kidney transplant recipients.
The study by Prasad et al. confirms a faster and significant recovery in terms of mineral and bone disorders within the first year after transplantation in living-donor kidney transplant recipients when compared with deceased-donor kidney transplant recipients, perhaps as a result of a less marked bone and PTH derangement due to the shorter dialysis vintage (Figure 1).Normalization of phosphate and FGF23 levels and the reduced prevalence of hyperparathyroidism are likely to have an important impact on graft and patient survival through reduced progression of cardiovascular and bone disease and this needs to be confirmed in larger studies with a longer follow-up. Of note, the recovery of hyperphosphatoninism and hypophosphataemia seems particularly intriguing in living-donor kidney transplant recipients.
Chronically elevated FGF23 levels may be considered ultimately as a maladaptive process in patients with CKD, given the strong associations between higher FGF23 levels and increased risk of left ventricular hypertrophy, congestive heart failure, CKD progression and death [4, 25, 26]. In a prospective study of stable kidney transplant recipients, elevated FGF23 levels were independently associated with an increased risk of cardiovascular and all-cause mortality and allograft loss [10, 27]. There are several possible mechanisms that may explain this finding. In vitro and in vivo studies have shown that 1,25-dihydroxyvitamin D decreases T cell activation and proliferation and inhibits dendritic cell differentiation and maturation, while its supplementation may have beneficial effects on chronic allograft nephropathy [24]. FGF23-mediated suppression of 1,25-dihydroxyvitamin D is one possible mechanism through which high FGF23 levels could contribute to allograft loss. In kidney transplant recipients, phosphate depletion, in conjunction with high PTH levels, vitamin D deficiency and chronic steroid use, might worsen skeletal demineralization and contribute directly to fractures, which in turn could increase the risk of mortality. High levels of FGF23 may impair neutrophil recruitment, which could jeopardize antibacterial defence [28]. Moreover, if chronically increased FGF23 levels can directly stimulate FGF receptors in the kidneys and heart, independent of Klotho [29], FGF23 could mimic the known effects of FGF2 to induce glomerulosclerosis and cardiac hypertrophy and thereby contribute directly to chronic allograft nephropathy and death [30, 31, 32]. Finally, FGF23 is associated with endothelial dysfunction and atherosclerosis in patients with high, or even normal, serum phosphate levels [33, 34]. Post-transplant hypophosphataemia and hypercalcaemia were associated with calcium and phosphate deposition in renal allografts [35], although human studies have shown conflicting results on whether renal calcification contributes to worse allograft outcomes.
The study of Prasad et al. confirms once again that the post–kidney transplant period should be considered a unique phase in the natural history of disordered mineral metabolism associated with CKD that requires dedicated investigation. In living-donor kidney transplant recipients, faster normalization of FGF23 and phosphate levels, in addition to a lower prevalence of hyperparathyroidism, if confirmed in larger studies, could be one of the possible pathways towards better outcomes in living-donor kidney transplant recipients.
Conflict of interest statement
None declared.
References
- 1.Wolfe RA, Ashby VB, Milford EL et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Knoll G et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 3.Matas AJ, Smith JM, Skeans MA et al. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant 2013; 13(Suppl 1): 11–46 [DOI] [PubMed] [Google Scholar]
- 4.Isakova T, Wahl P, Vargas GS et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanekar H, Welch BJ, Moe OW et al. Post-renal transplantation hypophosphatemia: a review and novel insights. Curr Opin Nephrol Hypertens 2006; 15: 97–104 [DOI] [PubMed] [Google Scholar]
- 6.Evenepoel P, Meijers BK, de Jonge H et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol 2008; 3: 1829–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrin P, Caillard S, Javier RM et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant 2013; 13: 2653–2663 [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferro S, Pasquali M, Taggi F et al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol 2009; 4: 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz A, Mengel M, Gwinner W et al. Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int 2005; 67: 341–348 [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Molnar MZ, Amaral AP et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011; 22: 956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzaferro S, Pasquali M, Pugliese F et al. Distinct impact of vitamin D insufficiency on calcitriol levels in chronic renal failure and renal transplant patients: a role for FGF23. J Nephrol 2012; 25: 1108–1118 [DOI] [PubMed] [Google Scholar]
- 12.Sanchez Fructuoso AI, Maestro ML, Perez-Flores I et al. Serum level of fibroblast growth factor 23 in maintenance renal transplant patients. Nephrol Dial Transplant 2012; 27: 4227–4235 [DOI] [PubMed] [Google Scholar]
- 13.Olauson H, Lindberg K, Amin R et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet 2013; 9: e1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M. Post-transplant hypophosphatemia. Kidney Int 2001; 59: 2377–2387 [DOI] [PubMed] [Google Scholar]
- 15.Evenepoel P, Rodriguez M, Ketteler M. Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol 2014; 34: 151–163 [DOI] [PubMed] [Google Scholar]
- 16.Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol 2002; 13: 551–558 [DOI] [PubMed] [Google Scholar]
- 17.Sirilak S, Chatsrisak K, Ingsathit A et al. Renal phosphate loss in long-term kidney transplantation. Clin J Am Soc Nephrol 2012; 7: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf M, Weir MR, Kopyt N et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 2016; 100: 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawarazaki H, Shibagaki Y, Fukumoto S et al. The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphatemia and hypercalcemia after living donor kidney transplantation: a 1-year prospective observational study. Nephrol Dial Transplant 2011; 26: 2691–2695 [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu T, Kazama JJ, Yamashita T et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 2004; 44: 250–256 [DOI] [PubMed] [Google Scholar]
- 21.Prasad N, Jaiswal A, Agarwal V et al. FGF23 is associated with early post-transplant hypophosphataemia and normalizes faster than iPTH in living donor renal transplant recipients: a longitudinal follow-up study. Clin Kidney J 2016; 9: 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evenepoel P, Naesens M, Claes K et al. Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 2007; 7: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 23.Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis 2013; 61: 310–325 [DOI] [PubMed] [Google Scholar]
- 24.Cianciolo G, Galassi A, Capelli I et al. Vitamin D in kidney transplant recipients: mechanisms and therapy. Am J Nephrol 2016; 43: 397–407 [DOI] [PubMed] [Google Scholar]
- 25.Faul C, Amaral AP, Oskouei B et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fliser D, Kollerits B, Neyer U et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007; 18: 2600–2608 [DOI] [PubMed] [Google Scholar]
- 27.Baia LC, Humalda JK, Vervloet MG et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8: 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossaint J, Oehmichen J, Van Aken H et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 2016; 126: 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juppner H, Wolf M, Salusky IB. FGF-23: more than a regulator of renal phosphate handling? J Bone Miner Res 2010; 25: 2091–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez OM, Januzzi JL, Isakova T et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leifheit-Nestler M, Grosse Siemer R, Flasbart K et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant 2016; 31: 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floege J, Kriz W, Schulze M et al. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest 1995; 96: 2809–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez OM, Mannstadt M, Isakova T. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza MA, Larsson A, Melhus H et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 2009; 207: 546–551 [DOI] [PubMed] [Google Scholar]
- 35.Evenepoel P, Lerut E, Naesens M et al. Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant 2009; 9: 2470–2478 [DOI] [PubMed] [Google Scholar]