Abstract
Since angiotensin increases the expression of plasminogen activator inhibitor (PAI), mechanisms associated with an actively functioning renin–angiotensin–aldosterone system can be expected to be associated with increased PAI-1 expression. These mechanisms are present not only in common conditions resulting in glomerulosclerosis associated with aging, diabetes or genetic mutations, but also in autoimmune disease (like scleroderma and lupus), radiation injury, cyclosporine toxicity, allograft nephropathy and ureteral obstruction. While the renin–angiotensin–aldosterone system and growth factors, such as transforming growth factor-beta (TGF-β), are almost always part of the process, there are rare experimental observations of PAI-1 expression without their interaction. Here we review the literature on PAI-1 and its role in vascular, fibrotic and oxidative injury as well as work suggesting potential areas of intervention in the pathogenesis of multiple disorders.
Keywords: cardiovascular, end-stage renal disease, renin–angiotensin system, thrombosis, type 2 diabetes
Introduction
We have followed a cohort of diabetic patients with end-stage renal disease treated with hemodialysis or transplantation in an effort to identify potential predictors of cardiovascular disease in this population. We reported in a prospective study that several markers of hemostasis, inflammation or oxidative stress were related to a past history of myocardial infarction, stroke or cardiovascular surgery [1]. This was in line with concepts first demonstrated by the Framingham Heart Study, which documented relationships between serum cholesterol and progression of cardiovascular disease [2] and a reproducible association of a prior history of cardiovascular events (CVE) with subsequent CVE, as well as reports about the usefulness of blood tests of hemostasis, inflammation or oxidative stress in predicting cardiovascular outcomes [3].
After 13 years of follow-up in our cohort, however, neither the presence nor the absence of a prior history of CVE [4] or baseline blood test findings nor combinations of the two held up as predictors of subsequent CVE as long as data from all diabetic patients were treated as a single study group. When we analyzed differences between Type 1 (insulinopenic) and Type 2 (insulin-resistant) patients, we noted that event-free intervals in the Type 1 patient subgroup were significantly longer when PAI-1 levels were close to normal rather than low. Yet the type 2 diabetic patient subgroup had significantly longer event-free intervals if PAI-1 levels were close to the normal range rather than high. Thus it became clear that differences in event-free intervals for tertiles of PAI-1 would be mathematically canceled out when Type 1 data and Type 2 data are analyzed as a single group [5].
In this review of the literature about PAI-1, we have sought a better understanding of the cardiovascular significance of these opposing divergences from normal and possible areas for further research.
Intensive insulin therapy or statin lowering and PAI-1
Among type 1 diabetic study subjects with albuminuria treated with angiotensin-converting enzyme (ACE) inhibitors, we found that an intensive insulin regimen reversed increased results for hemoglobin A1C, advanced glycation end-products and fibrinogen [6] and that very low levels of PAI-1 were raised to the normal range after 6 months. Low levels of PAI-1 in type 1 diabetic patients have also been reported by Agren and colleagues [7]. The increase in PAI-1 levels with intensive insulin therapy is generally supported by additional studies.
The pathophysiologic relationship between higher biomarker levels and progression of subclinical atherosclerosis in type 1 diabetes remains unclear due to the obesity epidemic that currently involves teenagers and young adults with type 1 diabetes. This is in contradistinction to the literature on type 2 diabetic populations for whom body mass and insulin resistance are generally high. Based upon these observations, we hypothesized that plasma PAI-1 concentration in individuals with little adipose and no insulin production might affect type 1 and type 2 diabetic populations differently.
Since the initial Framingham study, numerous investigators have demonstrated that cardiovascular risk lowering by statins [8–11] and fibrates [12–15], alone or in combination [11], is also associated with suppression of PAI-1 levels (Table 1). On the other hand, supplementation with omega fatty acids, which reduces triglycerides, actually elevates PAI-1 and has not been demonstrated to reduce cardiovascular risk [29].
Table 1.
Effect of medications on blood levels of plasminogen activator inhibitor 1 (PAI-1)
| Medications | Increases PAI-1 | Decreases PAI-1 |
|---|---|---|
| Antihypertensive agents | ||
| Beta-blockers (atenolol [16], metoprolol [17]) | ↓ | |
| ACE inhibitors (captopril [18], quinapril, ramipril [19]) | ↓ | |
| Angiotensin receptor blockers (losartan [16,20], valsartan [21]) | ↓ | |
| Calcium channel blockers (verapamil [17], isradipine [22]) | ↑ | |
| Aldosterone receptor antagonist (spironolactone [23]) | ↓ | |
| Anti-renin (aliskiren [24]) | ↑ | |
| Immune system-related drugs | ||
| Glucocorticoids [25, 26] | ↑ | |
| Bleomycin [27, 28] | ↑ | |
| Lipid-lowering agents | ||
| Statins (simvastatin [8, 11], atorvastatin [9], cervistatin [10]) | ↓ | |
| Fibrates (gemfibrozil [13], fenofibrate [11]) | ↓ | |
| Omega fatty acids [29] | ↑ | |
| Blood glucose-lowering agents | ||
| Sulfonylureas | ||
| Chlorpropamide [30], glipazide [30,31] | ↓ | |
| Tolbutamide [30], tolazamide [30], glyburide [30] | ↓ | |
| Biguanides (metformin)[31] | ↓ | |
| Thiazolidinediones (troglitazone [32], rosiglitazone [33], pioglitazone [34]) | ↓ | |
| Insulin [25,35] | ↑ | |
| Miscellaneous | ||
| Acetylcysteine [36] | ↓ | |
| Ethyl alcohol [37] | ↑ | |
| L-thyroxine [38] | ↑ | |
| Vitamin D [39] | ↓ | |
The role of PAI-1 in angiogenesis and fibrosis
A significant relationship of CVE with both high and low concentrations of PAI-1 [5] opened the possibility of other instances of seemingly counterintuitive observations with particular interest in mechanisms outside of fibrinolysis, such as angiogenesis/fibrosis. Devy and colleagues [40] studied growth of micro-vessels of the aortic ring in mice, finding that angiogenesis was promoted at physiologic PAI-1 levels but impaired with graded increases above normal concentrations. No vessel growth was noted for PAI-1 (−/−) animals.
Equally striking reports linking PAI-1 with mechanisms of fibrosis in the heart, kidney, liver, lung and skin have been reviewed by Ghosh and Vaughn [41]. PAI-1 appears to have controlling influences upon tissue plasminogen activator, urokinase plasminogen activator, plasmin and matrix metalloproteinase. Whereas deficiency of PAI-1 may protect most tissues from scarring during wound healing, the heart may experience fibrosis in the presence or absence of PAI-1.
PAI-1 in suppression of the insulin receptor
Additional links to mechanisms have been described by Bernot and associates [42], who observed PAI-1 involvement in the suppression of the insulin receptor, which they suggest may be a part of the pathogenesis of type 2 diabetes. Another finding was suppression of ADAM 17, which involves a complex of PAI-1 with furin, a pro-protein convertase. Such studies have not been repeated in an insulinopenic model. The failure of ADAM 17 (a dimer and metalloproteinase with 17 thrombospondin repeats) to cleave large von Willebrand factor polymers is a key step in the initiation of intravascular coagulation that consumes platelets in certain hemolytic uremic syndromes [43]. Involvement of PAI-1 with suppression of ADAM 17 would be consistent with its pro-coagulant role in the inhibition of fibrinolysis.
Metabolic mechanisms interacting with PAI-1
The physiological impact of PAI-1 as drawn by Alessi and colleagues [35] begins with the proportional relationship between the mass of adipose tissue secreting PAI-1, its plasma concentration and the degree of inhibition of plasminogen activator in blood or urine. But nearly a decade later Liang and associates [44] refined thinking on these relationships by demonstrating inhibition of differentiation of adipocytes associated with overexpression of PAI-1 as well as enhanced differentiation of adipocytes with underexpression of PAI-1. In further studies Liang et al. [44] showed that PAI-1 (−/−) deficient mice increased efficiency of insulin uptake via the glucose transporter while higher amounts of PAI-1 had an inhibitory effect.
Beir and Arteel [37] found an example of duality of mechanisms involving PAI-1 in a liver injury model, involving use of both alcohol and lipopolysaccharide. In the early stage of injury, PAI-1 was found to be protective through regeneration of hepatocytes injured by accumulated levels of toxic triglycerides. But in subsequent stages of steatohepatitis (inflammation/isolated fibrosis) and cirrhosis (connected fibrosis), PAI-1 was found to promote inflammation and to inhibit degradation of collagen complexes. PAI-1 was thus active both beneficially and pathologically following alcohol (Table 1).
Two drugs with opposite effects on glycemia affect PAI-1 levels. Glucocorticoids and insulin increased output of PAI-1 from human adipose tissue in studies by Morange and associates [25]. Streptozotocin (active against the beta cell) was also associated with increased expression of PAI-1 in the experience of Tamura and colleagues [45]. In an important report from the Veterans' Administration Study by Koska and colleagues [46], two groups were treated with insulin prescriptions that would be expected to increase expression of PAI-1 (standard versus intensive insulin). Despite significantly greater diminution of hemoglobin A1C and triglycerides in the intensive insulin group, cardiovascular outcomes were not significantly different between the two insulin groups. It is assumed that this diabetic study population is composed of Type 2 individuals.
The most common relationship of PAI-1 to triglyceride concentrations in type 2 diabetes is a parallel increase or decrease, depending upon experimental conditions. The Cardiovascular Risk in Young Finns Study demonstrated this relationship in work by Raiko and colleagues [47]. Nagi et al. [48] in a long-term study of the Pima Indians of Arizona found that despite increased consumption of fast-food diets, neither the prevalence of type 2 diabetes nor coronary artery disease was increased. In this population, levels of both PAI-1 and triglycerides were directly related to insulin resistance. Boberg and associates [29] observed that the trajectories of PAI-1 and triglycerides became divergent when type 2 diabetic study subjects were given 3 (omega) fatty acids, corresponding with a decrease in triglyceride level and an increase in PAI-1 concentrations. From this small cluster of reports, one could conclude that triglyceride and PAI-1 in type 2 diabetes usually run in parallel but may also move in opposite directions, depending upon the experimental conditions.
Vascular mechanisms interacting with PAI-1
Several experimental approaches to vascular injury have been used to explore relationships with PAI-1: balloon angioplasty [49–52]; ligation [53]; inhibition of nitric oxide synthesis [23,54–56]; ferric chloride [57–59] and increased the expression of TGF-β [59].
Replication of vascular injury using balloon angioplasty
A spectrum of associations with PAI-1 has been demonstrated. Hasenstab and colleagues [49] reported that the inhibitor of tissue plasminogen activator increased to a peak several days after angioplasty, with resolution to baseline by the end of the first week. These investigations identified smooth muscle cells of the medial layer to be stimulated to proliferation/migration in relation to the expression of PAI-1. Complex results involving balloon angioplasty injury to rat aortic endothelium occurred in a study by Hamden and colleagues [50]. Three hours after initial injury, a peak of PAI-1 levels was seen to be independent of angiotensin, but 4 days later there was an angiotensin-dependent peak of PAI-1. The initial injury was to mature endothelium while the second injury was to the neo-intima in the growth phase. So in these two studies the increased expression of PAI-1 would be anticipated to provide a prothrombotic milieu.
Tschopl et al. [51] used tests of hemostasis and inflammation to predict re-stenosis within 6 months of transluminal angioplasty. Elevated levels of fibrinogen and C-reactive protein at baseline were associated with re-stenosis after injury, but baseline PAI-1 levels were not. When wild-type rats are compared with PAI-deficient (−/−) rats after balloon injury to the carotid, increased neo-intimal healing was noted. In this study published by DeYoung and associates [52], PAI-1 appeared to be associated with protection from re-stenosis via intimal healing. We can conclude that PAI-1 may be related to injury or to recovery, depending upon timing, location, concentration or relationship to angiotensin.
Replication of vascular injury using vascular ligation
Another mechanical force that has been used to pursue the role of PAI-1 in response to injury is the placement of a ligature effectively replicating occlusion. Results after ligation are similar to those of DeYoung and associates [52] after balloon angioplasty, in that normal concentrations of PAI-1 were associated with a healing result compared to that seen with the absence of PAI-1 in a state of vascular injury at risk for obstruction. An important role in vascular homeostasis at the site of arterial injury is played by vitronectin, which is present in atherosclerotic plaque and co-localizes with PAI-1 wherever there is vascular injury. Vitronectin stabilizes PAI-1 in its active conformation and mediates the binding of this inhibitor to fibrin clots. deWaard and associates [53] found that the complex of PAI-1 bound to vitronectin is capable of: inhibiting polymerization of fibrinogen to fibrin; inhibiting cleavage of fibrin to fibrin-split products by plasmin; and inhibiting the generation of new intima following injury. Thus inhibition of excessive smooth muscle proliferation after ligation seen in the wild-type mice was not found in PAI-1 (−/−) deficient mice.
Vascular injury and oxidation mechanisms
Inhibition of nitric oxide synthesis has been shown to be followed by thickening of the muscular layer of the aorta with fibrosis by Katoh and co-workers [54], who exposed the Wistar-Kyoto rat to N (omega) nitro-l-arginine methyl ester (L-NAME) in a setting of angiotensin stimulation of both hypertension and enhanced expression of PAI-1. Use of an ACE inhibitor was found to eliminate both the hypertension and the excess expression of PAI-1 that were instrumental in the pathogenesis of vascular fibrosis. Kaikita et al. [55] and Boe et al. [56] from the Vaughn lab provided additional insight into the nitric oxide synthesis inhibition model, with data indicating that the absence or inhibition of PAI-1 would be sufficient to block both hypertension and vascular fibrosis/senescence. In a mouse model using inhibition of nitric acid synthase and high-salt diet, fibrosis of the kidney and heart was potentiated by aldosterone and PAI-1. Oestreicher et al. [23] reduced organ injury with spironolactone, which inhibited the increased expressions of aldosterone and PAI-1 that may be critical for the expression of fibrosis (Table 1).
Ferric chloride, an oxidizing agent, has been used in PAI-1 (−/−) deficient versus wild-type mice by Shafer, Konstantinides and associates [57–59] to identify immediate versus later responses. Within the first hour after exposure to adventitial ferric chloride, thrombosis was associated with the anti-fibrinolysis function of PAI-1, followed 3 weeks later by an increase in tissue plasminogen contributing to a decrease in occlusive thrombosis.
Replication of vascular injury with transforming growth factor models
Overexpression of TGF-β leading to vascular injury has been studied in the mouse by Otsuka and colleagues [60], using a carotid artery experimental preparation. Using wild-type and PAI-1 (−/−) deficient mice, they were able to demonstrate the dependence of TGF-β-induced vessel injury on the presence of PAI-1. Their model explored the rate of growth of the intima, the volume of matrix material accumulated, and patterns of cell proliferation/migration following exposure to TGF-β.
Cardiac, pulmonary and renal fibrosis
Prolonged hypertension promotes overexpression of collagen fibrils with fibrosis and scarring as well as cardiomyocyte hypertrophy and cell death. There appears to be an interplay between PAI-1 and hypertension in the formation/modulation of cardiac fibrosis. Following increased hemodynamic stress and prior to the generation of fibrotic scars, an intermediate process of angiotensin-induced expression of both TGF-β and PAI-1 has been identified. As a result, some investigations [20] and reviews [61] of ACE inhibition or angiotensin receptor blockade indicate a specific anti-fibrotic outcome.
Banding of the aorta in rats has been used to observe the effect of high blood pressure on ventricular muscle anatomy and function. Doering and colleagues [62] demonstrated accumulation of collagen without injury to muscle in the first few weeks of hypertension, but in the second month collagen fibrils between hypertrophied muscle showed signs of stress injury (necrotic fibril disruption with edema). Pressure/volume measurements indicated stiffness during cardiac diastole. This same group (Weber et al. [63]), using a non-human primate model, was able to identify three phases in the heart pathology. During the first month, type III collagen increased in the left ventricle with no sign of necrosis. After 35 weeks, thick strands of collagen (septae) were noted running through muscle, at which point the sub types of collagen were back in normal proportion. After 88 weeks, muscle necrosis was being repaired with fibrosis. Adaptive pressure/volume relationships were associated with lesser degrees of muscle necrosis/fibrosis. These studies by Doering et al. [62] and Weber et al. [63] were forerunners to a series of studies that found roles for the renin–angiotensin system [64, 65], endothelin [66] and growth factors [60, 67–70] in tissue injury/fibrosis, which eventually assigned a role for PAI-1.
Farivar and associates [64] used phenylephrine to generate cardiac hypertrophy in Wistar rats. Fibroblast proliferation was inhibited by both losartan and prazosin. In this model, the key contributor to heart failure was fibrosis. Linen and colleagues [65] found dose-dependent relationships between angiotensin and hypertrophied cardiac fibroblasts that could be blocked by telmisartan. Widyantoto et al. [66] used endothelin to demonstrate similar findings. Linen and colleagues [65] identified a step between angiotensin II and cardiac fibrosis to be the expression of tissue growth factor Beta.
Studies by Otsuka and associates [60] have also found TGF-β to be a stimulator of PAI-1 expression. Thus PAI-1 would be expected to be positively related to the generation of collagen from activated fibroblasts. Despite the fact that Otsuka [60], Stempien-Otero et al. [67] and Liu et al. [68–70] have demonstrated connections between PAI-1 and growth factors for fibrosis, many research groups do not mention PAI in similar studies. Recent observations by Hu and colleagues [71], with an editorial by Floege and Fliser [72], have identified deficiency of the gene Klotho to be associated with high fibroblast growth factor 23 in a mouse model where both cardiac and renal fibrosis were associated with TGF-β1, angiotensin II and a high phosphate diet. Unfortunately there were no comments on interaction with PAI-1, and a recent review on the subject of cardiac muscle maladaptation to stress by Hill [73] does not include any mention of PAI-1. A thorough review relating PAI-1 to tissue injury secondary to fibrosis has been presented by Ghosh and Vaughn [41].
Summary and conclusions
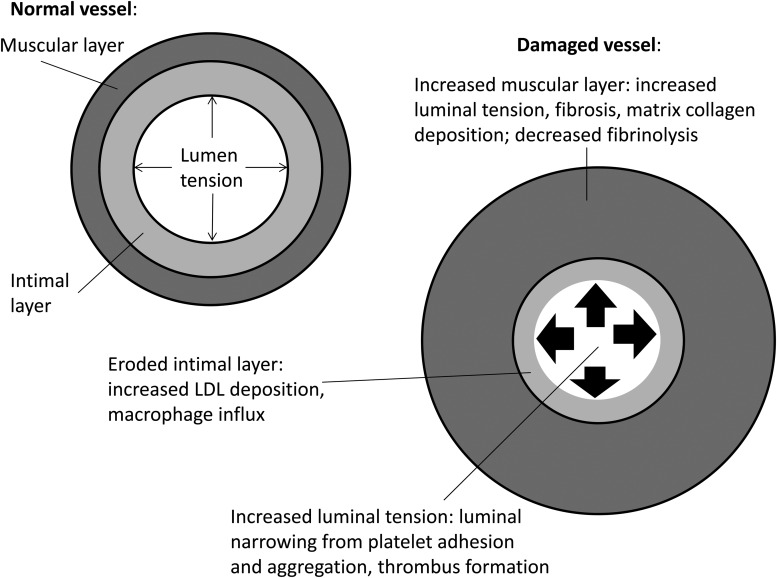
Table 1 lists medications that have an effect on the expression of PAI-1. Most are included in the text, but several were beyond the scope of the discussion. Drugs that directly address glycemia control include insulin, which increases the expression of PAI-1. On the other hand, metformin, glipizide, troglitazone, rosiglitazone and pioglitazone are associated with decreased expression of PAI-1. Several sulfonylurea drugs (chlorpropamide, glyburide, tolazamide, tolbutamide) increase PAI-1 expression. Agents for treatment of elevated lipids lower PAI-1 expression as do ACE inhibitors, angiotensin receptor blockers and beta-blockers. Figures 1 and 2 summarize the role of PAI-1 in vascular injury and fibrosis.
Fig. 1.
Inflammation of renal insufficiency in vascular injury. *Increased low-density lipoprotein cholesterol. **Increased platelet adhesion, aggregation. FGF 23, fibroblast growth factor 23.
Fig. 2.
Pathologic vascular changes associated with PAI-1. LDL, low-density lipoprotein.
PAI-1 expression promoted by TGF-β and the renin–angiotensin–aldosterone system has been noted in clinical conditions including nephrosclerosis of aging [74], diabetic nephropathy [75], focal sclerosis [76], scleroderma [77], radiation injury [78], cyclosporine toxicity [79] and transplant allograft nephropathy [80]. The same phenomenon has been observed in experimental models: 5/6 nephrectomy [20], protein overload [81], salt and angiotensin exposure [82], bleomycin-induced pulmonary sclerosis [83], nitric oxide synthase inhibitor [84], TGF-β overexpression [85] and unilateral ureteral obstruction [86].
Our attempts to identify biomarkers that might help predict CVE in type 1 and type 2 diabetic patients noted significant increases in CVE in type 1 diabetics with low levels of PAI-1 compared to those with normal levels and in type 2 diabetics with high levels versus those with normal levels. This may be related to the degree of adiposity (low in type 1 diabetics, high in type 2 diabetics) and resulting plasma PAI-1 levels. The link between PAI-1 and aldosterone is clear in vascular injury and organ fibrosis, with a role demonstrated for renin/angiotensin/aldosterone blockers and inhibitors that goes beyond the hemodynamic effects.
Further studies are needed to assess the contribution of PAI-1 activity to inflammation, adiposity, circulating insulin level and vascular injury. More work is needed to understand the pathophysiologic mechanisms more clearly. Only then will we know whether levels of PAI-1 in type 1 and type 2 diabetes can be used to predict future CVE and whether we can somehow intervene in the process to prevent those events from occurring. Reduction of the risk of CVE by strict control of blood pressure, blood sugar and lipids has been demonstrated, while testing of PAI-1 levels has not been shown to improve healthcare outcomes. Recent studies have emphasized that genetic polymorphisms may underlie cardiovascular risks associated with PAI-1 in patients with type 2 diabetes undergoing hemodialysis [87]. Investigation of the interaction of PAI-1 with stress factors for aging-related dysfunction of the heart and kidneys could be a worthwhile further addition to our understanding of the role PAI-1 in health and disease.
Conflict of interest statement
The authors report no conflicts of interest and confirm that this work has not been published or submitted for publication to any other journal.
Acknowledgements
The authors would like to acknowledge the expert assistance of the research librarian Diane Young of the Beth Israel Deaconess Medical Center Information Services. Also, we thank the Pat Covelli Foundation and the Amgen Corporation for funding investigator-initiated research.
References
- 1.D'Elia JA, Weinrauch LA, Gleason RE et al. Risk factors for thromboembolic events in renal failure. Int J Cardiol 2005; 101: 19–25 [DOI] [PubMed] [Google Scholar]
- 2.Mahmood SS, Levy D, Vasan RS et al. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014; 383: 999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, D'Agostino RB, Wilson PW et al. Diabetes, fibrinogen, and risk of cardiovascular disease: the Framingham experience. Am Heart J 1990; 120: 672–676 [DOI] [PubMed] [Google Scholar]
- 4.Bayliss G, Roshan B, Ventrapragada S et al. The impact of a prior history of cardiovascular events on outcomes in patients on renal replacement therapy. Int J Cardiol 2012; 157: 146–148 [DOI] [PubMed] [Google Scholar]
- 5.Bayliss GP, Weinrauch LA, Gleason RE et al. Do biologic markers predict cardiovascular endpoints in diabetic end stage renal disease? A prospective longitudinal study. Clin Kidney J 2013; 6: 599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Elia JA, Weinrauch LA, Gleason RE et al. Fibrinogen and factor VII levels improve with glycemic control in type 1 diabetic patients with microvascular complications. Arch Intern Med 2001; 161: 98–101 [DOI] [PubMed] [Google Scholar]
- 7.Agren A, Jorneskog G, Elgue G et al. Increased incorporation of antiplasmin into the fibrin network in patients with type 1 diabetes. Diabetes Care 2014; 37: 2007–2013 [DOI] [PubMed] [Google Scholar]
- 8.Ni XQ, Zhu JH, Yao NH et al. Statins suppress glucose-induced plasminogen activator inhibitor-1 expression by regulating RHOA and nuclear factor-kB activities in cardiac microvascular endothelial cells. Exp Biol Med 2013; 238: 37–46 [DOI] [PubMed] [Google Scholar]
- 9.Van de Ree MA, DeMat MP, Kluft C et al. Decrease of hemostatic cardiovascular risk factors by aggressive versus conventional atorvastatin treatment in patients with type 2 diabetes mellitus. J Thromb Haemost 2003; 1: 1753–1757 [DOI] [PubMed] [Google Scholar]
- 10.Takeshita Y, Takamura T, Hamaguchi E et al. Tumor necrosis factor-alpha- induced production of plasminogen activator inhibitor-1 and its regulation by pioglitazone and cervistatin in a nonmalignant human hepatocyte cell line. Metabolism 2006; 55: 1464–1472 [DOI] [PubMed] [Google Scholar]
- 11.Derosa G, Maffioli P, Salvadeo SA et al. Fenofibrate simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr Med Res Opin 2009; 25: 1972–1983 [DOI] [PubMed] [Google Scholar]
- 12.Arts J, Kocksx M, Princen HM et al. Studies on the mechanism of fibrate-inhibited expression of plasminogen activator inhibitor-1 in cultured hepatocytes from Cynomolgus monkey. Arterioscler Thromb Vasc Biol 1997; 17: 26–32 [DOI] [PubMed] [Google Scholar]
- 13.Nordt TK, Lutzi S, Ruef J et al. Attenuation by fibrates of plasminogen activator inhibitor type 1 expression in human arterial smooth muscle cells. Thromb Haemost 2001; 86: 1305–1313 [PubMed] [Google Scholar]
- 14.Kocksx M, Princen HM, Kooistra T. Fibrate modulated expression of fibrinogen, plasminogen activator inhibitor-1 and apolipoprotein A-1 in cultured Cynomolgus monkey hepatocytes—role of the peroxisome proliferator activated receptor-alpha. Thromb Haemost 1998; 80: 942–948 [PubMed] [Google Scholar]
- 15.Zirlik A, Ernst S, Leugers A et al. Inhibition by fibrates of plasminogen activator inhibitor type 1 expression in human adipocytes and pre-adipocytes. Thromb Haemost 2009; 101: 1060–1069 [PubMed] [Google Scholar]
- 16.Boman K, Boman JH, Andersson J et al. Effects of atenolol or losartan on fibrinolysis and von Willebrand factor in hypertensive patients with left ventricular hypertrophy. Clin Appl Thromb Hemost 2010; 16: 146–162 [DOI] [PubMed] [Google Scholar]
- 17.Held C, Hjemdahl P, Rehnqvist N et al. Fibrinolytic variables and cardiovascular prognosis inpatients with stable angina pectoris treated with verapamil or metoprolol. Results from the angina pectoris study in Stockholm. Circulation 1997; 20: 2380–2386 [DOI] [PubMed] [Google Scholar]
- 18.Wright RA, Flapan AD, Alberti KGMM et al. Effects of captopril therapy on endogenous fibrinolysis in men with recent uncomplicated myocardial infarction. J Am Coll Cardiol 1994; 24: 67–73 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan DE, Rouleau JL, Ridker PM et al. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation 1997; 96: 442–447 [DOI] [PubMed] [Google Scholar]
- 20.Ma LJ, Nakamura S, Aldigier JR et al. Regression of glomerulosclerosis with high dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol 2005; 16: 966–976 [DOI] [PubMed] [Google Scholar]
- 21.Sironi L, Calvio AM, Arnaboldi L et al. Effect of valsartan on angiotensin 2-induced plasminogen activator inhibitor-1 biosynthesis in arterial smooth muscle. Hypertension 2001; 37: 961–966 [DOI] [PubMed] [Google Scholar]
- 22.Ranieri G, Filliti V, Bonfantino MV et al. Effects of isradipine sustained release on platelet function in essential hypertension with or without other risk factors. Cardiovasc Drugs Ther 1996; 10: 119–123 [DOI] [PubMed] [Google Scholar]
- 23.Oestreicher EM, Martinez-Vasquez D, Stone JR et al. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation 2003; 108: 2517–2523 [DOI] [PubMed] [Google Scholar]
- 24.Irons BK, Trujillo A, Selfert CF et al. Effects of direct renin inhibition on atherosclerotic biomarkers in patients with stable coronary artery disease and type 2 diabetes mellitus. J Cardiovasc Pharmacol Ther 2013; 18: 427–432 [DOI] [PubMed] [Google Scholar]
- 25.Morange PE, Aubert J, Peiretti F et al. Glucocorticoids and insulin promote plasminogen activator inhibitor-1 production by human adipose tissue. Diabetes 1999; 48: 890–895 [DOI] [PubMed] [Google Scholar]
- 26.Sartori TM, Maurizio PG, Sara P et al. Relation between long-term steroid treatment after heart transplantation, hypofibrinolysis and myocardial microthrombi generation. J Heart Lung Transplant 1999; 18: 693–700 [DOI] [PubMed] [Google Scholar]
- 27.Matsushita M, Yamamoto T, Nishioka K. Plasminogen activator inhibitor-1 is elevated but not essential in the development of bleomycin-induced murine scleroderma. Clin Exp Immunol 2005; 139: 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senoo T, Hattori N, Tanimoto T et al. Suppression of plasminogen activator inhibitor by RNA interference attenuates pulmonary fibrosis. Thorax 2010; 65: 334–340 [DOI] [PubMed] [Google Scholar]
- 29.Boberg M, Pollare T, Seigbahn A et al. Supplementation with n-3 fatty acids reduces triglycerides, but increases PAI-1 in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1992; 22: 645–650 [DOI] [PubMed] [Google Scholar]
- 30.Kuo BS, Korner G, Bjornsson TD. Effects of sulfonylureas on bovine aortic endothelial cells. J Clin Invest 1988; 81: 730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cefalu WT, Schneider DJ, Carlson HE et al. Effect of combination glipizide GITS/metformin on fibrinolytic and metabolic parameters in poorly controlled type 2 diabetic subjects. Diabetes Care 2002; 25: 2123–2128 [DOI] [PubMed] [Google Scholar]
- 32.Kruszynska YT, Yu JG, Olevsky JN et al. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor in patients with type 2 diabetes and in lean normal subjects. Diabetes 2000; 49: 633–639 [DOI] [PubMed] [Google Scholar]
- 33.Liu HB, Hu YS, Medcalf RL et al. Thiazolidinediones inhibit TNF alpha induction of PAI-1 independent of PPAR-gamma activation. Biochem Biophys Res Commun 2005; 334: 30–37 [DOI] [PubMed] [Google Scholar]
- 34.Saremi A, Schwenke DC, Buchanan TA et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2013; 33: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alessi MC, Peiretti F, Morange P et al. Production of plasminogen activator inhibitor by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997; 46: 860–867 [DOI] [PubMed] [Google Scholar]
- 36.Martina V, Masha A, Gigliardi VR et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 2008; 31: 940–944 [DOI] [PubMed] [Google Scholar]
- 37.Brier J, Arteel G. Alcoholic liver disease and the potential role of plasminogen activator-1 and fibrin metabolism. Exp Biol Med 2012; 237: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horne MK III, Singh KK, Rosenfeld KG et al. Is thyroid hormone suppression therapy prothrombotic? J Clin Endocrinol Metab 2004; 89: 4469–4473 [DOI] [PubMed] [Google Scholar]
- 39.Wu-Wong JR, Nakane M, Ma J. Effects of vitamin D analogs on the expression of plasminogen activator inhibitor-1 in human vascular cells. Thromb Res 2006; 118: 709–714 [DOI] [PubMed] [Google Scholar]
- 40.Devy L, Blacher S, Grignet-Debrus C et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor-1 is dose dependent. FASEB J 2002; 16: 147–154 [DOI] [PubMed] [Google Scholar]
- 41.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol 2012; 227: 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernot D, Stalin J, Stocker P et al. Plasminogen activator inhibitor-1 is an intracellular inhibitor of furin proprotein convertase. J Cell Sci 2011; 124: 1224–1230 [DOI] [PubMed] [Google Scholar]
- 43.D'Elia JA, Aslani M, Schurmer S et al. Hemolytic-uremic syndrome and acute renal failure in metastatic adenocarcinoma treated with mitomycin. Ren Fail 1987; 10: 107–113 [DOI] [PubMed] [Google Scholar]
- 44.Liang X, Talergsak K, Mao S-L et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab 2006; 290: E103–E113 [DOI] [PubMed] [Google Scholar]
- 45.Tamura Y, Kawao N, Okada K et al. Plasminogen activator inhibitor-1 is involved in streptozotocin-induced bone loss in female mice. Diabetes 2013; 62: 3170–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koska J, Saremi A, Bahn G et al. The effect of intensive glucose lowering on lipoprotein particle profiles and inflammatory markers in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2013; 36: 2408–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raiko J, Oikonen M, Wendelin-Saarenhovi M et al. Plasminogen activator inhibitor-1 associates with cardiovascular risk factors in healthy young adults in the Cardiovascular Risk in Young Finns Study. Atherosclerosis 2012; 224: 208–212 [DOI] [PubMed] [Google Scholar]
- 48.Nagi DK, Tracy R, Pratley R. Relationship of hepatic and peripheral insulin resistance with plasminogen activator inhibitor-1 in Pima Indians. Metabolism 1996; 45: 1243–1247 [DOI] [PubMed] [Google Scholar]
- 49.Hasenstab D, Forough R, Clowes AW. Plasminogen activator-1 and metalloproteinase-2 increase after arterial injury in rats. Circ Res 1997; 80: 490–496 [DOI] [PubMed] [Google Scholar]
- 50.Hamdan AD, Quist WC, Gagne JB et al. Angiotensin-converting enzyme inhibition suppresses plasminogen activator inhibitor-1 expression in the neointima of balloon-injured rat. Circulation 1996; 93: 1073–1078 [DOI] [PubMed] [Google Scholar]
- 51.Tschopl M, Tsakiris DA, Marbet GA et al. Role of hemostatic risk factors for restenosis in peripheral arterial occlusive disease after transluminal angioplasty. Arterioscler Thromb Vasc Biol 1997; 17: 3208–3214 [DOI] [PubMed] [Google Scholar]
- 52.DeYoung MD, Tom C, Dichek DA. Plasminogen activator inhibitor type 1 increases neointimal formation of balloon-injured rat carotid arteries. Circulation 2001; 104: 1972–1981 [DOI] [PubMed] [Google Scholar]
- 53.DeWaarde V, Arkenbult EK, Carmeliet P et al. Plasminogen activator inhibitor-1 and vitronectin protect against stenosis in a murine carotid artery ligation model. Arterioscler Thromb Vasc Biol 2002; 22: 1978–1983 [DOI] [PubMed] [Google Scholar]
- 54.Katoh M, Egashira K, Matsui T et al. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J Mol Cell Cardiol 2000; 32: 73–83 [DOI] [PubMed] [Google Scholar]
- 55.Kaikita K, Fogo AB, Ma L et al. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation 2001; 104: 839–844 [DOI] [PubMed] [Google Scholar]
- 56.Boe AE, Eren M, Murphy SB et al. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates N (omega)-N-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 2013; 128: 2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konstantinides S, Schafer K, Thinnes T et al. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation 2001; 104: 576–583 [DOI] [PubMed] [Google Scholar]
- 58.Schafer K, Konstantinides S, Riedel C et al. Different mechanisms of increased luminal stenosis after arterial injury in mice deficient for urokinase or tissue-type plasminogen activator. Circulation 2002; 106: 1847–1852 [DOI] [PubMed] [Google Scholar]
- 59.Schafer K, Schroeter MR, Dellas C et al. Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol 2006; 26: 1254–1259 [DOI] [PubMed] [Google Scholar]
- 60.Otsuka G, Agah R, Frutkin AD et al. Transforming growth factor beta-1 induces neointimal formation through plasminogen activator inhibitor-1 dependent pathways. Arterioscler Thromb Vasc Biol 2006; 26: 737–743 [DOI] [PubMed] [Google Scholar]
- 61.D'Elia JA, Bayliss G, Roshan B et al. Diabetic microvascular complications: Possible targets for improved macrovascular outcomes. Int J Nephrol Renovasc Dis 2011; 4: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doering CW, Jalil JE, Janicki JS et al. Collagen network remodeling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res 1988; 22: 686–695 [DOI] [PubMed] [Google Scholar]
- 63.Weber KT, Janicki JS, Shroff SG et al. Collagen remodeling of the pressure-overload hypertrophy nonhuman primate myocardium. Circ Res 1988; 62: 757–765 [DOI] [PubMed] [Google Scholar]
- 64.Farivar RS, Crawford DC, Chobanian AV et al. Effect of angiotensin II on the blockade on the fibroproliferative response to phenylephrine in the rat heart. Hypertension 1995; 25: 809–813 [DOI] [PubMed] [Google Scholar]
- 65.Linen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by angiotensin II. Methods Find Exp Clin Pharmacol 2000; 22: 709–723 [DOI] [PubMed] [Google Scholar]
- 66.Widyantoro B, Emoto N, Nakayama K et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010; 121: 2407–2418 [DOI] [PubMed] [Google Scholar]
- 67.Stempien-Otero A, Plawman A, Meznarich J et al. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. J Biol Chem 2006; 281: 15345–15351 [DOI] [PubMed] [Google Scholar]
- 68.Liu RM. Oxidative stress, plasminogen activator inhibitor-1, and lung fibrosis. Antioxid Redox Signal 2006; 10: 303–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu RM, Gaston-Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med 2010; 48: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu RM, Choi J, Wu JH et al. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase-1 is involved in transforming growth factor beta-1-induced expression of plasminogen activator inhibitor-1 in fibroblasts. J Biol Chem 2010; 285: 16239–16247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu MC, Shi M, Cho HJ et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015; 26: 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Floege J, Fliser D. Klotho deficiency and the cardiomyopathy of advanced CKD. J Am Soc Nephrol 2015; 26: 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill JA. Braking bad hypertrophy. N Engl J Med 2015; 372: 2160–2162 [DOI] [PubMed] [Google Scholar]
- 74.Fogo AB. Mechanisms in nephrosclerosis. J Nephrol 2001; Suppl 4: S63–S69 [PubMed] [Google Scholar]
- 75.Zhang J, Laurence BA, Cheung AK et al. A plasminogen activator type 1 mutant retards diabetic nephropathy in db/db mice by protecting podocytes. Exp Physiol 2014; 99: 802–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fogo AB. Mesangial matrix modulation and glomerulosclerosis. Exp Nephrol 1999; 7: 147–159 [DOI] [PubMed] [Google Scholar]
- 77.Kessler-Becker D, Smota S, Krieg T et al. High plasminogen activator inhibitor type 2 expression is a hallmark of scleroderma fibroblasts in vitro. Exp Dermatol 2004; 13: 708–714 [DOI] [PubMed] [Google Scholar]
- 78.Rossini M, Naikto T, Yang H et al. Sulodexide ameliorates early, but not late, kidney disease in models of radiation nephropathy and diabetic nephropathy. Nephrol Dial Transplant 2010; 25: 1803–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira MG, Camara NO, Campaholle G et al. Pioglitazone limits cyclosporine nephrotoxicity in rats. Int Immunopharmacol 2006; 6: 1943–1951 [DOI] [PubMed] [Google Scholar]
- 80.Revelo MP, Federspiel C, Helderman H et al. Chronic allograft nephropathy: expression and localization of PAI-1 and PPAR-gamma. Nephrol Dial Transplant 2005; 20: 2812–2819 [DOI] [PubMed] [Google Scholar]
- 81.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int 1995; 47: 1546–1557 [DOI] [PubMed] [Google Scholar]
- 82.Schreier B, Rabe S, Schneider B et al. Aldosterone/NaCl-induced renal and cardiac fibrosis is modulated by TGF Beta responsiveness of T cells. Hypertens Res 2011; 34: 623–629 [DOI] [PubMed] [Google Scholar]
- 83.Zhang YP, Li WB, Wang WL et al. si-RNA against plasminogen activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis in rats. Acta Pharmacol Sin 2012; 33: 897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parra ER, Aguiar AC, Silva LO et al. Morphometric evaluation of nitric oxide synthase isoforms and their cytochrome regulators predict pulmonary dysfunction and survival in systemic sclerosis. Braz J Med Biol Res 2013; 46: 881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moll S, Menoud PA, Fulpius T et al. Induction of plasminogen activator inhibitor type 1 in murine lupus-like glomerulonephritis. Kidney Int 1995; 48: 1459–1468 [DOI] [PubMed] [Google Scholar]
- 86.Matsuo S, Lopez-Guisa JM, Cal X et al. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1 overexpressing mice. Kidney Int 2005; 67: 22221–22238 [DOI] [PubMed] [Google Scholar]
- 87.De Carvalho SS, Simoes E Silva AC, Sabino Ade P et al. Influence of ACE I/D polymorphism on circulating levels of plasminogen activator inhibitor 1, d-dimer, ultrasensitive c-reactive protein and transforming growth factor beta 1 in patients undergoing hemodialysis. PLoS One 2016; 11: e0150613. [DOI] [PMC free article] [PubMed] [Google Scholar]