This meta-analysis provides strong evidence that opioid substitution therapy improves several key outcomes of the HIV care continuum among people who inject drugs, including recruitment onto antiretroviral therapy, retention in care, adherence, and viral suppression.
Keywords: HIV, people who inject drugs (PWID), medication-assisted therapy for opioid dependence (MAT), antiretroviral treatment (ART), systematic review
Abstract
Background. Human immunodeficiency virus (HIV)–infected people who inject drugs (PWID) frequently encounter barriers accessing and remaining on antiretroviral therapy (ART). Some studies have suggested that opioid substitution therapy (OST) could facilitate PWID's engagement with HIV services. We conducted a systematic review and meta-analysis to evaluate the impact of concurrent OST use on ART-related outcomes among HIV-infected PWID.
Methods. We searched Medline, PsycInfo, Embase, Global Health, Cochrane, Web of Science, and Social Policy and Practice databases for studies between 1996 to November 2014 documenting the impact of OST, compared to no OST, on ART outcomes. Outcomes considered were coverage and recruitment onto ART, adherence, viral suppression, attrition from ART, and mortality. Meta-analyses were conducted using random-effects modeling, and heterogeneity assessed using Cochran Q test and I2 statistic.
Results. We identified 4685 articles, and 32 studies conducted in North America, Europe, Indonesia, and China were included. OST was associated with a 69% increase in recruitment onto ART (hazard ratio [HR], 1.69; 95% confidence interval [CI], 1.32–2.15), a 54% increase in ART coverage (odds ratio [OR], 1.54; 95% CI, 1.17–2.03), a 2-fold increase in adherence (OR, 2.14; 95% CI, 1.41–3.26), and a 23% decrease in the odds of attrition (OR, 0.77; 95% CI, .63–.95). OST was associated with a 45% increase in odds of viral suppression (OR, 1.45; 95% CI, 1.21–1.73), but there was limited evidence from 6 studies for OST decreasing mortality for PWID on ART (HR, 0.91; 95% CI, .65–1.25).
Conclusions. These findings support the use of OST, and its integration with HIV services, to improve the HIV treatment and care continuum among HIV-infected PWID.
Although harm reduction programs for people who inject drugs (PWID) are being scaled up globally, coverage remains low [1, 2]. Approximately 30% of new infections outside sub-Saharan Africa are attributed to injecting drug use [3], and the epidemic is growing in Central Asia and Eastern Europe [4, 5]. In recent years, increased injecting drug use has been documented in several countries with high human immunodeficiency virus (HIV) prevalence, such as Kenya and Tanzania [6, 7], and new HIV outbreaks have occurred in PWID in other settings, including the United States [8, 9].
Worldwide, new goals of achieving high coverage of HIV testing, treatment, and viral suppression have been proposed, with the aim of dramatically reducing global HIV transmission and morbidity [10]. However, PWID often have poor HIV treatment coverage [1, 11] and antiretroviral therapy (ART) outcomes [12, 13], which could hamper progress toward these goals. To achieve these targets in this group, interventions are needed to improve treatment outcomes. One such intervention could be opioid substitution therapy (OST), also known as medication-assisted therapy for opioid dependence (MAT), which could potentially enhance the uptake and retention of PWID on ART [14], their adherence to ART [15], and possibly their treatment outcomes [16].
To synthesize available evidence, we conducted a systematic review and meta-analysis to quantify the impact of concurrent OST use on the following ART outcomes among PWID: recruitment onto ART, adherence to ART, viral suppression and immune recovery, attrition from ART, individual mortality, and coverage at the programmatic level.
METHODS
Search Strategy
A systematic literature search was performed. The search identified studies describing the impact of OST on ART outcomes (search 1), and studies of PWID taking ART regardless of whether OST status was mentioned in the title or abstract (search 2). We searched Medline, Global Health, Embase, Social Policy and Practice, the Cochrane Library, and Web of Science including conference proceedings, from 1996 to November 2014 (see Supplementary Appendix 1 for search terms). We included experimental and observational studies that drew on quantitative methods, without any language or sample size restrictions. For additional sources, reference lists were screened, and known cohorts of PWID and corresponding author names were searched in Google Scholar.
Study Definitions and Outcomes
Interventions of Study
OST or MAT includes psychoactive medications administered under supervision with the aim of reducing opioid dependence and withdrawal symptoms; opioid agonists include methadone (methadone maintenance therapy), and buprenorphine; antagonists include naloxone or naltrexone. Older agents include codeine, levo-α-acetylmethadol, and morphine. ART was defined as highly active or combined HIV treatment, including ≥3 agents.
Study Outcomes
The primary outcome was the impact of OST in PWID on each step of the HIV treatment and care continuum following diagnosis [17, 18]. The primary outcomes are as follows: (1) ART coverage is the proportion of eligible HIV-infected PWID prescribed ART at a fixed point in time, and recruitment is the cumulative proportion who initiated ART during follow-up; (2) adherence to ART is the proportion of PWID on ART achieving a defined threshold of ART adherence, whether self-reported or objectively measured; (3) viral suppression is the proportion of those on ART with undetectable plasma HIV viral loads, based on the threshold of detection in each study (<500, <400 or <50 copies/mL); (4) CD4 count response to ART is the median or mean CD4 cell count increase over time on ART; (5) attrition from ART is the proportion of those on ART who were lost to follow-up or discontinued ART during follow-up, excluding those who died if data was available; and (6) mortality rate on ART is the HIV-related mortality rate during follow-up or, if this was not available, all-cause mortality rate.
Inclusion Criteria
Included studies presented outcomes of interest in adult (>15 years old) PWID populations where data were available to compare outcomes for PWID receiving OST to those not receiving OST. All studies describing active or former PWID were included, but, when available, data for active PWID were preferentially used (Table 1). Active PWID were those who injected drugs within a defined recent time period, as described in the studies.
Table 1.
Characteristics of Included Studies and Reported Outcomes
| Author and Year | Study Perioda | Location (City, Country) | Cohort: Recruitment Site and Method, Inclusion Criteria | No. on OST/Total (no./No.) | Type of OST | Comparison Population | Median Follow-up, mo | ART Outcomes |
|---|---|---|---|---|---|---|---|---|
| Abellan 1999 | 1997–1998 | Madrid, Spain | NS: People infected through IDU starting PI-containing ART | 18/28 | MMT | Active IDU at baseline | 4 | VL CD4 |
| Achmad 2009 | 2006–2009 | West Java, Indonesia | Hospital clinic recruitment Age >18, ICD-9 OD in previous 12 mo, attempt to stop opioids at least once |
25/140 | MMT | Former IDU starting ART, matched for date of ART initiation | 14 | VL AT MO |
| Altice 2011 | 2004–2009 | 10 sites, USA | BHIVES cohort: Provider referral, word of mouth, community outreach Age >18, DSM-IV OD, excluded for alcohol or benzodiazepine abuse, mental illness or increased AST/ALT, pregnant |
84/266 | BUP/NX | PWID not retained on BUP/NX | 12 | Cov UP VL CD4 |
| Celentano 1998 | 1996–1997 | Baltimore, USA | ALIVE cohort: Drug abuse treatment centers, STD & HIV clinics, parole officers, street outreach Age >18, injected drugs between 1977 and study entry, CD4 count < 500 in 1996 |
97/404 | MMT | Not in drug treatment in past 6 mo. 52% overall current drug use | CS | Cov |
| Celentano 2001 | 1996–1999 | Baltimore, USA | ALIVE cohort: Drug abuse treatment centers, STD & HIV clinics, parole officers, street outreach Age >18, injected drugs within past 10 y |
115/528 | MMT | PWID abstinent from IDU | 42 | UP |
| Escaffre 2000 | 1996–1998 | Marseilles, Nice, and Paris, France | MANIF 2000 cohort: Hospital clinic recruitment Age >18, infected through injecting drug use |
145/429 | BUP | Not in DMT, 48% IDU in past 6 mo overall | CS | Cov |
| Kapadia 2008 | 1998–2002 | 6 sites, USA | WIHS cohort: Clinic referrals, community outreach, participant word of mouth Women who had engaged in drug use at baseline or during follow-up on ART |
74/136 | MMT | No current MMT | 60 | AD |
| Kavasery 2009 | 1996–2006 | Baltimore, USA | ALIVE cohort: Drug abuse treatment centers, STD & HIV clinics, parole officers, street outreach Age >18, injected drugs between 1977 and study entry, AIDS-free at enrollment |
82/269 | MMT | No MMT and no IDU in past 6 mo | 31 | AT |
| Kerr 2005 | 2001–2002 | Vancouver, Canada | VIDUS cohort: Self-referral, street outreach Age >18, illicit drug use in past month, reside in Vancouver region, had taken or were taking ART |
78/160 | MMT | No MMT in past 6 mo | CS | AT |
| Knowlton 2010 | 2001–2005 | 4 sites, USA | INSPIRE cohort: Community venues, shelters, medical clinics and methadone clinics Injected drugs in past year, heterosexual act in past 3 mo | 223/1225 | MMT | No current methadone treatment | 6 | Cov AT |
| Lucas 2006 | 2001–2003 | Baltimore, USA | Methadone clinics, HIV care provider referral Age >18, baseline HIV-1 RNA > 500 copies/mL, no triple-class drug resistance |
75/319 | MMT | PWID not receiving MMT at time of ART initiation | 6 12 |
VL CD4 |
| Michel 2009 | 1996–2003 | Marseilles, Nice, and Paris, France | MANIF 2000 cohort: Hospital clinic recruitment Age >18, infected through injecting drug use |
100/294 | Both | Not on OST; 92% abstinent in the prior 6 mo | 48 | MO |
| Moatti 2000 | 1995–1998 | Marseilles, Nice, and Paris, France | MANIF 2000 cohort: Hospital clinic recruitment Age >18, infected through injecting drug use, CD4 count > 300 in visit before enrollment, no OIs |
32/51 | BUP | Active IDU in past 6 mo, not on BUP at baseline | CS | AD |
| Palepu 2006 | 1996–2003 | Vancouver, Canada | VIDUS cohort: Self-referral, street outreach Age >18, illicit drug use in past month, reside in Vancouver region; 25% weekly heroin use at baseline |
161/278 | MMT | Not on MMT | 58 | AD VL CD4 |
| Reddon 2014 | 1996–2008 | Vancouver, Canada | ACCESS cohort: Self-referral, street outreach Age >18, illicit drug use other than cannabinoids in past month, on ART during study period |
198/408 | MMT | Not on MMT in past 6 mo | 15 | AT |
| Richardson 2014 | 1996–2010 | Vancouver, Canada | ACCESS cohort: Self-referral, street outreach Age >18, illicit drug use other than cannabinoids in past month; 90% with IDU in past 6 mo overall |
186/666 | MMT | No MMT in past 6 mo | 51 | MO |
| Roux 2009 | 1995–2007 | Marseilles, Nice, and Paris, France | MANIF 2000: Hospital clinic recruitment Age >18, infected through injecting drug use, on ART for ≥6 mo, OD during study |
80/153 | Both | No OST in previous 6 mo | 24 | VL |
| Sambamoorthi 2000 | 1996 | New Jersey, USA | Surveillance data of Medicaid records for PWID with drug abuse claims Age >18, AIDS diagnosis; receiving Medicaid services for >90 d |
276/1109 | MMT | Current drug use based on ICD-9 codes | CS | Cov |
| Schinkel 1998 | 1996–1997 | Amsterdam, The Netherlands | ACSA cohort: At methadone posts and STD clinics PWID with CD4 count < 500 starting PI-based ART |
97/103 | MMT | Not on MMT; active drug use in past 6 mo at the last visit in 75% overall | CS | Cov |
| Springer 2012 | 2005–2010 | Connecticut, USA | Prisoners transitioning to the community Age >18, DSM-IV OD, returning to New Haven or Hartford, not pregnant |
50/94 | Both | Not on OST | 6 | VL |
| Strathdee 1998/1999 | 1996–1997 | Vancouver, Canada | VIDUS cohort: Self-referral, street outreach Injected drugs in past month; resident in Vancouver region |
40/177 | MMT | Not enrolled in MMT | 11 | Cov |
| Tapp 2011 | 1996–2008 | Vancouver, Canada | ACCESS cohort: Self-referral, street outreach Age >18, illicit drug use other than cannabinoids in past month |
169/545 | MMT | Not on MMT | 24 | AD |
| Ti 2014 | 1996–2012 | Vancouver, Canada | ACCESS cohort: Self-referral, street outreach Age >18, illicit drug use other than cannabinoids in past month |
211/587 | MMT | Not on MMT; 76% IDU overall | 32 | VL |
| Turner 2001 | 1996–1998 | New York State, USA | Surveillance data of Medicaid records Age >18, nonpregnant women with a live-born delivery within 5 y |
255/412 | MMT | Illicit drug use in past 6 mo | CS | Coverage |
| Uhlmann 2010 | 1996–2008 | Vancouver, Canada | ACCESS cohort: Self-referral, street outreach Injected drugs in past month; resident in Vancouver region |
55/231 | MMT | Not on MMT in past 6 mo | 24 | UP |
| Usukula 2012 | 2010 | Tartu, Estonia | Hospital clinic recruitment Age >18, receiving ART, ever PWID, 33% report illicit DU in past year overall |
66/92 | MMT | Ever IDU | CS | AD |
| Vallecillo 2010 | 1997–2007 | Barcelona, Spain | Referral to hospital detoxification units from outpatient clinics Age >18, DSM-IV OD with relapse or severe addiction |
380/673 | MMT | Not on MMT at admission; 68% active IDUs overall at admission | CS | Cov |
| Vlahov 2005 | 1996–2002 | Baltimore, USA | ALIVE cohort: Community outreach Age >18, injected drugs between 1977 and study entry, ≥1 visit with CD4 count < 200; 40% IDU in past 6 mo overall |
77/295 | MMT | No MMT in past 6 mo | 26 | MO |
| Weber 2009 | 1997–2006 | Multiple, Switzerland | SHCS cohort: Referral from outpatient clinics of 7 hospitals ≥2 biannual cohort visits during study period and CD4 count and HIV-1 RNA measured at baseline visit |
1348/1489 | Both | Active IDU in the past 6 mo | 65 | Cov VL AT MO |
| Westergaard 2013 | 1998–2011 | Baltimore, USA | ALIVE cohort: Drug abuse treatment centers, STD & HIV clinics, parole officers, street outreach Age >18, injected drugs between 1977 and study entry, attended ≥2 study visits |
165/740 | MMT | No MMT in past 6 mo | 104 | VL |
| Wood 2005 | 1996–2003 | Vancouver, Canada | BART cohort: Self-referral, street outreach Injected drugs in past month; resident in Vancouver region, never on ART at baseline |
171 234 | MMT | Not on MMT at baseline | 24 | UP |
| Zhao 2013 | 2002–2011 | China | Enrollment in China's national ART program Infected through injecting drug use and starting ART during the study period |
5161/23813 | MMT | No MMT at any time while on ART | 21 | AT MO |
All references are available in the Supplementary Appendix.
Abbreviations: AD, adherence; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; AT, treatment discontinuation/attrition; BUP, buprenorphine; CD4, CD4 cell count response to ART; Cov, ART coverage; CS, cross-sectional; DMT, drug maintenance therapy including OST; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; DU, drug use; HIV, human immunodeficiency virus; ICD-9, International Classification of Diseases, Ninth Revision; IDU, injecting drug use; MMT, methadone maintenance therapy; MO, mortality; NS, not specified within the text; NX, naloxone; OD, opioid dependence; OI, opportunistic infection; OST, opioid substitution therapy; PI, protease inhibitor; PWID, people who inject drugs; STD, sexually transmitted disease; UP, ART uptake; VL, viral load suppression.
a If the end year of the study is not reported, it was assumed to be 2 years earlier.
Studies of monotherapy or dual ART were excluded because of their limited relevance. We excluded review papers, modeling studies, commentaries, or editorials without primary data, but their reference lists were screened for additional sources. Studies describing substance abuse support, harm reduction services, or other forms of therapy without including OST as a core component were excluded. Studies were also excluded if the majority of drug users had never injected illicit drugs, or were only crack cocaine, alcohol, or methamphetamine users and therefore not eligible for OST.
Duplicate references were removed. Two reviewers conducted the search, screened titles and abstracts, and reviewed full-text articles or conference posters for inclusion. Disagreements on study eligibility were resolved with P. V., M. T. M., and N. J. W. Data were extracted from selected articles, including study design, recruitment methodology, outcome measurement, years of study, follow-up time and months of ART, number of participants, and measures of effect with 95% confidence intervals (CIs) where available. If not reported, CIs were calculated from raw data.
Data Analysis
Search results were summarized for each outcome, including the number of studies and separate cohorts represented. Where there were multiple publications from the same study or cohort for the same outcome, we included the most comprehensive in the meta-analysis, as defined by number of participants, years covered, and comparability of outcome measure with other studies, to minimize bias [19]. The proportion of women in each study was stratified as <25% or ≥25%. Geographical region was defined as North America, Europe, or Asia. For studies that did not report the final year of measurement, it was assumed to be 2 years before publication.
To provide a summary estimate for each outcome by OST status, crude odds ratios (ORs) or hazard ratios (HRs) and their standard error were log-transformed. Adjusted estimates were also pooled and the effect size compared to the pooled crude estimate. Outcomes were summarized using the predominant type of effect estimate (OR or HR), with conversion of differing estimates to maintain consistency when data allowed (Supplementary Appendix 2). Increases in CD4 counts were pooled using standardized mean difference (SMD) using the Hedges g adjustment, to account for the large variation in scale of measurement across studies [20]. Where studies reported a proportion with a predefined increase in CD4 count, the proportion was converted to an SMD by assuming a logistic distribution for CD4 counts, so that the SMD was estimated by multiplying the log OR and its standard error by √3/π [21].
Summary effect measures were obtained using random-effects meta-analysis, as we expected high between-study variability. Between-study heterogeneity was evaluated using the I2 statistic and the P value for heterogeneity (Cochran Q statistic) [22]. For outcomes with data from ≥10 studies and high levels of heterogeneity, we performed random-effects meta-regression analyses to explore potential sources of heterogeneity, including a priori the method of outcome measurement for adherence, median length of follow-up or time on ART if available, and proportion of active PWID in the comparison group.
Univariable and multivariable meta-regression analyses were performed: Time on ART or median follow-up time were included a priori in multivariable models, and any variables from the univariable analysis with P ≤ .20. Those variables that remained P ≤ .05 were retained in the final model. Meta-regression was performed using Stata software version 13.0. For those outcomes with fewer eligible studies, subgroup analyses were performed to compare summary effects. We assessed the risk of bias of each study for each included outcome against different domains, using recommended criteria [23]. Studies were judged as having low, high, or unclear risk of bias for each domain.
RESULTS
Search Results
The searches identified 4685 records, 3558 of which were not duplicates (Supplementary Figure 1), with 32 meeting the inclusion criteria (Table 1). This included 36 327 participants in 19 cohorts from 9 countries. The median number of PWID per study was 294 (range, 28–23 813), and the median follow-up period was 24 months (range, 4–104 months) for longitudinal studies (n = 24). Few studies were from lower-income countries, mainly Asia.
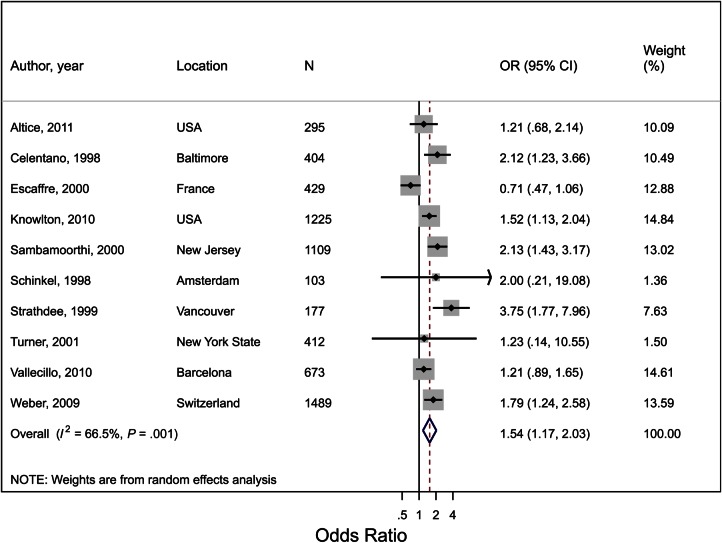
Coverage and Recruitment Onto ART
Ten studies reported on ART coverage, including 3 cross-sectional studies, 1 case-control study, 4 longitudinal studies, and 2 surveillance studies presenting coverage at baseline or a fixed time point (Supplementary Table 1). OST use was associated with a 54% increase in the odds of being on ART (OR, 1.54; 95% CI, 1.17–2.03; P = .002; Figure 1; Supplementary Table 2) with moderate heterogeneity (I2 = 67%; P = .001). In the meta-regression analysis, 28% of the between-study variance was explained by the different regions (North America vs Europe), with a larger effect in North America (OR, 1.84; 95% CI, 1.40–2.43; I2 = 37%) than Europe (OR, 1.19; 95% CI, .75–1.88; I2 = 74%).
Figure 1.
Forest plot of the effect of opioid substitution therapy on coverage of antiretroviral therapy (ART) among people who inject drugs (PWID), defined as the proportion of PWID on ART at a given time point. I2 and P value are measures of between-study heterogeneity. Abbreviations: CI, confidence interval; N, total sample size of PWID; OR, odds ratio.
Estimates of recruitment onto ART were provided by 4 studies, with follow-up time ranging from 12 to 42 months (Supplementary Figure 2; Supplementary Table 1). Three other studies were excluded: 1 duplicate, 1 for only providing a time ratio, and 1 for providing results as an OR that could not be converted to an HR. All excluded studies found a positive effect of OST on ART initiation. The pooled estimate for the effect of OST was an 87% increase (HR, 1.87; 95% CI, 1.50–2.33; P < .001) in ART uptake, with no heterogeneity (I2 = 0%; P = .55). There was no difference in the pooled estimate if a study where inactive PWID were the comparator was removed.
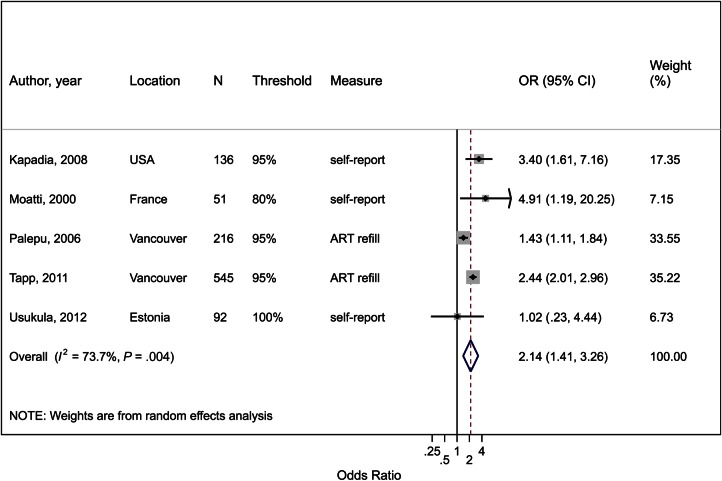
ART Adherence
Five studies reported on the effect of OST on adherence (Figure 2; Supplementary Table 3). Only 2 studies used an objective measure of adherence (pharmacy refills), whereas others used self-report, with adherence thresholds ranging from 80% to 100% of doses. In the pooled estimate, OST use was associated with a 2-fold increase in adherence (OR, 2.14; 95% CI, 1.41–3.26; P < .001), with high between-study heterogeneity (I2 = 74%; P = .004). In a subgroup analysis, the effect was higher for studies using self-reported adherence (OR, 2.86; 95% CI, 1.35–6.04; n = 3) compared with using pharmacy refill (OR, 1.88; 95% CI, 1.11–3.17; n = 2), although both the latter studies were from Vancouver. In other subgroup analyses, there was no effect of the adherence threshold, proportion of females in the study, or geographical region.
Figure 2.
Forest plot of the effect of opioid substitution therapy on adherence to antiretroviral therapy (ART) among people who inject drugs (PWID), defined as the proportion achieving a defined threshold of ART adherence, whether self-reported or objectively measured. I2 and P value are measures of between-study heterogeneity. Abbreviations: CI, confidence interval; measure, method of measurement of adherence; N, total sample size of PWID; OR, odds ratio; threshold, percentage of doses taken to indicate adherence.
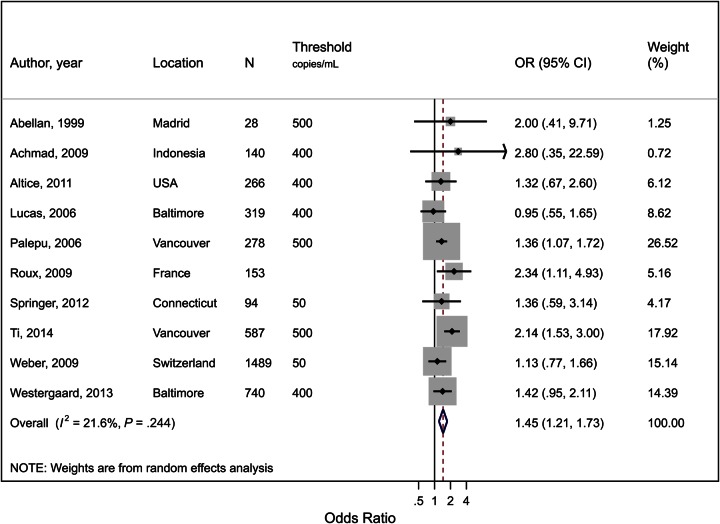
Viral Suppression
Ten studies were included that described the impact of OST on viral suppression (detection threshold being 500, 400, or 50 copies/mL; Figure 3; Supplementary Table 4). Three studies were excluded due to presenting time to viral suppression, where an OR could not be calculated, 1 for presenting the impact on viral loads as a continuous outcome, 3 for being duplicate cohorts, and 1 for presenting the number of visits with suppressed viral loads. The use of OST was associated with a 45% increase in the odds of viral suppression (OR, 1.45; 95% CI, 1.21–1.73; P < .001). The results were fairly homogeneous (I2 = 22%; P = .24).
Figure 3.
Forest plot of the effect of opioid substitution therapy on plasma viral suppression among people who inject drugs (PWID) on antiretroviral therapy (ART), defined as the proportion of those on ART with undetectable plasma human immunodeficiency virus (HIV) RNA loads, based on the threshold of detection in each study (<500, <400 or <50 copies/mL). I2 and P value are measures of between-study heterogeneity. Note that Roux used multiple thresholds depending on clinical site. Abbreviations: CI, confidence interval; N, total sample size of PWID; OR, odds ratio; threshold, number of HIV-1 RNA copies/mL used as threshold for HIV-1 RNA detection in that study.
Increase in CD4 Count
Information on CD4 count gains following ART initiation was available for 4 studies (Supplementary Table 5). The association of OST with increases in CD4 count was difficult to assess because duration of follow-up varied between studies. For 2 studies, the median increase was marginally smaller in those on OST at 4 months (79 vs 111 cells/mm3) and 6 months (39 vs 44 cells/mm3) following ART initiation, compared with those off OST. However, the increase was substantially larger for those on OST at 12 months in the latter study and another study (65 vs 14 cells/mm3 and 46 vs 1 cell/mm3). At 58 months, 1 study found more patients on OST had achieved immune restoration (gain of >100 cells/mm3) than those not on OST.
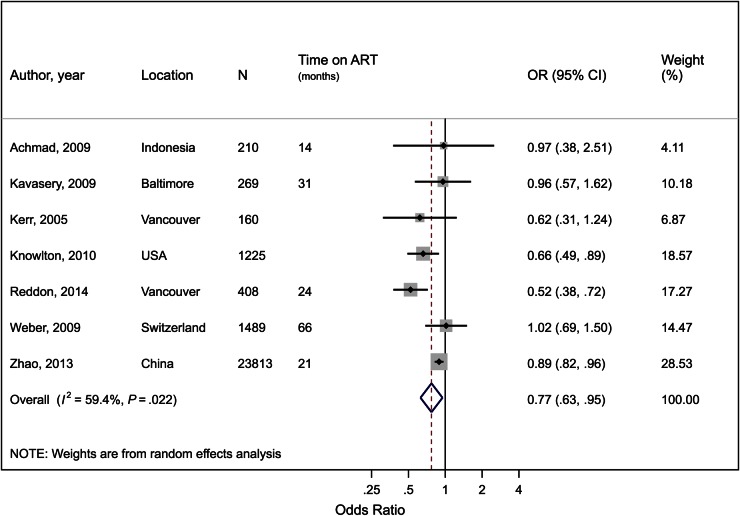
Attrition or Treatment Discontinuation
There were 7 eligible studies describing ART discontinuation/attrition (Supplementary Table 6). The average time on ART exceeded 12 months for all 5 studies reporting it. The pooled estimate found a 23% reduction in the odds of ART discontinuation if on OST (OR, 0.77; 95% CI, .63–.95; P = .01) with moderate heterogeneity (I2 = 59%; P = .02; Figure 4). There was no significant difference in effect by sex, duration of ART, or when studies with inactive PWID were removed.
Figure 4.
Forest plot of the effect of opioid substitution therapy on antiretroviral therapy (ART) discontinuation or attrition among people who inject drugs (PWID), defined as the proportion of those on ART who were lost to follow-up or discontinued ART during follow-up. I2 and P value are measures of between-study heterogeneity. Abbreviations: CI, confidence interval; N, total sample size of PWID; OR, odds ratio; time on ART, average time on ART for study participants.
Mortality
Six studies were included in the analysis of the impact of OST on mortality while on ART (Supplementary Figure 3; Supplementary Table 7). Only 1 study presented the impact on HIV-related mortality. There was no evidence for a reduction in all-cause mortality rates among those on OST while on ART (HR, 0.91; 95% CI, .65–1.25; P = .55) compared to those just on ART, although there was substantial heterogeneity (I2 = 74%; P = .002). The study reporting HIV-related mortality had comparable results. In a subgroup analysis, OST had a larger effect on mortality in 2 Asian studies (HR, 0.63; 95% CI, .57–.70; n = 2) than non-Asian studies (HR, 1.04; 95% CI, .77–1.40; n = 4). However, the Asian studies had fewer female participants and shorter follow-up than non-Asian studies.
Publication Bias
Some studies were judged to be at high risk of selection bias, for instance, due to poor representativeness if the cohorts consisted of volunteers, or if they did not ensure the outcomes were not present before exposure to OST (Supplementary Table 8). Some studies did not adjust for known confounders; thus, the results could be biased in terms of comparability of cohorts. However, sensitivity analyses limited to adjusted estimates did not substantially alter our results. Last, some studies had a high risk of bias due to their method of outcome assessment, mainly due to the outcome being self-reported or the study not accounting for differential loss to follow-up.
DISCUSSION
This is the first systematic review and meta-analysis summarizing evidence on the effect of OST, or MAT, on ART outcomes among PWID living with HIV. We found strong evidence of a positive impact on most outcomes: OST increased the coverage of ART by 54%, recruitment onto ART by 87%, ART adherence 2-fold, viral suppression by 45%, and reduced ART discontinuation by 23%. There was weak evidence that OST improves CD4 count gains, with greater benefits at ≥12 months, and little evidence of OST improving mortality among PWID on ART, although the results had considerable heterogeneity. Despite data being limited, there was little evidence that the effects of OST on ART outcomes varied by geographic region, except for ART coverage, where OST was associated with higher ART coverage in North America than in Europe, and mortality, which was reduced among those on OST in Asia but not elsewhere. There were insufficient studies to examine other geographical regions. These findings support a strong policy recommendation to provide integrated OST and ART care to PWID to improve individual clinical benefits, and the potential benefit of HIV treatment as prevention in this group.
The lack of observed impact on mortality in North America and Europe is likely due to the small number of studies. Several studies have shown that OST reduces mortality among PWID independent of ART [24, 25], mainly as a result of reducing opiate overdose [26, 27]. A more recent study from Vancouver found that OST conferred additional benefits to ART in reducing both all-cause and HIV-related mortality [28]. This study benefited from linkage to relevant outcomes through a provincial database, thereby capturing more events accurately and providing more rigor to the mortality outcome, which is particularly vulnerable to attrition bias.
As the studies included in this review are observational, it is possible that there were differences between the populations on and off OST that could partially explain differences in outcomes. Unfortunately, few studies presented relevant participant characteristics, such as sex, mental illness, alcohol use, or homelessness stratified by OST status, which made it difficult to assess whether these characteristics contributed to the observed effects. Studies have shown that PWID on OST are considered by practitioners to be more engaged and reliable than active PWID not on OST [29, 30]. It is possible that the increased uptake and coverage of ART in PWID on OST may result from practitioners' increased willingness to recruit them into HIV care [31]. Although improved adherence and viral suppression might reflect a more motivated patient, and may not be due to the assumed benefits of OST in reducing injecting drug use, a recent study using linked population-level data found that much of the improvement in adherence could be attributed to OST [32, 33]. It is also likely that integration of ART into OST care increases ART access for those on OST through increased convenience and reduced out-of-pocket costs, while those not on OST face additional challenges in navigating HIV care systems (A. Guise et al, unpublished data).
The limitations of this review include its dependence on a small number of studies for some outcomes, which reduced our ability to conduct meta-regression analyses to determine factors contributing to heterogeneity in our outcomes, and to control for potential confounding variables. We instead focused on measurable study characteristics and their associations with effect estimates. In addition, only comparative studies were included in our review; this was done to reduce the heterogeneity that would result from comparing ART outcomes across different populations.
Limited data came from 6 key countries (Ukraine, Russia, Vietnam, Malaysia, the United States, and China) that contain 50% of all PWID [34, 35], despite not limiting our search by language or publication source. Furthermore, except for Indonesia and China, all studies were conducted in high-income settings. Estimates depended greatly on a few cohorts of PWID, likely skewing the data toward those particular settings. This is especially relevant given that the context in which OST is delivered is likely to vary and could affect the benefits of OST. Unfortunately, few studies included details of how OST and ART were provided. This emphasizes the need for further studies in lower- or middle-income countries, including models of integrated care, and how to optimize potential benefits by co-locating ART and OST services, mitigating both drug use and HIV stigma, and reorienting OST and ART care philosophies to be more client-centered (Guise et al, unpublished data).
In conclusion, this systematic review found strong evidence to support the use of OST and its inclusion in routine HIV care for improving the treatment and care continuum among HIV-infected PWID. It supports the need for policy and health system reforms to accelerate the integration of OST and HIV treatment services. The review provides evidence for the multiple potential benefits of OST, and its pivotal importance in a combination approach to harm reduction [36]. This is particularly important for those countries with a significant or increasing prevalence of injecting drug use, and policy restrictions limiting expansion of harm reduction interventions [37, 38].
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. P. V. and G. M. conceived of the study. A. J. L. and P. V. provided overall leadership for the study, but all authors were involved in study design, data analysis, and interpretation of the data. A. J. L., P. V., G. M., C. F. D., C. F., K. M. T., and K. L. performed the systematic review and extracted all relevant data. A. J. L., M. T. M., and N. J. W. performed the statistical analyses. A. J. L., P. V., C. F., C. F. D., and K. L. performed the assessment of bias. M. T. M., H. C., S. M. L., L. P., M. H., and A. G. provided input on study design and analysis. A. J. L. wrote the first draft. All authors contributed to interpreting the results and to writing subsequent versions of the manuscript.
Disclaimer. The views expressed are those of the author(s) and not necessarily those of the University of Bristol, UK National Health Service, the UK National Institute for Health Research, or the UK Department of Health.
Financial support. This work was supported by the International Human Immunodeficiency Virus/AIDS Alliance (AIDS Alliance 711); the National Institute on Drug Abuse (grant number R01 DA037773-01A1) to P. V. and M. H.; National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at University of Bristol to P. V., C. F., K. M. T., K. L., H. C., and M. H.; NIHR HPRU in sexually transmitted infections and blood-borne viruses at University College London to P. V.; UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and the EDCTP2 program supported by the European Union to M. T. M.; and Bill & Melinda Gates Foundation HIV modeling consortium to P. V.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mathers BM, Degenhardt L, Ali H et al. . HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 2010; 375:1014–28. [DOI] [PubMed] [Google Scholar]
- 2.Petersen Z, Myers B, van Hout MC, Pluddemann A, Parry C. Availability of HIV prevention and treatment services for people who inject drugs: findings from 21 countries. Harm Reduct J 2013; 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS. The gap report 2014: people who inject drugs. Available at: http://www.unaids.org/en/resources/documents/2014/Peoplewhoinjectdrugs.pdf Accessed 20 March 2015.
- 4.Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS 2004; 18:2295–303. [DOI] [PubMed] [Google Scholar]
- 5.Oprea C, Ceausu E, Ruta S. Ongoing outbreak of multiple blood-borne infections in injecting drug users in Romania. Public Health 2013; 127:1048–50. [DOI] [PubMed] [Google Scholar]
- 6.Okal J, Geibel S, Muraguri N et al. . Estimates of the size of key populations at risk for HIV infection: men who have sex with men, female sex workers and injecting drug users in Nairobi, Kenya. Sex Transm Infect 2013; 89:366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid SR. Injection drug use, unsafe medical injections, and HIV in Africa: a systematic review. Harm Reduct J 2009; 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzakis A, Sypsa V, Paraskevis D et al. . Design and baseline findings of a large-scale rapid response to an HIV outbreak in people who inject drugs in Athens, Greece: the ARISTOTLE programme. Addiction 2015; 110:1453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad C, Bradley HM, Broz D et al. . Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS. Fast track: ending the AIDS epidemic by 2030. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf Accessed 12 November 2015.
- 11.World Bank. The global HIV epidemics among people who inject drugs. Available at: http://www.worldbank.org/content/dam/Worldbank/document/GlobalHIVEpidemicsAmongPeopleWhoInjectDrugs.pdf Accessed 2 February 2015.
- 12.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav 2010; 14:731–47. [DOI] [PubMed] [Google Scholar]
- 13.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy 2007; 18:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celentano DD, Galai N, Sethi AK et al. . Time to initiating highly active antiretroviral therapy among HIV infected injection drug users. AIDS 2001; 15:1707–15. [DOI] [PubMed] [Google Scholar]
- 15.Kapadia F, Vlahov D, Wu Y et al. . Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse 2008; 34:161–70. [DOI] [PubMed] [Google Scholar]
- 16.Krusi A, Milloy MJ, Kerr T et al. . Ongoing drug use and outcomes from highly active antiretroviral therapy among injection drug users in a Canadian setting. Antivir Ther 2010; 15:789–96. [DOI] [PubMed] [Google Scholar]
- 17.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnighausen T. The HIV treatment cascade and antiretroviral impact in different populations. Curr Opin HIV AIDS 2015; 10:391–4. [DOI] [PubMed] [Google Scholar]
- 19.Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ 1997; 315:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- 21.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000; 19:3127–31. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O'Connell D et al. . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 11 July 2015.
- 24.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ 2013; 91:102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health 2001; 91:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimber J, Copeland L, Hickman M et al. . Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 2010; 341:c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davoli M, Bargagli AM, Perucci CA et al. . Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction 2007; 102:1954–9. [DOI] [PubMed] [Google Scholar]
- 28.Nosyk B, Min JE, Evans E et al. . The effects of opioid substitution treatment and highly active antiretroviral therapy on the cause-specific risk of mortality among HIV-positive people who inject drugs. Clin Infect Dis 2015; 61:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassetti S, Battegay M, Furrer H et al. . Why is highly active antiretroviral therapy (HAART) not prescribed or discontinued? J Acquir Immune Defic Syndr 1999; 21:114–9. [PubMed] [Google Scholar]
- 30.Chakrapani V, Velayudham J, Shunmugam M, Newman PA, Dubrow R. Barriers to antiretroviral treatment access for injecting drug users living with HIV in Chennai, South India. AIDS Care 2014; 26:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc 2012; 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouhnik AD, Chesney M, Carrieri P et al. . Nonadherence among HIV-infected injecting drug users: the impact of social instability. J Acquir Immune Defic Syndr 2002; 31:S149–53. [DOI] [PubMed] [Google Scholar]
- 33.Nosyk B, Min JE, Colley G et al. . The causal effect of opioid substitution treatment on HAART medication refill adherence. AIDS 2015; 29:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degenhardt L, Mathers BM, Wirtz AL et al. . What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy 2014; 25:53–60. [DOI] [PubMed] [Google Scholar]
- 35.United Nations Office on Drugs and Crime. World drug report 2014. Available at: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf Accessed 16 June 2015.
- 36.Strathdee SA, Beyrer C. Threading the needle—how to stop the HIV outbreak in rural Indiana. N Engl J Med 2015; 373:397–9. [DOI] [PubMed] [Google Scholar]
- 37.US President's Emergency Plan for AIDS Relief. Comprehensive HIV prevention for people who inject drugs, revised guidance. Available at: www.pepfar.gov/documents/organization/144970.pdf Accessed 10 June 2015.
- 38.Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A; Substance Use Scientific Committee of the HIVPTN. Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Curr Opin HIV AIDS 2012; 7:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.