Universal surgical mask usage by both patients and providers reduced the incidence of respiratory viral infections in patients following hematopoietic stem cell transplant, especially parainfluenza 3, in this single-site prospective study.
Keywords: hematopoietic stem cell transplant, infection, prevention, parainfluenza virus, surgical mask
Abstract
Background. Respiratory viral infections (RVIs) are frequent complications of hematopoietic stem cell transplant (HSCT). Surgical masks are a simple and inexpensive intervention that may reduce nosocomial spread.
Methods. In this prospective single-center study, we instituted a universal surgical mask policy requiring all individuals with direct contact with HSCT patients to wear a surgical mask, regardless of symptoms or season. The primary endpoint was the incidence of RVIs in the mask period (2010–2014) compared with the premask period (2003–2009).
Results. RVIs decreased from 10.3% (95/920 patients) in the premask period to 4.4% (40/911) in the mask period (P < .001). Significant decreases occurred after both allogeneic (64/378 [16.9%] to 24/289 [8.3%], P = .001) and autologous (31/542 [5.7%] to 16/622 [2.6%], P = .007) transplants. After adjusting for multiple covariates including season and year in a segmented longitudinal analysis, the decrease in RVIs remained significant, with risk of RVI of 0.4 in patients in the mask group compared with the premask group (0.19–0.85, P = .02). In contrast, no decrease was observed during this same period in an adjacent hematologic malignancy unit, which followed the same infection control practices except for the mask policy. The majority of this decrease was in parainfluenza virus 3 (PIV3) (8.3% to 2.2%, P < .001).
Conclusions. Requiring all individuals with direct patient contact to wear a surgical mask is associated with a reduction in RVIs, particularly PIV3, during the most vulnerable period following HSCT.
Respiratory viral infections (RVIs) are a significant complication of hematopoietic stem cell transplant (HSCT), affecting up to 30% of patients [1–3]. In addition to causing rhinitis, cough, and other symptoms, 25%–40% of cases progress to lower respiratory tract infection [4, 5], with associated mortality as high as 20%–40% [6–8].
Because HSCT recipients are immunocompromised and tightly cohorted, they are vulnerable to nosocomial spread. For example, there have been a number of reports of clusters or outbreaks of parainfluenza virus type 3 (PIV3) within HSCT units [5, 9–12]. In many of these cases, molecular epidemiologic analyses have traced these outbreaks to a single or handful of strains that spread through person-to-person transmission [13–18].
While standard infection control procedures serve an essential role in curtailing RVIs [7, 19]. it is hypothesized that they may be insufficient to prevent the nosocomial spread of PIV3 [10]. This is because patients (or caregivers or providers) with PIV3 may shed virus, yet be asymptomatic [10, 12, 14–16], and are therefore missed by standard droplet precautions that focus on symptomatic patients. Similarly, strategies that increase infection control measures during the winter influenza and respiratory syncytial virus (RSV) seasons neglect PIV3, which peaks in the summer [5, 9, 11–15, 17, 20].
In 2009, our HSCT unit experienced a higher-than-average incidence of RVI, prompting an exploration for means to better prevent transmission. We hypothesized that instituting an infection control protocol that requires universal surgical mask usage year-round by all individuals in contact with patients peritransplant would complement existing measures (eg, universal hand washing) and provide greater protection against RVI transmission.
MATERIALS AND METHODS
Infection Control and Mask Protocol
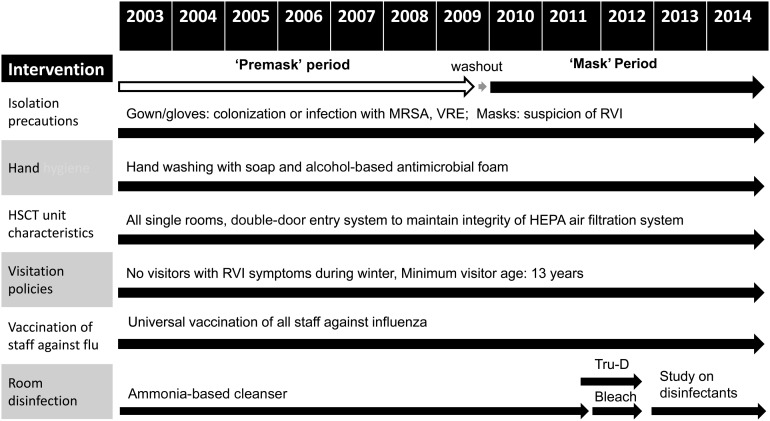
Following approval from the Duke University Health System institutional review board, we conducted a prospective interventional trial of the impact of universal surgical mask usage on RVIs. This policy was added to standard infection control practices as implemented and monitored by Duke Hospital Epidemiology and Infection Control (Figure 1). The universal mask policy required all individuals with direct patient contact—medical and support staff, visitors, caregivers, and patients—to wear surgical masks (3M Standard Earloop Mask 1826) when within 3 feet of an HSCT patient. Similar masks have been shown to reduce aerosol shedding of both coarse (>5 µm) and fine (≤5 µm) virus particles [21]. Patients were required to wear masks when traveling outside of their room. Universal surgical mask usage was instituted in both the HSCT inpatient (16 beds) and outpatient day hospital (40 beds) units and associated waiting rooms. Compliance was reinforced by nurses, who were trained during the designated “washout” period, and monitored periodically by independent observers using the same protocol as for monitoring hand hygiene compliance [22]. Because the focus was on reducing nosocomial transmission, patients or caregivers were not required to wear masks when alone at home or alone in their private rooms.
Figure 1.
Infection control practices during “premask” and “postmask” period are as follows: (1) isolation precautions including use of gown and gloves when patients have or are colonized with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), and use of gowns, gloves, and surgical masks if a patient has symptoms of an upper respiratory tract infection; (2) hand washing, which includes both washing with soap before entering the outpatient or inpatient hematopoietic stem cell transplant (HSCT) units as well as additional hand washing or hand sanitization with an antibacterial alcohol-based foam before patient contact (of note, a hospital-wide campaign for aggressive hand hygiene started in April 2009, although compliance in the bone marrow transplant unit has consistently been high, ie, 96% average [range, 94%–98%]); (3) all single rooms and double-door entry systems to maintain the integrity of the high-efficiency particulate air (HEPA) filtration system for the HSCT inpatient unit; in the outpatient unit/day hospital, patients are either seen in individual rooms if positive or suspected to have a communicable organism (eg, MRSA, VRE, respiratory viral infection [RVI]) or 1 of 2 open treatment areas separated by curtains; (4) visitation policies limiting visitors with RVI symptoms; (5) universal vaccination of staff against influenza; and (6) disinfection protocols with ammonia or bleach with or without Tru-D ultraviolet-C room disinfection. Of note, while the HSCT unit switched from ammonia to bleach in March 2011, further changes took place between April 2012 and August 2014 as part of a hospital-wide study examining room disinfection protocols for contact isolation rooms: ammonia was used from April 2012 to October 2012; bleach from November 2012 to May 2013; ammonia again from June 2013 to December 2013; and bleach again from January 2014 to August 2014 (however, rooms of patients with Clostridium difficile were always cleaned with bleach). The HSCT unit also began using Tru-D in February 2011; as part of the above study, hospital-wide use of this machine was also regulated from April 2012 to August 2014 as follows: Tru-D was used from April 2012 to October 2012, not used November 2012 to December 2013, and used again from January 2014 to July 2014.
Patient Population
Universal surgical mask usage was instituted 1 December 2009. Allowing 6 weeks for implementation and washout, the mask cohort included all patients transplanted between 12 January 2010 and 11 January 2014. This conservative washout period would be expected to be significantly greater than the up to 7-day incubation period for the respiratory viral pathogens of interest [23]. All patients who received a transplant between 1 December 2003 (the earliest date data were available in the electronic record) and 30 November 2009 were considered the premask cohort. Patients were observed from the start of conditioning (pretransplant preparative chemotherapy and/or radiation) to the completion of peritransplant care and discharge. Conditioning, prophylaxis for graft-vs-host disease (GVHD), discharge criteria, and other clinical protocols were unchanged over the course of the study. There was not a significant change in the number of beds in either the hematology/oncology unit or the bone marrow transplant unit through the course of the study.
Microbiologic Sampling, Diagnosis, and Definitions
All patients with respiratory symptoms were tested via nasopharyngeal wash, sputum culture, tracheal aspirate, or bronchoalveolar lavage. Presence of virus was determined by direct fluorescence antigen (DFA) or culture (2003–2009), polymerase chain reaction (PCR) (2011–present), or a combination of DFA, culture, and limited PCR (2009–2011) for influenza A and B, parainfluenza virus (PIV) types 1, 2, and 3, adenovirus, and RSV. Because testing for metapneumovirus, rhinovirus, and coronavirus was not performed until 2011, they were excluded from analysis. Death due to RVI was determined by blinded review of the medical record by 3 physicians.
Data was obtained from medical records, the Duke HSCT database, and the Duke Enterprise Data Unified Content Explorer database.
Statistical Analysis
Baseline characteristics were summarized as number (percentage) for categorical variables, and mean (standard deviation) and median (range) for continuous variables. Differences in continuous baseline characteristics between premask and mask cohorts were examined using the Wilcoxon rank-sum test for independent nonparametric samples, as all continuous variables were not normally distributed, and differences in categorical baseline characteristics, incidence of RVIs, percentage of positive test results, and deaths due to RVIs were examined using the χ2 test or Fisher exact test, as appropriate.
To further investigate the effects of seasonal and year-to-year variation in RVIs, we conducted a time-series analysis, in which patient observation time was broken down in a longitudinal way so that each interval signified a single season (spring, summer, fall, or winter) and a single location (inpatient or outpatient/day hospital). The number of intervals per patient ranged from 1 for a patient who had all procedures and treatment as an outpatient over the course of a single season, to 19 for a patient who was in and out of the hospital over the course of several seasons.
To account for unequal numbers of observation points and varying lengths of total observation time, a segmented negative binomial model with an exchangeable correlation structure and an exposure time of the total number of days of observation was used to examine the incidence of RVI. Quasi-Akaike Information Criterion analysis was used to determine the best correlation structure [24, 25]. A segmented regression model was used because this type of model may be able to explain apparent differences due to external effects that cannot be quantified using traditional multiple regression [26]. In this model, time was segmented into days from the start of the study, and days postintervention. Patients in the premask cohort were given a value of 0 for postintervention time. Patient demographic and clinical variables were examined as covariates in the multivariate model. Myeloablative conditioning, use of alemtuzumab, umbilical cord transplant, haploidentical transplant, and GVHD were only relevant for allogeneic HSCT (allo-HSCT), so a third level was added to these variables to indicate that an autologous HSCT (auto-HSCT) was performed. This level was excluded in the fitting of most models due to collinearity with the type of transplant variable, but by coding the variables like this, no patients were excluded in any model.
The primary endpoint was the incidence of RVI during the peritransplant period. Incidence of PIV3 was examined as a secondary endpoint because the majority of RVIs fell into this category. Model results are presented as incidence rate ratios with 95% confidence intervals (CIs). CIs were calculated using robust standard errors. No adjustments were made for multiple comparisons. Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, North Carolina) and Stata software, version 13.1 (StataCorp, College Station, Texas).
RESULTS
Patient Characteristics
Nine hundred twenty patients were included in the premask cohort and 911 in the mask cohort (Table 1). Groups were balanced in terms of sex, though the mask cohort tended to be older (mean age, 55 vs 50 years, P < .001) and included more patients with multiple myeloma (P < .001); as a result, a greater proportion received auto-HSCT (622/911 [68.3%] vs 542/920 [58.9%], P < .001). Among allo-HSCT recipients, patients in the mask cohort were less likely to have received mismatched donor transplants (73/911 [25.3%] vs 133/920 [35.2%], P = .006), particularly human leukocyte antigen–haploidentical transplants, although they were more likely to have received myeloablative conditioning (172/289 [59.5] vs 196/378 [51.9%], P < .05).
Table 1.
Baseline Patient Characteristics
| Characteristic | Study Group |
P Valuea | |
|---|---|---|---|
| Premask (n = 920) | Mask (n = 911) | ||
| Male sex | 536 (58.3) | 525 (57.6) | .78 |
| Age, y, mean (SD) | 50 (12.7) | 54.7 (12.3) | <.001 |
| Age, y, median (range) | 52 (19–79) | 57 (18–81) | |
| Disease | <.001 | ||
| Leukemia | 237 (25.8) | 186 (20.4) | |
| Lymphoma | 264 (28.7) | 209 (22.9) | |
| PCD | 291 (31.6) | 428 (47) | |
| MDS/MPD | 62 (6.7) | 51 (5.6) | |
| Other | 66 (7.2) | 37 (4.1) | |
| Previous transplant | 15 (1.6) | 12 (1.3) | .58 |
| Type of transplant | <.001 | ||
| Autologous HSCT | 542 (58.9) | 622 (68.3) | |
| Allogeneic HSCT | 378 (41.1) | 289 (31.7) | |
| Myeloablativeb | 196 (51.9) | 172 (59.5) | .05 |
| Alemtuzumabb | 152 (40.2) | 99 (34.3) | .12 |
| Mismatchb | 133 (35.2) | 73 (25.3) | .006 |
| Umbilical cordb | 73 (19.3) | 51 (17.6) | |
| Haploidenticalb | 60 (15.9) | 22 (7.6) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: cord, cord blood donor; haploidentical, human leukocyte antigen–haploidentical donor; HSCT, hematopoietic stem cell transplant; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; PCD, plasma cell dyscrasia; SD, standard deviation.
a Wilcoxon rank-sum test for continuous variables and χ2 test for categorical variables.
b Presented as percentage of patients who received allogeneic HSCT.
Impact of Universal Mask Usage
The overall incidence of RVI dropped from 95 of 920 (10.3%) in the premask cohort to 40 of 911 (4.4%) in the mask cohort (P < .001; Table 2). This decrease was primarily in PIV3, which dropped from 76 of 920 (8.3%) to 20 of 911 (2.2%) (P < .001). There was also a decrease in RSV (16/920 [1.7%] to 11/911 [1.2%]), although this was not significant. The incidence of influenza A and B, PIV1 and PIV2, and adenovirus were also too low (<1%) to make meaningful comparisons.
Table 2.
Incidence of Respiratory Viral Infections
| All Patients (N = 1831) | Study Group |
P Valuea | |
|---|---|---|---|
| Premask (n = 920) | Mask (n = 911) | ||
| Any virus (excluding metapneumovirus) | 95 (10.3) | 40 (4.4) | <.001 |
| Influenza A | 5 (0.5) | 4 (0.4) | |
| Influenza B | 1 (0.1) | 1 (0.1) | |
| Parainfluenza (any) | 78 (8.5) | 22 (2.4) | <.001 |
| Parainfluenza 1 | 0 (0) | 1 (0.1) | |
| Parainfluenza 2 | 3 (0.3) | 1 (0.1) | |
| Parainfluenza 3 | 76 (8.3) | 20 (2.2) | <.001 |
| Adenovirus | 0 (0) | 5 (0.5) | |
| Respiratory syncytial virus | 16 (1.7) | 11 (1.2) | .35 |
| Allo-HSCT Patients (n = 667) | Study Group |
P Value | |
| Premask (n = 378) | Mask (n = 289) | ||
| Any virus (excluding metapneumovirus) | 64 (16.9) | 24 (8.3) | .001 |
| Influenza A | 4 (1.1) | 3 (1) | |
| Influenza B | 0 (0) | 0 (0) | |
| Parainfluenza (any) | 51 (13.5) | 14 (4.8) | <.001 |
| Adenovirus | 0 (0) | 3 (1) | |
| Respiratory syncytial virus | 14 (3.7) | 5 (1.7) | |
| Study Group |
|||
| Auto-HSCT Patients (n = 1164) | Premask (n = 542) | Mask (n = 622) | P Value |
| Any virus (excluding metapneumovirus) | 31 (5.7) | 16 (2.6) | .007 |
| Influenza A | 1 (0.2) | 1 (0.2) | |
| Influenza B | 1 (0.2) | 1 (0.2) | |
| Parainfluenza (any) | 27 (5) | 8 (1.3) | <.001 |
| Adenovirus | 0 (0) | 2 (0.3) | |
| Respiratory syncytial virus | 2 (0.4) | 6 (1) | |
Data are presented as No. (%).
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplant; auto-HSCT, autologous hematopoietic stem cell transplant.
a χ2 test.
The decrease in RVIs was significant among both allo-HSCT (64/378 [16.9%] to 24/289 [8.3%], P = .001) and auto-HSCT patients (31/542 [5.7%] to 16/622 [2.6%], P = .007). Similarly, both allo-HSCT and auto-HSCT patients experienced large decreases in PIV3 (allo-HSCT: 49/378 [13.0%] to 12/289 [4.2%], P < .001; auto-HSCT: 27/542 [5.0%] to 8/622 [1.3%], P < .001). Concurrent with this decrease, the rate of death secondary to RVI dropped in the mask cohort (all patients: 11/920 [1.2%] to 0/911 [0%], P = .001; allo-HSCT: 9/378 [2.4%] to 0%, P = .006, auto-HSCT: 2/542 [0.4%] to 0%, P = .217).
The decision to test for viral pathogens was dependent on patient signs and symptoms and clinician concern for viral infection. It was not surprising, therefore, that concurrent with the decrease in RVIs, fewer viral tests were performed in the mask period. However, despite the increased sensitivity of PCR-based tests in the mask period, the proportion of positive test results was lower: 29% of tests were positive premask (95 positive of 328 tests), compared with 15% (25/162) from 12 January 2010 to 11 January 2013 (P = .002; data on total tests performed were not available for the last year, and thus are not included in this calculation). This suggests that testing practices for RVIs was more aggressive in the mask period, and the rate of RVIs was truly lower.
Time-Series Analysis to Adjust for Seasonal and Yearly Variation and Other Potential Confounders
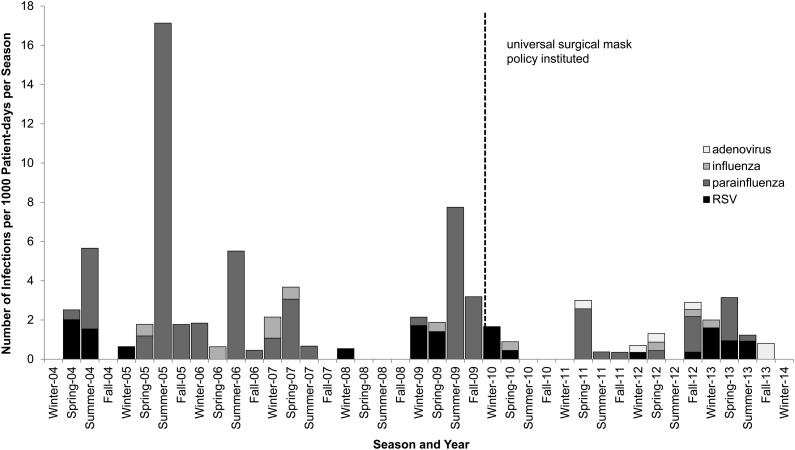
There were substantial seasonal and yearly variations in the incidence of RVI (Figure 2). For example, PIV tended to peak in the summer, whereas RSV and influenza peaked in the winter. Some years (eg, 2005) had particularly severe outbreaks of PIV whereas other years (eg, 2008) saw few or no cases. To rule out the possibility that seasonal or yearly variations confounded our analysis, we conducted a time-series analysis to adjust for the covariates of season and year, as well as age, location, type of transplant, and, for allo-HSCT, conditioning intensity, T-cell depletion, donor, and GVHD (Table 3). Using this model, mask use was estimated to reduce the risk of RVI by 60% after adjusting for covariates (P = .017). Other significant variables were season, conditioning intensity, and GVHD.
Figure 2.
The incidence of respiratory viral infections (RVIs) after hematopoietic stem cell transplant by season and year. The dotted line represents when the universal surgical mask policy was instituted, after which the number of RVIs dropped significantly. Parainfluenza virus predominated and tended to cluster in the summertime. Abbreviation: RSV, respiratory syncytial virus.
Table 3.
Negative Binomial Model Results for the Incidence of Any Viral Infection
| Predictor | IRR (95% CI) | P Value |
|---|---|---|
| Age at HSCT, y | 1.000 (.988–1.012) | .97 |
| Season | ||
| Spring vs fall | 2.597 (1.286–5.245) | .008 |
| Summer vs fall | 3.137 (1.735–5.674) | <.001 |
| Winter vs fall | 2.186 (1.062–4.502) | .03 |
| Year of HSCT | 1.106 (.505–2.423) | .80 |
| Location: inpatient vs outpatient | 1.436 (.913–2.259) | .12 |
| Type of transplant: allo-HSCT vs auto-HSCT | 1.112 (.596–2.076) | .74 |
| For allo-HSCT | ||
| Conditioning intensity: myeloablative vs not myeloablative | 0.394 (.226–.689) | .001 |
| T-cell depletion: alemtuzumab vs no alemtuzumab | 0.904 (.479–1.705) | .76 |
| Donor: umbilical cord vs not umbilical cord | 1.037 (.599–1.794) | .90 |
| Donor: haploidentical vs not haploidentical | 1.037 (.642–1.675) | .88 |
| GVHD vs no GVHD | 1.418 (1.010–1.990) | .04 |
| Mask use: mask vs premask (adjusted) | 0.398 (.187–.848) | .02 |
| Mask use: mask vs premask (unadjusted) | 0.484 (.347–.675) | <.001 |
The adjusted model also controls for the time from the start of the study (1 December 2003) and the time from the start of the intervention (12 January 2010) in days. The unadjusted model includes only mask use. After controlling for potential year-to-year variation, the season, conditioning intensity, presence of GVHD, and mask use had a statistically significant impact on RVI (bolded).
The bolded P values are for those variables that are significantly associated with incidence of any viral infection.
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplant; auto-HSCT, autologous hematopoietic stem cell transplant; CI, confidence interval; GVHD, graft-vs-host disease; HSCT, hematopoietic stem cell transplant; IRR, incidence rate ratio; RVI, respiratory viral infection.
We also used this model to evaluate the interaction between season and mask use and found that the impact of the mask intervention was greatest in the summer (P = .001; Supplementary Table 1). We found no interaction between location (inpatient vs outpatient) and mask use (Supplementary Table 2), suggesting that the impact of mask use was not dependent on location. We also found no interaction between location and type of transplant (allo- vs auto-HSCT) (Supplementary Table 3). Analyses were also performed looking at PIV3 as the endpoint of interest (Supplementary Table 4): mask use was estimated to reduce the risk of PIV3 by 69% after adjusting for covariates (P = .016).
Comparisons With the Rest of the Hospital
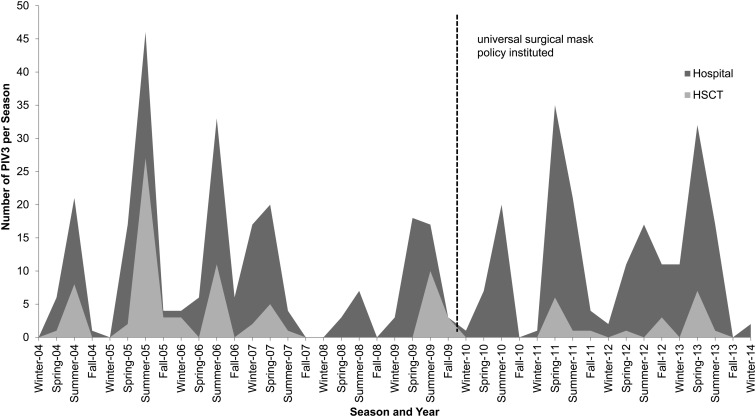
To evaluate the impact of the addition of universal mask usage to standard infection control procedures, we compared the occurrence of PIV3 in the HSCT population to that of the rest of the hospital (which only used masks as part of standard droplet precautions with symptomatic patients) (Figure 3). In the premask period, PIV3 among HSCT patients tracked closely with the PIV3 in the rest of the hospital. However, after the universal surgical mask policy was instituted, the number of PIV3 infections dropped significantly among HSCT patients, whereas it remained high in the rest of the hospital. Before universal mask usage, HSCT patients accounted for 32% (76/233) of all hospital-wide PIV3 infections; this dropped to just 10.4% (20/192) after implementation (P < .001).
Figure 3.
Parainfluenza virus 3 (PIV3) infections after hematopoietic stem cell transplant (HSCT) vs all other hospitalized patients (hospital). The dotted line represents when universal surgical mask policy was instituted. Prior to the mask policy, PIV3 infections among HSCT patients tracked closely with those in the rest of the hospital; after intervention, the number of PIV3 cases dropped dramatically among HSCT patients but remained elevated in the rest of the hospital (P < .001).
We also compared the HSCT population to a neighboring hematologic malignancy unit that had identical infection control practices with the exception of universal surgical mask usage (Supplementary Figure 1): RVI and PIV3 in the neighboring unit actually increased over time, whereas they decreased in the HSCT population (data from the neighboring unit were not available prior to 1 July 2006).
Compliance
Compliance with universal surgical mask usage was extremely high (>99% among healthcare providers [414 compliant per 415 observations] and 98% among patients/caregivers [193/197]). Compliance with hand hygiene was also consistently high (96% among healthcare providers [5672/5934]; data not available for patients/caregivers).
DISCUSSION
Although modern infection control practices have helped decrease viral infections and curtail outbreaks, RVIs remain problematic after HSCT [9, 11, 12]. Standard preventive methods include strict hand hygiene, vaccination, active and early surveillance, and contact and droplet isolation of symptomatic patients [27, 28]. However, strategies that focus on symptoms do not address the prolonged asymptomatic shedding of RVIs in immunocompromised patients, including PIV3 [10, 12, 14–16], which can range from 5 to 121 days [10, 12, 14, 20]. It is therefore not surprising that symptom-based surveillance and isolation policies have been reported with mixed results [11, 12, 16]. In one prolonged outbreak of PIV3 at the Fred Hutchinson Cancer Research Center, droplet isolation of aggressively screened symptomatic patients did not impact the duration or severity of the outbreak [16], as was the case at our institution in 2005. In contrast, more success was seen when universal surgical mask usage was instituted in response to a PIV3 outbreak at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center [12].
Given its effectiveness in controlling an outbreak, universal surgical mask usage shows promise as an inexpensive and easy-to-implement preventative. In addition to directly stopping transmission of viral particles, universal usage may drive a cultural change in providers and patients, heightening awareness of and attention to the importance of infection control. This intervention may be readily applied to other HSCT programs, and in fact, some institutions already require everyone to wear surgical masks during the peak winter influenza season [12]. However, as PIV3 is most common in the spring and summer months, and nosocomial outbreaks of PIV3 can be prolonged and extend beyond the typical season [11, 17], focus on the winter neglects a significant proportion of RVIs.
By requiring universal surgical mask usage year-round, we were able to significantly reduce RVIs after HSCT. The biggest impact was on PIV3, which made up the majority of RVIs. Consistent with the reported literature [2], we also found that GVHD was associated with more RVIs (GVHD patients receive additional immunosuppression including corticosteroids), and myeloablative conditioning was associated with fewer RVIs (these patients are generally healthier, ie, able to tolerate myeloablative conditioning). Interestingly, our model did not find a significant difference between allo-HSCT and auto-HSCT; however, our model controls for inpatient/outpatient stay, taking into account the length of the interval. Because incidence of RVI after allo-HSCT is 3 times that of auto-HSCT, and the length of stay for allo-HSCT is 3 times that of auto-HSCT, controlling for length of stay minimizes the impact of type of transplant.
A limitation of this study is the before-and-after design. There were significant differences between cohorts, such as the increased prevalence of plasma cell dyscrasia, and consequently auto-HSCT, in the mask cohort. However, universal surgical mask usage had a significant protective effect within both the auto-HSCT and allo-HSCT subgroups. Whereas seasonal and yearly variations in RVIs may also bias results, we found no parallel decrease in the rest of the hospital. Thus, it seems unlikely that natural variation in the rate of RVIs in the community accounts for the observed decrease. This is supported by our time-series analysis, which found a 60% decrease in the risk of RVIs in the mask period after accounting for the covariates of season and year, in addition to disease, type of transplant, and other variables. Additionally, differences in detection methods in the premask period (predominantly viral culture and DFA) vs the postmask period (PCR) could impact the results. However, as PCR is known to be at least 30% more sensitive at detecting viral pathogens compared with DFA and culture, depending on the pathogen, without sacrificing specificity [29, 30], we would expect a bias toward the null. The true effect size of the mask intervention thus may be underrepresented in the current study.
Our interventional study was designed in direct response to a rise in RVI in 2009, which could render it susceptible to effects seen due to regression to the mean. To mitigate this possibility, we collected data over an extended period of 4 full years following implementation of universal mask usage, rather than examining simply 1 season or 1 year. We considered the possibility of testing bias resulting in reduced vigilance in screening for RVIs during the mask period. However, the lower proportion of positive test results in the mask period suggests that testing is more frequent in the mask period—that is, undertesting would not explain the decrease in RVIs. We also considered the possibility that, over the course of the study period, improved hospital-wide infection control efforts over the 10-year span of the study were responsible for the reduction in RVI rates in HSCT patients. However, a concurrent reduction in RVIs was not observed in either an adjacent non-HSCT hematologic malignancy unit or hospital-wide. Moreover, increasing use of more sensitive and specific PCR-based viral diagnostic testing would be expected to increase the detection of RVI during the universal mask period, as was seen in the adjacent hematologic malignancy unit. In contrast, we observed a decrease in RVIs despite this increased sensitivity, raising the possibility that we are underestimating the protective effect of universal mask usage.
In conclusion, we have shown that universal surgical mask usage, which requires all individuals in inpatient and outpatient HSCT facilities with direct patient contact to wear surgical masks regardless of symptoms or season, is associated with a significant decrease in clinically significant RVI, particularly PIV3. This suggests that universal surgical mask usage could be a potent adjunct to standard infection control practices in bone marrow transplant units.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Mary Althaus, Duke University Medical Center, for her help with viral assays; and Cameron R. Wolfe, MBBS, Division of Infectious Diseases, Duke University Medical Center, and Emily R. M. Syndor, MD, Division of Infectious Diseases, University of Utah Health Care, for their advice with the manuscript.
Author contributions. M. E. H., N. J. C., G. L., C. G., D. R., and K. M. S. were responsible for study protocol development and implementation. K. C. provided database management expertise. J. A. M. S., A. D. S., and M. E. H. provided data analysis and performed chart reviews. S. T., G. B., and T. H. provided statistical expertise and analysis. A. D. S. and J. A. M. S. drafted the manuscript, and all authors contributed.
Financial support. This work was supported by the National Institutes of Health (2P-01 CA47741 to the project; T32 HL007057-37 and 5KL2TR001115-03 to A. D. S.; and T32 AI007001 and 1KL2TR001109, to J. A. M. S.) and the American Society of Hematology (Research Training Award for Fellows to A. D. S.).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Martino R, Porras RP, Rabella N et al. . Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant 2005; 11:781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfromm A, Porcher R, Legoff J et al. . Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant 2014; 20:1238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano F, Campbell AP, Guthrie KA et al. . Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010; 115:2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YJ, Guthrie KA, Waghmare A et al. . Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014; 209:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun C, Slaby J, Shanley RM et al. . Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant 2012; 18:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med 2007; 28:222–42. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whimbey E, Champlin RE, Couch RB et al. . Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 1996; 22:778–82. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98:573–8. [DOI] [PubMed] [Google Scholar]
- 10.Peck AJ, Englund JA, Kuypers J et al. . Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood 2007; 110:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maziarz RT, Sridharan P, Slater S et al. . Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 2010; 16:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sydnor ER, Greer A, Budd AP et al. . An outbreak of human parainfluenza virus 3 infection in an outpatient hematopoietic stem cell transplantation clinic. Am J Infect Control 2012; 40:601–5. [DOI] [PubMed] [Google Scholar]
- 13.Karron RA, O'Brien KL, Froehlich JL, Brown VA. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis 1993; 167:1441–5. [DOI] [PubMed] [Google Scholar]
- 14.Zambon M, Bull T, Sadler CJ, Goldman JM, Ward KN. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol 1998; 36:2289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez KJ, Erdman DD, Peret TC et al. . Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis 2001; 184:1093–7. [DOI] [PubMed] [Google Scholar]
- 16.Nichols WG, Erdman DD, Han A, Zukerman C, Corey L, Boeckh M. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biol Blood Marrow Transplant 2004; 10:58–64. [DOI] [PubMed] [Google Scholar]
- 17.Jalal H, Bibby DF, Bennett J et al. . Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. J Clin Microbiol 2007; 45:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piralla A, Percivalle E, Di Cesare-Merlone A, Locatelli F, Gerna G. Multicluster nosocomial outbreak of parainfluenza virus type 3 infection in a pediatric oncohematology unit: a phylogenetic study. Haematologica 2009; 94:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan KM, Dykewicz CA, Longworth DL et al. . Preventing opportunistic infections after hematopoietic stem cell transplantation: the Centers for Disease Control and Prevention, Infectious Diseases Society of America, and American Society for Blood and Marrow Transplantation Practice Guidelines and beyond. Hematology Am Soc Hematol Educ Program 2001; 392–421. [DOI] [PubMed] [Google Scholar]
- 20.Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clin Infect Dis 2001; 32:413–8. [DOI] [PubMed] [Google Scholar]
- 21.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 2013; 9:e1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sax H, Allegranzi B, Chraiti MN, Boyce J, Larson E, Pittet D. The World Health Organization hand hygiene observation method. Am J Infect Control 2009; 37:827–34. [DOI] [PubMed] [Google Scholar]
- 23. Center for Disease Control. Human Parainfluenza Viruses. Available at: http://www.cdc.gov/parainfluenza/about/symptoms.html . Accessed 1 June 2016. [Google Scholar]
- 24.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics 2001; 57:120–5. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Hafner C, Tavzarashvili K, Vahldieck R. Metallic and dielectric photonic crystal filter design using multiple multipole program and model-based parameter estimation methods. J Opt Soc Am A 2007; 24:1761–6. [DOI] [PubMed] [Google Scholar]
- 26.Gebski V, Ellingson K, Edwards J, Jernigan J, Kleinbaum D. Modelling interrupted time series to evaluate prevention and control of infection in healthcare. Epidemiol Infect 2012; 140:2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chemaly RF, Ghosh S, Bodey GP et al. . Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine 2006; 85:278–87. [DOI] [PubMed] [Google Scholar]
- 28.Chakrabarti S, Collingham KE, Holder K, Oyaide S, Pillay D, Milligan DW. Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy. Clin Infect Dis 2000; 31:1516–8. [DOI] [PubMed] [Google Scholar]
- 29.Cho CH, Chulten B, Lee CK et al. . Evaluation of a novel real-time RT-PCR using TOCE technology compared with culture and Seeplex RV15 for simultaneous detection of respiratory viruses. J Clin Virol 2013; 57:338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J Hematol Oncol 2013; 6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.