Elevated cholesterol and APOE ε4 genotype were independent risk factors for cognitive decline in antiretroviral therapy–adherent human immunodeficiency virus (HIV)-infected men aged 50–65 years, whereas higher high-density lipoprotein attenuated cognitive decline. Treatment of dyslipidemia may reduce midlife cognitive decline among HIV-infected individuals.
Keywords: HIV-1, aging, APOE, cholesterol, cognitive decline
Abstract
Background. Dyslipidemia and apolipoprotein E4 (APOE ϵ4) allele are risk factors for age-related cognitive decline, but how these risks are modified by human immunodeficiency virus (HIV) infection is unclear.
Methods. In a longitudinal nested study from the Multicenter AIDS Cohort Study, 273 HIV type 1–infected (HIV+) men aged 50–65 years with baseline HIV RNA <400 copies/mL and on continuous antiretroviral therapy (ART) in ≥95% of follow-up visits were matched by sociodemographic variables to 516 HIV-uninfected (HIV–) controls. The association between lipid markers (total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides), APOE genotype, and cognitive decline in HIV infection was examined using mixed-effects models.
Results. The median baseline age of participants was 51, 81% were white, and 89% had education >12 years. HIV+ men had similar baseline total cholesterol and LDL-C, but lower HDL-C and higher triglycerides than controls (P < .001). Higher total cholesterol and LDL-C were associated with faster rates of cognitive decline (P < .01), whereas higher HDL-C attenuated decline (P = .02) in HIV+ men. In HIV+ men with elevated cholesterol, statin use was associated with a slower estimated rate of decline (P = .02). APOE ϵ4 genotype accelerated cognitive decline in HIV+ but not HIV– men (P = .01), with trajectories diverging from HIV– ε4 carriers after age 50. Total cholesterol levels did not modify the association of ϵ4 genotype with decline (P = .9).
Conclusions. Elevated cholesterol and APOE ϵ4 genotype are independent risk factors for cognitive decline in ART-adherent HIV+ men aged >50 years. Treatment of dyslipidemia may be an effective strategy to reduce cognitive decline in older HIV+ individuals.
The population of human immunodeficiency virus type 1 (HIV-1)–infected (HIV+) individuals over age 50 is growing due to effective antiretroviral therapies (ART), and focus has shifted to prevention and management of age-related comorbidities. Dyslipidemia is common among people living with HIV (PLWH) in the current ART era. Persistent elevations in triglycerides and total cholesterol, and reductions in high-density lipoprotein cholesterol (HDL-C) levels, are detected in HIV+ cohorts, whereas elevations in low-density lipoprotein cholesterol (LDL-C) are less consistent [1, 2]. Previous studies suggest that elevated total cholesterol or low HDL-C levels are associated with increased risk of late-onset dementia in the general population [3, 4]. Furthermore, high total cholesterol was implicated as a risk factor for lower cognitive scores in PLWH and worsening HIV-1–associated neurocognitive disorders (HAND) [5], but the longitudinal effects of lipid levels on cognitive decline in ART-treated older HIV+ individuals are unknown.
The main cholesterol transporter in the central nervous system is apolipoprotein E (APOE), a structural component of very low-density lipoproteins and HDL-C [6]. Three major APOE isoforms are encoded by the ϵ2, ϵ3, and ϵ4 alleles, with worldwide frequencies of approximately 8%, 78%, and 14%, respectively [7]. The ϵ4 allele is the most important genetic risk factor for Alzheimer's disease, and is a risk factor for age-related cognitive decline in the general population [8]. The relationship between APOE genotype and HAND is unclear due to conflicting results [9–22]. While some cross-sectional studies suggest that the ϵ4 allele increases risk for HAND over age 50 [12, 19], others found no significant cognitive effect of the ϵ4 allele in HIV+ adults [9, 11, 13, 20, 22]. The ϵ4 allele has been associated with hypercholesterolemia, but no studies have examined whether ϵ4 genotype interacts with cholesterol levels to influence cognitive decline in aging PLWH.
It is critical to understand when lipids and APOE ε4 status modify cognitive performance among ART-treated HIV+ adults, as these factors can guide clinical practice and trial design. Here, we examined the effect of lipid profiles and APOE ϵ4 allele on cognitive decline in a cohort of ART-adherent HIV+ and HIV– men aged 50–65 years. We then modeled the interaction between elevated cholesterol levels and statin use or ϵ4 genotype to estimate their combined effects on the rate of cognitive decline.
METHODS
Data Source
This was a prospective study using data from the Multicenter AIDS Cohort Study (MACS), an observational cohort of HIV+ and HIV– men who have sex with men. Interviews, physical examinations, and biological specimens were collected in biannual visits; neuropsychological examinations began in 1986. Details of the study design and enrollment patterns have been previously described [23] (Supplementary Methods). The institutional review boards at each of the clinical sites approved the research, and subjects signed a written statement of informed consent. The MACS public data is released annually (http://www.statepi.jhsph.edu/macs/macs.html) [23]; the p23 release was translated to a local SQL database and used in these analyses.
Study Population
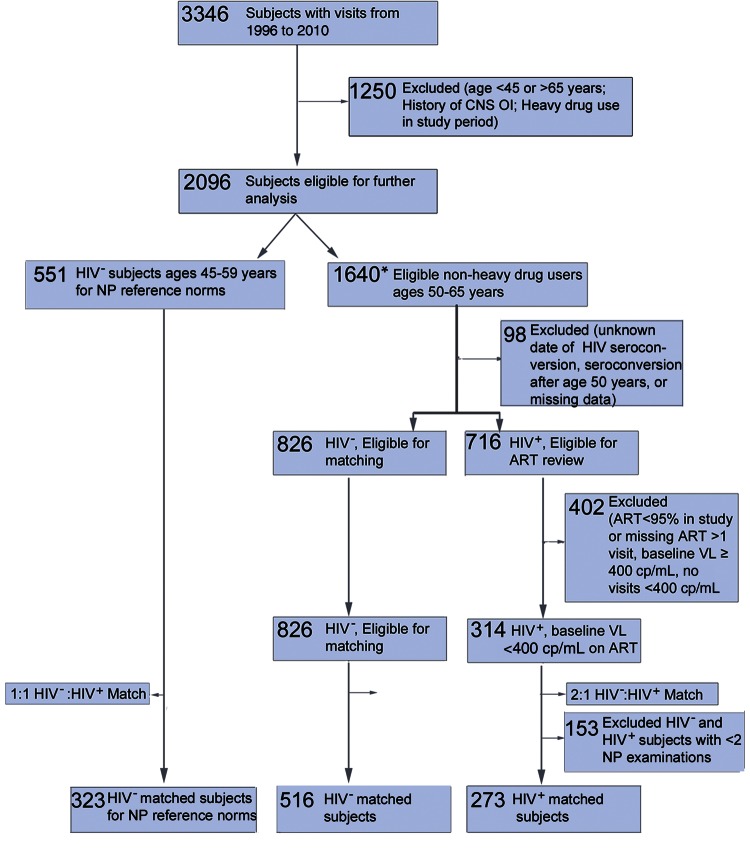
This study was restricted to MACS visits between January 1996 and December 2010. A sequential process was performed to define the study cohort of 789 men aged 50–65 years (Figure 1; Supplementary Methods). Among 3346 men with visits from 1996 to 2010, 1250 were outside the age for eligibility, had a history of CNS opportunistic infections, or reported cocaine, crack, or heroin use at >50% of visits during the study period, while 653 were excluded due to ART adherence <95% in follow-up and other exclusion criteria (Supplementary Methods). For inclusion, HIV+ participants had to be on ART for ≥1 year prior to baseline visit and have plasma viral load <400 copies/mL at baseline. HIV– controls were matched to HIV+ cases with the MatchIt package in R (version 2.4–21; http://gking.harvard.edu/matchit) [24]. Subjects were matched irrespective of the number of neurocognitive visits to minimize bias that may have been associated with neuropsychological substudy entry; matched covariates included age at study entry, black race, education level, alcohol use, and smoking. Matched subjects with at least 2 neurocognitive visits were included in the final study cohort.
Figure 1.
Selection of the human immunodeficiency virus (HIV)–infected and HIV-uninfected study cohort. Subject enrollment and sequential application of inclusion and exclusion criteria to define the study population. *Subjects aged 45–49 years and 50–65 years with neuropsychological scores were counted toward both groups. Heavy drug use was defined as crack, cocaine, or heroin use >50% of visits during study period. Abbreviations: ART, antiretroviral therapy; CNS, central nervous system; HIV+, human immunodeficiency virus infected; HIV−, human immunodeficiency virus uninfected; NP, neuropsychological; OI, opportunistic infection (lymphoma, progressive multifocal leukoencephalopathy, toxoplasmosis, or Cryptococcus); VL, HIV-1 RNA load.
Measures of Cognitive Function
A battery of 15 neuropsychological tests measuring cognitive domains related to HAND was used to generate a composite cognitive summary score [25]. Individual tests were converted to z scores using the test's mean and standard deviation (SD) from HIV– and hepatitis C virus–antibody negative men aged 45–49 years stratified by education level as reference norms. The age range of the normative group (45–49 years) was selected based on proximity to the cohort median age at the baseline visit, and relatively stable cognitive performance within this narrow age window. The cognitive summary score created to capture performance heterogeneity included (1) executive function (trail-making part B, Stroop interference); (2) perceptual speed (Symbol Digit Modalities Test, Stroop color naming and word naming, trail-making part A); (3) attention and working memory (CalCAP reaction time measures); (4) verbal learning and memory (Rey Auditory Verbal Learning test [RAVLT] sum of trials 1–5; RAVLT immediate recall; RAVLT delayed recall); (5) motor (Grooved Pegboard, both scores) (Supplementary Table 1). The following covariates with potential for confounding were used in adjusted models: baseline age (years), Shipley WAIS IQ-Equivalent score (IQ), Centers for Epidemiologic Studies Depression (CES-D) score, smoking, and CD4 cell count.
Genotyping
Genomic DNA extraction and genotyping of APOE single-nucleotide polymorphisms rs429358 [C/T] and rs7412 [C/T] from individuals within the MACS has been described [22]. Genotyping was conducted using TaqMan OpenArray technology. Arrays were imaged after amplification on OpenArray NT images, genotypes ascribed after clustering VIC and FAM signals (Stata 12.1; StataCorp, College Station, Texas) and used to determine APOE alleles. APOE genotype was available for 350 participants.
Statistical Methods
Cohort characteristics were described using means and SDs or median and interquartile range (IQR) depending on the distribution of variables. Simple univariate/bivariate tests were conducted using t tests, Wilcoxon rank-sum tests, analysis of variance, and Pearson χ2 or Fisher exact tests. The association between total cholesterol, HIV infection, and change in cognitive score was examined using mixed-effects models with interaction terms for cholesterol with time, HIV infection with time, and their joint interaction with time; cholesterol was analyzed as a time-varying covariate. Statin use was examined in a separate mixed-effects model. A quadratic term (time * time) was used to estimate accelerated rates of decline. Continuous variables included baseline age at study entry, CES-D score, and IQ score, and binary variables were smoking and HIV infection; CD4 cell count and statin use were examined as time-varying covariates. Backward elimination was used to identify significant longitudinal relationships among predictors (P < .05 cutoff). The effect of APOE ϵ4 allele was explored in an independent mixed-effects model; ε4 status was modeled as a categorical covariate (ϵ4 carrier, ϵ4 noncarrier, and unknown/ϵ2 homozygotes). The decision to categorize the ϵ2 allele separately was made prior to analysis given its protective cognitive effects, which may falsely underestimate cognitive decline in ϵ4 noncarriers [6]. All models included a random intercept and slope. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Clinical Characteristics
Clinical characteristics of the study cohort are shown in Table 1 (n = 789 men; 273 HIV+, 516 HIV–). The median age was 51 years at study entry (IQR, 50–55 years) with a mean follow-up of 6.3 years. Eighty-one percent self-identified as non-Hispanic white, and 14% as black. HIV+ and HIV– men had similar proportions with >12 years of education (P = .35). HIV+ men had higher mean baseline CES-D scores (9.7 vs 8.5) and greater proportion with scores ≥16 (22% vs 18%; P = .08), a cutoff for high depressive symptoms. The median CD4 count was 514 at baseline; 70% maintained viral suppression (<50 copies/mL with ≤2 blips, blip ≥400 copies/mL). Baseline cholesterol and LDL-C levels were similar between groups, whereas HIV+ men had higher triglycerides and lower HDL-C than controls (P < .001). Among HIV+ men, 51% were on a statin for at least 1 year.
Table 1.
Cohort Characteristics
| Characteristic | All Subjects | HIV− | HIV+ | P Value |
|---|---|---|---|---|
| No. of patients | 789 | 516 | 273 | NA |
| Length of follow-up, y, mean (SD) | 6.3 (3.2) | 6.0 (3.2) | 6.8 (3.1) | <.01 |
| Demographics | ||||
| Baseline age, y, median (IQR) | 51.0 (50–55) | 51.5 (50–55) | 50.0 (50–52) | <.001 |
| Race | ||||
| White | 636 (80.6) | 429 (83.1) | 207 (75.8) | <.01 |
| Black | 112 (14.2) | 62 (12.0) | 50 (18.3) | |
| Hispanic or Latino | 21 (2.7) | 15 (2.9) | 6 (2.2) | |
| Other | 15 (1.9) | 10 (1.9) | 5 (1.8) | |
| Education >12 years | 702 (89.0) | 463 (89.7) | 239 (87.5) | .35 |
| Shipley WAIS IQ-Equivalent, mean (SD) | 109.5 (8.9) | 109.1 (10.7) | 106.5 (11.3) | <.01 |
| Depression profile | ||||
| Baseline CES-D score, mean (SD) | 8.9 (9.6) | 8.5 (9.5) | 9.7 (9.8) | .13 |
| Baseline CES-D score ≥16 | 151 (19.1) | 92 (17.8) | 59 (21.6) | .08 |
| Substance use | ||||
| Smoking (highest use on ≥2 visits) | .26 | |||
| None | 574 (72.8) | 386 (74.8) | 188 (68.9) | |
| <1/2 pack per day | 66 (8.4) | 38 (7.4) | 28 (10.3) | |
| 1/2–2 packs per day | 146 (18.5) | 90 (17.4) | 56 (20.5) | |
| Alcohol (highest use on ≥2 visits)a | .07 | |||
| None/light | 111 (14.1) | 71 (13.8) | 40 (14.7) | |
| Occasional/moderate | 566 (71.7) | 379 (73.4) | 187 (68.5) | |
| Heavy/binge | 100 (12.7) | 55 (10.7) | 45 (16.5) | |
| Baseline lipid profile, mg/dL, mean (SD) | ||||
| Total cholesterol | 197.1 (40.4) | 195.1 (36.3) | 201.1 (47.4) | .11 |
| LDL-C | 115.4 (34.8) | 117.2 (33.7) | 111.9 (36.8) | .08 |
| HDL-C | 47.6 (13.3) | 49.0 (12.4) | 44.8 (14.4) | <.001 |
| Triglycerides | 161.0 (123.1) | 136.3 (95.5) | 212.7 (154.8) | <.001 |
| Lipid-lowering medicationb | ||||
| Statin | 304 (38.5) | 164 (31.8) | 140 (51.3) | <.001 |
| Fibrate | 78 (9.9) | 23 (4.5) | 55 (20.1) | <.01 |
| Niacin | 38 (4.8) | 21 (4.1) | 17 (6.2) | .24 |
| HCV antibody positive | 74 (9.4) | 35 (6.8) | 39 (14.3) | <.01 |
| HIV disease characteristics, median (IQR) | ||||
| Baseline CD4, cells/µLc | 805 (569–1055) | 951 (743–1174) | 514 (333–684) | <.001 |
| CD4 nadir in study, cells/µL | 623 (431–840) | 726 (580–916) | 387 (265–536) | <.001 |
| Baseline HIV-1 RNA VL, copies/mLc | 40 (40–40) | |||
| Baseline CPE scorec | 7.0 (6–9) | |||
| Antiretroviral medicationb | ||||
| Azidothymidine | 95 (34.8) | |||
| Efavirenz | 114 (41.8) | |||
| Protease inhibitor | 190 (69.6) | |||
| ddI, d4T, ddC | 106 (38.8) | |||
| Abacavir | 96 (36.2) | |||
Data are presented as No. (%) unless otherwise indicated. P values <.05 were considered significant.
Abbreviations: CES-D, Center for Epidemiological Studies Depression Scale; CPE, central nervous system penetration effectiveness; d4T, stavudine; ddC, zalcitabine; ddI, didanosine; HCV, hepatitis C virus; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; HIV+, human immunodeficiency virus infected; HIV−, human immunodeficiency virus uninfected; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NA, not applicable; SD, standard deviation; VL, viral load.
a Alcohol use was defined as follows: light, <1 drink/week; occasional to moderate, 1–14 drink(s)/week; heavy, >14 drinks/week; binge, ≥5 drinks in 1 sitting per month.
b Self-reported lipid-lowering medication and antiretroviral therapy medication used on ≥2 visits.
c Baseline values: first visit or within 6 months of study period. See [38] for CPE details.
Lipids and Cognitive Decline
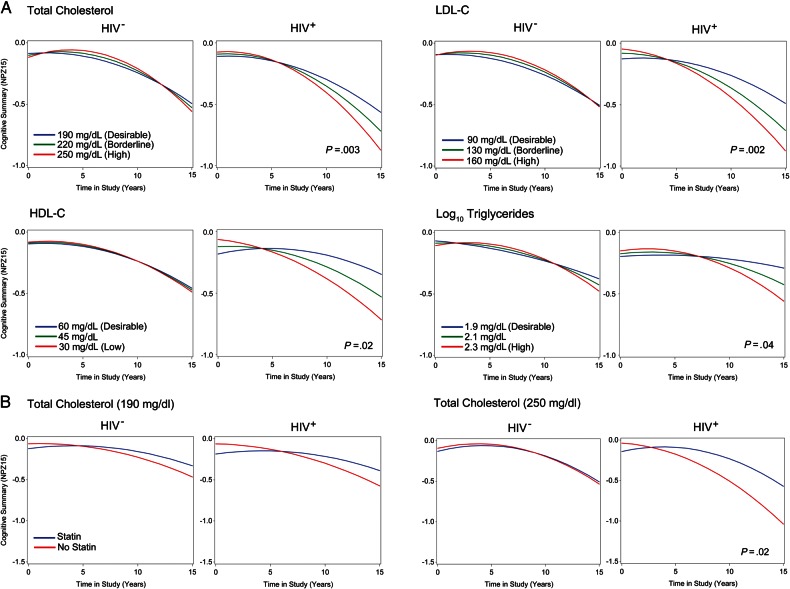
Time-related terms reflecting the association between total cholesterol levels and cognitive decline between ages 50–65 years are summarized in Table 2. While the estimated rate of cognitive decline accelerated with increasing cholesterol levels in both groups, HIV+ men had a faster rate of decline compared with HIV– controls (estimate = −0.0034, P = .003; Figure 2). Figure 2A depicts the estimated annual rate of decline for a 50-year-old man with and without HIV infection, illustrating 2 main findings: (1) On average, HIV+ men with higher cholesterol levels have faster rates of cognitive decline than HIV+ men with lower levels; and (2) the rate of cognitive decline in HIV+ men aged 50–65 years is differentially modified by cholesterol compared with HIV– men of the same age, IQ, baseline CES-D score, smoking status, and CD4 count. Higher cholesterol levels in HIV+ men were marginally associated with better cognitive scores at the intercept (total cholesterol * HIV+: estimate 0.01058; P = .05), suggesting that cognitive decline associated with elevated cholesterol most likely occurred after age 50. Older age and baseline CES-D scores correlated with lower cognitive scores; IQ was associated with higher scores (Supplementary Table 2).
Table 2.
Associated Effect of Lipids on the Annual Rate of Cognitive Decline
| Model | Estimate | SE | P Value |
|---|---|---|---|
| Model 1a | |||
| Total cholesterol | |||
| HIV+* years in study | 0.0613 | 0.0226 | .007 |
| Total cholesterol (10 mg/dL)* Years in study | 0.0040 | 0.0016 | .112 |
| Total cholesterol (10 mg/dL)* Years in study * Years in study | −0.0003 | 0.0001 | .043 |
| Total cholesterol (10 mg/dL)* HIV+* Years in study | −0.0034 | 0.0011 | .003 |
| LDL-C | |||
| HIV+* Years in study | 0.0423 | 0.0170 | .013 |
| LDL-C (10 mg/dL)* Years in study | 0.0022 | 0.0018 | .995 |
| LDL-C (10 mg/dL)* Years in study* Years in study | −0.0002 | 0.0002 | .371 |
| LDL-C (10 mg/dL)* HIV+* Years in study | −0.0043 | 0.0014 | .002 |
| HDL-C | |||
| HIV+* Years in study | −0.0460 | 0.0202 | .024 |
| HDL-C (10 mg/dL)* Years in study | −0.0006 | 0.0053 | .390 |
| HDL-C (10 mg/dL)* Years in study* Years in study | 0.0001 | 0.0005 | .819 |
| HDL-C (10 mg/dL)* HIV+* Years in study | 0.0098 | 0.0043 | .022 |
| Triglycerides (log10 mg/dL) | |||
| HIV+* Years in study | 0.0949 | 0.0450 | .036 |
| Triglycerides* Years in study | 0.0599 | 0.0259 | .121 |
| Triglycerides* Years in study* Years in study | −0.0047 | 0.0023 | .041 |
| Triglycerides* HIV+* Years in study | −0.0424 | 0.0205 | .039 |
| Model 2b: Total cholesterol model 1 + Statin use | |||
| Total cholesterol (10 mg/dL)* HIV+* Years in study | −0.0053 | 0.0015 | .004 |
| Statin use* Years in study | 0.0400 | 0.0347 | .913 |
| Statin use* HIV+* Years in study | −0.0739 | 0.0372 | .048 |
| Statin use* Total cholesterol (10 mg/dL)* Years in study | −0.0014 | 0.0017 | .612 |
| Statin use* HIV+* Total cholesterol (10 mg/dL)* Years in study | 0.0043 | 0.0018 | .019 |
All models were adjusted for age, Shipley WAIS IQ-Equivalent Score, Center for Epidemiological Studies Depression Scale at study entry, smoking status, and CD4 count. Model 2 was also adjusted for statin use. Lipid estimates except triglyceride levels were interpreted in 10-mg/dL increments. R2 is the squared Pearson correlation between predicted values from fixed or fixed and random effects vs actual values and represents the variance in the cognitive summary score accounted for by terms in the model.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HIV+, human immunodeficiency virus infected; LDL-C, low-density lipoprotein cholesterol; SE, standard error.
a Model 1 total cholesterol: R2 for fixed effects = 0.25, P < .001; R2 including random terms = 0.94, P < .0001.
b Model 2: R2 for fixed effects = 0.25, P < .001; R2 including random terms = 0.95, P < .0001.
* Indicates an interaction.
Figure 2.
Higher total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides are associated with faster rates of cognitive decline, whereas high-density lipoprotein cholesterol (HDL-C) levels attenuate decline in antiretroviral therapy–treated human immunodeficiency virus–infected (HIV+) men. A, Estimated slopes in neurocognitive scores according to total cholesterol, LDL-C, HDL-C, and log10 triglyceride levels stratified by HIV infection, and categorized by National Cholesterol Education Program guidelines are shown. The slopes are estimated for a man with study entry age of 50, and cohort mean IQ score 108, baseline Center for Epidemiological Studies Depression Scale score 9, and CD4 count held at 800 cells/mL. The x-axis is time in study (years) centered at zero for the first visit after age 50, and y-axis is the change in cognitive performance from the baseline score. There is an accelerated rate of age-related decline in the cognitive score as total cholesterol and triglycerides levels increase in human immunodeficiency virus–uninfected (HIV–) and HIV+ men, an effect not observed for LDL-C or HDL-C levels. Higher total cholesterol (P = .003), LDL-C (P = .002), and triglyceride (P = .04) levels in HIV+ men are associated with a steeper slope of cognitive decline during the study, whereas higher HDL-C levels attenuated the rate of decline (P = .02). B, Estimated slopes for cognitive scores according to statin use by total cholesterol levels. The association between elevated total cholesterol and faster rate of decline was attenuated in HIV+ men on a statin medication (P = .02).
In post hoc analyses, cholesterol was replaced with time-varying LDL-C, HDL-C, or log10 triglycerides, and associations with cognitive scores examined. Higher LDL-C and triglyceride levels were associated with a steeper slope of cognitive decline, while elevated HDL-C levels attenuated the rate of cognitive decline in HIV+ men (Figure 2 and Table 2). The association between cholesterol and decline in specific cognitive domains was examined in secondary analyses for composite scores of executive function, perceptual speed, verbal memory, attention and working memory, and motor speed. Higher cholesterol was associated with a steeper slope of decline in attention and working memory (P < .001), and marginal significance for verbal memory (P = .05; Supplementary Table 2). In sensitivity analyses, the association between cholesterol level and the rate of decline among HIV+ subjects remained significant after the exclusion of 2 subjects with baseline cognitive z score < −2 (data not shown) and 145 HIV+ subjects who did not maintain viral suppression (<50 copies/mL) at all study visits (estimate −0.0028; P = .04).
In a separate analysis, the association between total cholesterol, statin use, and rate of cognitive decline was examined. In this model, statin use was not associated with a change in the baseline cognitive score in HIV– or HIV+ men (P = .43 and .61, respectively) between the ages of 50 and 65 years. In models adjusted for statin use, the association between elevated cholesterol and steeper estimated rate of cognitive decline in ART-adherent HIV+ men remained significant (P = .004; Table 2), but the estimated decline associated with elevated total cholesterol was attenuated with statin use (Figure 2B; P = .019).
APOE ϵ4 Allele and Cognitive Decline
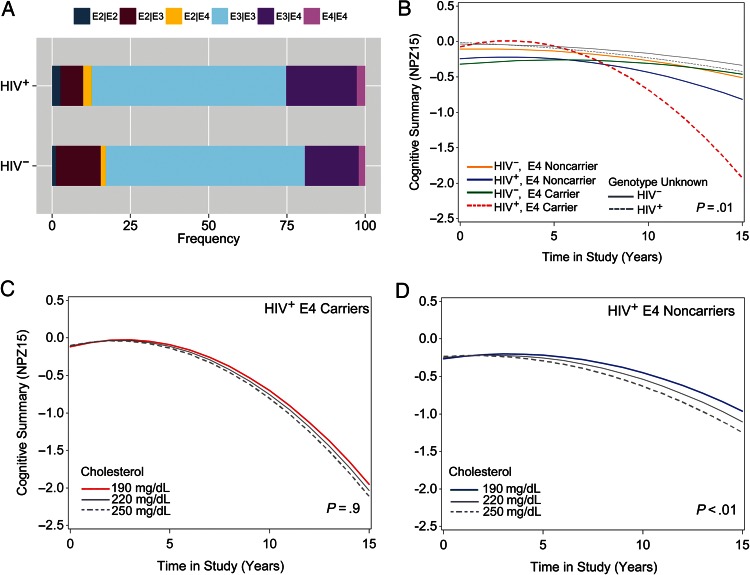
Cohort characteristics of APOE ϵ4 carriers and noncarriers were similar to the larger study cohort (Supplementary Table 3), and ε4 genotype frequencies were comparable (Figure 3A). Among HIV+ ϵ4 carriers vs noncarriers, there were no differences in median baseline CD4 count or ART medications used, but HIV+ ϵ4 carriers had higher baseline triglyceride levels (P < .001). While longitudinal decline in cognitive scores was observed among all HIV+ individuals, the rate of decline accelerated among HIV+ APOE ϵ4 carriers (P = .01; Table 3). Divergent estimated slopes in Figure 3B illustrate that the estimated cognitive trajectory for HIV+ ϵ4 carriers deviates rapidly from HIV+ ϵ4 noncarriers and HIV– controls aged 50–65 years. Given that there were no significant differences in the intercept between HIV+ carriers and noncarriers at study entry (Supplementary Table 4), cognitive decline for HIV+ ϵ4 carriers is expected to start after age 50. In post hoc analyses accelerated rate of decline in perceptual speed, but no other cognitive domains, was estimated for HIV+ ϵ4 carriers (P = .03; Supplementary Table 4). Given that cholesterol levels and APOE ϵ4 genotype were associated with cognitive decline in HIV+ men, we next examined whether these covariates interact to influence the rate of decline. The 3-way interaction term between HIV infection, cholesterol, and time (P = .002; Table 3) remained significant for cognitive decline among HIV+ ϵ4 carriers. While accelerated rates of decline were estimated among HIV+ APOE ϵ4 carriers vs noncarriers (P < .01), the annual rate of decline among ϵ4 carriers was not further modified by cholesterol levels (P = .9; Figure 3C and 3D and Table 3). Thus, cholesterol levels and presence of the ϵ4 allele have independent effects on cognitive decline in HIV+ subjects, and do not substantially influence their respective associations.
Figure 3.
APOE ϵ4 allele and total cholesterol have independent effects on cognitive decline in antiretroviral therapy–treated human immunodeficiency virus–infected (HIV+) men. The distribution of APOE genotypes among HIV+ and human immunodeficiency virus–uninfected (HIV–) subjects (A), and estimated slopes for cognitive scores for APOE ϵ4 allele and HIV infection status (B) are shown. Cognitive scores for subjects with unknown or ϵ2/ϵ2 genotypes are shown in gray (B). Among those with ϵ4 genotype, cognitive decline for HIV+ ϵ4 carriers (C) and noncarriers (D) modified by total cholesterol are shown. The annual rate of decline is estimated for a man with baseline age 50 years, and cohort mean IQ score 108, baseline Center for Epidemiological Studies Depression Scale score 9, and CD4 count held at 800 cells/mL. APOE ϵ4 carriers had lower baseline cognitive scores than noncarriers (P = .03), an association that was not modified by HIV infection (P = .14). HIV+ APOE ϵ4 carriers showed accelerated decline in cognitive scores between ages 50 and 65 years, and the rate of accelerated decline was faster than predicted for HIV+ noncarriers (P = .01). Elevated total cholesterol levels were associated with faster rates of decline among HIV+ ϵ4 noncarriers (P < 0.01), while the accelerated rate of decline in HIV+ ϵ4 carriers was not further modified by cholesterol (P = .9).
Table 3.
Effect of APOE ϵ4 Allele, Total Cholesterol, and Human Immunodeficiency Virus Infection on the Rate of Cognitive Decline
| Model | Estimate | SE | P Value |
|---|---|---|---|
| Model 1a (n = 542) | |||
| HIV+* Years in study | −0.0104 | 0.0168 | .266 |
| HIV+* Years in study* Years in study | 0.0002 | 0.0016 | .003 |
| APOE ε4 carrier* Years in study | 0.0227 | 0.0243 | .022 |
| APOE ε4 carrier* Years in study* Years in study | −0.0008 | 0.0022 | .002 |
| HIV+* APOE ε4 carrier* Years in study | 0.0519 | 0.0355 | .300 |
| HIV+*APOE ε4 carrier* Years in study* Years in study | −0.0106 | 0.0035 | .010 |
| Model 2b (n = 245) | |||
| APOE ε4 carrier* Years in study | −0.02388 | 0.06127 | .8136 |
| HIV+* Years in study | 0.1452 | 0.04568 | .0005 |
| Total cholesterol (10 mg/dL)* Years in study | 0.00214 | 0.00125 | .8417 |
| Total cholesterol (10 mg/dL)* HIV+* Years in study | −0.0056 | 0.00184 | .0021 |
| APOE ε4 carrier* HIV+* Years in study | 0.0266 | 0.07136 | .71 |
| APOE4 carrier* HIV+* Years in study* Years in study | −0.01099 | 0.003421 | .0058 |
| APOE ε4 carrier* Total cholesterol (10 mg/dL)* Years in study | 0.00192 | 0.00252 | .3598 |
| APOE ε4 carrier* HIV+* Total cholesterol (10 mg/dL)* Years in study | −0.00004 | 0.000308 | .897 |
All models were adjusted for age, Shipley WAIS IQ-Equivalent Score, Center for Epidemiological Studies Depression Scale at study entry, smoking status, and CD4 count. Total cholesterol was interpreted in 10 mg/dL increments. APOE ε4 was modeled as a categorical variable (ϵ4 carrier, ϵ4 noncarrier or unknown/ϵ2 homozygous) in model 1. Model 2 included subjects with known APOE ϵ4 genotype.
R2 is the squared Pearson correlation between predicted values from fixed or fixed and random effects vs actual values and represents the variance in the cognitive summary score accounted for by terms in the model.
Abbreviations: APOE, apolipoprotein E; HIV+, human immunodeficiency virus infected; SE, standard error.
a Model 1: R2 for fixed effects = 0.26, P < .001; R2 including random terms = 0.97, P < .0001.
b Model 2: R2 for fixed effects = 0.28, P < .001; R2 including random terms = 0.93, P < .0001.
* Indicates an interaction.
DISCUSSION
In this prospective study, elevated cholesterol, LDL-C, and triglyceride levels were associated with faster rates of cognitive decline in ART-adherent HIV+ men ages 50–65, while higher HDL-C attenuated cognitive decline. The estimated rate of cognitive decline associated with elevated cholesterol was attenuated in ART-adherent HIV+ men on statins. The APOE ϵ4 genotype was associated with accelerated cognitive decline in HIV+ ε4 carriers older than 50 years, approximately a decade earlier than reported for HIV– ϵ4 carriers [7], suggesting that the interaction between treated HIV-infection and the ϵ4 genotype is a significant risk factor for earlier onset of cognitive decline. Cholesterol levels and the APOE ϵ4 genotype had independent effects on the rate of decline among treated HIV+ but not HIV– men, and are therefore unlikely to be redundant risk factors. In aggregate, these findings suggest that control of dyslipidemia may reduce the risk of midlife cognitive decline in aging PLWH on ART, and the APOE ϵ4 genotype likely influences cognitive trajectories via mechanisms distinct from its effects on lipid metabolism.
HIV+ individuals are at increased risk for dyslipidemia due to HIV infection and ART, and have higher rates of cardiovascular disease and metabolic syndrome [26, 27]. We tested the relationship between time-varying cholesterol levels and cognitive decline, and showed that for every 10 mg/dL increase in cholesterol or LDL-C between ages 50 and 65, the rate of cognitive decline among HIV+ men increased. We also demonstrated a positive relationship between time-varying HDL-C levels and longitudinal cognitive performance in HIV+ subjects. While published reports on the relationship between lipids and cognitive decline in the general population are mixed [28], the association between HDL-C and higher cognitive scores in midlife HIV+ men is similar to findings in older HIV– cohorts [29, 30]. HDL-C-like lipoproteins are found in cerebrospinal fluid (CSF), are lower in those with Alzheimer's disease or APOE ϵ4 allele, and may be protective against cognitive decline [28]. HDL-C is proposed to play a role in mitigating oxidative stress, metabolizing oxidized lipids, and reducing LDL-C-induced inflammation [31]. Together with findings from preceding studies demonstrating altered CSF lipid metabolism among HIV+ adults [32, 33], these analyses highlight the importance of identifying mechanisms by which lipids affect cognitive aging and potential strategies for therapeutic intervention.
While our findings suggest that the APOE ϵ4 allele has a substantial effect on cognitive decline in older men with treated HIV infection, accelerating a downward trajectory after age 50, they differ from those of 2 previous longitudinal studies. Burt et al [11] did not identify an association between the APOE ϵ4 allele and HIV-associated dementia in subjects on early ART regimens, and Becker et al [22] recently reported no association between the ϵ4 allele and time to impairment or death. However, there are key methodological differences between study designs that should be taken into account when comparing the aforementioned results to the present study. We studied ART-adherent HIV+ men over age 50, included HIV– controls well-matched for demographics and lifestyle factors, and allowed for acceleration in the rate of decline. Our model predicts that while all groups are estimated to decline over time, there is a complex, nonlinear relationship between time and cognitive performance among older HIV+ ε4 carriers. Statistical models in prior longitudinal studies relied on assumptions that the relationship between predictors, time, and risk for cognitive impairment remains constant. As such, time or age-dependent effects of the ϵ4 allele may have been underestimated in later follow-up.
In addition to its role in Aβ homeostasis, APOE modulates neuroinflammation and oxidative injury in an isoform-specific manner [34, 35]; these effects may be augmented in aging PLWH, especially given that HIV-related metabolic syndrome and abdominal obesity are associated with CSF immune activation markers and cognitive impairment [27, 36]. Superimposed cognitive aging effects related to dyslipidemia or ε4 genetic susceptibility, HIV-related neuroinflammation, and oxidative injury may increase vulnerability to midlife cognitive decline among ART-suppressed HIV+ individuals. Cholesterol levels did not further moderate decline in HIV+ APOE ϵ4 carriers, suggesting that cholesterol and ϵ4 allele have independent effects on cognitive decline via mechanisms that may involve cerebrovascular disease, in addition to other mechanisms.
This study has several limitations, including those inherent to longitudinal observational studies such as selection, survivorship, and severity bias reflected in characteristics of the MACS study population. These findings require replication in populations with other demographic characteristics. The study was limited to men, predominantly with >12 years of education. Epidemiological studies report greater risk of clinical conversion from healthy aging to mild cognitive impairment or Alzheimer's disease in female APOE ϵ4 carriers compared with males [37], highlighting the need for similar analyses in HIV+ women. Low education level is a known predictor for decline to symptomatic HAND [25], and higher educational attainment may provide some protection against effects of the ϵ4 allele by increasing cognitive reserve. Nonetheless, despite high education levels, HIV+ men remained vulnerable to faster rates of decline compared with HIV– controls in the presence of high cholesterol or the ϵ4 allele.
Our findings suggest that clinical management of dyslipidemia with statins in ART-adherent HIV+ individuals may reduce the risk of midlife cognitive decline, and a window of opportunity likely occurs between ages 50 and 65 years. Statins block conversion of 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor for cholesterol, and have pleiotropic effects that include reducing inflammatory responses and improving endothelial function. In the present study, the association between statin use and estimated rate of cognitive decline was dependent on cholesterol levels in HIV+ men, suggesting that the relationship is likely to be mediated through effects of statins on lipid metabolism. Given impressive efforts that have improved survival among HIV+ individuals, this study underscores the importance of lipid profiles and APOE ϵ4 allele to midlife cognitive health in aging HIV+ adults and suggests that clinical management of dyslipidemia may be an effective adjunctive strategy to reduce cognitive decline in ART-treated HIV+ individuals.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors are grateful to Drs Dorene Rentz, Reisa Sperling, Anthony Hollenberg, and Rebecca Betensky for review of the data and manuscript. The data for this manuscript were obtained by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (principal investigator [PI]), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, David Ostrow, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-PI), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D'Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan.
Financial support. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (grant numbers U01-AI35039, U01-AI35040; U01-AI35041; U01-AI35042; and UM1-AI35043), with additional co-funding from the National Cancer Institute, National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH) at the National Institutes of Health (NIH). MACS data collection is also supported by UL1-TR000424 (Johns Hopkins University Clinical and Translational Science Award). The website is located at http://www.statepi.jhsph.edu/macs/macs.html. This work was supported by the NIMH (RO1 MH097659) and NIDA (RO1 DA028994) to D. G. Training and educational support for S. S. M. and A. D. was provided by National Institute on Aging award T32-AG000222. Additional support for S. S. M. included the Harvard Catalyst Master's Program in Clinical and Translational Investigation funded by the NIH Clinical and Translational Science Award Program (1UL1-TR001102), and Catalyst Biostatistical Consultation with contributions from Harvard Medical School and affiliated hospitals.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hadigan C, Meigs JB, Corcoran C et al. . Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis 2001; 32:130–9. [DOI] [PubMed] [Google Scholar]
- 2.Riddler S, Li X, Chu H et al. . Longitudinal changes in serum lipids among HIV‐infected men on highly active antiretroviral therapy. HIV Med 2007; 8:280–7. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Laakso MP et al. . Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001; 322:1447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005; 64:277–81. [DOI] [PubMed] [Google Scholar]
- 5.Sacktor N, Skolasky RL, Seaberg E et al. . Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988; 240:622–30. [DOI] [PubMed] [Google Scholar]
- 7.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9:106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caselli RJ, Dueck AC, Osborne D et al. . Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 2009; 361:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop O, Goplen AK, Liestol K et al. . HIV dementia and apolipoprotein E. Acta Neurol Scand 1997; 95:315–8. [DOI] [PubMed] [Google Scholar]
- 10.Corder EH, Robertson K, Lannfelt L et al. . HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med 1998; 4:1182–4. [DOI] [PubMed] [Google Scholar]
- 11.Burt TD, Agan BK, Marconi VC et al. . Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A 2008; 105:8718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valcour V, Shikuma C, Shiramizu B et al. . Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol 2004; 157:197–202. [DOI] [PubMed] [Google Scholar]
- 13.Joska JA, Combrinck M, Valcour VG et al. . Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol 2010; 16:377–83. [DOI] [PubMed] [Google Scholar]
- 14.Spector SA, Singh KK, Gupta S et al. . APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS 2010; 24:1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Andres M, Sadino J et al. . Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. NeuroImage 2011; 58:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andres MA, Feger U, Nath A, Munsaka S, Jiang CS, Chang L. APOE epsilon 4 allele and CSF APOE on cognition in HIV-infected subjects. J Neuroimmune Pharmacol 2011; 6:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soontornniyomkij V, Moore DJ, Gouaux B et al. . Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS 2012; 26:2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoare J, Westgarth-Taylor J, Fouche JP et al. . Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. Eur Arch Psychiatry Clin Neurosci 2013; 263:189–95. [DOI] [PubMed] [Google Scholar]
- 19.Panos SE, Hinkin CH, Singer EJ et al. . Apolipoprotein-E genotype and human immunodeficiency virus-associated neurocognitive disorder: the modulating effects of older age and disease severity. Neurobehav HIV Med 2013; 5:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan EE, Woods SP, Letendre SL et al. . Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol 2013; 19:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Jiang C, Cunningham E et al. . Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology 2014; 82:2213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker JT, Martinson JJ, Penugonda S et al. . No association between apoE4 alleles, HIV infection, age, neuropsychological outcome, or death. J Neurovirol 2015; 21:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker JT, Kingsley LA, Molsberry S et al. . Cohort profile: recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol 2015; 44:1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42:1–28. [Google Scholar]
- 25.Antinori A, Arendt G, Becker JT et al. . Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker JT, Kingsley L, Mullen J et al. . Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCutchan JA, Marquie-Beck JA, Fitzsimons CA et al. . Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitz C. Dyslipidemia and dementia: current epidemiology, genetic evidence and mechanisms behind the associations. J Alzheimers Dis 2012; 30(suppl 2):S127–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Exel E, de Craen AJ, Gussekloo J et al. . Association between high‐density lipoprotein and cognitive impairment in the oldest old. Ann Neurol 2002; 51:716–21. [DOI] [PubMed] [Google Scholar]
- 30.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. Role of lipoproteins and inflammation in cognitive decline: do they interact? Neurobiol Aging 2012; 33:196 e1–12. [DOI] [PubMed] [Google Scholar]
- 31.Baker J, Ayenew W, Quick H et al. . High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis 2010; 201:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutler RG, Haughey NJ, Tammara A et al. . Dysregulation of sphingolipid and sterol metabolism by ApoE4 in HIV dementia. Neurology 2004; 63:626–30. [DOI] [PubMed] [Google Scholar]
- 33.Bandaru VV, McArthur JC, Sacktor N et al. . Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology 2007; 68:1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet 1996; 14:55–61. [DOI] [PubMed] [Google Scholar]
- 35.Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997; 388:878–81. [DOI] [PubMed] [Google Scholar]
- 36.Sattler FR, He J, Letendre S et al. . Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr 2015; 68:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE‐related risk of developing Alzheimer disease. Ann Neurol 2014; 75:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammond ER, Crum RM, Treisman GJ et al. . The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol 2014; 180:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.