ABSTRACT
Measuring thermal behavior in smaller insects is particularly challenging. In this study, we describe a new horizontal thermal gradient apparatus designed to study adult thermal behavior in small insects and apply it using D. melanogaster as a model and case study. Specifically, we used this apparatus and associated methodology to examine the effects of sex, geographic origin, and developmental rearing temperature on temperature preferences exhibited by adults in a controlled laboratory environment. The thermal gradient established by the apparatus was stable over diurnal and calendar time. Furthermore, the distribution of adult flies across thermal habitats within the apparatus remained stable following the period of acclimation, as evidenced by the high degree of repeatability across both biological and technical replicates. Our data demonstrate significant and predictable variation in temperature preference for all 3 assayed variables. Behaviorally, females were more sensitive than males to higher temperatures. Flies originating from high latitude, temperate populations exhibited a greater preference for cooler temperatures; conversely, flies originating from low latitude, tropical habitats demonstrated a relative preference for higher temperatures. Similarly, larval rearing temperature was positively associated with adult thermal behavior: low culture temperatures increased the relative adult preference for cooler temperatures, and this response was distinct between the sexes and for flies from the temperate and subtropical geographic regions. Together, these results demonstrate that the temperature chamber apparatus elicits robust, predictable, and quantifiable thermal preference behavior that could readily be applied to other taxa to examine the role of temperature-mediated behavior in a variety of contexts.
KEYWORDS: behavior, Drosophila, geographical variations, thermal gradient, thermal plasticity
Introduction
Organisms routinely face a variety of environmental stresses in their natural habitats. The levels of these stresses are often correlated with a variety of factors, including latitude and altitude.1,2 Variation in fitness traits across these scales (clines) are often correlated with covarying environmental factors.3-16 Many of these clines reflect evolutionary adaptive differentiation in response to different climates, while others reflect simple to complex aspects of demography. 17-22 The mechanistic basis of many clines remains elusive, and a major emphasis in the dissection of clines has been to understand how the observed variation in fitness-associated traits reflects the adaptive response to specific environmental conditions.23,24 A key component in the potential adaptation to climatic variation is the behavioral response to temperature and temperature variation experienced in natural habitats, which may have pronounced effects on organismal performance and fitness.25 Thermal behavior has been incorporated in numerous studies of climatic adaptation, albeit in larger animals.29 However, the analysis of variation in thermal-mediated behavior in drosophilids and other smaller insects has not been widely incorporated in studies of climatic adaptation, potentially due to the lack of adequate methods to quantify behavioral responses in smaller insects. 26-28
Ectotherms exploit a range of natural habitats that vary greatly with respect to a number of environmental parameters. Temperature is a ubiquitous environmental cue that has widespread effects on physiology, performance, and fitness. 29 To find favorable thermal habitats, insects exhibit a variety of behaviors. An insect's ability to avoid lethal temperatures, as well as sub-optimal temperatures that affect performance, can have observable effects on its physiology and fitness.30,31 This raised a concern among physiologists to measure the preferred temperatures of organisms in relation to body temperatures experienced in the field.29,32 Measurements of thermal preference for small insects are important because many serve as model organisms (e.g., fruit flies, mosquitoes, wasps, etc.) in a variety of biological disciplines. Many of these model organisms are used to study molecular and neuronal aspects of thermal sensation and thermal behavior. 33-41 In addition, these model organisms can facilitate the evaluation of the effects of climate and climate change at the phenotypic, molecular and species distribution levels. 42,43
The existence of parallel latitudinal clines across multiple continents has been interpreted as adaptation to local climatic conditions.17,26,44,45 For example, on the Indian subcontinent several Drosophila species show parallel opposing clines for desiccation tolerance and starvation resistance.17,46,47 Furthermore, temperature shifts associated with recent climate change have affected the shape or slope of latitudinal clines.43,48,49 Despite the emphasis on temperature as a selective agent resulting in local adaptation.24,26,42,47,49,50 The potential role of thermal behavior in local adaptation has not been explored. Here, we develop an apparatus designed to examine thermal preference exhibited by flies collected from natural populations. We demonstrate that there is significant variation within and among populations in thermal preference, and that this varies predictably with geography: flies from high latitude, temperate origins avoid high temperatures and prefer cool temperatures, whereas flies from low latitude, subtropical populations avoid cold temperatures and exhibit greater relative preference for higher temperatures. In addition, our apparatus and methodology elucidate clear and predictable differences between sexes, and demonstrate pronounced effects of larval rearing temperature on the thermal behavior exhibited by adults. Our method and data focus on the thermally-mediated behavior of adults, which has greater relevance to understanding plastic and genetic responses to temperature variation.18,51
We suggest that the incorporation of thermal behavioral response traits is essential to a comprehensive evaluation of fitness and performance along environmental gradients. The interactions between stress, behavior and fitness could be assessed using the methods we describe here. It should be explored whether thermal preference exhibits variation among individuals within populations, among populations within a species, and among taxa. Such information may be of great utility in understanding the process of adaptation under various scenarios of climate change.24,52 Our results suggest that thermal behavior/preference is shaped by natural selection and is one component in a suite of traits that represent local adaptation to climate.
Material and methods
Thermal gradient apparatus design
A closed horizontal thermal gradient was constructed to examine aspects of thermal behavior in Drosophila (Fig. 1; see also Supporting file 1). The apparatus was built by mounting an aluminum rod (74.93 cm length; 3 cm in diameter), on thermoelectric peltier devices (TEC 127 cpl, 12 amp; Custom Thermoelectric, Bishopville, MD, USA) to generate heat or cold (depending on the direction of current) on either end. In order to create a thermal gradient along the aluminum rod, one end was heated and the other was cooled using specific current settings. The peltier devices were connected to a DC voltage supply (0–12 Volt; WEP DC. Power Supply PS 305-D). The entire thermal gradient assay region was encapsulated using a polycarbonate tube (58.42 cm in length; 6.35 cm in diameter) and no exchange of air was allowed. Peltier devices were mounted over a heat sink (HSB6; Custom Thermoelectric, Bishopville, MD, USA) for temperature dispersion. In addition, both ends of the setup were placed in a circulating water bath in which the water level was kept to half the height of the heat sink. To create the hot (32°C) and cold ends (12°C) of the apparatus, 1.5 and 3 amps of current were allowed to pass through the peltier devices at the hot and cold ends, respectively. We then calibrated the setup for temperature stability along the gradient.
Figure 1.
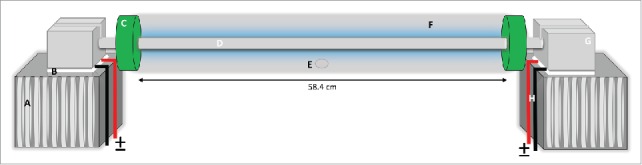
Artistic rendition of the thermal gradient design. At either end of the aluminum rod, peltier devices were connected and mounted over heat sinks, which were then submerged in circulating water baths. The aluminum rod was encased in a polycarbonate tube and sealed using docking adapters. The desired thermal gradient was created by adjusting the power input to the peltier devices. A=heat sink; B = peltier device; C = polycarbonate tube adapter-connector; D = aluminum rod; E = fly loading aperture; F = plexiglas tube around aluminum rod; G = supporting aluminum adapter block over the peltier device; H = peltier device power cables. For details see methods section. Temperature probes were inserted at following locations- 2.6, 11.7, 20.4, 29.2, 38, 46.7, and 55.8 cm. The diameter of the polycarbonate tube was 6.5 cm. Actual image of apparatus is provided as supplementary file (Supporting file 1).
Calibration and temperature along the thermal gradient
A total of 7 thermal probes (Fine Gauge Wire Probe; Wire Dia 0.01″, 0.024″ with insulation; ThermoWorks, Utah, USA) were inserted along the thermal gradient tube (C3, C2, & C1, Mid-Point, H1, H2, H3) where C3 is the cold end and H3 is the hot end of the tube (see Fig. 2). Once the temperature gradient is established in the apparatus (approximately 30 minutes for a thermal range of 12–32°C), it is constant over >50 min. Supporting file 2 and Supporting file 3 describe data on temperature stability and repeatability along the thermal gradient (Fig. 3). Supporting file 2 contains data on aluminum rod temperatures whereas Supporting File 3 gives data on mid-air temperatures, under the aluminum rod temperatures, and polycarbonate tube inner surface temperatures. The various analyses presented in this paper are based on the temperature range shown in Supporting file 2. Temperature measurements were recorded for every second using an 8 channel thermocouple temperature recorder (OctTemp2000; ThermoWorks, Utah, USA). Our experimental assays were conducted over a period of 20 min following establishment of the thermal gradient.
Figure 2.
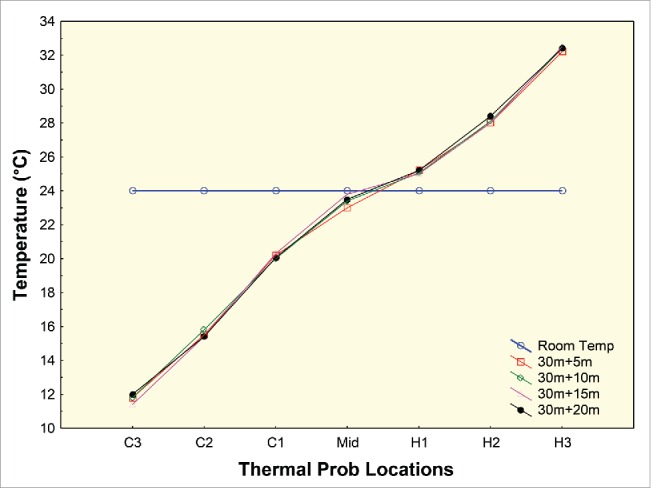
The thermal gradient temperature calibration over the duration of the behavioral assays. Temperature probe locations are indicated as C3, C2, C1, Midpoint, H1, H2, H3 (ordered from lower to higher temperature; temperature range for the assays was 12°C – 32°C). The solid black line indicates temperature in the assay chamber when power is off and the thermal gradient is absent. Once power is turned on, establishment of the temperature gradient takes 30 minutes. The thin and dashed lines indicate subsequent temperature across the apparatus during next 20 minutes in 5 minute intervals (30 m+5 m, 30 m+10 m, 30+15 m and 30 m+20 m). A Lowess fit was performed to smooth the curves. This method uses locally weighted linear regression to smooth data.
Figure 3.
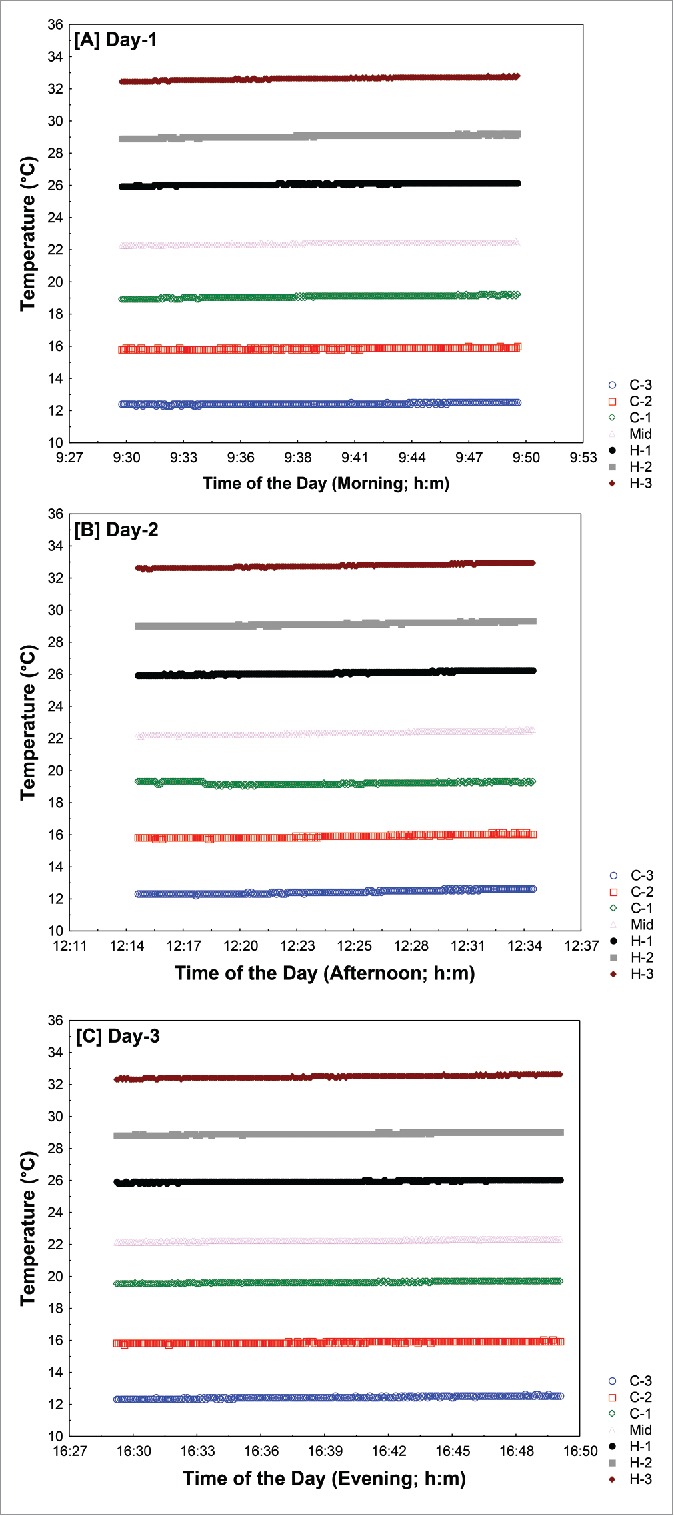
Measure of temperature repeatability along the thermal gradient. Temperature probe locations are indicated as C3, C2, C1, Mid, H1, H2, H3 (ordered from lower to higher temperature; see Fig. 2 also). Temperature along the thermal gradient was collected for 3 different days in the morning (A), in the afternoon (B) and in the evening (C). Different colors correspond to different probes.
Stocks and husbandry
In this study we used 3 natural populations collected from orchards located along the latitudinal gradient of the east coast of the USA (Homestead, Florida 25.46 N, 80.45 W; Media, Pennsylvania 40.04 N, 76.30 W; Bowdoin, Maine 44.03 N, 73.20 W; Fig. 4). We collected gravid females from the above listed locations in September 2012 by direct aspiration on wind fallen fruit. Individual isofemale lines were established in the field on standard cornmeal-molasses medium; species identity was confirmed by examining resulting male progeny (as described earlier).53 Isofemale lines were maintained in narrow Drosophila culture vials on a regular corn-agar-molasses media at room temperature. All the collections described above were maintained under common garden conditions for several generations before using them for various experimental purposes.
Figure 4.
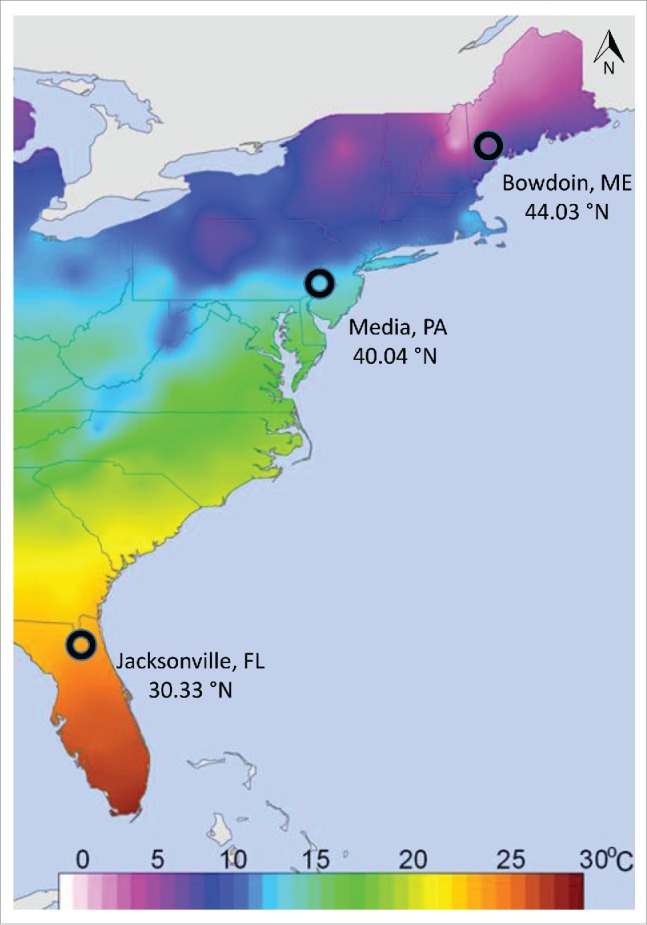
Temperature map of the east coast of the USA. The three collection sites for natural D. melanogaster populations are denoted by circles (Jacksonville, FL 30.33 °N, 81.66 °W; Media, PA 40.04 °N, 76.30 °W; Bowdoin, ME 44.03 °N, 73.20 °W). To study sex differences in thermal preference the Media, PA populations were used. Thermal plasticity experiments were performed on Jacksonville, FL and Bowdoin, ME populations only.
Establishment of population cages
To establish natural population cages we pooled 25–30 isofemale lines in 12 × 12 × 12 inch insect enclosures (Live Monarch Foundation, Boca Raton, Florida, USA). We created 2 cages for each of the geographical locations (Florida, Pennsylvania, and Maine). Each cage was created using independent sets of isofemale lines, thus establishing biological replicates for each population. Ten mated females from each line were collected and pooled in above specified cages, and females were subsequently allowed to oviposit in regular Drosophila culture bottles to establish the next generation. Flies were allowed to eclose over a period of one week and then provided with fresh media bottles; after a period of 3 d for oviposition, culture bottles were capped, progeny allowed to develop and eclose, and adults from the preceding generation discarded. Each cage was maintained for 5 generations to allow outcrossing among lines, after which time experimental samples were collected for the various assays (described below).
Experiments
We performed 3 separate experiments in this study to evaluate thermal behavior: the first examined the effects of sex, the second the effects of geographic origin of the source populations, and the third the effects of developmental temperature. We used the mid-latitudinal Pennsylvania population to examine sex-related differences for thermal preference, and the Florida and Maine populations to examine geographical differences and thermal plasticity.
To study sex related differences in thermal behavior we exposed a mid-latitude Pennsylvania population to the thermal gradient. For this experiment we placed 20 regular narrow size Drosophila culture vials in the replicate Pennsylvania population cages for a period of 4 hours. After 4 hr vials with eggs were removed and controlled for density (30–40 eggs per vial). Vials were immediately transferred to a 25°C incubator under a 12L:12D photoperiod regime. On emergence virgin males and females were collected using mild CO2 anesthesia and aged for 4–5 days before subjecting them to the thermal gradient tube to study thermal preference. All the assays were run in the morning hours (9 am – 12 noon). Sexes were run separately. This eliminated any reproductive interactions between males and females (e.g. time spent in courtship, pheromonal mediation of behavior). In order to remove pheromonal artifacts, after each replicate run we thoroughly cleaned the thermal gradient apparatus components (aluminum rod, adapters, and polycarbonate tube) that directly came in contact with flies. A total of 3 experimental assays were performed for each replicate cage (N = 6 trials per sex).
Geographical variation in thermal behavior was examined using the populations derived from Maine and Florida, which represent the climatic extremes on the US east coast. The experimental procedures conducted were as described above. In this experiment flies from the 2 geographic populations (ME and FL) were released together and a total of 3 experimental runs were conducted for each replicate cage. We used different color fluorescent dust to mark flies (BioQuip Products, Inc. Gardena, California, USA), and dust colors were randomized among technical replicate runs.
In order to examine effects of developmental temperature on thermal behavior we cultured flies at 2 different temperatures (18°C and 25°C). On emergence, virgin males and females were collected and kept at the respective temperature until they attained 4–5 days age. The experimental procedures were the same as described in the preceding 2 paragraphs.
Sample loading
No anesthesia (CO2 gas) was used during preparations of samples and/or while releasing flies in the thermal gradient tube. For each run 150 males and females were released through the sample loading aperture using a 2 channel wide opening syringe (Lab crafted). All the behavioral assays were performed under dark conditions; the thermal gradient apparatus was kept inside a photo dark room. A camera (16 MP and 8x Optical Zoom; Canon) and a UV lamp (Model UV-5NF; 365/254 nm; Spectroline.com) were placed over the tube to take pictures under UV light. Experimental flies were released once a stable thermal gradient was achieved inside the polycarbonate tube. Flies were released in the center of the tube through a ¼ cm diameter opening, which was sealed using thermostable tape. We used fluorescent dust to mark flies (BioQuip Products, Inc. Gardena, California, USA). Just before the release, groups of 150 flies were transferred into a regular plastic Drosophila culture bottle with 0.0020 (±0.0005) g of fluorescent micronized dust and lightly shaken. Dust colors were randomly assigned, and assignments were changed between releases. No anesthesia was used over the last 3 days prior to experiments or during any of the subsequent transfers.
Data collection and statistics
Temperature along the gradient was linear. This allowed us to determine the temperature perceived by the fly on the gradient and thus create a thermal distribution. To facilitate data collection and analysis, we initially grouped the entire thermal gradient (C3, C2, C1, Mid-Point, H1, H2, H3) into 3 major habitat regions: 1) cold (C3 & C2), 2) medium (C1, Mid-point, & H1), and 3) hot (H2 & H3). This represents the coarsest level of resolution for thermally mediated behavior that could be generated using the apparatus.
Data for each replicate assay were collected in the form of digital pictures. Pilots were done taking pictures at multiple time points to ensure that thermal distributions of flies were stable (15, 20, 25, 30 min). We observed significantly stable dispersion across the measurement interval in all pilot assays as well as the replicate trials analyzed. For the experiments conducted in this study, pictures were taken 20 minutes after releasing the flies in the tube. Pictures of the entire thermal gradient were taken from top, lateral and bottom views in the presence of UV light without using a camera flash, so that only the fluorescent signature was captured. The UV lamp was turned on just for the time required for image acquisition. Images were viewed in WINDOWS image viewer application and flies were counted in each thermal region based on their dust color signatures.
For the statistical analyses, the thermal gradient was converted to 3 major temperature bins or thermal habitats (cold, medium, and hot). Categorical logistic regression was used to examine the effects of predictor variables (thermal habitat, developmental temperature, biological replicate, experimental replicate) on the log odds of sampling different categories of the response variable (sex, geographic origin). To examine the effects of temperature on difference in preferences between the sexes, we used thermal habitat as the predictor, modeling the log odds for females relative to males. To examine the effects of geographic origin, developmental temperature, and their interaction on thermal preference, we used these factors as predictors and modeled the log odds for flies from the temperate relative to the subtropical populations. Separate models were constructed for females and males. Categorical logistic regressions were implemented in JMP v.11 (SAS Institute, Cary, NC).
Results
In this work we describe a new horizontal design to measure thermal preference in smaller insects (Fig. 1). We evaluate the efficacy of this design using a case study in D. melanogaster. We collected data on sex differences, geographical variation and developmental plasticity-induced changes for thermal preferences in adults. We observed repeatable, quantifiable, and significant differences in thermal behavior at all 3 levels.
Calibration and repeatability
The stability of the temperature gradient along the tube, as well as the repeatability of the organismal distribution along the gradient, are crucial to this technique. The stability of the thermal gradient was examined using temperature-sensing probes inserted at different locations along the tube. Figure 2 depicts data on temperature along the thermal gradient for the 7 described probe locations. A highly significant correlation was observed between different probe locations (C3, C2, C1, Mid-Point, H1, H2, & H3) and observed temperature over the duration of 30 minutes (r=0.98; Fig. 2). This demonstrated that temperature along the thermal gradient was stable throughout the duration of the assay. To test the repeatability of thermal gradient temperature over diurnal and calendar time, we collected temperature data along the gradient on 3 different days; on each day, data were collected at different time points (morning, afternoon, and evening; Supporting File 2).
To test the stability and repeatability of fly distributions across the gradient, we examined thermal distributions across independent trials for both males and females; 3 assays were conducted per replicate cage for each sex, and were done at different times and on different days. For this experiment we used flies from a mid-latitude population (Fig. 4). Figure 5 shows the distribution of males and females in each of the 6 replicate trials across the 7 thermal zones. One -way ANOVAs were used to measure variation within and between groups, and demonstrated high repeatability in distribution of flies along the gradient (Supporting file 4)
Figure 5.
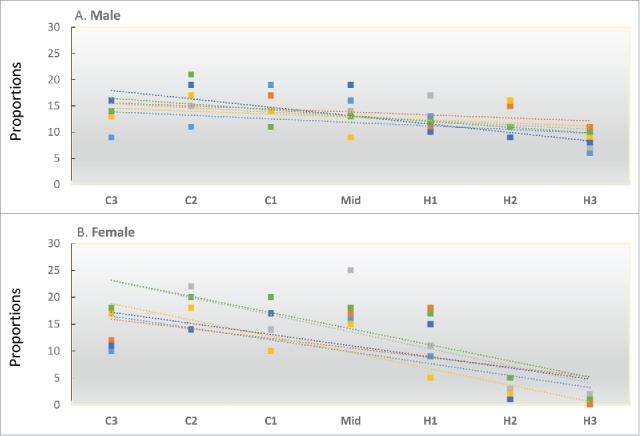
Measure of fly distribution repeatability along the gradient. Six independent runs were performed for males (A) and females (B) separately over the experimental period of 3 days. Different colors correspond to different runs. Unidirectional and overlapping regression lines for 6 independent runs clearly demonstrated high repeatability in fly distribution along the thermal gradient.
Sex-specific differences in temperature sensing
Figure 6 depicts distribution of males and females in the 3 different thermal zones. Both males and females tended to stay in the portion of the apparatus that was characterized by intermediate temperatures (20–24°C). However, thermal habitat had a significant effect on the relative distribution of females and males in the thermal assay (Table 1). Relative to males, females were more likely to be found in the cold habitats and less likely to be observed in the hot thermal habitats (Table 2). No significant effects were observed for biological replicate cages or technical replicate assays, again demonstrating the consistency and stability of organismal distributions across the thermal gradient.
Figure 6.
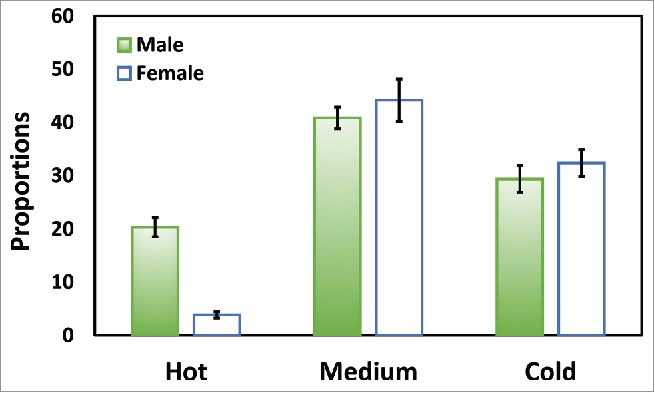
Dispersion of male (filled) and female (open) D. melanogaster along the thermal gradient (T range: 12–32°C). Relative to males, females were more behaviorally sensitive to higher temperatures. Data were presented as means.
Table 1.
Effect likelihood ratio tests from the nominal logistic regression of the effects of thermal habitat, biological replicate, and technical replicates on the log odds of females/males. Significant at p≤0.05.
| Source | Parameters | d.f. | ChiSquare | Prob>ChiSq |
|---|---|---|---|---|
| Habitat | 2 | 2 | 71.4870162 | <0.0001* |
| Biol. Repl. | 1 | 1 | 0.00272406 | 0.9584 |
| Replicate [Biol. Rep.] | 4 | 4 | 4.45921506 | 0.3474 |
Table 2.
Odd Ratios for odds of sampling females relative to males, comparing level 1 to level 2. Significant at p≤0.05.
| Level 1 | /Level 2 | Odds Ratio | Prob>Chisq |
|---|---|---|---|
| hot | cold | 0.172 | <.0001* |
| hot | medium | 0.174 | <.0001* |
| medium | cold | 0.984 | <.910 |
Geographic and developmental variation in thermal behavior
For both females and males, thermal habitat had a significant effect on the relative dispersion of flies from the temperate relative to the subtropical populations (Table 3). Flies from the northern, temperate population were more likely to be observed in the cold thermal habitat relative to flies from the southern, subtropical population; similarly, the log odds of sampling a fly from the temperate relative to the subtropical population were significantly lower in the hot thermal habitat (Table 4). Developmental rearing temperature did not have a significant main effect for either male or female thermal preferences, but the interaction between culture temperature and geographic origin was significant for both sexes. These relative preferences are plotted in Figure 7, which depicts relative abundance in the hot and cold thermal habitats. For both males and females, geographic differences in relative thermal preference were exacerbated when flies were cultured at 18°C, and differences reduced when cultured at 25°C.
Table 3.
Effect likelihood ratio tests from the nominal logistic regression of the effects of the predictors on the log odds of temperate vs. subtropical populations. Sexes were analyzed separately. Significant at p≤0.05.
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Source | Parameters | d.f. | ChiSquare | Prob>ChiSq | ChiSquare | Prob>ChiSq |
| Habitat | 2 | 2 | 39.7270409 | <0.0001* | 10.038799 | 0.0398* |
| Dev. Temp. | 1 | 1 | 0.50744842 | 0.4762 | 0.0074357 | 0.9313 |
| Dev. Temp. x Habitat | 2 | 2 | 56.1973429 | <0.0001* | 8.52534512 | 0.0141* |
| Biol. Repl. | 1 | 1 | 0.29482696 | 0.5871 | 3.36242047 | 0.0667 |
| Replicate [Biol. Repl.] | 4 | 4 | 0.88699223 | 0.9264 | 65.7359944 | <0.0001 |
Table 4.
Odd Ratios for odds of sampling temperate relative to subtropical populations, comparing level 1 to level 2. Significant at p≤0.05.
| Sex | Level1 | /Level2 | Odds Ratio | Prob>Chisq |
|---|---|---|---|---|
| Male | hot | cold | 0.30 | <.0001* |
| medium | cold | 0.72 | 0.0064* | |
| medium | hot | 2.38 | <.0001* | |
| Female | hot | cold | 0.13 | <0001* |
| medium | cold | 0.55 | <.0001* | |
| medium | hot | 4.19 | <0001* |
Figure 7.
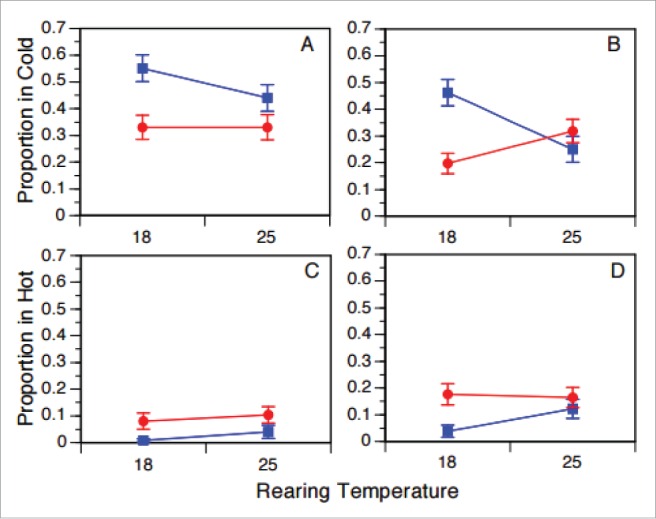
The plots depict the proportion of flies in the cold habitat (panels A, B) and the hot habitat (C, D) of the thermal gradient when reared under culture temperatures of 18°C and 25°C. Females are shown in panels A and C, males in panels B and D. The blue lines/rectangles depict the temperate (ME) populations and the red lines/circles denote the subtropical (FL) populations. Plotted are means.
As with the analysis of sex, the geographical patterns of response to thermal variation were robust across both technical and biological replicates; this is evident from the statistical analysis, which includes both levels of replication as predictors (Tables 3, 4). The two levels of replication address repeatability of the assays and stability of the fly distributions over time. Furthermore, the patterns of thermal preference observed at all 3 levels (gender, geography, rearing temperature) are fully consistent with a priori predictions based on the extensive information available for D. melanogaster; the method is therefore also robust with respect to biological interpretation in this ecological, evolutionary, and plasticity context. Our context-based validation clearly demonstrates the effectiveness of the apparatus and method.
Discussion
Ectotherm body temperature changes rapidly in response to environmental temperature. It makes thermoregulation challenging for them particularly in smaller size insects like D. melanogaster. Several efforts have been made to understand this complex regulatory mechanism.33-41,54
With our method and thermal gradient apparatus, we demonstrated that there is significant variation within and among populations in thermal preference, and that this varies predictably with geography: flies from high latitude, temperate origins avoided high temperatures and preferred cool temperatures, whereas flies from low latitude, subtropical populations avoided cold temperatures and exhibited greater relative preference for higher temperatures. Given the biological and technical validation, we propose that this new method and thermal-gradient apparatus can be used to examine thermal preferences in natural populations of Drosophila and other insect taxa of similar size with free living adult stages (mosquitos, wasps etc.). This method could also be used in screening of mutants and naturally occurring allelic variants at candidate genes in a variety of organisms, as well as the identification of genes underlying fundamental aspects of thermal behavior.
Thermal dependence of motion has largely been ignored in analyses of thermal behavior of small ectotherms. 55 It is key to assess thermal behavior along a temperature gradient. Neglecting this could lead to misinterpretations to thermal preference data.29,56-59 Therefore, actual distribution of animals along a thermal gradient can only be evaluated with the combination of thermal preference and thermal dependence of motion. The design of the apparatus and methodology presented here allows a 3 dimensional space for movement and clearly avoids even 2-dimensional patch model constraints (Fig. 1). 60,61 Our design satisfies all requirements for a new method including thermal stability along the gradient, stability of the thermal gradient across diurnal and calendar time, and repeatability of distribution of flies across thermal habitats (see Figs. 2, 3, and 5) This design also satisfies the recommendations of Dillon et al.,56 which explicitly incorporates the effects of the thermal dependence of motion on estimates of thermal preference.
Males and females exhibited significant differences in thermal preference in natural populations of D. melanogaster (Fig. 6; Table 1). Differences between the sexes were most pronounced in the hot zone of the thermal gradient rather than the cold zone, suggesting that males are more stable with respect to thermal behavior across a wider thermal range than expected (Table 2). However, males go sterile at temperatures greater than 32°C. 62 One possible explanation for female avoidance of high temperature regions may be the selection of suitable oviposition substrates based on temperature: high temperature habitats/substrates could compromise subsequent larval performance and survivorship, and thus thermally mediated behavior could have a direct effect on individual fitness. This hypothesis is supported by earlier work. Feder 63 reported that a full size decomposing apple under direct sun light can reach temperatures as high as 48°C. Further, his work suggested that females clearly avoided decomposing fruit characterized by elevated temperature. Females can detect and avoid oviposition sites that are warm at the time of oviposition.63-65 This indicates that temperature can be a significant stress for the resultant offspring and can result in the evolution of stress tolerance.63
Temperate D. melanogaster populations tended to avoid the hot end of the thermal gradient whereas subtropical populations tended to avoid the cold end (Fig. 7; Table 3 and 4). These differences were comparatively more significant in populations grown at lower temperatures (18°C) than higher temperatures (25°C). Merging both the trends clearly indicated the presence of genetic variation and plasticity for this trait in natural populations. Furthermore, the behavioral response of the temperate and subtropical populations matches the temperature profiles experienced in their natural habitats (Fig. 8). It remains to be tested whether the phenotypic variation observed here may be driven, in part, by naturally occurring allelic variation segregating at candidate genes such as those encoding trp channels. It would be of particular interest to extend the methodology described here to experimental manipulation of specific genes and molecular variants in Drosophila and other taxa.
Figure 8.
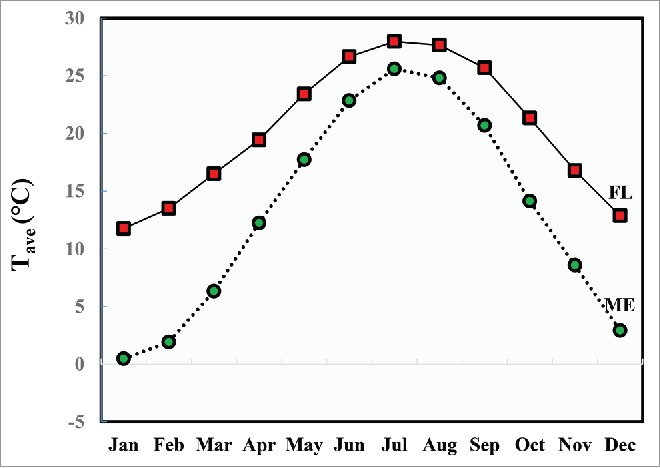
Twelve-month average temperature patterns for the sites of origin for the assayed populations. Jacksonville (30.33 N), and Bowdoin (44.03 N) station Tave is shown in solid (with rectangles), and dotted (with circles) lines respectively. Projections displayed here are the averages of 30 years (1980–2010). Climatic data was obtained from National Oceanic & Atmospheric Administration (NOAA; www.ncdc.noaa.gov).
In geographical populations of various Drosophila species, studies have been done to assess latitudinal and altitudinal variation in traits directly related to temperature stress, such as heat knockdown and chill-coma recovery. For example, in Australia and India, D. melanogaster populations living at higher latitudes recover more quickly from chill-coma than populations living in lower latitudes whereas the opposite was observed for heat-knockdown. 26 In India, similar patterns were observed in heat knockdown and chill-coma recovery in D. ananassae. 66 Drosophila species show clear physiological climatic limits and exploit a range of habitats that vary in temperature and associated conditions. 24 Our study supports the hypothesis that stress and behavioral responses are physiologically linked. Our data and analyses suggest that behavior is another component of the adaptive response to temperature/climate and should be quantified and examined in a variety of contexts. Such analysis of thermal behavior may also be essential in the assessment of performance and fitness connections among different types of traits. We suggest that incorporating behavioral aspects of fitness could facilitate the comprehensive evaluation of the adaptive response to climate and climate change in ectotherms.
Conclusions
The method described here in this study is highly consistent across both technical and biological replication, and is effective at discerning predicted behavioral differences in thermal preference associated with sex, geographic origin, and developmental rearing temperature. Although the molecular genetic basis of geographical variation for complex phenotypes is poorly understood, genome sequencing of natural populations may identify variants and regions of the genome that respond non-randomly to environmental gradients; such studies may identify candidate genes that underlie variation in temperature sensation/behavior in natural populations.67,68 A simple and standardized methodology for the evaluation of thermal sensation and behavior, such as the one presented here, would be invaluable in assessing the functional significance of identified genes and variants on thermal behavior in natural populations.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Frank van Bruklin, University of Nevada, Las Vegas for his valuable comments on the design. We would also like to thank Fred Letterio and Mike Suplick from the University of Pennsylvania Machine Shop, Philadelphia, USA for their help in crafting some of the parts of the design.
Funding
The funding for this work was supported by the National Institute of Health grant NIH RO1GM100366.
Author contributions
PSS and SR conceived the idea. SR designed the thermal gradient apparatus. SR executed experiments and collected data. PSS and SR analyzed data. SR and PSS wrote the manuscript.
References
- [1].Spicer JI, Gaston KJ. Physiological diversity and its ecological implications. Oxford: Blackwell Science; 1999. [Google Scholar]
- [2].Klok CJ, Chown SL, Gaston KJ. The geographical range structure of the Holly Leaf-miner. III. Cold hardiness physiology. Funct Ecol 2003; 17:858-68; http://dx.doi.org/ 10.1111/j.1365-2435.2003.00794.x [DOI] [Google Scholar]
- [3].Ray C. The application of Bergmann's and Allen's rules to the poikilotherms. J Morphol 1960; 106:85-108; PMID:14436612; http://dx.doi.org/ 10.1002/jmor.1051060104 [DOI] [PubMed] [Google Scholar]
- [4].Fleming IA, Gross MT. Latitudinal clines: a tradeoff between egg number and size in Pacific Salmon. Ecology 1990; 71:1-11; http://dx.doi.org/ 10.2307/1940241 [DOI] [Google Scholar]
- [5].Green DM, Sharbel TF, Kearsley J, Kaiser H. Postglacial range fluctuation, genetic subdivision and speciation in the western North American spotted frog complex, Rana pretiosa. Evolution 1996; 50:374-90; http://dx.doi.org/ 10.2307/2410808 [DOI] [PubMed] [Google Scholar]
- [6].Chown SL, Gaston KJ. Exploring links between physiology and ecology at macro-scales: The role of respiratory metabolism in insects. Biol Rev 1999; 74:87-120; http://dx.doi.org/ 10.1017/S000632319800526X [DOI] [Google Scholar]
- [7].Jonas CS, Geber MA. Variation among populations of Clarkia unguiculata (Onagraceae) along altitudinal and latitudinal gradients. Am J Bot 1999; 86:333-43; PMID:10077496; http://dx.doi.org/ 10.2307/2656755 [DOI] [PubMed] [Google Scholar]
- [8].Jenkins AR. Hockey PAR. Prey availability influences habitat tolerance: an explanation for the rarity of Peregrine Falcons in the tropics. Ecography 2001; 24:359-67; http://dx.doi.org/ 10.1111/j.1600-0587.2001.tb00209.x [DOI] [Google Scholar]
- [9].Weeks AR, McKechnie SW, Hoffmann AA. Dissecting adaptive clinal variation: markers, inversions and size/stress associations in Drosophila melanogaster from a central field population. Ecol Lett 2002; 5:756-63; http://dx.doi.org/ 10.1046/j.1461-0248.2002.00380.x [DOI] [Google Scholar]
- [10].Hutchison DW. Testing the central/peripheral model: analyses of microsatellite variability in the eastern collared lizard (Crotaphytus collaris collaris). Am Midland Nat 2003; 149:148-62; http://dx.doi.org/ 10.1674/0003-0031(2003)149%5b0148:TTCPMA%5d2.0.CO;2 [DOI] [Google Scholar]
- [11].Blanckenhorn WU, Demont M. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr Comp Biol 2004; 44:413-24; PMID:21676727; http://dx.doi.org/ 10.1093/icb/44.6.413 [DOI] [PubMed] [Google Scholar]
- [12].Halsall M, Walters RJ, Telfer M, Hassall MRJ. Why does a grasshopper have fewer, larger offspring at its range limits? J Evol Biol 2005; 19:267-76; http://dx.doi.org/ 10.1111/j.1420-9101.2005.00967.x [DOI] [PubMed] [Google Scholar]
- [13].Heibo E, Magnhagen C, Vøllestad LA. Latitudinal variation in life-history traits in Eurasian perch. Ecology 2005; 86:3377-86; http://dx.doi.org/ 10.1890/04-1620 [DOI] [Google Scholar]
- [14].Cooper CB, Hochachka WM, Phillips TB, Dhondt AA. Geographical and seasonal gradients in hatching failure in Eastern Bluebirds Sialia sialis reinforce clutch size trends. IBIS 2006; 148:221-30; http://dx.doi.org/ 10.1111/j.1474-919X.2006.00500.x [DOI] [Google Scholar]
- [15].Collinge JE, Hoffmann AA, McKechnie SW. Altitudinal patterns for latitudinally varying traits and polymorphic markers in Drosophila melanogaster from eastern Australia. J Evol Biol 2006; 19:473-82; PMID:16599923; http://dx.doi.org/ 10.1111/j.1420-9101.2005.01016.x [DOI] [PubMed] [Google Scholar]
- [16].Rajpurohit S, Nedved O. Clinal variation in fitness related traits in tropical drosophilids of the Indian subcontinent. J Ther Biol 2013; 38:345-54; http://dx.doi.org/ 10.1016/j.jtherbio.2013.04.004 [DOI] [Google Scholar]
- [17].Karan D, Dahiya N, Munjal AK, Gibert P, Moreteau B, Parkash R, David JR. Desiccation and starvation tolerance of adult Drosophila: opposite latitudinal clines in natural populations of three different species. Evolution 1998; 52:825-31; http://dx.doi.org/ 10.2307/2411276 [DOI] [PubMed] [Google Scholar]
- [18].Hoffmann AA, Shirriffs J, Scott M. Relative importance of plastic vs genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct Ecol 2005; 19:222-7; http://dx.doi.org/ 10.1111/j.1365-2435.2005.00959.x [DOI] [Google Scholar]
- [19].Gilchrist GW, Jeffers LM, West B, Folk DG, Suess J, Huey RB. Clinal patterns of desiccation and starvation resistance in ancestral and invading populations of Drosophila subobscura. Evol Appl 2008; 1:513-23; PMID:25567732; http://dx.doi.org/ 10.1111/j.1752-4571.2008.00040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Frentiu FD, Yuan F, Savage WK, Bernard GD, Mullen SP, Briscoe AD. Opsin clines in butterflies suggest novel roles for insect photopigments. Mol Biol Evol 2014; 32:368-79; PMID:25371434; http://dx.doi.org/ 10.1093/molbev/msu304 [DOI] [PubMed] [Google Scholar]
- [21].Campo D, Lehmann K, Fjeldsted C, Souaiaia T, Kao J, Nuzhdin SV. Whole-genome sequencing of two North American Drosophila melanogaster populations reveals genetic differentiation and positive selection. Mol Ecol 2013; 22:5084-97; PMID:24102956; http://dx.doi.org/ 10.1111/mec.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bergland AO, Tobler R, Gonzalez J, Schmidt P, Petrov D. Secondary contact and local adaptation contribute to genome-wide patterns of clinal variation in Drosophila melanogaster. Mol Ecol 2015; 25(5):1157-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gaston KJ, Chown SL, Evans KL. Ecogeographical rules: elements of a synthesis. J Biogeogr 2008; 35:483-500; http://dx.doi.org/ 10.1111/j.1365-2699.2007.01772.x [DOI] [Google Scholar]
- [24].Hoffmann AA. Physiological climatic limits in Drosophila: patterns and implications. J Exp Biol 2010; 213:870-80; PMID:20190112; http://dx.doi.org/ 10.1242/jeb.037630 [DOI] [PubMed] [Google Scholar]
- [25].Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. Predicting organismal vulnerability to climate warming: roles of behavior, physiology and adaptation. Philos Trans Royal Soc B: Biol Sci 2012; 367:1665-79; http://dx.doi.org/ 10.1098/rstb.2012.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoffmann AA, Anderson AR, Hallas R. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett 2002; 5:614-8; http://dx.doi.org/ 10.1046/j.1461-0248.2002.00367.x [DOI] [Google Scholar]
- [27].Parkash R, Sharma V, Kalra B. Correlated changes in thermotolerance traits and body color phenotypes in montane populations of Drosophila melanogaster: analysis of within-and between-population variations. J Zool 2009; 280:49-59; http://dx.doi.org/ 10.1111/j.1469-7998.2009.00641.x [DOI] [Google Scholar]
- [28].Loeschcke V, Hoffmann AA. Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. Am Nat 2007; 169, 175-83; PMID:17211802; http://dx.doi.org/ 10.1086/510632 [DOI] [PubMed] [Google Scholar]
- [29].Angilleta MJ., Jr Thermal Adaptation: A Theoretical and Empirical Synthesis. New York: Oxford University Press, 2009. [Google Scholar]
- [30].Kingsolver JG, Watt WB. Thermoregulatory strategies in Colias butterflies: Thermal stress and the limits to adaptation in temporally varying environments. Am Nat 1983; 121:32-55; http://dx.doi.org/ 10.1086/284038 [DOI] [Google Scholar]
- [31].Kingsolver JG, Buckley LB. Climate variability slows evolutionary responses of Colias butterflies to recent climate change. Proc Royal Soc B 2015; 282:20142470; http://dx.doi.org/ 10.1098/rspb.2014.2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Denlinger DL, Richard EL Jr. Low Temperature Biology of Insects. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- [33].Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 1975; 72, 4061-5; http://dx.doi.org/ 10.1073/pnas.72.10.4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygro-sensation in Drosophila. Proc Natl Acad Sci U S A 1996; 93:6079-84; http://dx.doi.org/ 10.1073/pnas.93.12.6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev 2005; 19:419-24; PMID:15681611; http://dx.doi.org/ 10.1101/gad.1278205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Luo L, Clark DA, Biron D, Mahadevan L, Samuel ADT. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J Exp Biol 2006; 209:4652-62; PMID:17114399; http://dx.doi.org/ 10.1242/jeb.02590 [DOI] [PubMed] [Google Scholar]
- [37].Ito H, Inada H, Mori I. Quantitative analysis of thermotaxis in the nematode Caenorhabditis elegans. J Neurosci Methods 2006; 154:45-52; http://dx.doi.org/ 10.1016/j.jneumeth.2005.11.011 [DOI] [PubMed] [Google Scholar]
- [38].Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A 2008; 105:14668-73; http://dx.doi.org/ 10.1073/pnas.0805041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hong S-T, Bang S, Hyun S, Kang J, Jeong K, Paik D, Chung J, Kim J. cAMP signaling in mushroom bodies modulates temperature preference behavior in Drosophila. Nature 2008; 454:771-5; PMID:18594510 [DOI] [PubMed] [Google Scholar]
- [40].Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008; 454:217-20; PMID:18548007; http://dx.doi.org/ 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev 2010; 24:2365-82; PMID:21041406; http://dx.doi.org/ 10.1101/gad.1953710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Umina PA, Weeks AR, Kearney MR, Mckechnie SW, Hoffmann AA. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 2005; 308, 691-3; PMID:15860627; http://dx.doi.org/ 10.1126/science.1109523 [DOI] [PubMed] [Google Scholar]
- [43].Rajpurohit S, Parkash R, Singh S, Ramniwas S. Climate change, boundary increase and elongation of a pre-existing cline: A case study in Drosophila ananassae. Entomolog Res 2008; 38:268-75; http://dx.doi.org/ 10.1111/j.1748-5967.2008.00186.x [DOI] [Google Scholar]
- [44].Oakeshott JG, Wilson SR, Gibson JB. An attempt to measure selection coefficients affecting the alcohol dehydrogenase polymorphism in Drosophila melanogaster populations maintained on ethanol media. Genetics 1983; 61:151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paaby AB, Blacket MJ, Hoffmann AA, Schmidt PS. Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol Ecol 2010; 19:760-74; PMID:20074316; http://dx.doi.org/ 10.1111/j.1365-294X.2009.04508.x [DOI] [PubMed] [Google Scholar]
- [46].Parkash R, Munjal AK. Phenotypic variability of thoracic pigmentation in Indian populations of Drosophila melanogaster. J Zool Syst Evol Res 1999; 37:133-40; http://dx.doi.org/ 10.1111/j.1439-0469.1999.tb00975.x [DOI] [Google Scholar]
- [47].Rajpurohit S, Nedved O, Gibbs AG. Meta-analysis of geographical clines in desiccation tolerance of Indian drosophilids. Comp Biochem Physiol, Part A 2013; 164:391-8; http://dx.doi.org/ 10.1016/j.cbpa.2012.11.013 [DOI] [PubMed] [Google Scholar]
- [48].Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci U S A 2001; 98:14509-11; http://dx.doi.org/ 10.1073/pnas.241391498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chahal J, Kataria SK, Parkash R. Invasion and adaptation of a warm-adapted species to montane localities: effect of acclimation potential. J Exp Biol 2013; 216:1578-86; PMID:23348937; http://dx.doi.org/ 10.1242/jeb.080200 [DOI] [PubMed] [Google Scholar]
- [50].Parkash R, Sharma V, Kalra B. Correlated changes in thermotolerance traits and body color phenotypes in montane populations of Drosophila melanogaster: analysis of within-and between-population variations. J Zool 2010; 280:49-59; http://dx.doi.org/ 10.1111/j.1469-7998.2009.00641.x [DOI] [Google Scholar]
- [51].Fischer K, Karl I. Exploring plastic and genetic responses to temperature variation using ccopper butterflies. Climate Res 2010; 43:17-30; http://dx.doi.org/ 10.3354/cr00892 [DOI] [Google Scholar]
- [52].Overgaard J, Kearney MR, Hoffmann AA. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Global Change Biol 2014; 20:1738-50; PMID:24549716; http://dx.doi.org/ 10.1111/gcb.12521 [DOI] [PubMed] [Google Scholar]
- [53].Schmidt PS, Matzkin LM, Ippolito M, Eanes W. Geographic variation in diapause incidence, life history traits and climatic adaptation in Drosophila melanogaster. Evolution 2005; 59:1721-32; PMID:16331839; http://dx.doi.org/ 10.1111/j.0014-3820.2005.tb01821.x [DOI] [PubMed] [Google Scholar]
- [54].Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci 2013; 92:394-403; http://dx.doi.org/ 10.1016/j.lfs.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dillon ME, Rongsong L, Wang G, Huey RB. Disentangling thermal preference and the thermal dependence of movement in ectotherms. J Ther Biol 2012; 37:631-9; http://dx.doi.org/ 10.1016/j.jtherbio.2012.07.004 [DOI] [Google Scholar]
- [56].Hong S-T, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 2006; 26:7245-56; PMID:16822982; http://dx.doi.org/ 10.1523/JNEUROSCI.5426-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yamada Y, Ohshima Y. Distribution and movement of Caenorhabditis elegans on a thermal gradient. J Exp Biol 2003; 206:2581-93; PMID:12819265; http://dx.doi.org/ 10.1242/jeb.00477 [DOI] [PubMed] [Google Scholar]
- [58].Heath J. Reptilian thermoregulation: evaluation of field studies. Science 1964; 146:784; PMID:14197571; http://dx.doi.org/ 10.1126/science.146.3645.784 [DOI] [PubMed] [Google Scholar]
- [59].Hertz PE, Huey RB, Stevenson RD. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am Nat 1993; 142:796-818; PMID:19425957; http://dx.doi.org/ 10.1086/285573 [DOI] [PubMed] [Google Scholar]
- [60].Gustafson E, Gardner R. The effect of landscape heterogeneity on the probability of patch colonization. Ecology 1996; 77:94-107; http://dx.doi.org/ 10.2307/2265659 [DOI] [Google Scholar]
- [61].Hanski I. Metapopulation dynamics. Nature 1998; 396:41-9; http://dx.doi.org/ 10.1038/23876 [DOI] [Google Scholar]
- [62].David JR. Male sterility at high and low temperatures in Drosophila. J de la Societe de biologie 2008; 202:113-7; PMID:18547508; http://dx.doi.org/ 10.1051/jbio:2008014 [DOI] [PubMed] [Google Scholar]
- [63].Feder ME. Necrotic fruit: A novel model system for thermal ecologists. J Ther Biol 1997; 22:1-9; http://dx.doi.org/ 10.1016/S0306-4565(96)00028-9 [DOI] [Google Scholar]
- [64].Fogleman JC. Oviposition site preference for substrate temperature in Drosophila melanogaster. Behav Genet 1979; 9:407-12; PMID:120186; http://dx.doi.org/ 10.1007/BF01066978 [DOI] [PubMed] [Google Scholar]
- [65].Schnebel EM, Grossfield J. Oviposition temperature-range in 4 Drosophila species triads from different ecological backgrounds. Am Midland Nat 1986; 116:25-35; http://dx.doi.org/ 10.2307/2425934 [DOI] [PubMed] [Google Scholar]
- [66].Sisodia S, Singh BN. Resistance to environmental stress in Drosophila ananassae: latitudinal variation and adaptation among populations. J Evol Biol 2010; 23:1979-88; PMID:20695963; http://dx.doi.org/ 10.1111/j.1420-9101.2010.02061.x [DOI] [PubMed] [Google Scholar]
- [67].Fabian DK, Kapun M, Nolte V, Kofler R, Schmidt PS, Schlotterer C, Flatt T. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol Ecol 2012; 21:4748-69; PMID:22913798; http://dx.doi.org/ 10.1111/j.1365-294X.2012.05731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bergland AO, Behrman EL, O'Brien KR, Schmidt PS, Petrov DA. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet 2014; 10(11):e1004775; PMID:25375361; http://dx.doi.org/ 10.1371/journal.pgen.1004775 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


