TO THE EDITOR
Schneckenbecken dysplasia [OMIM 269250] is an autosomal recessive, perinatal lethal skeletal dysplasia that was first characterized by [Borochowitz et al., 1986], and is phenotypically similar to affected individuals in a family described by Knowles et al. [1986]. Clinical findings include relative macrocephaly, a very flat midface, cleft palate, a short neck, a narrow thorax, brachydactyly, and a high incidence of polyhydramnios. The most characteristic radiographic abnormality, as viewed on an anterior-posterior radiograph, is a medial projection from the ilia that resembles a snail (thus snail pelvis or Schneckenbecken in German). Additional findings include handlebar clavicles, a hypoplastic scapula, generalized platyspondyly with small, rounded vertebral bodies, very short long bones with relatively widened metaphyses and precociously ossified carpal and tarsal bones [Borochowitz et al., 1986; Nikkels et al., 2001].
Schneckenbecken dysplasia has been classified among the severe spondylodysplastic disorders that also include the autosomal recessive disorders achondrogenesis type 1A [OMIM 200600], spondylometaphyseal dysplasia, Sedaghatian type [OMIM 250220], and opsismodysplasia [OMIM 258480] [Warman et al., 2011]. Mutations in SLC35D1 [OMIM 610804], the gene encoding the Solute Carrier Family 35 UDP-Glucuronic Acid/ UDP-N-Acetylgalactosamine Dual Transporter, Member D1, were found in 2 of 6 cases of Schneckenbecken dysplasia [Hiraoka et al., 2007]. In a subsequent report SLC35D1 mutations were identified in another three of five Schneckenbecken dysplasia cases but were absent among 15 cases of other severe spondylodysplastic skeletal dysplasias [Furuichi et al., 2009]. Most of the SLC35D1 mutations were predicted to lead to absence of the protein. SLC35D1 has been localized to the endoplasmic reticulum (ER) where it translocates two nucleotide sugars, UDP-glucuronic acid and UDP-N-acetylgalactosamine, into microsomal membrane vesicles to be used in the synthesis of chondroitin sulfate [Muraoka et al., 2001]. A mouse deficient for Slc35d1 showed a phenotype similar to Schneckenbecken dysplasia and had defective chondroitin sulfate synthesis on the core protein of cartilage proteoglycans [Hiraoka et al., 2007].
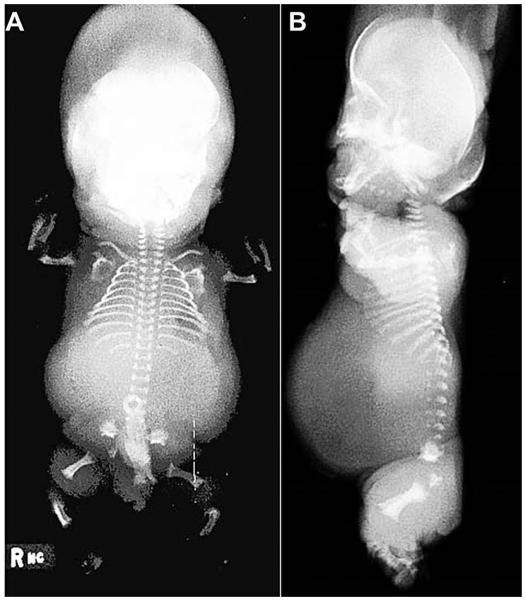
Under an approved human subjects protocol, exome sequence analysis using a previously described pipeline [Lee et al., 2012] was carried out in a case of Schneckenbecken dysplasia (International Skeletal Dysplasia Registry reference number R02–170) in which SLC35D1 was previously excluded (subject 4 in [Hiraoka et al., 2007]). The case presented as a 33 week gestation hydropic stillborn. The radiographic findings were characteristic for Schneckenbecken dysplasia and included midface hypoplasia, handlebar clavicles, short ribs, hypoplastic vertebrae with rounded anterior ends, short long bones with widened metaphyseal ends and a snail-like medial projection in the ileum (Fig. 1).
FIG. 1.
ISDR case R02-170, a 33 week stillborn with Schneckenbecken dysplasia. (A) AP full body radiograph showing a small thorax, micromelia, metaphyseal widening of the long bones, and characteristic snail-shaped iliac wings. (B) Lateral radiograph showing characteristic ovoid anterior vertebral body ossification with non-ossified posterior portions, as well as midface hypoplasia.
Analysis of the exome identified homozygosity for a variant, c.2415+1G>A in intron 21 of INPPL1 [OMIM 600829], which encodes Inositol Polyphosphate Phosphatase-like 1. The mutation predicts skipping of exon 21, which would lead to a frameshift and a premature termination codon. Recessively inherited mutations in INPPL1 also produce opsismodysplasia [OMIM 258480], another severe autosomal recessive spondylodysplastic disorder [Below et al., 2013; Huber et al., 2013]. Opsismodysplasia is generally non-lethal and is characterized by dysmorphic craniofacial features, a large anterior fontanelle, short stature and small hands and feet. Radiographic findings include very delayed bone maturation particularly at the knee, marked shortness of the metacarpals/ metatarsals and phalanges with metaphyseal cupping and markedly flat vertebral bodies [Cormier-Daire et al., 2003; Maroteaux et al., 1984]. Some affected individuals have severe renal phosphate wasting and there are variable degrees of respiratory insufficiency [Below et al., 2013] which may be secondary to the skeletal manifestations. A variety of other clinical findings have been described but an insufficient number of cases have been studied to know whether these are consistently associated with INPPL1 mutations. The c.2415+1G>A mutation found in the homozygous state in this case of Schneckenbecken dysplasia was also identified in a surviving opsismodysplasia case in compound heterozygosity with a frameshift mutation. Thus, apparent loss of INPPL1 function can produce either the perinatal lethal Schneckenbecken dysplasia phenotype or non-lethal opsismodysplasia.
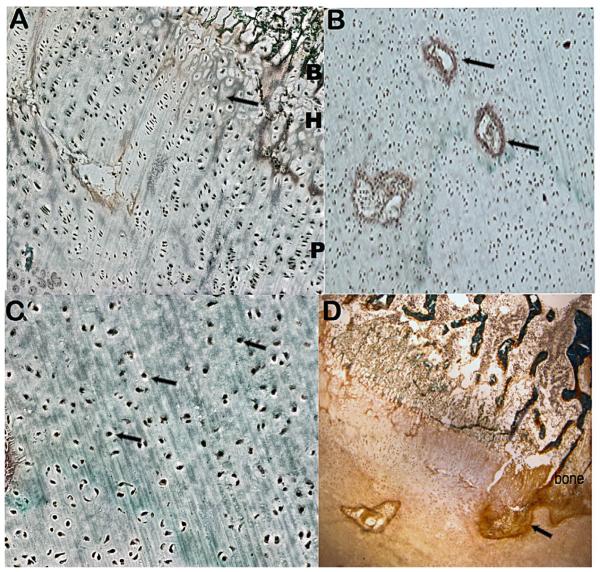
Histomorphology on the cartilage growth plate was performed by staining the tissue with toluidine blue and Goldner’s reagent. The findings included poor hypertrophic column formation (Fig. 2A) with fibrous septae between the columns, areas of hypercellularity with decreased extracellular matrix and hypervascularity (Fig. 2B), chondrocytes with central round nuclei (Fig. 2C), as well as thickened primary trabeculae and a fibrous band entering the growth plate from the bone collar (Fig. 2D). With the exception of the dense fibrous band in the growth plate, these findings have been previously described in Schneckenbecken dysplasia [Varkey and Jones, 2004], but the gene(s) involved were unknown. Similar findings were described in the Slc35d1 knockout mice as well as a Schneckenbecken dysplasia case due to defects in SLC35D1 [Hiraoka et al., 2007]. The histopathology in opsismodysplasia [Cormier-Daire et al., 2003] is similar, showing poorly organized growth plates, numerous vascular canals, increased chondrocyte density, and many round, ballooned chondrocytes and thickened trabeculae in the mineralized matrix. Thus the growth plate findings, while not necessarily pathognomonic for either Schneckenbecken dysplasia or opsismodysplasia, do show significant overlap. The only finding in this case that is distinct is the fibrous band that extends from the bone collar.
FIG. 2.
ISDR case R02-170 cartilage growth plate histology. (A) Cartilage growth plate (10X) showing poor hypertrophic column formation (arrow) with fibrous septae between the columns; B, bone, H, hypertrophic zone, P, proliferative zone. (B) Proliferative zone (10X) showing increased vascularity (arrows), decreased extracellular matrix and hypercellularity. (C) Reserve chondrocytes (40X) with arrows identifying chondrocytes with central round nuclei. (D) Cartilage stained with Goldner’s reagent showing a fibrous band at the distal end of the growth plate (arrow) and thickened trabeculae in the primary zone of ossification.
The similarities in the growth plates between this case of Schneckenbecken dysplasia due to homozygosity for an INPPL1 mutation and those due to SLC35D1 mutations could suggest possible mechanistic overlap. Defects in both genes could lead to reduced extracellular matrix synthesis and a hypercellular matrix containing chondrocytes with central nuclei. In cases due to SLC35D1 mutations, reduced post-translational modification of matrix proteoglycans appears to be the underlying abnormality. How defects in INPPL1 affect extracellular matrix production is unknown, but signaling cascades mediated by phosphatidylinositols including PI(3,4,5)P3 might contribute to matrix production and regulation.
The functional role of INPPL1 in skeletal biology is unknown. The protein is an inositol-1,4,5-trisphosphate 5-phosphatase and through its phosphatase activity converts phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) to PtdIns(3,4)P2. PtdIns-(3,4,5)P3 is widely expressed and acts as second messenger that participates in numerous cellular functions [Riehle et al., 2013]. Mouse models show that Inppl1 is a negative regular of insulin sensitivity and signaling and mice deficient for Inppl1 are small and have craniofacial abnormalities [Sleeman et al., 2005; Dubois et al., 2012], implying a yet uncharacterized effect on skeletogenesis.
Our findings identify a second locus for Schneckenbecken dysplasia and demonstrate that the phenotype is part of an INPPL1 allelic spectrum of disease. It remains to be determined whether the remaining SLC35D1 negative cases harbor mutations in INPPL1 or whether there is further locus heterogeneity in this disorder.
ACKNOWLEDGMENTS
We thank the family for their participation in this study. This work was supported by NIH grants RO1 DE019567 and R01 AR062651 to DHC, RO1 AR066124 to DK and 1U54 HG006493 to Drs. Debbie Nickerson, Jay Shendure, and Michael Bamshad. We also thank the March of Dimes Birth Defects Foundation, the Joseph Drown Foundation and the Orthopaedic Institute for Children/Orthopaedic Hospital Research Center for their support. Support was also provided by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Grant sponsor: NIH; Grant numbers: RO1 DE019567, R01 AR062651;
Grant sponsor: NIH/National Center for Advancing Translational Science; Grant number: UL1TR000124.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
REFERENCES
- Below JE, Earl DL, Shively KM, McMillin MJ, Smith JD, Turner EH, Stephan MJ, Al-Gazali LI, Hertecant JL, Chitayat D. Whole-genome analysis reveals that mutations in inositol polyphosphate phosphatase-like 1 cause opsismodysplasia. Am J Hum Genet. 2013;92:137–143. doi: 10.1016/j.ajhg.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochowitz Z, Jones K, Silbey R, Adomian G, Lachman R, Rimoin D, Opitz JM, Reynolds JF. A distinct lethal neonatal chondrodysplasia with snail-like pelvis: Schneckenbecken dysplasia. Am J med Genet. 1986;25:47–59. doi: 10.1002/ajmg.1320250107. [DOI] [PubMed] [Google Scholar]
- Cormier-Daire V, Delezoide A, Philip N, Marcorelles P, Casas K, Hillion Y, Faivre L, Rimoin D, Munnich A, Maroteaux P. Clinical, radiological, and chondro-osseous findings in opsismodysplasia: Survey of a series of 12 unreported cases. J Med Genet. 2003;40:195–200. doi: 10.1136/jmg.40.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E, Jacoby M, Blockmans M, Pernot E, Schiffmann SN, Foukas LC, Henquin J-C, Vanhaesebroeck B, Erneux C, Schurmans S. Developmental defects and rescue from glucose intolerance of a catalytically-inactive novel Ship2 mutant mouse. Cell Signalling. 2012;24:1971–1980. doi: 10.1016/j.cellsig.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Kayserili H, Hiraoka S, Nishimura G, Ohashi H, Alanay Y, Lerena J, Aslanger AD, Koseki H, Cohn DH. Identification of loss-of-function mutations of SLC35D1 in patients with Schneckenbecken dysplasia, but not with other severe spondylodysplastic dysplasias group diseases. J Med Genet. 2009;46:562–568. doi: 10.1136/jmg.2008.065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka S, Furuichi T, Nishimura G, Shibata S, Yanagishita M, Rimoin DL, Superti-Furga A, Nikkels PG, Ogawa M, Katsuyama K. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med. 2007;13:1363–1367. doi: 10.1038/nm1655. [DOI] [PubMed] [Google Scholar]
- Huber C, Faqeih EA, Bartholdi D, Bole-Feysot C, Borochowitz Z, Cavalcanti DP, Frigo A, Nitschke P, Roume J, Santos HG. Exome Sequencing Identifies INPPL1 Mutations as a Cause of Opsismodysplasia. Am J Hum Genet. 2013;92:144–149. doi: 10.1016/j.ajhg.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S, Winter R, Rimoin D, Opitz JM, Reynolds JF. A new category of lethal short-limbed dwarfism. Am J Med Genet. 1986;25:41–46. doi: 10.1002/ajmg.1320250106. [DOI] [PubMed] [Google Scholar]
- Lee H, Graham JM, Jr, Rimoin DL, Lachman RS, Krejci P, Tompson SW, Nelson SF, Krakow D, Cohn DH. Exome Sequencing Identifies PDE4D Mutations in Acrodysostosis. Am J Hum Genet. 2012;90:746–751. doi: 10.1016/j.ajhg.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux P, Stanescu V, Stanescu R, Le Marec B, Moraine C, Lejarraga H. Opsismodysplasia: A new type of chondrodysplasia with predominant involvement of the bones of the hand and the vertebrae. Am J Med Genet. 1984;19:171–182. doi: 10.1002/ajmg.1320190117. [DOI] [PubMed] [Google Scholar]
- Muraoka M, Kawakita M, Ishida N. Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 2001;495:87–93. doi: 10.1016/s0014-5793(01)02358-4. [DOI] [PubMed] [Google Scholar]
- Nikkels P, Stigter RH, Knol IE, van der Harten HJ. Schneckenbecken dysplasia, radiology, and histology. Pediatr Radiol. 2001;31:27–30. doi: 10.1007/s002470000357. [DOI] [PubMed] [Google Scholar]
- Riehle RD, Cornea S, Degterev A. Role of Phosphatidylinositol 3, 4, 5–Trisphosphate in Cell Signaling. Adv Exp Med Biol. 2013;991:105–139. doi: 10.1007/978-94-007-6331-9_7. [DOI] [PubMed] [Google Scholar]
- Sleeman MW, Wortley KE, Lai K-MV, Gowen LC, Kintner J, Kline WO, Garcia K, Stitt TN, Yancopoulos GD, Wiegand SJ. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med. 2005;11:199–205. doi: 10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- Varkey J, Jones R. Perinatally lethal, short-limbed dwarfism with distinct features—Schneckenbecken dysplasia. Ultrasound Obstetrics Gynecol. 2004;24:575–577. doi: 10.1002/uog.1115. [DOI] [PubMed] [Google Scholar]
- Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, LeMerrer M, Mortier G, Mundlos S, Nishimura G, Rimoin DL, Robertson S, Savarirayan R, Sillence D, Spranger J, Unger S, Zabel B, Superti-Furga A. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet Part A. 2011;155A:943–968. doi: 10.1002/ajmg.a.33909. [DOI] [PMC free article] [PubMed] [Google Scholar]