Abstract
Memories are consolidated and strengthened during sleep. Here we show that memories can also be weakened during sleep. We used a fear-conditioning paradigm in mice to condition footshock to an odor (conditioned stimulus (CS)). Twenty-four hours later, presentation of the CS odor during sleep resulted in an enhanced fear response when tested during subsequent wake. However, if the re-exposure of the CS odor during sleep was preceded by bilateral microinjections of a protein synthesis inhibitor into the basolateral amygdala, the subsequent fear response was attenuated. These findings demonstrate that specific fear memories can be selectively reactivated and either strengthened or attenuated during sleep, suggesting the potential for developing sleep therapies for emotional disorders.
Keywords: amygdala, anisomycin, behavioral freezing, extinction therapy, PTSD
Phobias, post-traumatic stress disorder (PTSD) and other psychiatric and emotional disorders are treated using extinction therapy in which the patient is repeatedly asked to recall a traumatic event or to re-experience a fear-inducing stimulus. Such reactivation of fearful memories in a safe context is used to eliminate fear responses by creating a new, less emotional and safe representation of the original traumatic experience.1,2 In addition to being emotionally demanding, a major limitation of extinction therapy is that it does not alter the original memory but rather forms a new memory that is associated with the therapeutic context in which it was introduced.3,4 Moreover, some memories are extended to include new associations,5 resulting in stimuli that were not formerly associated with the original trauma but which can trigger the fearful memory. These new associations can be resistant to classical extinction therapy.
Many studies have shown that memories are stabilized and consolidated during sleep.6 This processing of memory during sleep suggests that the memory trace becomes accessible to manipulation during this state, thus offering a new therapeutic environment. Accessing a specific memory during sleep is particularly challenging. Recent studies have demonstrated that specific declarative memories can be reactivated and strengthened during sleep using an associated cue.7–13 For example, a study in which human subjects learned a memory task while awake in the presence of rose odor showed that memory was strengthened when the rose odor was presented during sleep.11 Auditory cues were also shown to strengthen specific memories during sleep.7,12,13 These studies indicate that specific memories can be selectively reactivated and enhanced during sleep.
It has also been shown that if fear memories associated with a conditioned stimulus (CS) are reactivated in awake rodents, the memory becomes labile14 and can be destabilized by injection of a protein synthesis inhibitor (PSI) into the basolateral amygdala (BLA).14
On the basis of these studies, we hypothesized that by delivering the CS during sleep, a fear memory could be strengthened. However, if such reactivation (by the CS exposure during sleep) occurs in the presence of a PSI, the subsequent fear response to that stimulus would be weakened. To test this hypothesis, we performed two independent experiments using fear conditioning in mice. In the first experiment, we attempted to strengthen the fear memory and in the second to weaken it.
We used a fear-conditioning paradigm in which the unconditioned stimulus was footshock and the CS was an odor (amyl acetate; AA). Thus, when the mice experienced the conditioned odor in a novel context, they exhibited observable behavioral freezing, the amount of which reflects the strength of the reactivated fear memory.
For these experiments, the mice were housed in specialized cages that enabled electroencephalographic and electromyographic (EEG/EMG) cables to pass through the lid and also allowed air to be drawn unidirectionally through the cage. Odors could then be pulsed into the airstream. The fear-conditioning sessions occurred in the early light phase. After the conditioning session (Figure 1a), the mice were returned to their specialized home cages (Figure 1b) for 24 h. This 24-h interval after conditioning assured that the memory was consolidated and stabilized before its reactivation during sleep.15 The delay between conditioning and treatment is also significant for considerations of therapeutic potential, as it is unlikely that most patients could receive treatment immediately after a traumatic event.
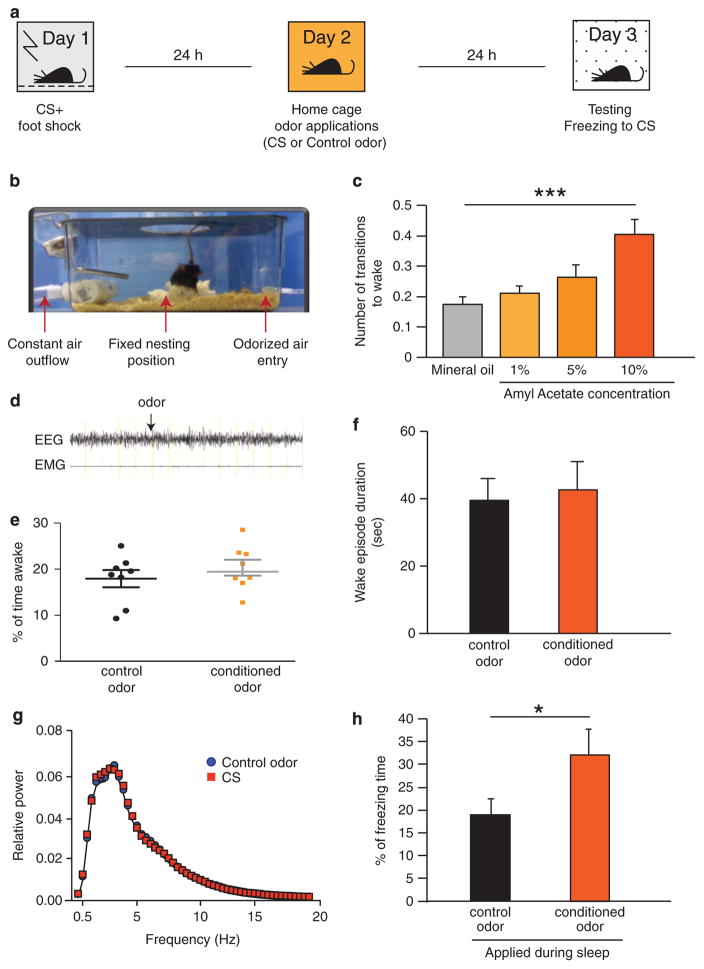
Figure 1.
Strengthening consolidated fear memory during sleep (a) Experimental paradigm. Day 1: 10 s conditioning odor AA (conditioned stimulus; CS) followed by 2 s footshock in the conditioning environment. Day 2 (24 h after FC): CS or control odor pulses (20 × 10 s) applied during NREM sleep in the home cage. Day 3 (48 h after FC): re-exposure to CS in a novel environment. A mild footshock (3 × 0.4 mA) was used because we expected to increase the freezing response. (b) The odor application set-up. An air purification system was connected to the home cage of each mouse. The top of the cage was designed to contain an air-tight opening for the EEG cable to be threaded through allowing the mouse to move freely. A glass dish with Nestlet was placed in the center of the cage, prompting most mice to nest in roughly the same position of the cage. Constant air inflow was maintained by a pump pulling air from one end of the cage (80 L air per minute capacity, cycling air every 3.7 s, total clearance every 13 s, verified by microstream capnography). On the basis of online observations of EEG/EMG trace, we added odorized air to the airflow during NREM sleep. (c) Titration of conditioned stimulus odor (amyl acetate) compared with mineral oil was done by measuring average wakening responses to increasing concentrations of amyl acetate (ANOVA followed by Dunnett’s multiple comparisons test; F (3, 21) = 11.24; P <0.0001). (d) Sample trace of EEG and EMG during application of CS odor, start point indicated by black arrow. (e) Percent of waking time during the 2 h period of odor application (unpaired t-test; t = 0.9419 df = 14; P = 0.362). (f) Duration of wake episodes during the 2 h odor application period (unpaired t-test; t = 0.7950; df = 12; P = 0.442) (g) Comparison of FFT power spectra for NREM sleep during odor application between the two groups; (repeated measure ANOVA; interaction = 0.583. F = 0.94). (h) Percent of freezing duration in response to CS presentation (3 × 60 s) in the novel environment. We compared two groups: mice that were exposed to the CS or to the control odor during sleep (Student’s t-test; t = 2.417 df = 15; P = 0.0289; ***P<0.001; *P<0.05). AA, amyl acetate; ANOVA, analysis of variance; CS, conditioned stimulus; EEG/EMG, electroencephalographic and electromyographic; FFT, Fast Fourier Transformation.
To determine the concentration of the odor to be used in these experiments, we tested increasing doses of the CS odor (AA; Figures 1c and d). We identified a dose (1%) that did not induce a significant increase in arousals (ANOVA followed by Dunnett’s multiple comparisons test significant only for 10%; F (3, 21) = 11.24; P<0.0001).
The CS was pulsed into the home cage airflow specifically during NREM sleep episodes (20 applications over 2 h of sleep). Control mice received a different unconditioned odor (beta-ionone; BI) during sleep to control for the specificity of the CS and for the effects of sensory stimulation during sleep. There was no significant difference between the experimental and control groups for total sleep duration (Figures 1e–f), and there was no difference in delta power during NREM sleep between the two groups of mice during odor applications (Figure 1g).
Following the odor applications, the mice were left uninterrupted in their home cages for another 24 h. We then placed the mice in a new testing chamber and assessed the duration of freezing behavior in response to the CS to indicate the strength of the fear memory (48 h after the FC). As shown in Figure 1h, duration of freezing in response to the CS was increased after exposure to the CS during sleep (Student’s t-test P = 0.0289; t = 2.417; df = 15). This experiment validated the hypothesis that specific fear memories in rodents could be strengthened during sleep. Of note, similar exposure to the conditioned cue during wake is known as extinction3,4,16 and it results in attenuation of the fear response. Here we show that application of the conditioned cue during sleep strengthens the fear response, emphasizing the difference between wake and sleep manipulations of the memory.
In contrast to the first experiment, the second experiment was designed to test whether fear memories reactivated during sleep could be weakened. To leave latitude for a weakening of the fear memory, we used a stronger conditioning stimulus than in the first experiment. To weaken the fear memory, we bilaterally injected the PSI, anisomycin into the BLA (AP, 1.5 mm; ML, 3.0 mm; DV, 4.5 mm) before applying the CS and control odors during sleep (Figure 2a). We applied odors for 2 h during the peak activity of the drug.17 We included two control groups: (1) injection of vehicle and CS application to determine the specific effect of anisomycin, (2) injection of anisomycin and control odor application to determine the specific effect of the CS.
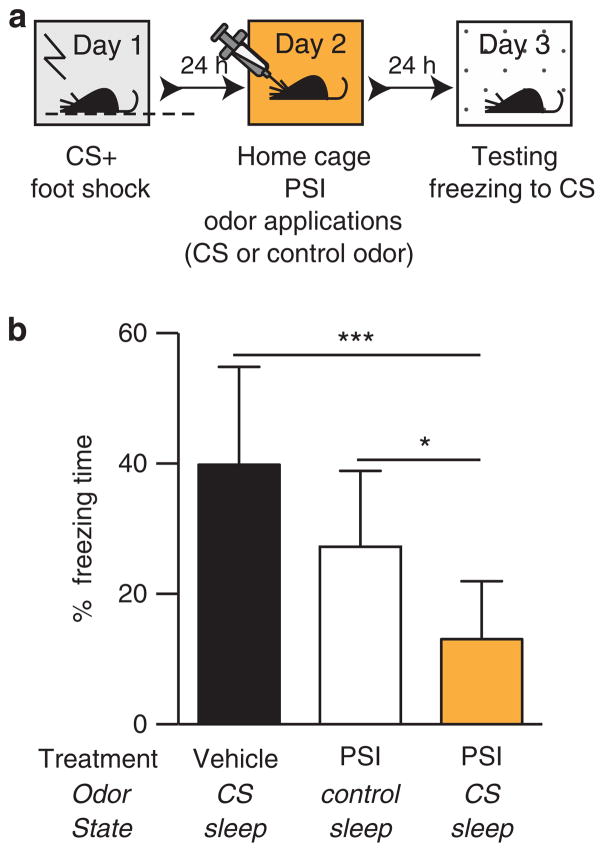
Figure 2.
Attenuating consolidated fear memory during sleep. (a) Conditioning paradigm for experiment 2. In order to maximize our difference in freezing response between treatment conditions, we used a stronger footshock paradigm (4 × 0.6 mA) than in experiment 1. (b) Percent of freezing duration in response to CS presentation (3 × 60 s) in the novel environment. We compared three conditions: (1) mice that received vehicle treatment and were exposed to conditioned odor (2) mice treated with the PSI (BLA injections of anisomycin prior to odor treatment during sleep) and received control odor and (3) mice receiving PSI treatment and exposed to CS odor (ANOVA followed by Tukey’s multiple comparison test F (2, 25) = 14.22; ***P<0.001; *P<0.05). BLA, basolateral amygdala; CS, conditioned stimulus; PSI, protein synthesis inhibitor.
As in the previous experiment, we applied the PSI and the odors 24 h after fear conditioning, after the memory was already consolidated. As shown in Figure 2b, mice that received an anisomycin injection coupled with the control odor did not show any attenuation of the fear memory. Likewise, mice injected with anisomycin but receiving control odor also did not show fear attenuation. This indicates that neither the anisomycin itself nor the sensory stimulation during sleep affected the memory trace.
As hypothesized, the application of the CS during sleep in the presence of a PSI produced a reduction in the freezing response (one-way ANOVA F(2,25) = 14.22; P<0.001 followed by Bonferoni’s multiple comparison test), suggesting that the fear memory trace was attenuated. Importantly, as the reduced freezing response was tested in a novel environment (response to cue), the effect of the treatment was not limited to a specific treatment environment.
We see these findings as proof of the concept that memories can be manipulated during sleep and that such manipulation offers diverse therapeutic potential. Although there are significant challenges for the development of sleep therapies for treating fear memories, there are also potential advantages. First, many extinction treatments terminate prematurely because of the difficulty of the patients to re-experience the fearful memories. Sleep therapy potentially offers an alternative method for treating memory-related disorders in a less stressful environment. Second, during sleep there is limited input from the external environment, and therefore the effects of the therapy are context independent. Furthermore, the sleeping brain may grant access to additional components of a fear memory that may be inaccessible during waking states.
METHODS
Animals
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male C57BL/6 mice, aged 8–10 weeks at the start of experimental procedures, were housed in individual standard rodent cages with constant airflow (the air in the cage was replaced five times every minute). The temperature was 24 ± 1 °C, humidity 40–60% and light cycle 12 h/12 h with lights on at 0900 hours. Food and water were available ad libitum. Mice were habituated to the cages and airflow for 7 days before the experiments.
Equipment
Home cages were approved by Stanford University’s APLAC to be modified from the standard cages. Several small holes were drilled into one end of the cage for airflow intake and connected to tubing (Nalgene 380, 3/16″ × 5/16″ × 1/16″) leading to an air pump with odor receptacles (Figure 1b). Air was drawn out on the opposite end of the cage through a series of holes connected to tubing leading to an air purification system. Cages were fitted with a novel cage top design to allow an EEG cable to be threaded through which facilitated mouse mobility within the home cage. For fear-conditioning, a modular testing chamber (Med Associates) was placed in a large rodent cage with a sealed lid to contain the stimulus odors introduced during the footshock applications. The air from this chamber was exhausted constantly by an air purification system using an enclosed pump (AP-100 108 Watt, Danner Mfg, Inc) leading to charcoal filters (Bickford Inc, Omnicon f/Air, Wales Center, NY, USA).
Odor calibration
Mice were tested for their sensitivity to the conditioned and control odors during sleep by measuring transitions from sleep to wakefulness in response to increasing concentrations of amyl acetate and beta-ionone (1%, 5%, 10% vol/vol diluted in mineral oil), compared with odorless mineral oil after fear conditioning. We used a concentration of amyl acetate (1% vol/vol diluted in mineral oil) that was associated with a waking pattern similar to that seen when pure mineral oil or beta-ionone (1% vol/vol diluted in mineral oil) were applied.
Fear-conditioning apparatus
On day 1 at zeitgeber time (ZT) 1, we placed C57BL/6 mice in a conditioning chamber with constant airflow using ambient air. A cotton pad with one drop of jasmine essential oil (Aura Cacia) provided a specific contextual odor for each conditioning experiment. After 3 min of habituation the CS odor amyl acetate (AA, 1%, Sigma) was inserted into the constant airflow for 10 s immediately followed by a footshock (Experiment 1: 0.4 mA, 2 s; Experiment 2: 0.6 mA, 2 s). We repeated this procedure for a total of three shocks in Experiment 1, and four shocks in Experiment 2. The mice were then returned to their home cages.
Surgery
All animals were equipped with custom-made EEG/EMG implants placed caudally on the skull under ketamine/xylazine anesthesia (80 and 16 mg kg −1, i.p., respectively). The EEG signals were recorded from electrodes placed over the frontal (AP, +1 mm; ML, 1 mm) and temporal (AP, −2 mm; ML, 1.5 mm) cortices. EMG signals were recorded from two electrodes inserted in the neck musculature. For Experiment 2, we also surgically implanted cannula guide tubes bilaterally (Plastics One, VA, USA). Using a small animal stereotaxic frame (David Kopf Instruments, CA, USA), the cannula guide tubes were placed above the left and right BLA (AP, 1.5 mm; ML, 3.0 mm; DV, 4.5 mm) and also affixed to the skull with C&B Metabond (Parkell; Edgewood, NY, USA) and dental acrylic. For the injection procedure, we used cannulae that projected 0.5 mm below the guide tubes. Cannulae placements for all experimental mice were verified by light microscopy on brain slices after perfusion at the end of the experiments (Supplementary Figure 1).
Polysomnographic recording data acquisition
EEG and EMG signals derived from the surgically implanted electrodes were collected using commercial hardware (Embla; Broomfield, CO, USA), digitized at 256 Hz and visualized using the sleep recording software Somnologica-3 (Medcare, Reykjavik, Iceland). For odor applications, we monitored the EEG online and applied the odors during NREM sleep. We defined NREM sleep as synchronized, high-amplitude, low-frequency (0.25–4 Hz) EEG and highly reduced EMG activity compared with wakefulness with no phasic bursts. Conversely, we defined REM sleep as a combination of a pronounced theta rhythm (4–9 Hz) and a flat EMG (muscle atonia).
Sleep analysis
We scored the EEG/EMG traces using the sleep analysis software (SleepSign for Animals; Kissei Comtec, Matsumoto, Nagano, Japan), and mean episode duration and number of transitions were automatically generated for each vigilance state. Values represent the mean ± s.e.m. for all mice in each group. All scoring was performed manually based on the visual signature of the EEG and EMG waveforms in 4 s epochs and verified by two independent investigators. Figure 1d shows an example of an EEG trace during an odor application (start indicated by arrow). Neither EEG nor EMG shows a noticeable change verified by FFT analysis of NREM sleep, demonstrating that the odor application did not disturb the mouse’s sleep, (Figure 1g).
Fast fourier transformation
The digitally filtered signals were also spectrally analyzed by FFT using the Sleep Sign program.
Odor administration during Experiments 1 and 2
On day 2, for 2 h starting at ZT1, mice received either AA or the control odor beta-ionone (BI, 1%, Sigma) for ~20 times in total. Each odor application lasted for 10 s while mice were in NREM (verified by simultaneous EEG/EMG recording with at least 20 s of stable NREM). Tubing connected to a pump (Coralife Luft 5 Watt, Carson, CA, USA) and Falcon tubes containing the volatile odor solution (AA or BI) were used to pulse odorized air into the constant ambient airflow of the home cages.
Anisomycin administration during Experiment 2
Before odor application, mice were bilaterally injected (via a cannula inserted into a previously implanted guide tube that ended just above the basolateral amygdala) with anisomycin (Sigma; 50 μg per side) dissolved in 1 N HCl adjusted with NaOH to a neutral pH. After the anisomycin/vehicle injections, the mice were allowed to sleep and the same procedure as stated earlier was followed for pulsing odors (AA or BI) into the home cages during sleep. Exclusion criteria were as follows: mice that woke up during odor application more than 25% of the time were excluded (five mice in the AA and four mice in the control BI group). A total of three mice were excluded because they did not sleep enough during the 2 h of application to receive all 20 applications.
Fear response testing
On day 3, mice were placed in a novel testing chamber, a clear Plexiglas tank with constant airflow of ambient air. After 3 min of habituation (baseline freezing was not different between the control and the odor application group (Student’s t-test P = 0.13)), the conditioned odor AA was pulsed into the airstream for 1 min, followed by a minute of ambient air, then repeated twice more, for a total of three 1-min applications of AA. A video camera recorded all behavior during this test for off-line scoring at a later time.
Behavioral video scoring
We used VCode software (open source Social Spaces project at University of Illinois, IL, USA) to determine the duration of each freezing event during the baseline (3 min before first odor application) and during the periods of odor application. We then calculated the total freezing time by subtracting baseline freezing times from total freezing duration during odor applications.
Acknowledgments
The authors are grateful to Drs Chuluun Bayarsaikhan and Antoine Adamantidis for their help. AR was supported by NARSAD Young Investigator Award. Other grant support that contributed to this study are an NSF predoctoral fellowship to MM; grants from NIMH and the Klarman Family Foundation to LdL; and grants from NIH as well as DSRTF, and RDSF to HCH.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Quirk GJ, et al. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 4.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 5.Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956): Twenty-five years of research on stimulus generalization. J Exp Anal Behav. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 9.Diekelmann S, Biggel S, Rasch B, Born J. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiol Learn Mem. 2012;98:103–111. doi: 10.1016/j.nlm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Paller KA, Voss JL. Memory reactivation and consolidation during sleep. Learn Mem. 2004;11:664–670. doi: 10.1101/lm.75704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 12.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dongen EV, et al. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc Natl Acad Sci USA. 2012;109:10575–10580. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 15.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. J Exp Psychol Anim Behav Process. 2000;26:174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- 17.Wanisch K, Wotjak CT. Time course and efficiency of protein synthesis inhibition following intracerebral and systemic anisomycin treatment. Neurobiol Learn Mem. 2008;90:485–494. doi: 10.1016/j.nlm.2008.02.007. [DOI] [PubMed] [Google Scholar]