Abstract
Researchers have proposed a variety of behavioral traits that may lead to weight gain and obesity; however, little is known about the neurocognitive mechanisms underlying these weight-related eating behaviors. In this study, we measured activation of reward circuitry during a task requiring response and inhibition to food stimuli. We assessed participants’ emotional eating, external eating, and two subscales of dietary restraint—routine restraint and compensatory restraint—using the Weight-Related Eating Questionnaire. For routine restraint, we found positive associations with activation in the insula, dorsolateral prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex and ventromedial prefrontal cortex in response to high-calorie versus low-calorie foods. For emotional eating, we found positive associations with insula and dorsolateral prefrontal cortex activation in response to high-calorie versus low-calorie foods. We also found positive associations between emotional eating and dorsolateral prefrontal cortex activation in response to approach versus inhibition towards high-calorie foods. Thus, our results demonstrate an increase in activation across brain regions related to self-control and urges in response to high-calorie food associated with both emotional eating and routine restraint. Overall, these results support the construct validity of both emotional eating and routine restraint and provide preliminary evidence that these subscales have similar neural correlates.
Keywords: Eating behaviors, Decision making, fMRI, Questionnaire, Prefrontal system, Interoceptive system
1. Introduction
Obesity rates in the USA have increased dramatically from 12% in 1991 [1] to over one third of adults in 2009-2010 [2]. Environmental factors including culture, technology, and food availability in developed countries have heavily contributed to the rise in obesity rates. However, even within the same environment, some individuals become obese while others do not. Individual characteristics and eating behaviors are critical to determining risk of weight gain. For some individuals, food elicits strong neurocognitive responses related to eating patterns and weight gain.
A growing body of research in neuroimaging supports a triple process framework of reward processing in the brain [3-5, for a broader review of the triple process framework, see 6]. First, the “impulsive” amygdala-striatal circuitry is responsive to external reward cues. These brain regions include the mesolimbic dopamine system, and they respond quickly and automatically to motivational stimuli and are critical to forming habits [7]. Second, neural circuitry involved in impulse control includes primarily prefrontal cortex structures, including the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), ventromedial prefrontal cortex (VMPFC), and dorsolateral prefrontal cortex (DLPFC). This circuitry has been called “reflective” [8] because it responds to hypothetical or remembered emotional triggers. Third, interoceptive circuitry, including the insula, modulates the activity of the other brain regions based on homeostatic signals [9, 10]. Specifically, the insula translates internal signals of hunger or satiety into subjective feelings such as the urge to eat.
These brain regions have been implicated in the few studies that have used neuroimaging to examine the neural correlates of theory-based indices of hedonic eating. For example, emotional eating has been associated with activity in the amygdala [11], ACC [12], OFC, and insula [13]. External eating has been associated with activation of the OFC and insula [11] and VMPFC [14] and differential connectivity patterns among the ventral striatum, amygdala, ACC, and premotor cortex [15]. Dietary restraint has been associated with increased activity in prefrontal, inhibitory brain regions [16-19] as well as the dorsal striatum [19], amygdala [20, 21], and nucleus accumbens [22].
Problematically, each of these studies used different methodologies and examined different individual weight-related eating behaviors. (An exception is [11], which examined external eating, emotional eating, and restrained eating, but in the specific context of diabetes management.) Additionally, many studies examining the same eating behavior have reported significant associations with different brain regions that serve different functions. These inconsistencies across studies have created challenges in drawing conclusions about the neurobiological similarities and differences across eating behaviors.
Here we address this gap in the literature by testing the neural correlates of different weight-related eating behaviors simultaneously. Our methodology also offers two additional advantages over prior neuroimaging studies on weight-related eating behaviors. First, participants performed an active task designed to engage impulsive food-related behavior. Conversely, in most prior neuroimaging studies of weight-related behaviors, participants have passively viewed images of food items or reported information about an image (e.g., “Is the image a food?” “How much do you like this food?”). In the present study, participants were scanned while performing a food-related go/nogo task. Additional data from this experiment has been reported elsewhere [4]. Using functional magnetic resonance imaging (fMRI), this study measured brain activation in regions of interest across impulsive, reflective, and interoceptive circuitry. The go/nogo task consisted of two trial types: participants needed to inhibit their responses to either high-calorie or low-calorie foods. Thus, the food-related go/nogo paradigm captures impulsive behavior (go trials) and inhibition (nogo trials) related to food stimuli.
Second, unlike the previous neuroimaging studies of weight-related eating behaviors, the present study used the Weight-Related Eating Questionnaire (WREQ). The WREQ has strong convergent validity with similar scales such as the Dutch Eating Behavior Questionnaire [23]. It is unique, however, from other similar measures in that the WREQ subscale of dietary restraint is separated into compensatory and routine restraint, reflecting more flexible and rigid aspects of dietary restraint, respectively. Therefore, the WREQ is able to highlight underlying differences between these two styles of restricted dietary intake.
The primary goal of this study was to determine the neural correlates of each eating behavior assessed by the WREQ: emotional eating, external eating, compensatory restraint, and routine restraint. By analyzing the relationship between brain activity and all of the eating behaviors in a single neuroimaging task, we were able to compare across eating behaviors for commonalities and differences. This approach allowed us to examine whether patterns of activation were overlapping or entirely distinct across eating behaviors. Moreover, this approach directly contrasts with past studies examining the association between specific eating behaviors and subsets of the brain’s reward circuitry (e.g., the association between emotional eating and amygdala-striatal regions or the association between restraint and the prefrontal cortex).
2. Methods
2.1 Participants
Twenty healthy, right-handed adults participated in this study (12 female, mean age = 19.8 years, SD = 1.0, range = 18-22)1. Their mean BMI was 22.6 kg/m2 (SD = 3.0, range = 18.5-31.3; 85% with BMI < 25 kg/m2). All participants had normal or corrected-to-normal vision and had no history of neurological or psychiatric disorders. All participants provided written, informed consent, and all study procedures were approved by the University of Southern California Institutional Review Board.
2.2 Measures
2.2.1 Questionnaire
The WREQ was used to assess theory-based eating behaviors [24, 25]. The WREQ consists of 16 items reflecting indices of hedonic eating: emotional eating (5 questions), external eating (5 questions), and two subscales of dietary restraint (compensatory restraint: 3 questions; and routine restraint: 3 questions). The WREQ was developed and validated for use in a diverse range of populations including young adults and has demonstrated good internal consistency and test-retest reliability among the subscales [24]. Subscales of the WREQ have demonstrated strong convergent validity with the Three Factor Eating Questionnaire R-18 [26] and the Dutch Eating Behavior Questionnaire [23], but the WREQ is unique in that it assesses two subscales of dietary restraint [25]. The WREQ subscales are associated with body weight status, weight control practices, and consumption of fruits/vegetables and dietary fat [24, 25]. Notably, the compensatory restraint subscale has been associated with lower BMI and less adult weight gain over time [24, 25]; whereas the routine restraint subscale has been associated with higher BMI [25].
2.2.2 fMRI task
During scanning, participants were asked to perform two food-related go/nogo tasks: 1) a high-calorie food go and low-calorie food nogo task (HGo/LNogo task), and 2) a low-calorie food go and high-calorie food nogo task (LGo/HNogo task). In the HGo/LNogo condition, participants were instructed to press a button as quickly as possible when shown a picture of a snack food (“HGo”) and inhibit responding when shown a picture of a vegetable (“LNogo”). In the LGo/HNogo condition, the participants were instructed to respond to vegetable pictures (“LGo”) and inhibit their response to snack pictures (“HNogo”). Thus, our task provided an estimate of brain activity across four states: 1) responding to high-calorie foods, HGo; 2) responding to low-calorie foods, LGo; 3) inhibiting response to high-calorie foods, HNogo; and 4) inhibiting response to low-calorie foods, LNogo.
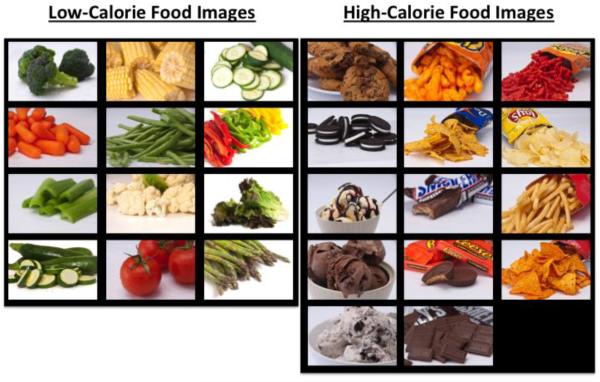
The food pictures that were used in the task are shown in Figure 1. The foods were chosen to accord with focus group data on snacks and vegetables that adolescents consume most frequently. The photographs of the foods were taken by a professional photographer to minimize differences in the lighting, background, and quality of the pictures. Images of each food were ranked internally from least appealing to most appealing by a dozen project staff. The most appealing version of each food was then used for the task. Each participant saw 11 low-calorie foods (all vegetables), and 11 high-calorie foods (six sweet and five savory foods) during the course of the task.
Figure 1.
The food images used in the food-specific go/nogo task. Each participant saw a randomly selected subset of these images during the task. Seven images of low-calorie foods and 4 images of high-calorie foods (2 sweet and 2 savory) were used for the LGo/HNogo trials. Seven different images of high-calorie foods (4 sweet and 3 savory) and 4 different images of low-calories foods were used for the HGo/LNogo trials.
Each condition lasted 8 minutes, and the order of the two conditions was counterbalanced across participants. In each condition, there were 120 go trials and 40 nogo trials. Nogo trials were presented in a pseudo-randomized order, such that nogo trials appeared with equal probability after 1–5 consecutive go trials. On each trial, the picture was presented for 500 ms, followed by an intertrial interval during which a fixation cross was presented for 1500–4000 ms (mean = 2500 ms).
Participants were asked not to eat for 15 hours before coming to the laboratory, and were asked to reschedule if they rated their hunger <5 on a 1–10 scale (from not hungry at all to very hungry). Prior to the scanning procedure, participants reviewed all stimuli used in the tasks and were informed of the category for each stimulus. During the fMRI scan, participants laid in the supine position on the scanner bed to view the task back-projected onto a screen through a mirror attached to the head coil. Foam pads were used to minimize head motion. Stimulus presentation and timing of all stimuli and response events were achieved using Matlab (Mathworks) and Psychtoolbox (www.psychtoolbox.org) on an IBM-compatible PC. Participants’ responses were collected online using an MRI-compatible button box.
2.2.3 fMRI protocol
fMRI imaging was performed using a 3T Siemens MAGNETOM Tim/Trio scanner in the Dana and David Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California. Blood oxygen level-dependent (BOLD) signals were obtained using a z-shimmed echo-planar imaging (EPI) sequence with TR/TE = 2000/25 ms, flip angle = 90°, and a 64 × 64 matrix size with an in-plane resolution of 3 × 3 mm2. Thirty-one 3.5-mm contiguous axial-oblique slices were aligned parallel to the AC-PC plane. The EPI sequence used Prospective Acquisition Correction (PACE), which helps reduce the impact of head motion during data acquisition. T1-weighted structural images were obtained for registration purposes (TR/TE = 1950/2.26 ms; flip angle 7°; 176 sagittal slices; spatial resolution = 1 × 1 × 1.95 mm).
2.2.4 fMRI analysis
fMRI analysis was performed using the fMRI Expert Analysis Tool, part of the FSL package (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included realignment using a 6-parameter rigid body transformation (to compensate for small residual head movements that were not captured by the PACE sequence), spatial smoothing (Gaussian kernel of 5-mm, full-width-half-maximum), and temporal filtering to remove low-frequency drift from the fMRI time series (using a nonlinear high pass filter with a 100-second cut-off). A two-step registration procedure was used whereby EPI images were first registered to the structural image, and then into standard MNI space, using affine transformations [27]. This registration was further refined using FNIRT nonlinear registration [28, 29].
Statistical analyses were performed in the native image space, with the statistical maps normalized to the standard space prior to higher-level analysis. We used a general linear model (GLM; FSL’s FILM module) to model the data at the single-subject level. Brain activation was modeled separately for go and nogo trials. Error-related trials (both go and nogo) were modeled separately as a nuisance variable. The event onsets were convolved with canonical hemodynamic response function to generate regressors. The GLM included covariates for the temporal derivatives to improve statistical sensitivity and the six movement parameters in the first-level general linear model.
A higher-level analysis created cross-run contrasts for each subject for a set of contrast images. For each subject, a task (go vs. nogo) × stimulus type (high-calorie vs. low-calorie) design was used. Next, higher level mixed effect models were used to estimate mean activation across all subjects (subject was modeled as a random effect) using FMRIB’s Local Analysis of Mixed Effect stage 1 only [30, 31] with automatic outlier detection [32]. Detailed results of the GLM analysis were reported elsewhere [4].
In this study, we were mainly interested in examining the correlation between WREQ scores and two contrasts of BOLD responses: HGo vs. LGo and HGo vs. HNogo. We focused on the HGo vs. LGo contrast because it allowed us to isolate the neural response to more rewarding food-stimuli—high-calorie foods—over less immediately rewarding food stimuli—low-calorie foods—when individuals were actively responding to the food images. Prior work has compared high reward go trials to low reward go trials to measure salience attribution in the scanner [4, 33]. The contrast between HGo vs. HNogo allowed us to examine neural activity associated with response vs. inhibition towards high-calorie foods.
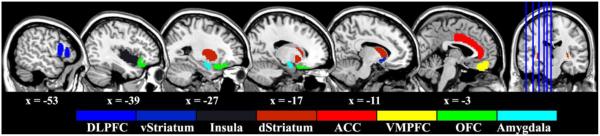
For each contrast, region of interest (ROI) analyses were performed by extracting parameter estimates (β) of each event type from the fitted model and averaging across all voxels in the ROI for each subject. The β estimates were then converted to percent signal change using a method suggested by Mumford (http://mumford.fmripower.org/perchange_guide.pdf). The following ROIs were selected a priori because they are part of the three-process model of reward processing: anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), ventromedial prefrontal cortex (VMPFC), amygdala, dorsal striatum, ventral striatum, and insula. The size and location of the ROIs (Figure 2) were obtained in MNI space using the Harvard-Oxford cortical and subcortical structural atlases (probabilistic atlases, 2mm resolution, 50% threshold) provided by the FSL package.
Figure 2.
The anatomical location of the ROIs. These ROIs were extracted using Harvard-Oxford atlas provided by FSL. Please note that we only illustrate the ROIs in the left hemisphere but the actual ROI includes both hemispheres.
3. Results
3.1 Eating behaviors
Scores on the WREQ subscales ranged from 1.0 to 4.8 (out of 5). The average score was similar across subscales (compensatory restraint: mean = 2.6, SD = 1.2; routine restraint: mean = 2.0, SD = 0.9; emotional eating: mean = 2.2; SD = 1.0; external eating: mean = 3.1; SD = 1.0). In this sample, routine restraint was significantly correlated with compensatory restraint (r = .702, p = .001) and with emotional eating (r = .661, p = .002). Males and females did not have significantly different scores for any subscale of the WREQ (independent t-tests, all Ps > .13).2
3.2 Go/nogo performance
For both go/nogo tasks, we measured reaction time for go trials, misses (errors of omission), false alarms (errors of commission), decision bias (C = −0.5 * [z(hit rate) + z(false alarm rate)]) and D-prime (d' = [z(hit rate) − z(false alarm rate)]). Decision bias is an index of response inhibition, with higher values indicating better inhibitory control. D-prime is a sensitivity index, with higher values indicating greater sensitivity to go trials. After Bonferroni correction (5 measures × 2 go/nogo tasks; p < 0.005), we found significant positive correlations between emotional eating and D-prime in the HGo/LNogo task (r = 0.659, p = 0.004), between compensatory restraint and misses on the LGo/HNogo (r = 0.676, p = 0.001), and between routine restraint and misses on the LGo/HNogo (r = 0.640, p = 0.002).3
3.3 Functional imaging results
3.3.1 HGo vs. LGo contrast
The correlations between each subscale of the WREQ and fMRI responses for the HGo vs. LGo contrast are presented in Table 1. The HGo vs. LGo contrast compares brain activation when responding to pictures of high-calorie foods versus responding to pictures of low-calorie foods. Thus, this contrast reflects approach to high-calorie over low-calorie foods. We hypothesized that the HGo vs. LGo contrast would be associated with the emotional eating and external eating subscales because both of these measures are related to eating as a reward-driven behavior. Additionally, we hypothesized that the HGo vs. LGo contrast would be associated with routine and compensatory restraint because brain regions related to inhibition and self-control would be recruited to compensate for reward salience in individuals who exercise greater restraint in eating.
Table 1.
Correlations between contrast of BOLD responses to HGo vs. LGo trials and each WREQ subscale.
| Brain Region | Hem | Emotional Eating |
External Eating |
Routine Restraint |
Compensatory Restraint |
||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| Anterior Cingulate Cortex | B | .51 | .02 | .07 | .78 | .57 | .009* | .32 | .17 |
| Dorsolateral Prefrontal Cortex | L | .65 | .002* | .24 | .30 | .59 | .006* | .43 | .06 |
| R | .61 | .004* | .12 | .61 | .60 | .005* | .34 | .14 | |
| Orbitofrontal Cortex | L | .29 | .22 | .04 | .86 | .64 | .002* | .39 | .09 |
| R | .24 | .30 | .19 | .42 | .40 | .08 | .11 | .65 | |
| Ventromedial Prefrontal Cortex | B | .36 | .12 | .16 | .51 | .59 | .006* | .27 | .24 |
| Amygdala | L | .29 | .22 | .08 | .75 | .26 | .27 | −.04 | .88 |
| R | .24 | .31 | .08 | .74 | .26 | .27 | .07 | .77 | |
| Dorsal Striatum | L | .24 | .30 | .15 | .52 | .25 | .29 | .11 | .63 |
| R | .17 | .46 | .25 | .29 | .18 | .44 | .01 | .97 | |
| Ventral Striatum | L | −.18 | .45 | .20 | .39 | −.03 | .91 | −.24 | .32 |
| R | −.24 | .31 | .19 | .42 | .02 | .94 | −.20 | .40 | |
| Insula | L | .56 | .01* | .28 | .23 | .59 | .006* | .32 | .16 |
| R | .41 | .07 | .29 | .22 | .54 | .01* | .23 | .34 | |
Note: Hem=Hemisphere, B=Bilateral, L=Left, R=Right. Bold indicates p < .05 and * indicates that the correlation remained significant after controlling the false discovery rate (FDR) at q < .05 for the correlations within each column (i.e., each questionnaire subscale was considered a family of tests).
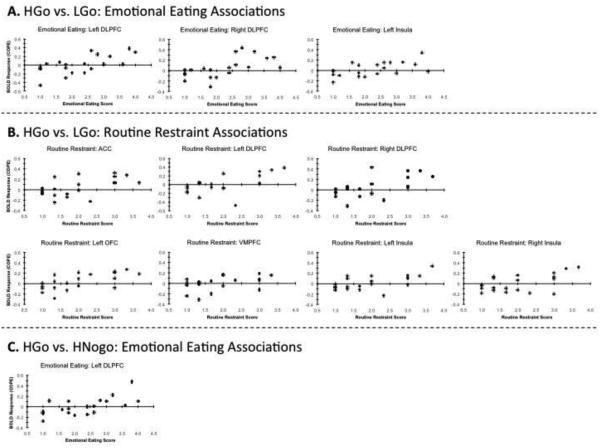
We controlled for multiple comparisons using a false discovery rate of q < 0.05. We chose to use a false discovery rate in order to balance the risk of committing a type I vs. type II error. There were significant positive correlations between BOLD responses to high- versus low-calorie food images and scores on the routine restraint and emotional eating subscales of the WREQ. For both subscales, significant positive correlations were found in the left and right DLPFC, and left insula (p’s ≤ 0.01). For the routine restraint subscale, there were also significant positive correlations for the ACC, right insula, left OFC, and the VMPFC (p’s ≤ 0.01). Figure 3 shows scatterplots of the significant correlations.
Figure 3.
The associations between the BOLD response (quantified as a contrast of parameter estimates; COPE) and the WREQ subscales. Only the associations that were significant after applying a false discovery rate of q < 0.05 are shown. The top (A) shows the associations between emotional eating and the BOLD response for the HGo vs. LGo contrast. The middle (B) shows the associations between routine restraint and the BOLD response for the HGo vs. LGo contrast. The bottom (C) shows the associations between emotional eating and the BOLD response for the HGo vs. HNogo contrast.
3.3.2 HGo vs. HNogo contrast
The correlations between each subscale of the WREQ and fMRI responses for the HGo vs. HNogo contrast are presented in Table 2. The HGo vs. HNogo contrast allowed us to isolate the approach versus inhibition response to high-calorie foods. We hypothesized that the HGo vs. HNogo contrast would be associated with routine and compensatory restraint scores because restrained eating requires exercising inhibition in response to high-calorie foods. We also hypothesized that the HGo vs. HNogo contrast would be associated with higher emotional eating and external eating because individuals with higher scores in these subscales have greater attraction than inhibition in response to high-calorie foods.
Table 2.
Correlations between contrast of BOLD responses to HGo vs. HNogo trials and each WREQ subscale.
| Brain Region | Hem | Emotional Eating |
External Eating |
Routine Restraint |
Compensatory Restraint |
||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| Anterior Cingulate Cortex | B | .33 | .16 | −.18 | .44 | .32 | .17 | .15 | .52 |
| Dorsolateral Prefrontal Cortex | L | .65 | .002* | .12 | .63 | .56 | .01 | .28 | .23 |
| R | .31 | .19 | −.06 | .80 | .44 | .06 | .24 | .31 | |
| Orbitofrontal Cortex | L | .14 | .56 | .14 | .56 | .30 | .20 | .11 | .66 |
| R | −.06 | .80 | .23 | .34 | .07 | .77 | −.09 | .71 | |
| Ventromedial Prefrontal Cortex | B | .40 | .08 | .37 | .11 | .41 | .07 | .16 | .51 |
| Amygdala | L | −.01 | .96 | −.03 | .91 | −.01 | .96 | −.16 | .50 |
| R | −.13 | .60 | −.06 | .81 | .04 | .86 | .00 | .99 | |
| Dorsal Striatum | L | .14 | .56 | −.06 | .81 | .22 | .34 | .15 | .52 |
| R | .02 | .94 | .05 | .83 | .20 | .39 | .09 | .69 | |
| Ventral Striatum | L | −.24 | .32 | .11 | .64 | .02 | .94 | −.12 | .62 |
| R | −.28 | .22 | .22 | .35 | .03 | .90 | −.11 | .63 | |
| Insula | L | .27 | .25 | −.06 | .81 | .42 | .07 | .12 | .61 |
| R | −.01 | .96 | .01 | .97 | .22 | .35 | −.02 | .93 | |
Note: Hem=Hemisphere, B=Bilateral, L=Left, R=Right. Bold indicates p < .05 and * indicates that the correlation remained significant after controlling the false discovery rate (FDR) at q < .05 for the correlations within each column (i.e., each questionnaire subscale was considered a family of tests).
We observed significant positive correlations between emotional eating and routine restraint with BOLD contrasts in the left DLPFC. Only the correlation between emotional eating and activation of the left DLPFC remained significant after applying a false discovery rate threshold of q < 0.05. Figure 3 shows a scatterplot of the significant correlation.
3.3.3 Controlling for BMI
BMI was considered as a covariate in the analysis based on previously reported associations with WREQ subscales [24, 25]. Preliminary analyses indicated that BMI was not correlated with the WREQ subscales in this sample (all Ps > .11). However, given the small sample size relative to behavioral studies of the WREQ (which have hundreds of participants) and the limited range of BMI in our participants, we would not expect to see significant associations between the WREQ subscales and BMI in this study.
Further, when BMI was included as a covariate in the analysis, all of the associations that were p < 0.05 in the original analysis remained p < 0.05. The pattern of significant associations between brain areas and WREQ subscales was completely unchanged after applying a false discovery rate of q < 0.05.4
4. Discussion
This study examined the brain activity associated with indices of hedonic eating behaviors. Participants underwent fMRI scanning while performing a food-related go/nogo task. When contrasting high-calorie versus low-calorie foods in an approach setting (i.e., the “Go” trials of the go/nogo task), emotional eating and routine restraint were associated with increased activity in the left and right DLPFC and left insula. Routine restraint was also associated with greater BOLD contrast in the left OFC, VMPFC, ACC, and right insula. When contrasting approach versus inhibition towards high-calorie foods (i.e., HGo vs. HNogo trials), emotional eating was associated with greater activity in the left DLPFC. These findings indicate that higher levels of both emotional eating and routine restraint are associated with differential neural activity in brain regions related to self-control (e.g., DLPFC, ACC, OFC, and VMPFC) and urges (e.g., the insula). These brain regions are part of a complex reward circuitry [6] that is also engaged in response to non-food rewards including money [34], alcohol [35], and drugs [8, 36].
Importantly, we found that routine restraint and emotional eating are associated with overlapping, rather than separate, patterns of activation. Both routine restraint and emotional eating were positively correlated with insula activity in our study. It is possible that emotional eating is rooted—at least in part—in an increased urge to eat, engaging the insula. Similarly, prolonged dieting due to routine restraint may gradually increase the urge to eat, engaging the insula. Both routine restraint and emotional eating were also positively correlated with DLPFC activation in the HGo vs. LGo trials (as well as the HGo vs. HNogo trials for emotional eating). DLPFC activation may reflect increased attention [37] to high-calorie foods or increased recruitment of inhibition. Specifically, individuals with higher routine restraint and emotional eating scores may be hyper-responsive to the reward of high-calorie foods and enlist greater DLPFC activation to modulate this response.
A critical difference between routine restraint and emotional eating was the presence of positive associations between routine restraint and OFC, VMPFC, and ACC activation. These brain regions are involved in the regulatory control of food intake. More generally, the OFC and VMPFC process long-term or hypothetical rewards [8], while the ACC is involved in conflict monitoring and error detection. Thus, differential activity across multiple brain regions related to self-control and inhibition is a critical signature of routine restraint.
Some researchers have proposed that overeating is a product of hypo-activity in brain regions like the DLPFC and ACC that are responsible for cognitive control and attention [3]. According to this hypothesis, overeating is partially related to a lack of cognitive control in response to food. Our results could be interpreted as providing support for this theory by showing that lower DLPFC activation is associated with higher emotional eating and routine restraint scores during LGo vs. HGo trials, and with higher emotional eating in the contrast of HNogo vs. HGo trials. Conversely, our results could also be interpreted as showing that greater DLPFC activation is associated with higher emotional eating and higher routine restraint scores in the contrast of HGo and LGo trials, and with higher emotional eating in the contrast of HGo and HNogo trials. The latter interpretation is consistent with other studies that have found a positive association between food-related DLPFC activation and dietary restraint [16-19].
Overall, our results support the neurobiological validity of emotional eating and routine restraint. Conversely, we did not detect significant associations between activation of the reward circuitry and external eating or compensatory restraint. There are at least two explanations for these null findings: (1) either subscale may elicit smaller changes in brain activity that require greater statistical power to detect, or (2) compensatory restraint and external eating may not elicit differential brain activation in response to a food-related go/nogo task. Behavioral evidence suggests that external eating and compensatory restraint are fundamentally different from emotional eating and routine restraint [24, 25]. For example, very few studies have reported independent associations between external eating and weight or weight change [24]. Moreover, flexible dimensions of dietary restraint, such as compensatory restraint, have been associated with lower BMI [38, 39].
An advantage of the WREQ is that it assesses correlated but theoretically opposing measures of dietary restraint that have inverse associations with weight and weight gain. Here we demonstrate that while compensatory restraint and routine restraint are correlated in our sample (r = .702, p = .001), only routine restraint, which is associated with greater weight and weight gain [24, 25], was associated with differential activation in reward circuitry. The discrepancy between routine restraint and compensatory restraint in the current study is consistent with behavioral findings of dissociation between rigid and flexible dimensions of dietary restraint [24, 25, 38, 39]. However, given that compensatory restraint is negatively correlated with BMI and weight gain in other studies, it is surprising that we did not detect negative associations with brain activity in the present task.
4.1 Directions for future research
One important question for future research is whether the effects observed in this study are specific to food or general responses to images of rewarding stimuli. Because the current study only used food images in the scanner, the present results cannot shed light on whether the patterns of activation associated with routine restraint and emotional eating are food-specific. These brain regions are also engaged by non-food rewards including money [34], alcohol [35], and drugs [8, 36]. Recent work using a monetary task to engage neural activity found associations between activation of brain regions involved in reward processing, self-control, and urges and participants’ actual consumption of vegetables and high-calorie foods [40]. Similarly, youth at risk for obesity show elevated reward circuitry responses to both food and monetary rewards [41]. Thus, altered patterns of activation in these brain regions may reflect increased sensitivity to rewards in general, and not responses that are specific to food.
Additionally, our study did not find any significant associations between eating behaviors and brain regions involved in habitual behavior and reward (e.g., the amygdala and striatum). These results may be due to our choice of task, since other studies have found connections between activity in these brain regions and indices of hedonic eating [e.g., 11, 15, 19, 22, 42, 43]. Go/nogo tasks require reflective control to inhibit responding to nogo items. Thus, using a food-related go/nogo task may have improved our ability to find associations between eating behaviors and inhibitory brain regions, relative to impulsive brain regions. Future studies on the neural correlates of eating behaviors could utilize tasks that more strongly recruit brain regions associated with habitual behavior and reward (e.g., an Implicit Attitude Test or the Iowa Gambling Task).
Finally, it would be interesting for future work to investigate gender differences in the neural correlates of eating behaviors. Significant differences between male and female scores on the WREQ have been reported in behavioral studies [24]. Thus, there may also be different associations between brain activation and eating behaviors in males and females. However, given the small sample size, the present study was not designed to test for these differences.
4.2 Conclusion
The present study is the first to compare neural responses to four different indices of hedonic eating—emotional eating, external eating, compensatory restraint, and routine restraint—simultaneously in a single task. Our findings extend the literature by showing that routine restraint and emotional eating share similar neural correlates in response to the same task. Specifically, both indices are associated with activation in brain regions related to urges, self-control, and reward salience. These brain regions are part of the same reward circuitry that responds to other rewarding, non-food stimuli. Therefore, higher levels of emotional eating and routine restraint may reflect an increased sensitivity to rewards. These results suggest that emotional eating and routine restraint have similar neurocognitive bases and may respond to similar interventions.
Highlights.
Weight-related eating behaviors associated with prefrontal and insula activation.
Differential activity found in response to high-calorie versus low-calorie foods.
Neural activity suggests a dissociation between two dietary restraint subscales.
Results support the construct validity of emotional eating and routine restraint.
Acknowledgements
We would like to thank Alexandra Hollihan and Stephanie Castillo who helped with the data collection. This research was supported by research grants from the National Cancer Institute (R01CA152062 and T32CA009492), the National Heart, Lung, & Blood Institute and the National Institute of Child Health & Human Development (U01HL097839), the National Institute on Drug Abuse (R01DA023051).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There were 30 participants in the fMRI portion of the experiment. The WREQ was added to expand the behavioral measures of the parent study. Study participants who were not given the option of completing the questionnaire at the time of their fMRI scan were contacted to voluntarily respond to the WREQ by online survey. We obtained WREQ data from 20 participants.
The average scores by gender for each subscale were as follows: compensatory restraint: female mean = 2.9, male mean = 2.1; routine restraint: female mean = 2.2, male mean = 1.7; emotional eating: female mean = 2.2, male mean = 2.2; external eating: female mean = 3.1, male mean = 3.3.
Correlations between decision bias and compensatory restraint (r = 0.574, p = 0.010) and routine restraint (r = 0.518, p = 0.023) in the LGo/HNogo task and between false alarms and external eating in the LGo/HNogo task (r = −0.496, p = 0.026) did not survive Bonferroni correction.
When gender was also included (in addition to BMI) as a covariate in the analysis, the pattern of results was also similar. All of the associations that were p < 0.05 without covariates were also p < 0.05 with gender and BMI covariates. The positive associations that were significant after applying a false discovery threshold of q < 0.05 were between emotional eating and left and right DLPFC in the HGo vs. LGo contrast; between routine restraint and ACC, left OFC, and left insula in the HGo vs. LGo contrast; and between emotional eating and left DLPFC in the HGo vs. HNogo contrast. However, we caution that, with only 8 male and 12 female participants, our study was not designed to account for gender differences.
References
- 1.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes. Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Q, Xiao L, Xue G, Wong S, Ames SL, Schembre SM, et al. Poor ability to resist tempting calorie rich food is linked to altered balance between neural systems involved in urge and self-control. Nutr. J. 2014;13:92. doi: 10.1186/1475-2891-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Q, Xiao L, Xue G, Wong S, Ames SL, Xie B, et al. Altered dynamics between neural systems sub-serving decisions for unhealthy food. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood SMW, Bechara A. The neuroscience of dual (and triple) systems in decision making. In: Reyna V, Zayas V, editors. The Neuroscience of Risky Decision Making. American Psychological Association; Washington, D.C.: 2014. [Google Scholar]
- 7.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 9.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 11.Chechlacz M, Rotshtein P, Klamer S, Porubska K, Higgs S, Booth D, et al. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia. 2009;52:524–33. doi: 10.1007/s00125-008-1253-z. [DOI] [PubMed] [Google Scholar]
- 12.Bohon C, Stice E, Spoor S. Female Emotional Eaters Show Abnormalities in Consummatory and Anticipatory Food Reward: A Functional Magnetic Resonance Imaging Study. Int. J. Eat. Disord. 2009;42:210–21. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killgore WDS, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. Int. J. Eat. Disord. 2006;39:357–63. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- 14.Eiler WJA, Dzemidzic M, Case KR, Considine RV, Kareken DA. Correlation Between Ventromedial Prefrontal Cortex Activation to Food Aromas and Cue-Driven Eating: An fMRI Study. Chemosens Percept. 2012;5:27–36. doi: 10.1007/s12078-011-9112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ. Personality Predicts the Brain's Response to Viewing Appetizing Foods: The Neural Basis of a Risk Factor for Overeating. J. Neurosci. 2009;29:43–51. doi: 10.1523/JNEUROSCI.4966-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011;55:233–9. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coletta M, Platek S, Mohamed FB, van Steenburgh JJ, Green D, Lowe MR. Brain activation in restrained and unrestrained eaters: an fMRI study. J. Abnorm. Psychol. 2009;118:598–609. doi: 10.1037/a0016201. [DOI] [PubMed] [Google Scholar]
- 18.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int. J. Obes. 2007;31:440–8. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 19.Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M, et al. Neural correlates of the volitional regulation of the desire for food. Int. J. Obes. (Lond.) 2012;36:648–55. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- 20.Born JM, Lemmens SG, Martens MJ, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. Am. J. Clin. Nutr. 2011;94:392–403. doi: 10.3945/ajcn.111.012161. [DOI] [PubMed] [Google Scholar]
- 21.Schur EA, Kleinhans NM, Goldberg J, Buchwald DS, Polivy J, Del Parigi A, et al. Acquired differences in brain responses among monozygotic twins discordant for restrained eating. Physiol. Behav. 2012;105:560–7. doi: 10.1016/j.physbeh.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demos KE, Kelley WM, Heatherton TF. Dietary Restraint Violations Influence Reward Responses in Nucleus Accumbens and Amygdala. J. Cogn. Neurosci. 2011;23:1952–63. doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanstrien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (Debq) for Assessment of Restrained, Emotional, and External Eating Behavior. Int. J. Eat. Disord. 1986;5:295–315. [Google Scholar]
- 24.Schembre SM, Geller KS. Psychometric Properties and Construct Validity of the Weight-Related Eating Questionnaire in a Diverse Population. Obesity. 2011;19:2336–44. doi: 10.1038/oby.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schembre SM, Greene G, Melanson K. Development and validation of a weight-related eating questionnaire. Eat Behav. 2009;10:119–24. doi: 10.1016/j.eatbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord. 2000;24:1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 28.Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. Practice. 2007 Jun; 2007a. [Google Scholar]
- 29.Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2007 [Google Scholar]
- 30.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 31.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 33.Van Holst RJ, Van Holstein M, Van Den Brink W, Veltman DJ, Goudriaan AE. Response inhibition during cue reactivity in problem gamblers: an fMRI study. PLoS One. 2012;7:e30909. doi: 10.1371/journal.pone.0030909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A. The Iowa gambling task in fMRI images. Hum. Brain Mapp. 2010;31:410–23. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, et al. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav. Brain Res. 2014;274:382–9. doi: 10.1016/j.bbr.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ames SL, Grenard JL, Stacy AW, Xiao L, He Q, Wong SW, et al. Functional imaging of implicit marijuana associations during performance on an implicit association test (IAT) Behav. Brain Res. 2013;256:494–502. doi: 10.1016/j.bbr.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb. Cortex. 2006;16:1679–89. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 38.Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. Int. J. Eat. Disord. 1999;26:53–64. doi: 10.1002/(sici)1098-108x(199907)26:1<53::aid-eat7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Shearin EN, Russ MJ, Hull JW, Clarkin JF, Smith GP. Construct validity of the three-factor eating questionnaire: Flexible and rigid control subscales. Int. J. Eat. Disord. 1994;16:187–98. doi: 10.1002/1098-108x(199409)16:2<187::aid-eat2260160210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 40.He Q, Xiao L, Xue G, Wong S, Ames SL, Xie B, et al. Altered dynamics between neural systems sub-serving decisions for unhealthy food. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of Neuroscience. 2011;31:4360–6. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, et al. Brain dopamine is associated with eating behaviors in humans. Int. J. Eat. Disord. 2003;33:136–42. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- 43.Schur EA, Kleinhans NM, Goldberg J, Buchwald DS, Polivy J, Del Parigi A, et al. Acquired differences in brain responses among monozygotic twins discordant for restrained eating. Physiol. Behav. 2012;105:560–7. doi: 10.1016/j.physbeh.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]