Abstract
Under many conditions spinal motoneurons produce plateau potentials, resulting in self-sustained firing and providing a mechanism for translating short-lasting synaptic inputs into long-lasting motor output. During the acute-stage of spinal cord injury (SCI), the endogenous ability to generate plateaus is lost; however, during the chronic-stage of SCI, plateau potentials reappear with prolonged self-sustained firing that has been implicated in the development of spasticity. In this work, we extend previous modeling studies to systematically investigate the mechanisms underlying the generation of plateau potentials in motoneurons, including the influences of specific ionic currents, the morphological characteristics of the soma and dendrite, and the interactions between persistent inward currents and synaptic input. In particular, the goal of these computational studies is to explore the possible interactions between morphological and electrophysiological changes that occur after incomplete SCI. Model results predict that some of the morphological changes generally associated with the chronic-stage for some types of spinal cord injuries can cause a decrease in self-sustained firing. This and other computational results presented here suggest that the observed increases in self-sustained firing following some types of SCI may occur mainly due to changes in membrane conductances and changes in synaptic activity, particularly changes in the strength and timing of inhibition.
Keywords: Motoneuron model, Self-sustained firing, Persistent inward current, Spinal cord injury model
1 Introduction
Shortly after spinal cord injury (SCI), a cascade of cellular responses is initiated. In addition to the immediate consequences of the impact on axonal damage, this cascade contributes to further tissue damage, including inflammation, accumulation of free radicals, and swelling. The acute-stage following SCI refers to the hours or days after the injury when continued deterioration or tissue damage may occur. In contrast, the chronic-stage refers to the months or years following the initial injury (Ramer et al. 2005). Spasticity, characterized by hyperreflexia, clonus and hypertonic musculature, often develops in the muscles innervated by nerves distal to the injury site during the chronic-stages after SCI and can interfere with normal motor function and strongly affect the quality of life (Adams and Hicks 2005). Spasticity is also observed in multiple sclerosis, cerebral palsy, ischemic brain damage, brain trauma and metabolic diseases, where the degree of spasticity may vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms (NINDS-NIH 2009).
To generate movements, spinal motoneurons must integrate received motor commands and generate sufficient output to produce muscular contraction, where the relation of input to output is determined by neuronal excitability. Spinal cord injury results in changes to both motoneuron morphology and electrical behavior; however, it is not clear how these changes are related or how these changes might interact to affect cell excitability and motor function including spasticity (Lynskey et al. 2008).
In intact animals, spinal motoneurons can produce plateau potentials or sustained depolarizations in order to amplify brief synaptic inputs. The plateau potentials not only amplify synaptic inputs but also provide sustained excitation, allowing motoneurons to exhibit repetitive firing following brief or reduced synaptic excitation (Hounsgaard et al. 1988a; Eken and Kiehn 1989). This bistable behavior endows motoneurons with a mechanism for translating short-lasting synaptic inputs into long-lasting motor output (Heckman et al. 2005) and occurs in normal motor behaviors such as locomotion (Shapiro and Lee 2007; Heckman et al. 2003). A characteristic feature of plateau potentials is that they can be readily interrupted by brief injection of hyperpolarizing current or by synaptic inhibition (Hounsgaard et al. 1988a; Kuo et al. 2003). It is known that plateau potentials are generated by persistent inward currents (PICs) which have been shown to amplify several different sources of sensory input including muscle spindle Ia activation (Lee and Heckman 2000) and inhibition (Kuo et al. 2003), cutaneous excitation (Prather et al. 2002) and recurrent inhibition via Renshaw cells (Hultborn et al. 2003). The effects of PICs on human motoneuron firing patterns are reviewed in Heckman et al. (2008). Functionally, PICs are important for bistable behavior, including production of plateau potentials and self-sustained firing in normal motoneurons (Lee and Heckman 1998a, b; Schwindt and Crill 1982) and chronic spinal animals (Bennett et al. 2001). In mammalian motoneurons, experiments suggest that calcium and sodium PICs both contribute to plateau potentials (Li and Bennett 2003; Heckman et al. 2005; Harvey et al. 2006c), whereas in other species, sodium PICs are thought to play a lesser role (e.g. turtle motoneurons Perrier and Hounsgaard 2003). During the chronic-stage of SCI, voltage-dependent nimodipine-sensitive calcium and TTX-sensitive sodium persistent inward currents are known to cause plateau potentials to reappear (Li and Bennett 2003; Powers and Binder 2003; Li et al. 2004). Data suggest that the ion channels responsible for plateau potentials are located primarily in the dendrites (Hounsgaard and Kiehn 1993; Lee and Heckman 1996; Bennett et al. 1998); however, the precise dendritic spatial distribution of the PIC channels has not been experimentally verified.
It has been suggested that large plateau potentials in motoneurons may play a primary role in the development of muscle spasms and spasticity in rats following SCI (Bennett et al. 1999, 2001). In normal motoneurons, the activation of PICs and associated self-sustained firing are regulated by neuromodulators and can occur due to direct facilitation of persistent calcium currents (Hounsgaard and Kiehn 1989), or by reduction of opposing outward potassium currents (Hounsgaard and Kiehn 1989; Hultborn and Kiehn 1992). During the acute-stage of SCI, the endogenous ability to generate plateaus is lost, likely due to the removal of descending neuromodulatory inputs from the brain stem. Plateaus occur in brain-stem intact decerebrate cats, but after acute spinalization, motoneurons caudal to the injury lose their ability to produce plateaus. However, plateaus reappear after application of external neuromodulators (Hounsgaard et al. 1988a; Hounsgaard and Kiehn 1989). During the chronic-stage of SCI, voltage-dependent nimodipine-sensitive calcium and TTX-sensitive sodium persistent inward currents cause plateau potentials to reappear (Li and Bennett 2003; Powers and Binder 2003; Li et al. 2004), and spasticity is observed (Schwindt and Crill 1980; Hounsgaard and Kiehn 1989; Li and Bennett 2003). A possible explanation for the redevelopment of substantial PICs after chronic SCI is an enhanced sensitivity of the PICs to residual monoamines such as serotonin (5-HT) or norepinephrine (NE) and associated peptides so that their residual transmitters facilitate the PICs (Heckman et al. 2005). Calcium and sodium PICs during chronic-stages are 30 fold supersensitive to 5-HT, appearing to compensate for the loss of brain-stem 5-HT (Harvey et al. 2006a; Li et al. 2007).
Besides changes in membrane currents and the relative balance among different synaptic currents, changes in motoneuron morphometry potentially could influence the development of spasticity after chronic SCI. Features of motoneuron morphology such as size, dendritic architecture, and the extent of arborization are known to affect excitability (Mainen and Sejnowski 1996; Johnston et al. 1997; Jaffe and Carnevale 1999). Morphological changes in motoneurons following SCI depend on the type of injury (transection, contusion or hemisection) and cell location. Experimental studies in rats after S2 level transection suggest that in the acute-stage after SCI there is a transient increase in the length of primary and secondary dendrites, which return to control levels during the chronic-stage. Irrespective of the type of injury, there is a progressive decrease in the average number of primary, secondary and tertiary dendrites during the chronic-stage and a significant decrease in the total dendritic arbor (Gazula et al. 2004; Kitzman 2005; Bose et al. 2005), although a qualitative increase in the primary dendrite diameter has been reported (Bose et al. 2005). For example, it is estimated that the total cell surface area decreases by 11 percent following a chronic thoracic transection (Hochman and McCrea 1994).
Previously, two-compartment conductance based models were used to examine bistable behavior of spinal motoneurons (Booth and Rinzel 1995; Booth et al. 1997). In a later study, a single-compartment spinal motoneuron model was developed to mimic the behavior of rat tail motoneurons after acute and chronic SCI (Graham et al. 2005); however, this model does not accurately replicate some of the frequency response characteristics observed in experimental data following chronic SCI. Data suggest that the ion channels responsible for plateau potentials are located primarily in the dendrites (Hounsgaard and Kiehn 1993; Lee and Heckman 1996; Bennett et al. 1998); hence, in this work a two-compartment model is constructed to investigate the dynamics of spinal motoneuron behaviors corresponding to experimental results for intact spinal cord, acute-stage SCI, and chronic-stage SCI (Lee and Heckman 1998a, b; Schwindt and Crill 1982; Bennett et al. 2001). In particular, parameters corresponding to the absence of plateaus (and absence of bistability) provide a model for the intact spinal cord, where dendritic plateaus are seen or unmasked only when calcium-dependent potassium currents in the dendrite are reduced or when PICs are directly facilitated (Zhang and Krnjevic 1987; Hounsgaard and Kiehn 1989; Li and Bennett 2007). During the acute-stage of SCI, the ability to generate plateaus is greatly diminished corresponding to a large reduction in PICs, which can be replicated in the model. For the chronic stage of SCI, model parameters are chosen so that plateau potentials are present with prolonged self-sustained firing. Published experimental data for injected ramp currents and injected pulse currents under various conditions are used to constrain the model parameters (Hounsgaard et al. 1988a; Bennett et al. 2001; Li and Bennett 2003, 2007; Li et al. 2004; Harvey et al. 2006a, b, c). The model is constructed such that the alterations in electrical and morphological properties seen after SCI that are discussed above can be examined by studying the parameter space of the model. In particular, we extend previous modeling studies of the physiological properties of motoneurons to characterize how dynamic properties depend on morphological parameters. Computational studies are used to systematically explore how the neuroanatomical and the neurophysiological properties of motoneurons interact to influence the generation of plateau potentials and cell excitabilty, with the goal of enhancing our understanding of the mechanisms that contribute to functional deficits following SCI.
2 Methods
Mathematical models for ion channel kinetics presented in this work follow the Hodgkin-Huxley model formalism for an excitable membrane (Hodgkin and Huxley 1952). Experimentally derived data were used to create kinetic models that capture the essential dynamics of specific ion channels known to exist in motoneurons. In general, each ionic current Iion is modeled as
| (1) |
where V is the cell membrane potential (in mV), m and h are the activation and inactivation gating variables, respectively, and y and z are small integers that determine the influence of the gating processes on the conductance. In the case of a non-inactivating current, there is no gating variable h. gion is the maximal conductance for the channel type (in mS/cm2), and Eion is the reversal (Nernst) potential for the corresponding active ion (in mV). The gating kinetics of the ionic conductances are governed by equations of the form
| (2) |
where w∞ (V) is the steady-state activation or inactivation function and τw (V) determines the time scale of the exponential decay or saturation of the dynamic gating processes. The steady state activation and inactivation functions are given by
| (3) |
where θw is the half-activation or inactivation voltage for the gating function (in mV), and kw is the activation or inactivation sensitivity (in mV). In some cases, τw may be independent of V. For more details, see Hille (2001).
Using currents modeled as discussed above, the current balance equation for the excitable membrane is
| (4) |
where Cm denotes the membrane capacitance (in μ F/cm2), Isyn is the synaptic current if present, and Iapp (in μA/cm2) simulates a current injection that mimics the application of injected current during neurophysiology experiments. The details of the synaptic current are described below in Section 2.2.
While the original Hodgkin-Huxley model characterizes voltage-gated ion channels, there are many types of ion channels which are controlled by factors other than membrane potential, including ligand-gated channels, which vary their conductance in response to changes in the intracellular concentration of some other molecule. The most common signaling molecule is calcium, and a widely-used simple model for the changes in intracellular calcium concentration assumes that the concentration increases due to an inward flux of calcium ions through calcium ion channels when calcium currents are activated and that a linear pumping process through the plasma membrane removes calcium ions from the cell (Fall et al. 2002). The total calcium in a cell or cellular compartment consists of free calcium and calcium bound to a buffer. Thus, the equation that represents the dynamics of the calcium concentration [Ca2+] (in μM) is
| (5) |
where λ is the percent of free to bound calcium, α converts the ionic current, ICa, to a rate of change in calcium concentration, and rCa is the removal rate constant due to calcium pumps. In the model, the conductance due to calcium dependent channels is modeled as an instantaneous function of [Ca2+] using a Hill equation in the standard manner. See for example Eq. (11).
2.1 Two-compartment model
The electrical activity of motoneurons is characterized by tonic firing, where the frequency depends on the synaptic activation or the strength of the current injected into the soma during an experiment. Here, a two-compartment model is constructed to aid our understanding of motoneuron dynamics. As in the Booth–Rinzel two-compartment motoneuron model, the two compartments represent the soma and the dendrites of the motoneuron (Booth et al. 1997). Electrotonic coupling between the compartments is modeled using the two parameters gc and p, where gc (in mS/cm2) represents the strength of the coupling and p represents the ratio of somatic surface area to the total surface area of the motoneuron (Pinsky and Rinzel 1994). Due to the lumped dendrite for this two compartment approximation, the soma and dendrite compartments must be considered as phenomenological compartments. Thus, the parameters gc and p cannot be derived directly from geometric and passive electrical neuronal properties. For further discussion of this issue, see Pinsky and Rinzel (1994).
The current balance equation for the soma is provided in Eq. (6). The soma compartment contains a sodium current (INa), delayed rectifier potassium current (IK–dr), an N-type calcium current (ICa–N), a calcium-dependent potassium current (IK(Ca)), and a leak current (IL). The current balance equation for the dendrite compartment is given below in Eq. (7), where ICa–P denotes a persistent calcium current and INa–P denotes a persistent sodium current. Throughout what follows, subscripts S and D correspond to the soma and dendrite compartments respectively.
| (6) |
| (7) |
The intrinsic currents are defined as
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
where the subscript x denotes S when the particular ion channel is in the soma and D when it is in the dendrite. is a dynamic variable representing the intracellular calcium concentration in the soma or dendrite compartment. In each case, the equation governing the changes in mimics Eq. (5). Calcium-activated potassium channels underlying the post-spike afterhyperpolarization are activated by N-type calcium currents and are likely to be located very close to the location of action potential initiation (Li and Bennett 2007). These currents are not affected by channel block that eliminates the persistent calcium currents. Thus, in our model, increases due to ICa–N in the soma and directly affects IK(Ca)S. In contrast, a separate calcium-activited potassium current is activated by the persistent inward calcium current, which are likely not very distant from the calcium PICs (Li and Bennett 2007). Thus, in our model increases due to ICa–P and affects IK(Ca)D only.
The details of the gating dynamics are discussed above in Section 2. Steady state activation and inactivation functions for gating follow the formalism described in Eq. (3). Time scales for the activation or inactivation of gating are independent of the membrane potential, with the exception that
| (15) |
and
| (16) |
Many of the parameter values for the model are derived from the two-compartment Booth–Rinzel motoneuron model (Booth et al. 1997); however, the activation and inactivation time constants for the N-type calcium current have been adjusted to mimic experimental data more closely (Hounsgaard et al. 1988a; Bennett et al. 2001). Since persistent sodium currents are known to contribute to the plateaus observed after spinal cord injury, we include a persistent sodium current with parameters derived from Li and Bennett (2003) and Li et al. (2004). Base parameter values and units for our motoneuron model are provided in Table 1. In Section 3, we vary many of these parameters to explore how the individual ionic currents and their interactions contribute to cell dynamics observed in intact animals and in the acute and chronic-stages following SCI. In many of these simulations, a current ramp is applied to the soma compartment that is governed by the equation
Table 1. Parameter values for the two-compartment model for a spinal motoneuron.
| Symbol | Parameter value | Description |
|---|---|---|
| Cm | 1 μF /cm2 | Membrane capacitance |
| gc | 0.1 mS/cm2 | Coupling conductance |
| gNa | 120 mS/cm2 | Sodium maximal conductance |
| gK–dr | 100 mS/cm2 | Potassium delayed-rectifier maximal conductance |
| gCa–N | 14 mS/cm2 | N-type calcium maximal conductance |
| gK(Ca)S | 3.136 mS/cm2 | Calcium-dependent potassium maximal conductance |
| gK(Ca)D | 0.69 mS/cm2 | Calcium-dependent potassium maximal conductance |
| gL | 0.51 mS/cm2 | Leak maximal conductance in soma and dendrites |
| gCa–P | 0.25 mS/cm2 | Persistent calcium maximal conductance |
| gNa–P | 0.1 mS/cm2 | Persistent sodium maximal conductance |
| ENa | 55 mV | Sodium reversal potential |
| EK | −80 mV | Potassium reversal potential |
| ECa | 80 mV | Calcium reversal potential |
| EL | −60 mV | Leak reversal potential in soma and dendrites |
| ϑmNa | −35 mV | Half activation voltage for sodium |
| ϑhNa | −55 mV | Half inactivation voltage for sodium |
| ϑn | −28 mV | Half activation voltage for potassium |
| ϑmCa–N | −30 mV | Half activation voltage for N-type calcium |
| ϑhCa–N | −45 mV | Half inactivation voltage for N-type calcium |
| ϑmCa–P | −40 mV | Half activation voltage for persistent calcium |
| ϑmNa–P | −25 mV | Half activation voltage for persistent sodium |
| kmNa | −7.8 mV | Activation sensitivity for sodium gating function |
| khNa | 7 mV | Inactivation sensitivity for sodium gating function |
| kn | −15 mV | Activation sensitivity for potassium gating function |
| kmCa–N | −5 mV | Activation sensitivity for N-type calcium gating function |
| khCa–N | 5 mV | Inactivation sensitivity for N-type calcium gating function |
| kmCa–P | −7 mV | Activation sensitivity for persistent calcium gating function |
| kmNa–P | −4 mV | Activation sensitivity for persistent sodium gating function |
| τmCa–N | 16 ms | Activation time constant for N-type calcium |
| τhCa–N | 160 ms | Inactivation time constant for N-type calcium |
| τmCa–P | 40 ms | Activation time constant for persistent calcium |
| τmNa–P | 40 ms | Activation time constant for persistent sodium |
| SCa | 0.2 μM | Half saturation of calcium concentration |
| p | 0.1 | Ratio of somatic surface area to total surface area |
| λ | 0.01 | Percent of free to bound calcium |
| α | 0.009 mol/C/cm | Calcium current to calcium concentration conversion factor |
| rCa | 2 ms−1 | Calcium removal rate |
Many of the parameter values are derived from the Booth–Rinzel motoneuron model (Booth et al. 1997). The activation and inactivation time constants of the N-type calcium current have been adjusted to mimic experimental data more closely (Hounsgaard et al. 1988a; Bennett et al. 2001). We also include a persistent sodium current with parameters derived from Li and Bennett (2003) and Li et al. (2004)
| (17) |
where ts (in ms) represents the time when the ramp switches from the up ramp to the down ramp and H(t) is the Heaviside function. The formulation of the two-compartment model is summarized in the schematic shown in Fig. 1.
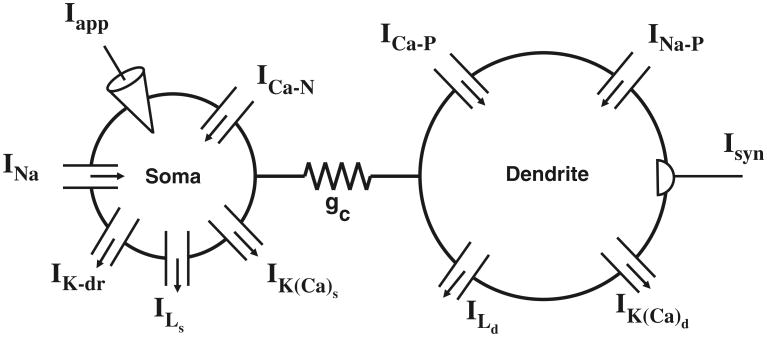
Fig. 1.
Schematic of two-compartment SCI motoneuron model. The densities of the persistent inward currents (ICa–P and INa–P) and densities of the calcium-dependent potassium currents (IK(Ca)S and IK(Ca)D) vary for the simulation studies described in the text
2.2 Modeling the effects of dendritic synaptic input
In addition to alterations in the intrinsic membrane properties of motoneurons, tonic synaptic inputs are also altered following SCI. In order to investigate the interactions between PICs and excitatory and inhibitory tonic synaptic inputs and the effects of these interactions on the firing characteristics of motoneurons, the model includes a synaptic current
| (18) |
The time varying synaptic conductance gsyn is defined by an alpha function (Rall 1967; Segev et al. 1990)
| (19) |
Here ḡsyn is the maximal synaptic conductance (in mS/cm2). Synaptic parameters are constrained by published experimental data such that Esyn = 0 mV and τsyn = 0.2 ms for excitatory synapses (Finkel and Redman 1983), and Esyn = −81 mV and τsyn = 0.65 ms for inhibitory synapses (Stuart and Redman 1990). In the model, ḡsyn = 0.1 mS/cm2 for both excitatory and inhibitory synapses, and the frequency of both excitatory and inhibitory tonic drive are set at 50 Hz (Bui et al. 2006, 2008a, b). Because these synaptic currents represent inputs from multiple synapses to a lumped dendrite compartment, it is not possible to relate the maximal synaptic conductances directly to experimentally derived values.
In what follows, the numerical solutions for the two-compartment model are computed using both MAT-LAB (Mathworks) and XPP (Ermentrout 2002). The MATLAB implementation uses the ode15s or ode23s solver, and the XPP simulations are performed using a time step of 0.05 ms with the QualRK method, which is an adaptive-step fourth order Runge-Kutta method. The steady-state analysis is done using MATCONT (Dhooge et al. 2003).
3 Results
Using this two-compartment model, we replicate the spiking behavior observed experimentally in intact, acute stage and chronic stage spinal cord injured animals (Hounsgaard et al. 1988a; Bennett et al. 2001). The model must be considered to be phenomenological due to its reduced nature; thus, parameters are chosen so that behavioral characteristics are reproduced rather than explicitly comparing results with quantitative experimental recordings. The basic firing properties for our two-compartment motoneuron model are similar to those reported for the two-compartment Booth–Rinzel motoneuron model (Booth et al. 1997). In the model, sodium (INa) and potassium (IK–dr) currents contribute to action potential generation in the standard manner. In addition, the inactivating high threshold, N-type calcium current allows calcium influx during action potentials and IK(Ca)S contributes to the slow afterhyperpolarization (AHP) observed following a spike. These four currents are responsible for action potential generation and the modulation of firing frequency. In the absence of synaptic input, the spike afterhyperpolarization determines spike timing during repetitive firing.
First, we show the effects of PICS and of calcium dynamics in the model in order to demonstrate that the model reproduces many of the known features of motoneurons in a realistic manner. This is followed by consideration of the effects of morphological changes and the interactions between morphological and electrical properties in the model. We also examine the effects of synaptic inputs and their interactions with PICs on model behavior.
3.1 Effects of PICs
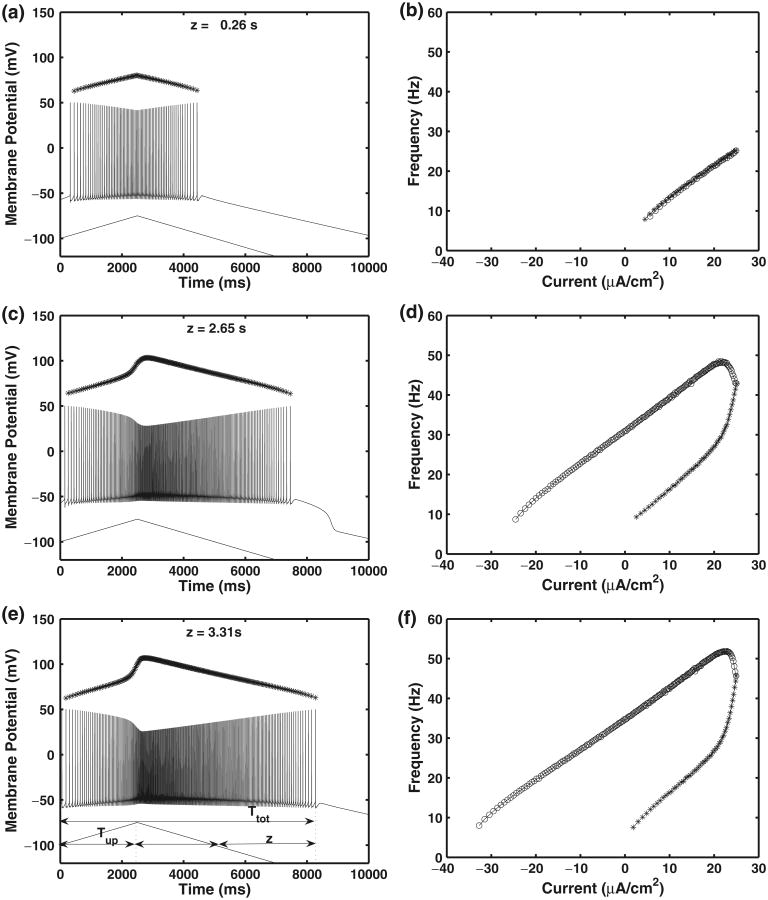
In this motoneuron model, plateau potentials are produced due to the presence of the PICs, ICa–P and INa–P, in the dendrites. However, these dendritic plateau potentials are seen or unmasked only when IK(Ca)D is reduced or the maximal conductances for the PICs (gCa–P and gNa–P) are increased enough to counter the effects of IK(Ca)D. Reduction of IK(Ca)D mimics the effects of the application of the bee venom apamin during experimental studies (Zhang and Krnjevic 1987; Li and Bennett 2007). Figure 2 demonstrates how the firing frequency varies with different values of applied current when the ramp current in Eq. (17) is applied to the soma compartment. These frequency-current response (f-I) characteristics are shown in the absence of plateaus (panels a and b) and when plateau potentials are unmasked (panels c–f). Each of the left panels shows the applied current ramp (bottom curve) which has been shifted by −100 units for clarity, the membrane potential in the soma (middle curve), and the firing rate or frequency (top curve) which has been shifted by +55 units for clarity. Currents are in μA/cm2, membrane potentials are in mV, and firing frequency is in Hz. In order to quantify sustained firing in the model, a measure of sustained firing time, z, is defined such that z = Ttot − 2 * Tup, where Ttot is the total firing time and Tup is the firing time during the up-ramp of the applied current as shown in Fig. 2(e). Thus, z = 0 corresponds to no self-sustained firing, and an increase in z represents a longer period of sustained firing, where z is measured in seconds.
Fig. 2.
Motoneuron model response to ramp current applied in the soma compartment. Each of the panels on the left show the applied current ramp (lower curve), the membrane potential (middle curve) and the firing rate response (upper curve), and the right panels show the frequency of firing response (f-I curves) in each case. Ramp characteristics are described in Eq. (17). In the figures, the ramp is shifted −100 μA/cm2 and the firing frequency is shifted +55 Hz for clarity. In the f-I curves, the asterisks (*) correspond to the upward ramp, and open circles (o) denote the downward ramp. Panels (a) and (b): Motoneuron model without plateau. Parameters values are provided in Table 1. Note the symmetry of response in relation to current. Panels (c) and (d): Model with plateau potential unmasked by decreasing dendritic gK(ca) values, which has been changed to 0.34 mS/cm2 respectively. Plateau results in a pronounced anti-clockwise hysteresis in f-I curve. Panels (e) and (f): Plateau revealed by increasing PIC maximal conductances. Parameter values are the same as in Table 1, except that gCa–P = 0.33 mS/cm2 and gNa–P = 0.2 mS/cm2. The presence of the plateau causes a sustained depolarization and discharge with a pronounced anti-clockwise hysteresis in the f-I curve. Sustained firing time, z, is measured in seconds as z = Ttot − 2 * Tup, where Ttot is the total firing time and Tup is the firing time during the up-ramp of the applied current. Compare to experimental results in Fig. 2 of Bennett et al. (2001) and Fig. 5 of Hounsgaard et al. (1988a)
For the results shown in panels (a) and (b) of Fig. 2, the motoneuron model parameters are set to the values provided in Table 1. In the absence of plateau potentials, there is a symmetry of response in relation to the applied current, and the f-I characteristics show little (clockwise) or no hysteresis. The same general results can be obtained if PICs are completely eliminated by setting gCa–P = 0 mS/cm2 and gNa–P = 0 mS/cm2. In the acute-stage after S2 sacral spinal level injury, the plateau potentials disappear in motoneurons caudal to the site of injury (Bennett et al. 2001); therefore, to model the acute-stage after SCI injury, we can simply decrease the maximal conductances of the PICs in the motoneuron model so that no plateau potentials are seen. Due to the absence of plateaus, the maximal conductances for the calcium-dependent potassium currents only affect the firing frequency.
In panels (c) and (d) of Fig. 2, the plateau potential is unmasked by reducing the maximal conductances of the calcium-dependent potassium current in the dendrite compartment so that gK(Ca)D = 0.34 mS/cm2. Note the pronounced anti-clockwise hysteresis. The upper curve in panel (c) shows the firing frequency, and the abrupt change in slope in this curve reveals the onset of the plateau potential. This behavior is representative of what is seen experimentally when apamin is applied in an intact spinal motoneuron (Zhang and Krnjevic 1987; Hounsgaard et al. 1988b; Li and Bennett 2007). In panels (e) and (f), the plateau is unmasked by increasing PIC maximal conductances. Parameter values are the same as in Table 1, except that gCa–P = 0.33 mS/cm2 and gNa–P = 0.2 mS/cm2. The presence of the plateau causes a sustained depolarization and sustained discharge with a pronounced anti-clockwise hysteresis in the f-I curve.
The voltage and f-I characteristics of the motoneuron model match published experimental data obtained from motoneurons under many experimental conditions, including after SCI. As an example, see Fig. 2 of Bennett et al. (2001) and Fig. 5 of Hounsgaard et al. (1988a). During the chronic-stage of SCI, in the presence of a plateau, the current value where spiking ends is lower than where it begins, which is referred to as self-sustained firing, and the firing frequency reveals an abrupt change in slope with the onset of the plateau potential as replicated in Fig. 2 panels (c) and (e). In addition, corresponding experimental f-I curves are nonlinear with large (anti-clockwise) hysteresis. This feature is also replicated in the motoneuron model as shown in Fig. 2 panels (d) and (f).
As introduced above, mammalian motoneurons experiments suggest that calcium and sodium PICs both contribute to plateau potentials (Li and Bennett 2003; Heckman et al. 2005; Harvey et al. 2006c), whereas in turtle motoneurons, sodium PICs are thought to play a lesser role (Perrier and Hounsgaard 2003). In all cases, the exact biophysically based parameters underlying plateau potentials and self-sustained firing in motoneurons in the chronic-stage of SCI are unknown. In what follows, we simply choose parameters that result in plateau potentials to model a chronic-stage SCI motoneuron. For the chronic-stage SCI motoneuron model, gCa–P = 0.33 and gNa–P = 0.2, where maximal conductances are in units of mS/cm2, and all other parameters are provided in Table 1.
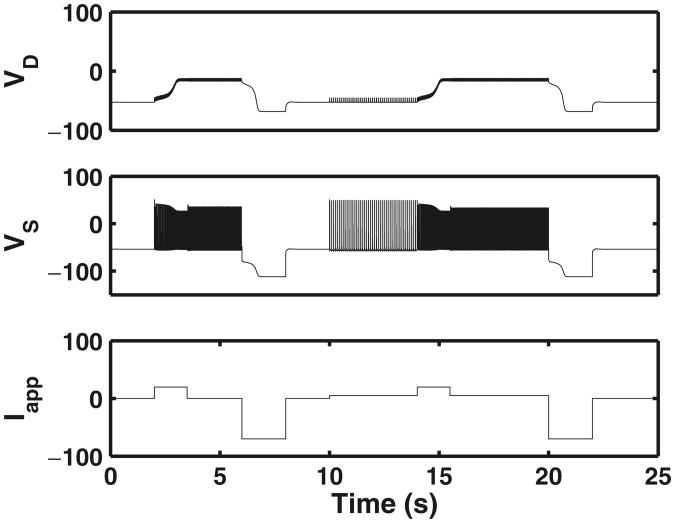
Figure 3 demonstrates the generation of plateau behavior and self-sustained firing with a depolarizing applied current pulse Iapp. The applied current pulse is varied to reveal bistable behavior. The plateau and self-sustained behavior are eliminated on application of a hyperpolarizing current pulse. At the left end of the figure, a depolarizing pulse of +20 μA/cm2 results in the plateau behavior and self-sustained firing. The firing terminates when a hyperpolarizing pulse of −70 μA/cm2 is applied. A pulse of +5 μA/cm2 results in only spiking behavior but no plateaus. On increasing the magnitude of pulse to +20 μA/cm2, plateau behavior and self-sustained firing are seen, which continue even after the pulse magnitude is reduced to +5 μA/cm2. A hyperpolarizing pulse of −70 μ A/cm2 terminates the plateau and self-sustained firing. Similar bistable behavior is seen in Fig. 2(c) of Booth and Rinzel (1995) for the two-compartment Booth–Rinzel model.
Fig. 3.
Bistable motoneuron model behavior in response to current injection in the soma. The lower panel shows applied current (Iapp), and membrane potentials as measured in soma (VS) and dendrite (VD) are shown in middle and upper panels, respectively. At the left end of the figure, a depolarizing pulse of +20 μ A/cm2 results in the plateau behavior and self-sustained firing. The firing terminates when a hyperpolarizing pulse of −70 μA/cm2 is applied. A pulse of +5 μA/cm2 results in only spiking behavior but no plateaus. On increasing the magnitude of pulse to +20 μA/cm2 we see plateau behavior and self-sustained firing which continues even after the pulse magnitude is reduced to +5 μA/cm2. A hyperpolarizing pulse of −70 μA/cm2 terminates the plateau and self-sustained firing. Similar bistable behavior is also shown in Fig. 2(c) of the two-compartment Morris–Lecar based Booth–Rinzel model Booth and Rinzel (1995)
It is important to note that there are two types of bistability in this model. Figure 4 demonstrates the two types of bistability seen during a ramp current. The first region, labeled bistability 1 in the figure, consists of resting and spiking states, where the spiking occurs due to self-sustained firing seen during the down-ramp current. The rest state occurs when the soma is at rest in the absence of a dendritic plateau; that is, when the dendrite is off. The spiking state of this region occurs when the dendrite is on; that is, the dendritic plateau appears and firing frequency is in the tertiary range of firing due to self-sustained firing. The second region, labeled bistability 2 in the figure, corresponds to the classical bistable behavior where two different stable firing states can be seen for the same level of injected current. This region consists of primary, secondary and tertiary frequency firing states. The primary range of firing occurs when the dendrite is off during the up-ramp, the secondary range occurs during the transition from off to on when the dendritic plateau is initiated, and the tertiary range of firing frequency occurs when the dendrite is on.
Fig. 4.
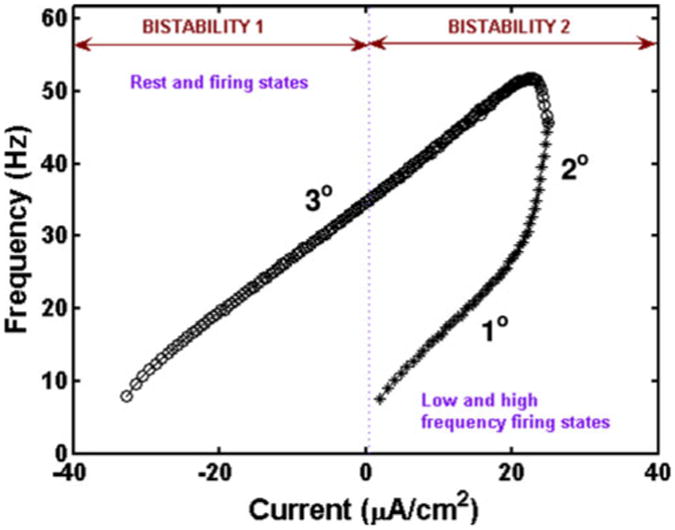
Bistability in SCI motoneuron model with applied ramp current in soma. Bistability 1 is obtained with dendrite OFF: soma at rest or dendrite ON: soma at 3° range of firing frequency. Bistability 2 is characterized by low and high frequency firing states. It is obtained with dendrite OFF: soma at 1° frequency range and with dendrite ON: soma at 3° frequency range. As before, the asterisks (*) correspond to the upward ramp, and open circles (o) denote the downward ramp
3.2 Calcium dynamics
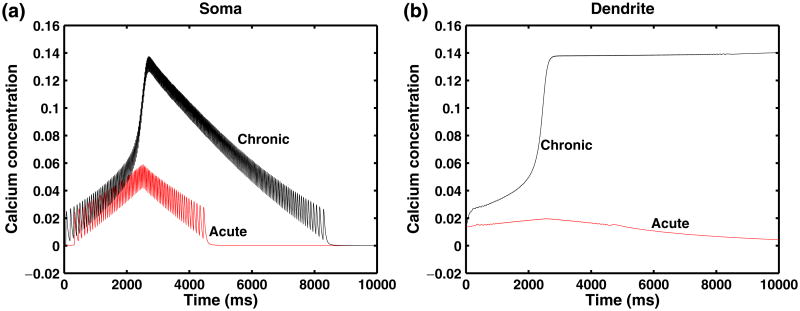
Examining the calcium dynamics during simulations based on the parameters in Table 1, we find that when gCa–P = 0 mS/cm2, calcium enters the cell during each spike due to N-type calcium channel in the soma, but there is no calcium accumulation over a longer time scale in the soma or the dendrites as shown in Fig. 5. However, in the chronic-stage SCI model, the influx of calcium due to the persistent inward calcium current in the dendrite leads to calcium accumulation (Fig. 5) in the dendritic compartment. The increased firing frequency due to the dendritic plateau leads to elevated concentrations of intracellular calcium in the somatic compartment as well, which causes increased activation of the calcium-dependent potassium current in both soma and dendrites. The intrinsic rate of calcium removal, while dependent on the calcium concentration, does not compensate for the increased calcium influx.
Fig. 5.
Calcium concentration in the SCI motoneuron model with application of somatic ramp current. Left panel shows the calcium concentration in the soma compartment, and right panel shows the calcium concentration in the dendrite compartment. In the absence of plateaus using parameters in Table 1, there is no buildup of calcium concentration (acute-stage). However, in the chronic-stage SCI motoneuron model with plateau potentials, there is calcium accumulation in both the soma and dendrite. Ramp characteristics are provided in Eq. (17) and are the same as in Fig. 2
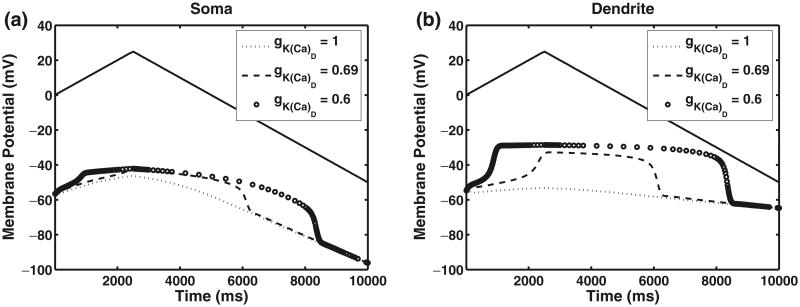
Increasing the maximal conductance for the persistent calcium current, gCa–P, increases the amplitude of the plateau potentials, resulting in increased hysteresis. Both the shape and the amplitude of the plateau are affected by the maximal conductance, and the effects are more pronounced in the dendrite compartment where the calcium PIC is located. The magnitude and duration of plateaus also depend on the hyperpolarizing IK(Ca)D current. The effect of changing dendritic calcium-dependent potassium conductance values in the chronic case is shown in Fig. 6. The left panel shows the effects in the soma compartment, while the right panel shows the effects of variation in the dendrite compartment. Decreasing the calcium-dependent potassium conductance value in the dendrite increases the plateaus. In the chronic-stage SCI model, increasing the hyperpolarizing effects of IK(Ca)D can cause the disappearance of plateaus. In addition, the accumulation of calcium which activates IK(Ca)D is dependent on the calcium PIC and the calcium removal rate. For example, increasing or decreasing the removal rate for calcium could affect the nature of plateaus, furthering them or annihilating them due to subsequent changes in the dynamics of IK(Ca)D.
Fig. 6.
Effects of variation of calcium-dependent potassium maximal conductance values. gNa = 0 mS/cm2 and gNa–P = 0 mS/cm2, mimicking the effects of TTX. gCa–P = 0.33 mS/cm2 in all figures, and all other parameters are provided in Table 1. The dashed line represents the applied ramp current in the soma. Ramp characteristics are provided in Eq. (17). The left panel shows the membrane potential in the soma compartment while the right panel shows the membrane potential in the dendrite compartment as gK(Ca)D is varied
3.3 Effects of morphological changes
As discussed in Section 1, changes in motoneuron morphology can occur after SCI, including a reduction in the dendritic arbor and changes in soma size (Gazula et al. 2004; Bose et al. 2005; Kitzman 2005). Dendrites make up more than 95% of the total membrane area of α-motoneurons, and over half of the area is greater than 500 μm from the soma (Cullheim et al. 1987). In earlier studies, equivalent cable models have been used to characterize α-motoneurons morphologically and electrophysiologically (Cullheim et al. 1987; Fleshman et al. 1988); however, it can be difficult to understand the effects of parameter variation in these models. With this two-compartment model we are able to understand some of the basic effects of cell morphology on cell excitability and how they affect sustained firing. In the two-compartment model, the parameter p, which represents the ratio of somatic surface area to the total surface area of the motoneuron, can be varied to mimic experimentally-observed changes in cell morphology. Slight increases in p correspond to small increases in the surface area of the soma and/or to small decreases in the dendritic surface area. Because gc represents the coupling conductance between the soma and dendrite compartments in the model, the effects of changes in p will depend on the value of gc. Note that changes in morphology may also affect gc since an increase in the diameter of the primary dendrite may increase the coupling conductance between the soma and the dendrite. Thus an exploration of the roles of p and gc and their interactions in the model will provide predictions about the effects of changes in morphology observed after SCI. As mentioned in the Methods section, due to the lumped dendrite approach of this two compartment approximation, the soma and dendrite compartments must be considered as phenomenological compartments. Thus, the parameters gc and p cannot be derived directly from geometric and passive electrical neuronal properties, and it is not possible to determine exact values for these parameters. Hence, attempts to classify the p parameter space into acute and chronic-stage SCI operating regions would not be accurate. Thus, the goal of these simulation studies with variation of p is to study the effects of relative changes in the size of soma and dendrites on self-sustained firing in order to understand interactions among the different currents and the role of the separation of currents. With this in mind, we find that all of the results described here are quite robust.
As described above, plateau potentials can be unmasked by either reducing the dendritic calcium-dependent potassium conductance value or increasing the persistent calcium and sodium conductance values. Figure 7 shows how self-sustained firing depends on both the morphological and electrical properties of the cell. Note that gCa–P must be above a threshold value for PIC activation and that self-sustained firing is only seen for lower values of p (upper panel). In contrast, gK(Ca)D must be below a threshold value in order to observe PIC activation, and self-sustained firing is only observed for lower p values (lower panel). As p varies, the relative number of the different channel types vary due to the changes in the relative surface areas of the somatic and dendritic compartments. In both the upper and lower panels of Fig. 7, the lack of self-sustained firing for higher values of p reflects the smaller influence of the dendrite on spiking dynamics for larger p.
Fig. 7.
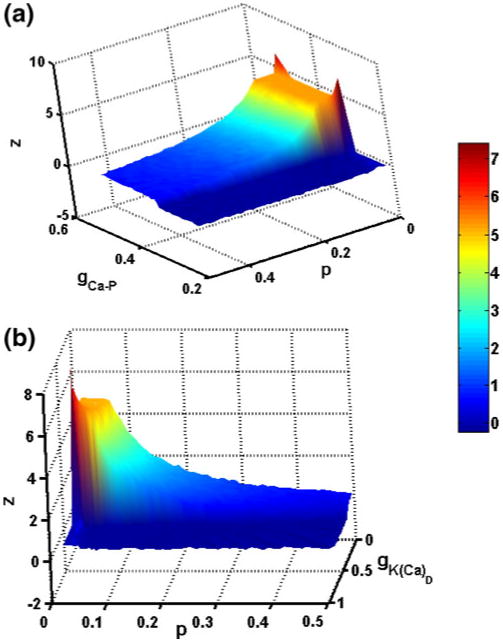
Sustained firing time z with different parameter values. Panel (a) shows z as gCa–P and p parameter values are varied in the chronic-stage SCI motoneuron model. Simulations were performed for gCa–P values between 0.21 and 0.5 mS/cm2 and p values between 0.01 and 0.5 at steps of 0.01. As p and gCa–P increase, the sustained firing time decreases. The gCa–P values have to be above a threshold for the plateau behavior to occur. Panel (b) shows z as gK(Ca)D and p parameter values are varied in the chronic-stage SCI motoneuron model. Simulations were performed for gK(Ca)D values between 0.11 and 0.74 mS/cm2 and p values between 0.01 and 0.5 at steps of 0.01. As p and gK(Ca)D increase, the sustained firing time decreases. The gK(Ca)D values have to be below a threshold for the plateau behavior to occur. In both panels, z = Ttot − 2 * Tup, where Ttot is the total firing time and Tup is the firing time during the up-ramp of the applied current. z values were interpolated between calculated values
A surface plot showing z as the two parameters gc and p are varied together in the chronic-stage SCI motoneuron model is provided in Fig. 8. Figure 9 depicts a two dimensional projection of this plot showing different firing regimes for the same parameter values. The firing regimes correspond to a sustained firing region, a region with spikes but no sustained firing, a region with no spiking, and a small region with a plateau potential but no sustained firing. The threshold for classifying motoneuron behavior as sustained firing is set at z = 0.067 s instead of zero to account for the few cases where an additional spike appears just before firing ceases. The panels below show examples of the membrane potential characteristics in the different firing regimes.
Fig. 8.
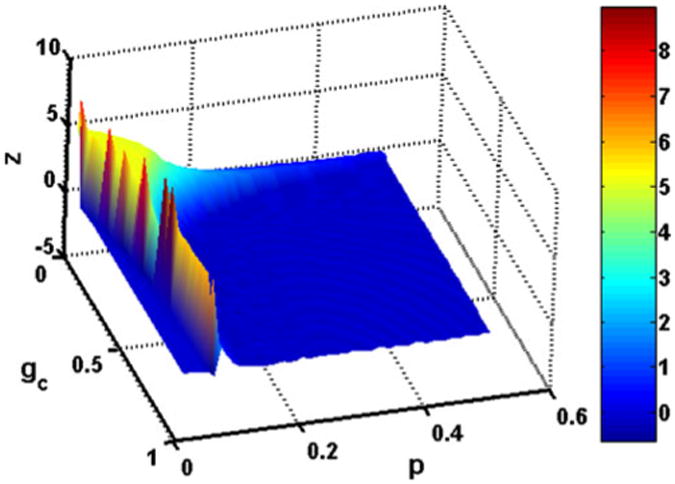
Sustained firing time z as gc and p parameter values are varied in the chronic-stage SCI motoneuron model. Simulations were performed for gc values at steps of 0.02 mS/cm2 and p values at steps of 0.01. z = Ttot − 2 * Tup, where Ttot is the total firing time and Tup is the firing time during the up-ramp of the applied current. z values were interpolated between calculated values. As p and gc increase, the sustained firing time decreases. Corresponding regions of different firing characteristics are shown in Fig. 9
Fig. 9.
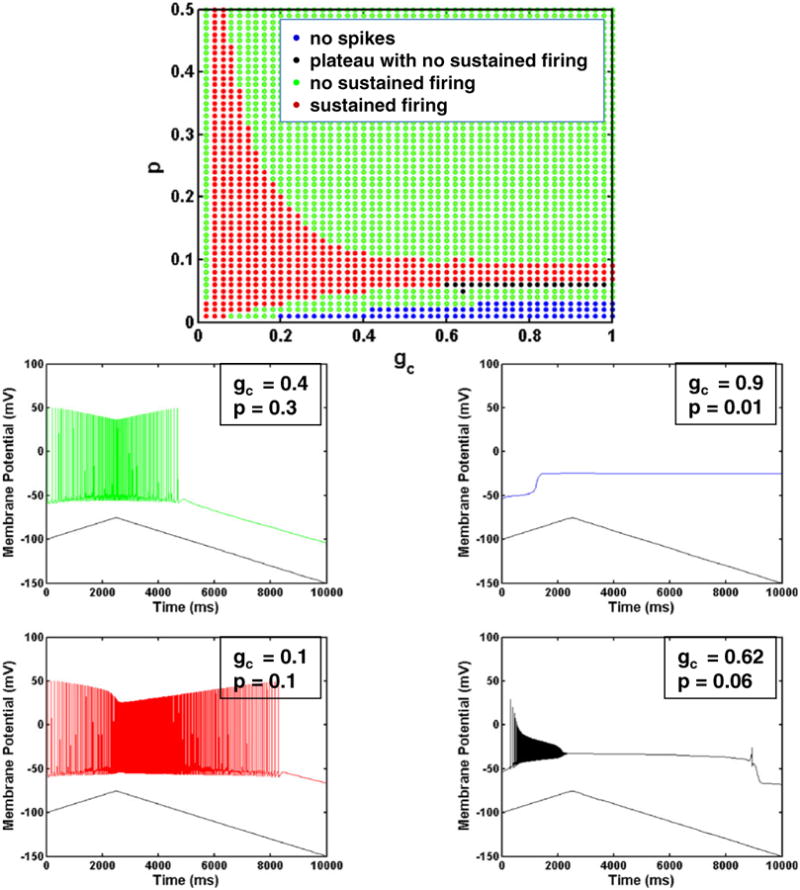
gc versus p plot. Figure shows firing characteristics of different regions as gc and p are varied in the chronic-stage SCI motoneuron model. The side panels show examples of somatic membrane potentials corresponding to the different parameter regions with different firing characteristics
Note that for extremely small values of p corresponding to very small relative values of somatic surface area, the model loses its ability to spike for a large range of gc values. This is due to the very limited surface area in the soma where spike generating currents are located. For slightly larger p (around 0.1), there is a broad range of gc values that result in self-sustained firing. Thus, for this narrow range of small p values, the dendritic membrane properties dominate, and self-sustained firing is seen for both weak and strong coupling between the compartments. Note that this result also implies that for a very limited parameter regime, only a single compartment model is needed to see self-sustained firing. The choice of p determines the relative values of the maximal conductances of currents in the soma and dendrite compartments.
As p increases and the relative size of the soma compartment increases, self-sustained firing can only be seen with small coupling conductance values. These small coupling conductance values ensure that the dendrite and soma compartments remain isolated so that the soma is not able to dominate the dynamics in the dendrite. Previous modeling studies have shown that only a weak coupling conductance value generates bistable firing behavior (Booth and Rinzel 1995; Lee and Heckman 1996). Note that in general, an increase in p with increased coupling conductance gc does not increase the sustained firing time, but rather has the opposite effect as shown in Fig. 8. Thus, these modeling results suggest that changes in morphology that are observed experimentally following some types of SCI such as increased soma size, decreases in the dendritic arbor, and increases in the diameter of the primary dendrites may not contribute to increases in self-sustained firing seen following SCI.
Note that the reduced nature of the two compartment approach is not able to accurately reflect the complexity of the changes in dendritic morphology seen after SCI. Although the simplicity of this approach allows for a complete characterization of the role of specific parameters, the details of branching patterns in the dendrites could have additional effects. Recent work demonstrates that both dendritic size and topology can affect cell firing characteristics (van Elburg and van Ooyen 2010). Studies of changes in dendritic topology will be the subject of future work.
3.4 Mathematical structure of the model
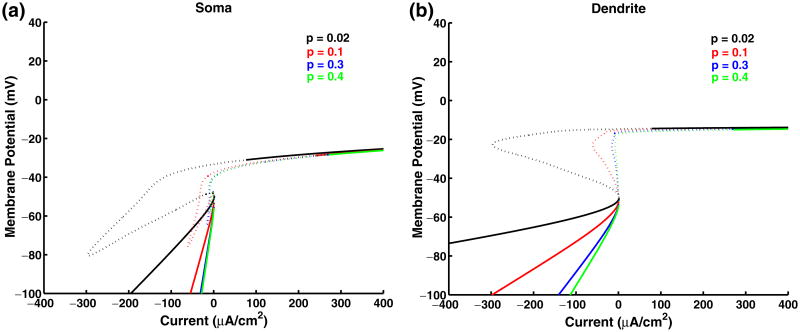
Even though the model has only two current balance equations, it consists of ten ordinary differential equations and 40 different parameter values. Due to the complicated nature of the model, a detailed bifurcation analysis was not performed. However, the bistable nature of the model can be understood by examining how the steady state varies with applied current. The steady-state curves for the somatic and dendritic compartments for the chronic-stage model with different p values are shown in Fig. 10. The solid portion of the curve corresponds to stable steady state values, while the dotted portion corresponds to unstable steady state values. The mathematical structure is similar to that of the minimal model of Booth and Rinzel for moderate coupling (as shown in Fig. 4(c) of Booth and Rinzel 1995). The response to the ramp input current illustrated in Fig. 2 panels (c) or (e) can be understood by examining the S shaped steady state curve seen in Fig. 10 for small p values. As in the Booth–Rinzel minimal model, if we plot the maximum and minimum membrane potentials during repetitive firing (not shown here for clarity), we see two distinct envelopes for low p values. The two envelopes correspond to the scenarios when the dendrite is off or on as described in Section 3.1. With the motoneuron model starting from rest, as the applied current increases, a bifurcation occurs at the lower knee of the curve and firing begins. Since the dendritic plateau has not been initiated, the dendrite is off. As the applied current continues to increase, the plateau is initiated, the dendrite is on, and the membrane potential moves to the upper envelope. When the down ramp begins, the applied current decreases and the hysteresis is seen as in the Booth–Rinzel model, resulting in self-sustained firing.
Fig. 10.
Steady-state characteristics. Panel (a) and (b) show the steady-state curves in the soma and dendrite respectively as the applied current in the soma is varied. gc = 0.1 in all the cases, and p varies as indicated in the legend. The solid curve corresponds to the stable region and the dotted curve the unstable region
It is interesting to note that there is a change in the mathematical structure of the model as p increases. As p increases, the steady state curves lose the characteristic S shape associated with hysteresis. In this case, the two envelopes overlap. The same result is seen for the model parameters associated with the acute-stage model. Thus, the difference in the spiking region with dendrite off and dendrite on gives an explanation for the hysteresis in the chronic-stage SCI motoneuron, while the overlapping envelopes explain the symmetry observed in the acute-stage SCI motoneuron or for larger values of p.
3.5 Effects of synaptic inputs
Most of the synaptic inputs to motoneurons occur in the dendrites (Fleshman et al. 1988; Fyffe 2001). The influence of dendritic synaptic input depends on the electrotonic characteristics of the dendritic trees which in turn depends on the morphology, specific resistivity, capacitance and cytoplasmic resistivity, where synapses effectively rescale the cable properties of the dendritic tree (Segev and London 2000). Several interesting experiments have focused on the interactions between tonic synaptic input and PICs. In particular, Bennett et al. (1998) obtained intracellular recordings from hindlimb motoneurons in decerebrate cats to investigate how synaptic inputs affect the threshold for activating plateau potentials when current is injected into the soma. They found that excitatory synaptic inputs significantly lowered the threshold for plateau activation, and conversely, synaptic inhibition raised the plateau threshold. Hence, PICs may play a critical role in modulating the effects of synaptic inputs.
In the presence of injected current ramps in the motoneuron model with the plateau unmasked, excitatory synaptic inputs decrease the threshold frequency for plateau activation while inhibitory synaptic inputs have the opposite effect as shown in Fig. 11 panels a–c. Plateau onset can be seen with the rapid rise in frequency along the up-ramps in the upper panels. If we account for the shift on the current axis due to the different synaptic biases as shown in panel d of Fig. 11, the duration of sustained firing is unaltered in the chronic-stage SCI motoneuron model when either excitatory or inhibitory synaptic inputs are included during the presentation of the current ramp. As in the experimental results described above, dendritic PICs provide substantial amplification of excitatory synaptic inputs and can be viewed as dramatically enhancing the efficacy of inhibition (Powers and Binder 2000). That is, when inhibitory inputs in the dendrite prevent the activation of PICs, they dramatically affect the firing frequency and presence of self-sustained firing of the cell.
Fig. 11.
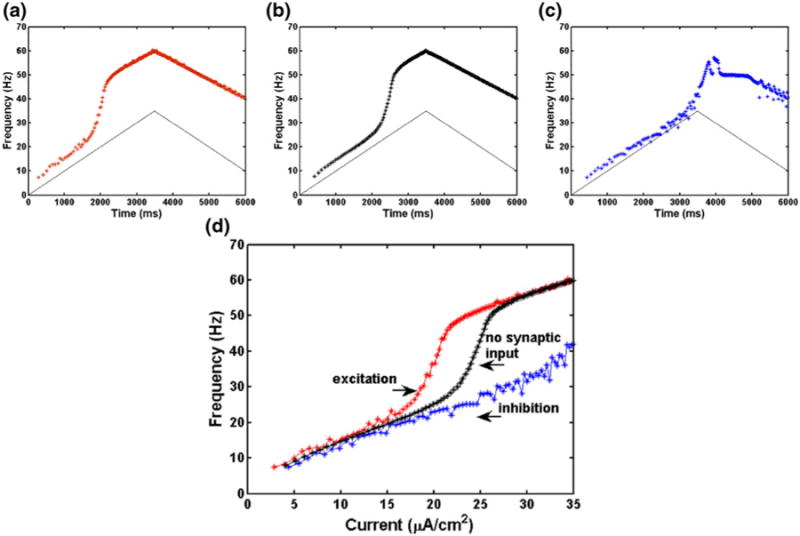
Results for chronic-stage SCI motoneuron model with both somatic applied ramp current and synaptic input in the dendrite compartment. Panel (a): 50 Hz excitatory synaptic input. Panel (b): no synaptic input. Ramp current only. Panel (c): 50 Hz inhibitory synaptic input. Panel (d): results for excitatory synaptic input (left red curve), no synaptic input (middle black curve), and inhibitory input (right blue curve) as a function of injected current for the increasing part of the ramp only. Compare results to Fig. 2 of Bennett et al. (1998). Panels (a)–(d): model parameters are the same as in Table 1 except ϑmNa = −34 mV. See text for synaptic parameters
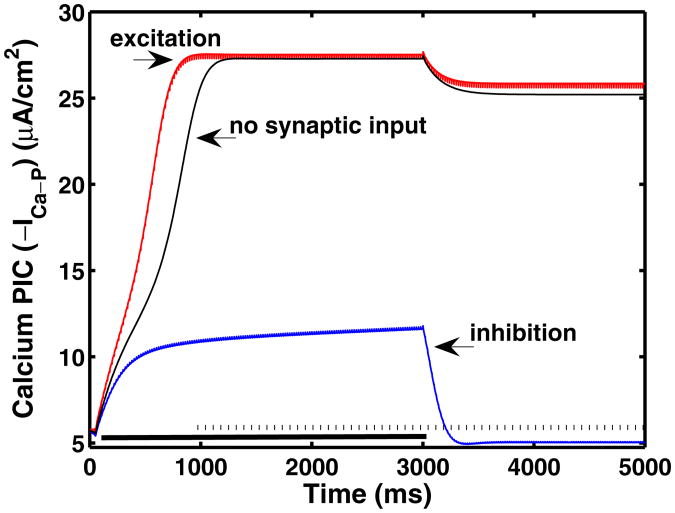
Figure 12 demonstrates the strong modulation of PICs by inhibition. In these simulations, a current pulse of 20 μA/cm2 is applied to the soma compartment for the first 3,000 ms during the period indicated by the solid bar along the time axis. The resulting persistent current (−ICa–P) is shown for three different simulations. One simulation (middle black curve) corresponds to the applied current in the soma with no synaptic input. The additional two curves show the persistent current when the applied current is supplemented by 50 Hz excitatory (left red curve) or inhibitory (right blue curve) synaptic input that begins at 1,000 ms as indicated by the dashed bar along the time axis. Note that without synaptic input, the current pulse that is applied to the soma is strong enough to induce a plateau potential, and thus self-sustained firing occurs after the applied current is removed. The excitatory input shifts the initiation of the plateau to the left indicating a slight advancement in time of onset. However, the inhibitory input keeps the persistent current below the threshold for plateau initiation, eliminating sustained firing. The timing of this small inhibitory input is critically important; if it begins after the plateau is initiated, the motoneuron still exhibits self-sustained firing.
Fig. 12.
Results for chronic-stage SCI motoneuron model with both somatic applied pulse current and synaptic input in the dendrite compartment showing −ICa–P μA/cm2 versus time. Solid bar indicates 20 μA/cm2 current applied to soma compartment. Dashed bar indicates 50 Hz synaptic input to dendrite compartment. For excitatory input, gSyn = 0.1 mS/cm2, while for inhibitory input gSyn = 0.05 mS/cm2. All other parameters are the same as in Fig. 11
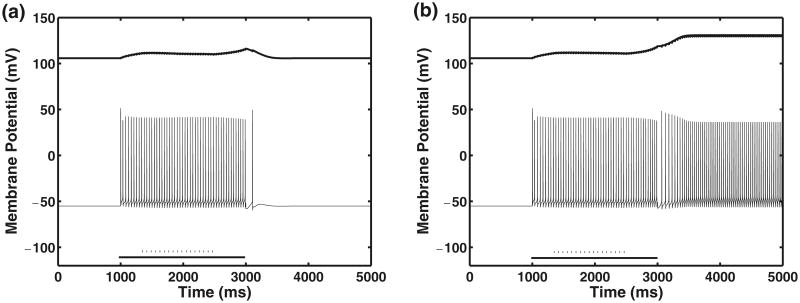
As the applied current in the soma increases, self-sustained firing occurs for increased range of inhibitory conductance values. Also, the amplitude of the PIC is inversely proportional to the strength of the inhibitory input as was shown experimentally (Kuo et al. 2003). Simulation studies also show the effects of the duration of inhibition on self-sustained firing by varying the the time at which the inhibition is switched on and switched off when a current pulse of 20 μA/cm2 is applied to the soma compartment. This two dimensional parameter variation reveals that the inhibitory synaptic input must be initiated before the plateau begins in order to prevent self-sustained firing. An example is shown in Fig. 13 where a 10 ms delay in the time at which the inhibitory pulse is initiated affects the occurrence and persistence of self-sustained firing. The inhibitory pulse is switched on at 1,340 ms in the left panel and at 1,350 ms in the right panel. The inhibitory pulse is switched off at 2,500 ms in both cases. In these simulations, a current pulse of 20 μA/cm2 is applied to the soma compartment from 1,000 to 3,000 ms during the period indicated by the solid bar along the time axis.
Fig. 13.
Effect of timing of inhibitory synaptic input on self-sustained firing. Figure shows results for chronic-stage SCI motoneuron model with somatic applied pulse current and synaptic input in the dendrite compartment. Panels (a) and (b) show −ICa–P (μA/cm2) versus time (upper curve) and membrane potential versus time (lower curve). In both panels, the persistent calcium current is shifted +100 μA/cm2 for clarity. Solid bar indicates 20 μA/cm2 current applied to soma compartment. Dashed bar indicates 50 Hz inhibitory synaptic input with gsyn = 0.05 mS/cm2 applied to dendrite compartment. The inhibitory pulse is switched on at 1,340 ms in the left panel, at 1,350 ms in the right panel and switched off at 2,500 ms in both panels. Panels (a) and (b): Model parameters are the same as in Table 1 except ϑmNa = −34 mV
4 Discussion
Motoneuron dendrites are very thin and are also populated with ion channels which furnish a rich repertoire of electrical behaviors. Thus, they function as electrically and chemically distributed nonlinear units which has important consequences for neuron behavior (Segev and London 2000). In addition, dendritic morphology, synapses and ion channels can undergo activity-dependent modulation. Following spinal cord injury, the sudden loss of descending input leads to substantial changes at the level of primary afferents, interneurons, and motoneurons, thus dramatically influencing sensorimotor interactions. At the cellular level, it is not known how these changes to motoneuron morphology, membrane properties, and synaptic inputs interact to affect motor function including spasticity; however, it is possible that the plateau potential and prolonged self-sustained firing may contribute to the increase in spasticity following sensory stimuli observed in human SCI patients (Gianano et al. 1998). In this work, we extend previous modeling studies of the physiological properties of motoneurons to characterize how dynamic properties depend on morphological parameters. Computational studies are used to systematically explore how the neuroanatomical and the neurophysiological properties of motoneurons interact to influence the generation of plateau potentials and cell excitabilty and to study the effects of changes in these properties following SCI.
The two-compartment motoneuron model presented here replicates many of the firing characteristics of motoneurons under a variety of conditions and contributes to our understanding of the role of individual ion channels in determining motoneuron behavior following SCI. The model also reveals the critical role of PICs in self-sustained firing. While earlier models focus on the role that monoaminergic inputs play in inducing PICs and bistable firing patterns (Gutman 1991; Lee and Heckman 1996; Booth and Rinzel 1995; Booth et al. 1997), in this work we show how these patterns depend on the interactions among PICs, intracellular calcium, and calcium-dependent potassium currents. In addition, simulation results suggest that the morphological changes seen in motoneurons in the chronic-stage following some types of SCI may tend to decrease self-sustained firing. The model also provides insight into the role of synaptic inputs in modulating PICs and how precisely timed inhibition can eliminate sustained firing. Thus, model results suggest that increases in self-sustained firing following SCI are likely to occur due to changes in membrane conductances and changes in synaptic activity, particularly changes in the strength and timing of inhibition.
4.1 Relation to previous modeling studies and assumptions
Previously, computational models of motoneurons were constructed to understand normal firing behavior in response to intracellular current injection or synaptic activation (Schwindt and Crill 1984; Binder et al. 1996; Vieira and Kohn 2007). Additional models have simulated the generation of excitatory postsynaptic potentials (Segev et al. 1990), investigated bistable firing patterns (Gutman 1991; Booth and Rinzel 1995; Lee and Heckman 1996), or examined complex firing patterns due to ion channel blockers and neurotransmitters (Booth et al. 1997). Like these earlier models, the model presented here explores the contribution of conductances in the generation of plateau potentials and bistable firing patterns; however these earlier modeling studies do not focus on changes that occur after spinal cord injury. Models of motoneurons following spinal cord injury include a single-compartment model with a limited repertoire of dynamic behaviors (Graham et al. 2005) and more complex multi-compartment models that replicate certain behaviors but are difficult to analyze mathematically (ElBasiouny et al. 2005; Bui et al. 2006).
Data suggest that the ion channels responsible for plateau potentials are located primarily in the dendrites (Hounsgaard and Kiehn 1993; Lee and Heckman 1996; Bennett et al. 1998). Thus, in this work a two-compartment model is constructed to investigate the dynamics of spinal motoneurons corresponding to experimental results for intact spinal cord, acute-stage SCI, and chronic-stage SCI. In the model, sodium and potassium currents in the somatic compartment contribute to action potential generation in the standard manner. In addition, an inactivating high threshold, N-type calcium current allows a calcium-dependent potassium current in the soma to contribute to the slow afterhyperpolarization observed following a spike, which also modulates firing frequency. As in mammalian motoneurons (Li and Bennett 2003; Heckman et al. 2005; Harvey et al. 2006c), calcium and sodium PICs in the dendrite both contribute to plateau potentials. Since the calcium-activated potassium current responsible for AHP is not affected by channel block that eliminates the persistent calcium currents (Li and Bennett 2007), in our model somatic calcium increases only due to the N-type calcium current in the soma compartment. Because it is known that a separate calcium-activited potassium current is activated by the persistent inward calcium current in the dendrite, dendritic calcium increases due to the calcium PIC and affects the calcium-dependent potassium current in the dendrite only. In general, the bistable nature of the model and the hysteresis in the threshold current required for repetitive firing can be understood by examining how the steady state varies with applied current, where the mathematical structure is similar to that of the minimal model of Booth and Rinzel with moderate coupling (Booth and Rinzel 1995).
4.2 Predicted effects of morphological changes
We examine the dependence of model behavior on morphological parameters, where the parameter p represents the ratio of somatic surface area to the total surface area of the motoneuron. Thus, slight increases in p correspond to small increases in the surface area of the soma or to small decreases in the dendritic surface area. Because gc represents the coupling conductance between the soma and dendrite compartments in the model, the effects of changes in p will depend on the value of gc. Note that changes in morphology may also affect gc since an increase in the diameter of the primary dendrite may increase the coupling conductance between the soma and the dendrite. Generally, the morphological changes associated with many types of chronic-stage spinal cord injury such as increased soma size, decreases in the dendritic arbor, and increases in the diameter of the primary dendrites, correspond to increases in p and gc. As mentioned above, due to the lumped dendrite for this two compartment approximation, the soma and dendrite compartments must be considered as phenomenological compartments. Thus, the parameters gc and p cannot be derived directly from geometric and passive electrical neuronal properties, and it is not possible to determine exact values for these parameters.
Note that for extremely small values of p corresponding to very small relative values of somatic surface area where spiking initiates, the model loses its ability to spike for a large range of gc values. This is due to the limited surface area in the soma where spikes are generated. For slightly larger p (around 0.1), there is a broad range of gc values that result in self-sustained firing. Thus, for this very small region of p values, the dendritic membrane properties dominate and self-sustained firing is seen for both weak and strong coupling between the compartments. This range is consistent with the fact that a single compartment model can exhibit self-sustained firing for a small range of parameters. As p increases and the relative size of the soma compartment increases, self-sustained firing can only be seen with small coupling conductance values. These small coupling conductance values ensure that the dendrite and soma compartments remain isolated so that the soma is not able to dominate the dynamics in the dendrite. Previous modeling studies have shown that only a weak coupling conductance value generates bistable firing behavior (Booth and Rinzel 1995; Lee and Heckman 1996). Note that in general, an increase in p with increased coupling conductance gc does not increase the sustained firing time, but rather has the opposite effect as shown in Fig. 8. The change in shape of the steady-state curves for the model revealed in Fig. 10 demonstrates the mathematical basis for this result. In particular, larger p values result in a loss of the characteristic “S” shape in the steady-state curve commonly associated with bistability. Thus, the modeling results suggest that changes in morphology that are observed experimentally following some types of SCI such as increased soma size, decreases in the dendritic arbor, and increases in the diameter of the primary dendrites may not contribute to increases in self-sustained firing.
Note that the reduced nature of the two compartment approach is not able to accurately reflect the complexity of the changes in dendritic morphology seen after SCI. Although the simplicity of this approach allows for a complete characterization of the role of specific parameters, the details of branching patterns in the dendrites could have additional effects. Recent work demonstrates that both dendritic size and topology can affect cell firing characteristics (van Elburg and van Ooyen 2010). Studies of changes in dendritic topology will be the subject of future work.
4.3 Predicted effects of synaptic changes
At high monoaminergic levels or after spinal cord injury, PICs dominate synaptic integration. The model precisely replicates the results of experiments that examine the influence of synaptic inputs on plateaus in motoneurons (Bennett et al. 1998). Further, we are able to examine the role of PICs in modulating excitability. We find that excitatory synaptic inputs significantly lower the threshold for plateau activation, and conversely, synaptic inhibition raises the plateau threshold. Hence, synaptic inputs may play a critical role in modulating the effects of PICs. Many studies suggest that inhibitory inputs have the ability to maintain the membrane below the threshold for activating dendritic PICs. By examining the persistent currents in the model during somatic pulse current injection, we show that excitatory inputs can cause an advancement in the time of plateau onset. However, inhibitory inputs that occur before the initiation of the plateau can keep the persistent current below the threshold for plateau initiation, eliminating sustained firing. We show that the timing of the inhibitory input is critically important, since the same level of inhibition does not eliminate the plateau if the input begins after the plateau is initiated. In this case, the motoneuron still exhibits self-sustained firing. Further studies will focus on the details of the interactions between persistent currents and inhibition in the dendrite.
Acknowledgments
Some of the results described here appeared previously in abstract form. Partial support for this work was provided by the National Institutes of Health R01-NS054282 and by the National Science Foundation through NSF IIS 0613404.
Contributor Information
Mini Kurian, School of Mathematical and Statistical Sciences, Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287, USA.
Sharon M. Crook, Email: sharon.crook@asu.edu, School of Mathematical and Statistical Sciences, Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287, USA; School of Life Sciences, Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287, USA.
Ranu Jung, School of Biological and Health Systems Engineering, Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85287, USA.
References
- Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini MA, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. Journal of Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini MA. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. Journal of Neurophysiology. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. Journal of Neurophysiology. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of physiology, exercise: Regulation and integration of multiple systems. Vol. 12. Oxford University Press; 1996. pp. 1–53. [Google Scholar]
- Booth V, Rinzel J. A minimal, compartmental model for dendritic origin of bistability of motoneuron firing patterns. Journal of Computational Neuroscience. 1995;2:299–312. doi: 10.1007/BF00961442. [DOI] [PubMed] [Google Scholar]
- Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. Journal of Neurophysiology. 1997;78:3371–3385. doi: 10.1152/jn.1997.78.6.3371. [DOI] [PubMed] [Google Scholar]
- Bose P, Parmer R, Reier PJ, Thompson FJ. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Experimental Neurology. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. Journal of Neurophysiology. 2008a;99:571–582. doi: 10.1152/jn.00717.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. Journal of Neurophysiology. 2008b;99:583–594. doi: 10.1152/jn.00718.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. Journal of Neurophysiology. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. Journal of Comparative Neurology. 1987;255:68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- Dhooge A, Govaerts W, Kuznetsov YA, Sautois B. Matcont: a MATLAB package for numerical bifurcation analysis of ODEs. ACM Transactions on Mathematical Software. 2003;29(2):141–164. [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiologica Scandinavica. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic Cav1.3 channels in cat lumbar motorneurons: Spatial distribution. Journal of Neurophysiology. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Ermentrout B. Simulating, analyzing, and animating dynamical systems: A guide to XPPAUT for researchers and students. Philadelphia: SIAM; 2002. [Google Scholar]
- Fall CP, Marland ES, Wagner JM, Tyson JJ. Computational cell biology. New York: Springer-Verlag; 2002. [Google Scholar]
- Finkel AS, Redman SJ. The synaptic current evoked in cat spinal motoneurons by impulse in single group Ia axons. Journal of Physiology. 1983;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman JW, Segev I, Burke RB. Electrotonic architecture of type-identified α-motoneurons in the cat spinal cord. Journal of Neurophysiology. 1988;60(1):60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- Fyffe RE. Spinal motoneurons: Synaptic inputs and receptor organization. In: Cope TC, editor. Motor neurobiology of the spinal cord. NY: CRC; 2001. [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motoneuron dendrite structure. Journal of Comparative Neurology. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Gianano JM, York MM, Pace JA, Schott S. Quality of life: Effect of reduced spasticity from intrathecal baclofen. Journal of Neuroscience Nursing. 1998;30:47–54. doi: 10.1097/01376517-199802000-00006. [DOI] [PubMed] [Google Scholar]
- Graham J, Booth V, Jung R. Modeling motoneurons after spinal cord injury: Persistent inward currents and plateau potentials. Neurocomputing. 2005;65–66:719–726. [Google Scholar]
- Gutman AM. Bistability of dendrites. International Journal of Neural Systems. 1991;1:291–304. doi: 10.1142/s0129065794000104. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. Journal of Neurophysiology. 2006a;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. Journal of Neurophysiology. 2006b;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. Journal of Neurophysiology. 2006c;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. The Neuroscientist. 2008;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyper-excitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends in Neurosciences. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. II Motoneuron electrical properties. Journal of Neurophysiology. 1994;77:1468–1479. doi: 10.1152/jn.1994.71.4.1468. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;4:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jesperson B, Kiehn O. Bistability of α-motoneurons in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. Journal of Physiology. 1988a;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurons in a slice preparation of the turtle spinal cord. Journal of Physiology. 1988b;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin induced bistability of turtle motoneurons caused by nifedipine sensitive calcium plateau potential. Journal of Physiology. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. Journal of Physiology. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurons by dendritic persistent inward current. Journal of Physiology. 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Current Opinion in Neurobiology. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Carnevale NT. Passive normalization of synaptic integration influenced by dendritic architecture. Journal of Neurophysiology. 1999;82:3268–3285. doi: 10.1152/jn.1999.82.6.3268. [DOI] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annual Review of Neuroscience. 1997;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Kitzman P. Alteration in axial motoneuronal morphology in the spinal cord injured spastic rat. Experimental Neurology. 2005;192(1):100–108. doi: 10.1016/j.expneurol.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ. Active dendritic integration of inhibitory synaptic inputs in vivo. Journal of Neurophysiology. 2003;90:3617–3624. doi: 10.1152/jn.00521.2003. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. Journal of Neurophysiology. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. Journal of Neurophysiology. 1998a;80:585–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: Systematic variations in rhythmic firing patterns. Journal of Neurophysiology. 1998b;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. Journal of Neuroscience. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. Journal of Neurophysiology. 2007;97:3314–3330. doi: 10.1152/jn.01068.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without spinal cord injury. Journal of Neurophysiology. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium currents and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. Journal of Neurophysiology. 2003;90(2):857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. Journal of Neurophysiology. 2004;91(2):767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. Journal of Rehabilitation Research and Development. 2008;42:229–240. doi: 10.1682/JRRD.2007.03.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model cortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- NINDS-NIH. NINDS-NIH. Spasticity information page. 2009 http://www.ninds.nih.gov/disorders/spasticity/spastic%ity.htm.
- Perrier JF, Hounsgaard J. Ca2+-activated nonselective cationic current (ICAN) in turtle motoneurons. Journal of Neurophysiology. 2003;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Pinsky PF, Rinzel J. Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. Journal of Computational Neuroscience. 1994;1:39–60. doi: 10.1007/BF00962717. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. Journal of Neurophysiology. 2000;83:483–500. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. Journal of Neurophysiology. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Prather JF, Clark BD, Cope TC. Firing rate modulation of motoneurons activated by cutaneous and muscle receptor afferents in the decerebrate cat. Journal of Neurophysiology. 2002;88:1867–1879. doi: 10.1152/jn.2002.88.4.1867. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different somadendritic distributions of synaptic input. Journal of Neurophysiology. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Ramer MS, Steeves JD. Setting the stage for functional repair of spinal cord injuries: A cast of thousands. Spinal Cord. 2005;43:134–161. doi: 10.1038/sj.sc.3101715. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. Journal of Neurophysiology. 1980;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage–clamp study. Journal of Neurophysiology. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Membrane properties of cat spinal motoneurons. In: Davidoff R, editor. Handbook of the spinal cord. NY: Dekker; 1984. pp. 199–242. [Google Scholar]
- Segev I, Fleshman JW, Burke RE. Computer simulation of Group Ia EPSPs using morphologically realistic models of cat α-motoneurons. Journal of Neurophysiology. 1990;64:648–660. doi: 10.1152/jn.1990.64.2.648. [DOI] [PubMed] [Google Scholar]
- Segev I, London M. Untangling dendrites with quantitative models. Science. 2000;290:744–750. doi: 10.1126/science.290.5492.744. [DOI] [PubMed] [Google Scholar]
- Shapiro NP, Lee RH. Synaptic amplification versus bistability in motoneuron dendritic processing: A top-down modeling approach. Journal of Neurophysiology. 2007;97:3948–3960. doi: 10.1152/jn.00084.2007. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Redman SJ. Voltage dependance of Ia reciprocal inhibitory currents in cat spinal motoneurons. Journal of Physiology. 1990;420:111–125. doi: 10.1113/jphysiol.1990.sp017903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elburg RAJ, van Ooyen A. Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Computational Biology. 2010;6:e1000781. doi: 10.1371/journal.pcbi.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira MF, Kohn AF. Compartmental models of mammalian motoneurons of types S, FR and FF and their computer simulation. Computers in Biology and Medicine. 2007;37:842–860. doi: 10.1016/j.compbiomed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Krnjevic K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neuroscience Letters. 1987;74:58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]