Abstract
Objectives
Access-related hand ischemia (ARHI) is a major complication after hemodialysis access construction. This study was designed to prospectively describe its incidence, predictors, interventions and associated access maturation.
Methods
The Hemodialysis Fistula Maturation Study is a multicenter prospective cohort study designed to identify predictors of autogenous arteriovenous access (AVF) maturation. Symptoms and interventions for ARHI were documented and participants who received interventions for ARHI were compared to other participants using a nested case-control design. Associations of ARHI with clinical, ultrasound, vascular function, and vein histological variables were each individually evaluated using conditional logistic regression and the association with maturation assessed by relative risk, Pearson's chi-square test, and multiple logistic regression.
Results
The study cohort included 602 participants with median follow-up 2.1 years (10th - 90th percentiles: 0.7 - 3.5 yrs). Mean age was 55.1 ± 13.4 (SD) years and the majority were male (70%), Caucasian (47%), diabetic (59%), smokers (55%), on dialysis (64%), and underwent an upper arm AVF (76%). Symptoms of ARHI occurred in 45 (7%) participants and intervention was required in 26 (4%). Interventions included distal revascularization with interval ligation (13), ligation (7), banding (4), revision using distal inflow (1), and proximalization of arterial inflow (1). Interventions were performed ≤ 7 days after AVF creation in 4 participants (15%), between 8 - 30 days in 6 (23%) and > 30 days in 16 (63%). Female gender (OR 3.17: 95% CI 1.27 - 7.91, p = 0.013), diabetes (13.62: 1.81 - 102.4, p = 0.011), coronary artery disease (2.60: 1.03 - 6.58, p = 0.044), higher pre-operative venous capacitance (per % /10mmHg: 2.76: 1.07 - 6.52, p = 0.021) and maximum venous outflow slope (per (mL/100 mL/min) /10 mmHg: 1.13: 1.03 - 1.25, p = 0.011) were significantly associated with interventions, while a lower carotid-femoral pulse-wave velocity and the outflow vein diameter in the early postoperative period (days 0 - 3) approached significance (p < .10). Intervention for ARHI was not associated with AVF maturation failure (unadjusted risk ratio 1.18: 0.69 - 2.04, p = 0.56, adjusted odds ratio 0.97: 0.41 - 2.31, p = 0.95).
Conclusions
Remedial intervention for ARHI after AVF construction is uncommon. Diabetes, female gender, capacitant outflow veins and coronary artery disease are all associated with an increased risk of intervention. These higher risk patients should be counseled preoperatively, their operative plans designed to reduce the risk of hand ischemia, and followed closely.
Introduction
The hemodynamic changes associated with the construction of an autogenous (AVF) or prosthetic arteriovenous (AVG) hemodialysis access in the upper extremity can lead to hand ischemia. This phenomenon, termed the “steal syndrome” or access-related hand ischemia (ARHI), can lead to significant disability. Symptoms of either acute or chronic ischemia have been reported in up to 20% of brachial artery-based procedures2-9 and 2% of radial artery-based procedures10-12 with up to half of the patients in the brachial artery group requiring some type of remedial intervention. The true incidence of ARHI associated with AVFs and AVGs is uncertain given the retrospective nature of the various studies, reported predominantly from referral centers. Multiple risk factors for ARHI have been identified and include female gender, advanced age, diabetes, peripheral artery disease, coronary artery disease, multiple previous access procedures, prior episodes of hand ischemia, anastomotic configuration and large outflow veins.5, 8, 13-16 The predictive value of these various risk factors has been insufficient to identify patients at such a high risk that the index access procedure should be avoided. A variety of remedial treatments have been reported including those designed to not only reverse the symptoms but to salvage the access. Unfortunately, there is no consensus about the optimal remedial treatment.
This study was designed to prospectively define the incidence, predictors and remedial treatments for ARHI, along with its impact on the maturation rate within the Hemodialysis Fistula Maturation Study (HFM).
Methods
Hemodialysis Fistula Maturation Study Design
the HFM was a multicenter, NIH-funded, prospective study conducted at 7 academic centers in the United States with a target enrollment of 600 participants and follow-up extending up to 4 years. 1 It was designed to identify predictors of AVF maturation within four domains: 1) anatomy; 2) biology; 3) clinical attributes; and 4) processes of care. The study was observational in that the clinical decisions regarding the AVF were dictated by the clinical team with the exception that no interventions to facilitate maturation were to be performed within the first 6 weeks after construction. Enrollment criteria included: 1) current or anticipated hemodialysis within 3 months; 2) life expectancy > 9 months; 3) age exceeding consent minimum (i.e. 18 – 21 years) and < 80 years unless on dialysis; and 4) single stage upper extremity AVF. The primary outcome measure was unassisted clinical maturation, defined as use of the AVF for dialysis over 4 weeks with specific, predefined criteria.1 Preoperative measurements included ultrasound mapping of the upper extremity arteries and veins, flow-mediated (FMD) and nitroglycerin-mediated (NMD) brachial artery dilation, arterial pulse wave velocity (PWV), and venous plethysmography. Postoperative measurements included ultrasounds within 3 days of the access procedure and at 2 weeks, 6 weeks, and prior to intervention or initial cannulation.
Access-related Hand Ischemia
ARHI was included as a secondary outcome measure.1 All symptoms (i.e. pain, paresthesia, motor dysfunction, tissue loss) and interventions for ARHI were documented throughout the perioperative and follow-up periods.
Participants
medical history and medications were defined at the time of enrollment. Peripheral artery disease was defined as prior amputation, carotid endarterectomy, carotid angioplasty, claudication, or lower extremity revascularization. Coronary artery disease was defined as prior angina, myocardial infarction, coronary artery bypass or percutaneous revascularization.
Ultrasound
preoperative mapping of the arteries and veins was performed along with the measurement of the blood flow in the brachial artery.17-19 Internal diameter measurements of the artery and veins were performed in the antero-posterior dimension on a transverse image with a linear transducer (9 MHz or higher). Measurements included the internal diameter of the brachial artery 2 cm cranial to the antecubital fossa and the radial artery 2 cm cranial to the wrist. The cephalic and basilic veins in the upper arm were measured at the antecubital fossa, mid and cranial upper arm, and cephalic vein in the forearm measured at the wrist, mid and cranial forearm. Arterial calcification was graded as absent, mild-to-moderate or severe (circumferential). Postoperative AVF evaluation was performed using a standardized protocol.20 The brachial or radial artery internal diameter measurements were obtained 2 cm cranial to the anastomosis. The AVF draining vein internal diameter measurements and blood flow were obtained 2, 5, 10 and 15 cm cranial to the anastomosis. The tests were performed by personnel trained by the Ultrasound Core and the studies were read at the Core.
Vascular Function Tests
the tests were performed to characterize endothelial–dependent (FMD) and endothelial-independent (NMD) arterial vasodilation, arterial stiffness (carotid-radial PWV – extremity, carotid-femoral PWV – central) and venous capacitance (venous occlusion plethysmography). They were performed within 45 days prior to the AVF procedure on the arm for the planned surgical procedure unless there was already a functional, ipsilateral access. The tests were performed by personnel trained by the Vascular Function Core and the studies were read at the Core.
Venous Occlusion Plethysmography
The Hokanson EC5 strain gauge plethysmography device with NIVP3 software was used for waveform acquisition and analysis (D.E. Hokansan, Inc, Bellvue, WA). A strain gauge of appropriate size was placed around the forearm at its greatest diameter. A straight segmental arm cuff (SC10D, Hokansan, Inc) was placed on the upper arm and inflated for 3 minutes to a designated pressure and deflated. Waveforms were acquired while the cuff was inflated and for 5 seconds after deflation. The procedure was successively performed at cuff inflations to 20, 30, 40, 50, and 60 mmHg, with maximum venous outflow and fractional change in forearm volume measured. Estimated slopes from their respective linear regressions on cuff pressure were used as measures of venous outflow and capacitance.
Pulse Wave Velocity
PWV (m/sec) was measured using the SphygmoCor system (Atcor Medical, Itasca, IL). Carotid-radial and carotid-femoral distances were taken as the lengths by which the distances from the sternal notch respectively to the radial or femoral pulse exceeded that from the sternal notch to the carotid pulse. Pulse waveforms were recorded using applanation tonometry at the carotid followed by the radial or femoral sites, respectively.
Brachial Artery Flow-mediated Dilation and Nitroglycerin-mediated Dilation
a custom 3.25” × 22” blood pressure cuff (Hokansan, Inc.) was placed on the upper arm. A 2-dimensional (2D) image of the brachial artery and pulsed wave Doppler signal was obtained with a high-resolution linear ultrasound probe (at least 7.5 MHz). For FMD, the cuff was then inflated to 200 mmHg and deflated after 5 minutes. Brachial artery Doppler signals were obtained 15 seconds after deflation. 2D images gated on the R-wave were obtained 55 - 65 seconds after deflation to determine flow-mediated dilation. For NMD, acquisition was repeated 3 minutes after administration of sublingual nitroglycerin (0.4 mg). Brachial artery diameters were extracted from the 2D images using customized software, and resting and hyperemic flow were determined from the Doppler signals. Flow-mediated dilation and nitroglycerin-mediated dilation were expressed as the post-ischemia and post-nitroglycerin percentage increases in brachial artery diameter, respectively.
Histology
a sample of the outflow vein near the region used for the anastomosis was harvested during the index AVF procedure and processed into paraffin in a standard manner. Sections were stained with Alizarin red S for the presence of calcium in the intima or media and classified as either “present” or “none”.
Statistical Analysis
participants requiring interventions for ARHI were taken as cases, andtheir times to intervention for ARHI were summarized by Kaplan-Meier plot. A nested case-control analysis was performed due to the statistical limitations of multivariable logistic regression modeling with few outcome cases per predictor. Cases were compared to respective sets of controls matched on sex, age (± 5 years), diabetes and AVF location (forearm vs. upper arm). Controls eligible for matching to any case were optimally partitioned into disjoint matched sets,21 with conditional logistic regression applied to the resulting matched sets to estimate and test associations with ARHI. To study association of each matching variable, we created new matched sets without matching on that variable, and treated it as a predictor as above. Variables examined for associations with hand ischemia (Appendix A) were preselected and identified in a formal statistical analysis plan. Vulnerability of results to confounding was assessed by studying associations of clinical center and other potential confounders to significant predictors. The association of ARHI with subsequent clinical maturation in the full HFM cohort was described by the crude relative risk and the odds ratio after adjustment for age, gender, African American race, access location and diabetes using multiple logistic regression. In view of concern over the ability of the conventional measures FMD% and NMD% to adequately normalize for variation in vessel size between individuals, we included alternative, exploratory allometric analyses by linearly regressing log (post-stimulus vessel diameter) on log (pre-stimulus vessel diameter).22 PROC LOGISTIC, the LGTPHCURV9 macro23 for spline fitting, and other SAS 9.4 components were used for computation, with two-sided 5% level tests to assess statistical significance.
The study was approved by each center's Institutional Review Boards and all participants provided informed consent. For additional details of methods see Appendix B and, for vascular function and ultrasound testing, the online supplement to Farber et al.24
Results
Cases and Matched Sets
the HFM Study cohort included 602 participants and follow-up ranged from 0.2 - 4.1 years with a median of 2.1 years (10th - 90th percentiles: 0.7 - 3.5 years). The mean age of the cohort was 55.1 ± 13.4 years with 37% of the participants being over 60 years of age. The majority of the participants were male (70%), Caucasian (Caucasian – 47%, African American – 44%, Hispanic – 13%), diabetic (59%), smokers (current or past – 55%) and on dialysis at the time of the index AVF (64%). The mean body mass index was 30.4 ± 7.6 (SD) and a significant proportion had coronary artery (26%) and/or peripheral artery disease (15%). The majority of the participants underwent an upper arm AVF (upper – 76%, forearm --24%) with the brachial-cephalic configuration accounting for most of these (brachial-cephalic – 62%, brachial-basilic – 38%). See Farber et al.24 for additional summary information on the cohort. Some degree of ARHI was diagnosed in 45 (7%) of participants and 26 (58% or 4% of total) required remedial surgical interventions (Figure 1). The remedial interventions were performed within 7 days of AVF creation surgery for 4 participants (15%), between 8 - 30 days for 6 (23%), and beyond 30 days for 16 (63%). These included distal revascularization with interval ligation (13), ligation (7), banding (4), revision using distal inflow (1), and proximalization of the arterial inflow (1). Two hundred thirty-five of the 576 HFM Study participants not treated for ARHI were matched to the ARHI cases that underwent remedial intervention with the number of controls varying from 1 to 45 per case (Table I).
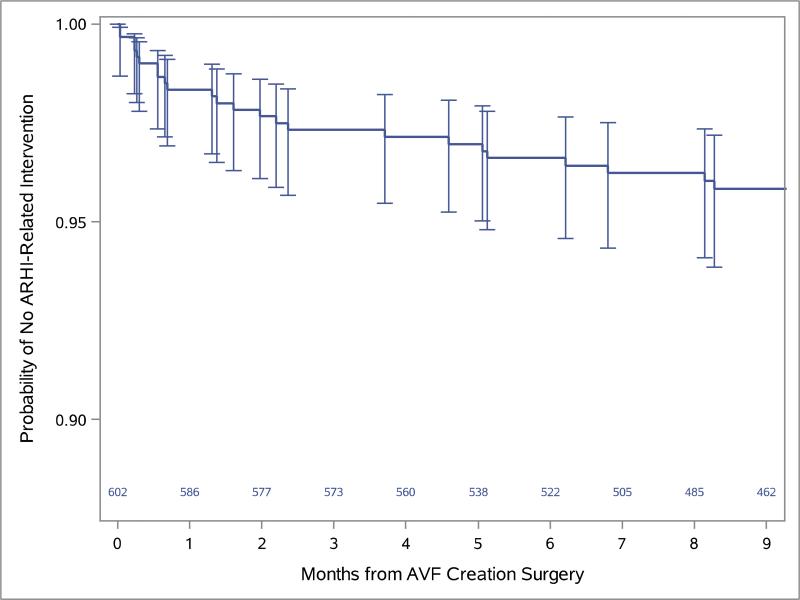
Figure.
the Kaplan-Meier curve for the time until the remedial intervention for ARHI is shown with the corresponding numbers of participants at risk at monthly intervals and 95% confidence intervals for proportions without ARHI-related intervention at the times such interventions occurred. Two additional interventions, respectively in months 14 and 21, are omitted and the vertical axis is truncated in order to better reveal detail in the first 9 post-operative months where 24 of the 26 interventions (92.3%) occurred, and in the 90-95% range where the curve and confidence intervals fall.
Table I.
Predictor summaries among participants with ARHI requiring intervention (cases), other HFM Study participants, and controls matched by age, gender, history of diabetes and AVF location.
| Variable | Subgroup |
||
|---|---|---|---|
| Participants without ARHI (N= 576) | Participants with ARHI (Cases) (N=26) | Weighted1, Matched Controls (N=235) | |
|
%, or mean ± SD | |||
| Age at time of consent (yrs) | 54.9 ± 13.5 | 59.6 ± 9.9 | 59.1 ± 9.5 |
| Female | 28.8% | 53.8% | 53.8% |
| Diabetes | 57.1% | 92.3% | 92.3% |
| AVF in upper arm | 75.5% | 92.3% | 92.3% |
| Peripheral artery disease | 15.5% | 30.8% | 22.0% |
| Coronary artery disease | 24.8% | 50.0% | 29.9% |
| Hypertension | 96.5% | 96.2% | 99.4% |
| Prior permanent hemodialysis access in the index AVF arm | 22.2% | 11.5% | 22.6% |
| Functional hemodialysis catheter ipsilateral to AVF | 33.6% | 38.9% | 22.2% |
| Current hemodialysis or peritoneal dialysis | 64.1% | 69.2% | 56.9% |
| Smoking status | |||
| - Never | 45.9% | 38.5% | 52.1% |
| - Former | 37.0% | 34.6% | 34.3% |
| - Current | 17.2% | 26.9% | 13.5 |
| Length of anastomosis (mm) | 7.8 ± 2.8 | 8.8 ± 2.1 | 7.7 ± 1.2 |
| Preoperative feeding artery diameter (mm) | 0.38 ± 0.12 | 0.40 ± 0.08 | 0.40 ± 0.07 |
| Preoperative radial artery diameter at wrist (mm) | 0.22 ± .053 | 0.21 ± 0.05 | 0.20 ± 0.30 |
| Mean preoperative draining vein diameter (mm) | 0.37 ± 0.12 | 0.40 ± 0.11 | 0.38 ± 0.04 |
| Minimum preoperative draining vein diameter (mm) | 0.30 ± 0.11 | 0.32 ± 0.11 | 0.30 ± 0.04 |
| AVF Configurations: | |||
| - Upper arm cephalic vein and antecubital/proximal forearm artery | 46.9% | 57.7% | 52.6% |
| - Upper arm basilic vein transposition and antecubital artery/proximal forearm artery | 26.7% | 30.8% | 35.9% |
| - Upper arm brachial vein transposition and antecubital artery | 1.9% | 3.9% | 3.8% |
| - Forearm cephalic vein and wrist radial artery | 22.7% | 7.7% | 7.7% |
| - Forearm basilic vein transposition and wrist radial artery | 1.7% | 0 | 0 |
| AVF classification based on feeding artery: | |||
| - Brachial artery | 62.7% | 84.6% | 78.3% |
| - Distal radial artery | 24.5% | 7.7% | 7.7% |
| - Antecubital artery | 10.6% | 7.7% | 8.1% |
| - Proximal radial artery | 2.3% | 0 | 5.9% |
Weighted statistics are simple averages of summaries (means or %s) within the sets of controls matched to each individual ARHI case.
Baseline Demographics, Clinical Characteristics and Comorbidities (Table II)
Table II.
Association of baseline demographics, clinical characteristics, and comorbidities with ARHI1
| Risk Factor | N2 | Odds Ratio (95% CI) | P-value3 |
|---|---|---|---|
| Factor | |||
| Age (per decade)4 | 470 | 1.16 (0.72, 1.86) | 0.55 |
| Female vs. male5 | 319 | 3.17 (1.27, 7.91) | 0.013 |
| Diabetes6 | 389 | 13.62 (1.81, 102.4) | 0.011 |
| Peripheral artery disease | 261 | 1.91 (0.75, 4.88) | 0.17 |
| Coronary artery disease | 261 | 2.60 (1.03, 6.58) | 0.044 |
| Hypertension | 261 | 0.35 (0.03, 4.55) | 0.42 |
| Smoking (never; former; current; per more current category) | 259 | 1.56 (0.90, 2.69) | 0.11 |
| Prior permanent hemodialysis access in the index AVF arm | 115 | 0.33 (0.071, 1.53) | 0.16 |
| Functional hemodialysis catheter ipsilateral to AVF | 113 | 0.79 (0.32, 1.94) | 0.60 |
| Current hemodialysis or peritoneal dialysis | 261 | 1.65 (0.63, 4.27) | 0.31 |
Unless otherwise noted, case-control comparisons are matched on baseline sex, age, fistula location (forearm vs. upper arm), and history of diabetes.
Number of participants in matched sets that are statistically informative, in the sense that data on the risk factor was present for both at least one case and one matched control.
All hypothesis tests are two-tailed with p ≤ 0.05 required for statistical significance.
Case-control comparisons matched on baseline sex, fistula location (forearm vs. upper arm), and history of diabetes.
Case-control comparisons matched on baseline age, fistula location (forearm vs. upper arm), and history of diabetes.
Case-control comparisons matched on baseline age, sex, and fistula location (forearm vs. upper arm).
ARHI requiring intervention was significantly more common in females (OR = 3.17, CI 1.27 - 7.91, P = 0.013), and participants with diabetes (OR = 13.62, CI 1.81 - 102.4, P = 0.011) and coronary artery disease (OR = 2.60, CI 1.03 - 6.58, P = 0.044).
Preoperative Ultrasound and Vascular Function Characteristics (Table III)
Table III.
Association of preoperative vascular anatomic and function characteristics with ARHI1
| Risk Factor | N2 | Odds Ratio (95% CI) | P-value3 |
|---|---|---|---|
| AVF configuration | |||
| Forearm vs. upper arm4 | 353 | 0.38 (0.085, 1.71) | 0.21 |
| High radial artery take-off | 261 | 1.41 (0.31, 6.38) | 0.66 |
| Cephalic vs. transpositions (both basilic and brachial vein) | 251 | 1.26 (0.48, 3.32) | 0.64 |
| Brachial transpositions vs. basilic transpositions | 38 | 0.32 (0.02, 5.84) | 0.44 |
| Surgical technique | |||
| Length of anastomosis (per 1 mm) | 107 | 1.12 (0.88, 1.42) | 0.34 |
| Vascular characteristics | |||
| Preoperative feeding artery diameter (per 1 mm) | 243 | 1.26 (0.006, 260) | 0.93 |
| Preoperative radial artery diameter (per 1 mm) | 245 | 1.45 (0.60, 3.49) | 0.41 |
| Mean preoperative draining vein diameter (per 1 mm) | 234 | 1.09 (0.76, 1.57) | 0.64 |
| Minimum preoperative draining vein diameter (per 1 mm) | 234 | 1.14 (0.78, 1.65) | 0.51 |
| Preoperative upper arm fistula feeding artery flow (per 10 mL/min) | 226 | 0.99 (0.93, 1.07) | 0.87 |
| Preoperative feeding artery ultrasound calcification (none or mild vs. moderate or severe) | 260 | 0.91 (0.45, 1.87) | 0.80 |
| Draining vein histological calcification (intimal and/or medial vs. none) | 222 | 2.46 (0.73, 8.28) | 0.14 |
| Brachial artery dilation measures | |||
| Brachial artery FMD% (per %) | 211 | 0.93 (0.83, 1.04) | 0.22 |
| Brachial artery NMD% (per %) | 125 | 0.98 (0.89, 1.08) | 0.72 |
| Arterial stiffness measures | |||
| Carotid-femoral PWV (per m/sec) | 124 | 1.14 (0.99, 1.32) | 0.080 |
| Carotid-radial PWV (per m/sec) | 124 | 1.02 (0.73, 1.44) | 0.90 |
| Vein function measures | |||
| Capacitance slope (per % /10 mmHg) | 234 | 2.76 (1.07, 6.52) | 0.021 |
| Maximum venous outflow slope (per (mL/100 mL/min) /10 mmHg) | 234 | 1.13 (1.03, 1.25) | 0.011 |
Unless otherwise noted, case-control comparisons are matched on baseline sex, age, fistula location (forearm vs. upper arm), and history of diabetes.
Number of participants in matched sets that are statistically informative, in the sense that data on the risk factor was present for both at least one case and one matched control.
All hypothesis tests are two-tailed with p ≤ 0.05 required for statistical significance.
Case-control comparisons matched on baseline age, sex, and history of diabetes.
a higher venous capacitance slope and the maximum venous output slope were associated with ARHI requiring intervention (OR = 2.76, CI 1.07 - 6.52, P = 0.021 and OR = 1.13, CI 1.03- 1.25, P = 0.011, respectively). The association with truncal artery stiffness, as assessed by carotid-femoral PWV, approached but did not attain statistical significance (OR = 1.14 per m/sec, CI 0.99 - 1.32, P = 0.08).
Postoperative Ultrasound and Vascular Function Characteristics (Table IV)
Table IV.
Association of postoperative vascular function with ARHI1
| Risk Factor | N2 | Odds Ratio (95% CI) | P-value3 |
|---|---|---|---|
| Average outflow vein diameter post-op day 0-3 (per 0.1 mm) | 226 | 1.50 (0.94, 2.38) | 0.088 |
| Minimum outflow vein diameter post-op day 0-3 (per 0.1 mm) | 226 | 1.61 (0.97, 2.66) | 0.066 |
| Inflow artery diameter at post-op day 0-3 (per 0.1 mm) | 214 | 0.72 (0.34, 1.53) | 0.39 |
| AVF flow rate day 0-3 (per 100 mL/min) | 219 | 1.09 (0.97, 1.23) | 0.14 |
| AVF flow rate week 2 (per 100 mL/min)4 | 162 | 1.01 (0.90, 1.13) | 0.89 |
Unless otherwise noted, case-control comparisons are matched on baseline sex, age, fistula location (forearm vs. upper arm), and history of diabetes.
Number of participants in matched sets that are statistically informative, in the sense that data on the risk factor was present for both at least one case and one matched control.
All hypothesis tests are two-tailed with p ≤ 0.05 required for statistical significance.
Analysis restricted to participants with patent AVF.
the mean and minimum diameters of the outflow vein, measured in the early postoperative period (postoperative days 0 – 3), approached but did not reach statistical significance for ARHI requiring intervention (per 0.1 mm increase: mean diameter: OR = 1.50, CI 0.76 - 1.02, P = 0.09; minimum diameter: OR = 1.61, CI 0.97 - 2.66, P = 0.07).
ARHI and AVF maturation
ARHI requiring intervention was not significantly associated with AVF clinical maturation failure, either unadjusted (RR 1.18: 0.69 - 2.04, p = 0.56) or after adjustment for age, gender, African American race, access location and diabetes (OR 0.97, 0.41 - 2.31, p = 0.95).
Assessment of confounding
The associations of statistically significant predictors with each other, clinical center, and marginally significant exposures (0.05 < p ≤ 0.10) suggested potential for confounding between capacitance and maximum vein outflow slopes, and that coronary artery disease might partially underlie the effects of diabetes and sex, but that any other notable confounding would have attenuated rather than exaggerated the observed effects.
Discussion
The study demonstrates that the incidence of ARHI after the construction of an autogenous arteriovenous hemodialysis access is relatively uncommon (7%) and that the incidence of severe symptoms sufficient to merit surgical interventions relatively low (4%). Remedial treatment was associated with female gender, diabetes, coronary artery disease, and more capacitant outflow veins while the association approached significance for the measures of truncal arterial stiffness and larger postoperative outflow veins. Remedial treatment for ARHI did not appear to affect the clinical maturation rate of the AVF. These observations are strengthened by the prospective study design and large sample size of the HFM Study that encompassed domains examining vascular anatomy, vascular wall biology, and clinical attributes.
The incidence of ARHI in the current study was lower than expected, based upon the predominance of brachial-artery AVFs. The literature has suggested that symptoms occur in up to 20% of brachial artery based procedures and that remedial interventions are required in roughly half or 10% of these cases.2-9 Keuters et al.3 prospectively analyzed the incidence of ARHI in a series (N = 61) of participants undergoing brachial-artery based AVFs and AVGs, reporting that some symptoms of ARHI developed within 28% of participants and that remedial intervention was required in 10%. There are multiple potential explanations for the discrepancy between the current study and the literature experience, including study design (i.e. prospective vs retrospective), clinical practice (i.e. primary vs referral), clinical center (i.e. academic vs non-academic), duration of follow-up, selection bias and type of access (i.e. AVF vs AVG). The HFM Study was performed in academic medical centers by care teams interested in hemodialysis access, and, thus, it is conceivable that this may partially account for the better outcomes. It is important to emphasize that the current study included only AVFs. The incidence and timing of ARHI is likely different between AVFs and AVGs. ARHI tends to occur earlier relative to the index access for patients receiving an AVG. Scheltinga et al.25 reported from their systematic review that ARHI associated with AVGs tend to be acute, both in timing (< 24 hrs) and symptoms (i.e. acute ischemia), while that associated with AVFs tended to be more chronic (i.e. > 1 mos, chronic ischemia). This time course is likely consistent with our own findings since 63% of the participants underwent remedial intervention > 30 days after their procedure and only 15% underwent intervention within a week.
Female gender and the presence of diabetes seem to be consistent predictors of ARHI although a variety of other factors have been incriminated. 5, 8, 13-16 Rocha et al.26 reported that female gender, diabetes, and side-to side anastomoses were predictors in their multivariate model with diabetes being the strongest predictor (OR 3.08, CI 1.2 – 8.2). The presence of coronary artery disease has been an inconsistent predictor of ARHI in contrast to our finding. Although our “negative” results need to be interpreted with caution given the small number of participants that required intervention and the potential for a Type II error, it is interesting that high take off of the radial artery, a proximal radial artery anastomosis, the length of the anastomosis, the diameter of the feeding artery, and the early access flow rates were not significantly associated with ARHI. Whittaker et al.27 and Gupta et al.28 have reported a 2% incidence of ARHI for proximal radial artery-based access procedures and have advocated this as an alternative to brachial-based procedures. The impact of the anastomotic length on the development of ARHI has been debated for some time despite the hemodynamic principle that the flow through a large arteriovenous fistula is independent of the communication between the vessels once the anastomotic length exceeds 75% of the arterial diameter.29 Bavare et al.30 reported that the mean volume flow rates were significantly higher in patients with brachial-cephalic AVFs that developed ARHI (1542 mL/min vs 1087 mL/min).
The associations of ARHI with the vascular function tests provide some insight into the hemodynamic changes that lead to ARHI. The construction of an AVF creates a low resistance, high flow communication. This results in a “pressure sink” or a gradual diminution in the pressure along the course of the inflow artery. Kopriva et al.31 measured the pressures at intervals along the inflow artery in a series of brachial artery-based AVFs in patients (N=10) with severe ARHI and reported that the pressures were significantly lower than the central pressures until a level of 20 – 25 cm from the anastomosis was reached (i.e. 20 cm proximal to the AVF anastomosis). Predictably, the creation of an AVF leads to a decrease in the distal perfusion and “physiological steal” phenomenon with 80% of patient developing a decrease in their digital pressures6. The normal compensatory responses include an increase in the cardiac output, arterial vasodilation and the development of arterial collaterals. Not surprisingly, arterial inflow (e.g. subclavian artery stenosis) and outflow (e.g. forearm occlusive disease) stenoses exacerbate these hemodynamic changes.32, 33. The association with higher pulse wave velocity suggests that the stiffer, non-compliant vessels may not be able to adapt and remodel in response to the AVF. This putative mechanism (i.e. arterial stiffness) may account for some of the strong association with diabetes although the degree of calcification within the inflow artery was not predictive. The associations with the venous plethysmography measures and the diameter of the outflow vein measured in the early postoperative period suggest that more compliant outflow veins exacerbate the outlined hemodynamic changes. The contribution of venous compliance is illustrated by the report from Huber et al.14 documenting their experience using the translocated femoral vein, a large compliant vein, as a conduit for a brachial-axillary access. In their series (N= 30), 43% of the patients developed symptoms consistent with ARHI and 27% required remedial intervention.
The important, outstanding question is how these data should be used to improve clinical care. Unfortunately, the list of factors significantly associated with ARHI is limited despite the large sample size and the small number of participants that required interventions precluded a reliable predictive model. Furthermore, the vascular function tests are not routinely available in the clinical setting, and we have limited follow-up regarding the definitive treatments for the ARHI. The data should likely be used to increase the clinical suspicion for ARHI, both in the preoperative and postoperative period, underscoring the fact that women, diabetics, patients with coronary artery disease and those with larger, compliant outflow veins are at increased risk. Strategies should be implemented during the preoperative evaluation in this higher-risk patient cohort to identify any contributory lesions, potentially including arterial noninvasive studies and arteriography (either CT- or catheter-based). The operative plan should be developed to minimize the risk of ARHI including correcting any inflow lesions, siting the arterial anastomosis more proximal (i.e. proximal brachial or axillary artery) or distal (i.e. proximal or distal radial artery) on the arterial tree, avoiding the use of large/compliant outflow veins, and or ligating all large venous side branches.28, 34, 35 Admittedly, these strategies may have a negative impact on AVF maturation. The most definitive operative plan to avoid ARHI is to perform a simultaneous remedial procedure (e.g. DRIL) although this is somewhat extreme and likely justified only in patients with pre-existing ischemic tissue loss or a prior ARHI in the ipsilateral extremity.36, 37 Higher-risk patients should be monitored closely in the postoperative period and counseled about the symptoms of ARHI. Lastly, a remedial operative plan should be generated before the index access procedures. Although the DRIL procedure appears to be the most accepted, the various remedial treatment options can be effective and should be viewed as complementary.36, 37
The results of the study should be interpreted with some caution given the inherent limitations. Although the HFMstudy was designed to prospectively define the incidence of ARHI, its primary objective was to define the predictors of AVF maturation. There was likely some inherent selection bias in that strategies were implemented preoperatively to reduce the risk of ARHI. The study was conducted at academic centers by clinical teams with an interest in hemodialysis access and may not be applicable across the country. Cases were restricted to HFM participants who required remedial intervention for ARHI, not the larger group of participants with any symptom related to ARHI. This was justified by the fact that remedial treatment is a well-defined endpoint although it is conceivable that the predictors for symptomatic ARHI are not identical to those severe enough to require remedial intervention. The results may not be applicable to patients undergoing AVGs. The time course of the symptoms of ARHI related to AVGs and AVFs is different, likely due to differences in the early hemodynamic changes related to the larger outflow conduit. The nested matched case-control analytic approach was chosen because of the limited number of cases although results could be distorted by unanticipated and unobserved confounders. Notably, the four factors selected for the matching were chosen prospectively based on a review of the literature. Lastly, the mean follow-up is relatively short and, thus, it is conceivable that a few additional participants will develop chronic symptoms of ARHI.
In conclusion, remedial intervention for ARHI after AVF construction is uncommon. Diabetes, female gender, capacitant outflow veins and coronary artery disease are all associated with an increased risk of intervention. These higher risk patients should be counseled preoperatively, their operative plans designed to reduce the risk of hand ischemia and followed closely throughout the postoperative period to avoid any longer term disability.
Appendix A - Pre-specified Candidate Predictors1
Baseline variables
Demographic
Age, sex, self-identified African-American vs. other race
Comorbidities
Diabetes, chronic dialysis. peripheral artery disease (any of amputation, carotid endarterectomy, carotid angioplasty, claudication or lower extremity angioplasty/bypass), coronary artery disease (any of myocardial infarction, coronary artery bypass, or percutaneous intervention), history of hypertension, other permanent access procedure, functional catheter on the surgery arm.
Vascular anatomy
Radial vs. brachial feeding artery, feeding artery diameter, draining vein diameter. (average & minimum) fistula configuration (brachial or antecubital vs. distal radial), high radial artery take-off among upper arm fistulas, cephalic vein vs. basilic vein transpositions vs. brachial vein transpositions (3 categories), within upper arm fistulas. Partition as cephalic vs. transpositions, and brachial transpositions vs. basilica transpositions,
Vascular function and pathology
Fistula arm brachial artery flow-mediated dilation (FMD), expressed as FMD%.
Fistula arm brachial artery nitrogen-mediated dilation (NMD), expressed as NMD%.
Feeding artery ultrasound calcification index (none, mild, moderate to severe).
Draining vein histological calcification (any intimal and/or medial vs. none)
Carotid-radial pulse-wave velocity.
Fistula arm carotid-femoral pulse wave velocity.
Fistula forearm vein capacitance slope.
Fistula forearm maximum vein output slope.
Rescaled alternatives to FMD%: log (Post to pre inflation vein diameter ratio); log scale post on pre inflation vein diameter regression residual; allometrically adjusted post to pre inflation vein diameter ratio.
Rescaled alternatives to NMD%, as above.
Cigarette smoking
Ever smoker
Intraoperative variables
Arteriotomy length
Postoperative vascular adaptation variables
Preoperative upper arm fistula feeding artery flow, fistula flow at day 1, inflow artery diameter at day 1, average outflow vein diameter at day 1, minimum outflow vein diameter at day 1
Appendix B - Details of Matching and Statistical Analysis
The set of all non-ARHI cases matched to any case within the prescribed tolerances was optimally partitioned into disjoint subsets matched to each individual case by optimal full matching21 which enforced matching of sex, diabetes, fistula location, and age within five years and ii) under this constraint, chose the partition minimizing the sum of the absolute case-control differences in age. Optimizations were performed by the R package optmatch.38
Relationships of ARHI to continuous predictors were initially examined for nonlinearity using natural cubic splines with internal knots at the first and third quartiles, and boundary knots at the observed extrema of the predictor, by comparing the fitted spline and linear logistic regression models based on the same predictor. If significant nonlinearity was found by this 2 degree of freedom (df) comparison, then significance of the predictor's association with ARHI was based on the 3 df test of the full spline model; otherwise, the single df linear regression Wald test was used. For ordinal predictors, we compared the model using category indicator variables to the pseudo-linear model based on equally-spaced category scores, and based a final test on the latter or a categorical model, depending on whether significant nonlinearity in the equally-spaced scores was found.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented to the Vascular Annual Meeting, June 17 – 20, 2015, Chicago, Illinois
Unless otherwise noted, hypothesis tests were two-tailed with p ≤ 0.05 required for statistical significance.
Reference List
- 1.Dember LM, Imrey PB, Beck GJ, Cheung AK, Himmelfarb J, Huber TS, et al. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis. 2014 Jan;63(1):104–12. doi: 10.1053/j.ajkd.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber TS, Ozaki CK, Flynn TC, Lee WA, Berceli SA, Hirneise CM, et al. Prospective validation of an algorithm to maximize native arteriovenous fistulae for chronic hemodialysis access. J Vasc Surg. 2002 Sep;36(3):452–9. doi: 10.1067/mva.2002.127342. [DOI] [PubMed] [Google Scholar]
- 3.Keuter XH, Kessels AG, de Haan MH, Van Der Sande FM, Tordoir JH. Prospective evaluation of ischemia in brachial-basilic and forearm prosthetic arteriovenous fistulas for hemodialysis. Eur J Vasc Endovasc Surg. 2008 May;35(5):619–24. doi: 10.1016/j.ejvs.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Lazarides MK, Staramos DN, Kopadis G, Maltezos C, Tzilalis VD, Georgiadis GS. Onset of arterial 'steal' following proximal angioaccess: immediate and delayed types. Nephrol Dial Transplant. 2003 Nov;18(11):2387–90. doi: 10.1093/ndt/gfg346. [DOI] [PubMed] [Google Scholar]
- 5.Morsy AH, Kulbaski M, Chen C, Isiklar H, Lumsden AB. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998 Jan;74(1):8–10. doi: 10.1006/jsre.1997.5206. [DOI] [PubMed] [Google Scholar]
- 6.Papasavas PK, Reifsnyder T, Birdas TJ, Caushaj PF, Leers S. Prediction of arteriovenous access steal syndrome utilizing digital pressure measurements. Vasc Endovascular Surg. 2003 May;37(3):179–84. doi: 10.1177/153857440303700304. [DOI] [PubMed] [Google Scholar]
- 7.Schanzer H, Eisenberg D. Management of steal syndrome resulting from dialysis access. Semin Vasc Surg. 2004 Mar;17(1):45–9. doi: 10.1053/j.semvascsurg.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Suding PN, Wilson SE. Strategies for management of ischemic steal syndrome. Semin Vasc Surg. 2007 Sep;20(3):184–8. doi: 10.1053/j.semvascsurg.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Tordoir JH, Dammers R, Van Der Sande FM. Upper extremity ischemia and hemodialysis vascular access. Eur J Vasc Endovasc Surg. 2004 Jan;27(1):1–5. doi: 10.1016/j.ejvs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Chemla E, Raynaud A, Carreres T, Sapoval M, Beyssen B, Bourquelot P, et al. Preoperative assessment of the efficacy of distal radial artery ligation in treatment of steal syndrome complicating access for hemodialysis. Ann Vasc Surg. 1999 Nov;13(6):618–21. doi: 10.1007/s100169900309. [DOI] [PubMed] [Google Scholar]
- 11.Miller GA, Khariton K, Kardos SV, Koh E, Goel N, Khariton A. Flow interruption of the distal radial artery: treatment for finger ischemia in a matured radiocephalic AVF. J Vasc Access. 2008 Jan;9(1):58–63. [PubMed] [Google Scholar]
- 12.Yu Q, Yu H, Chen S, Wang L, Yuan W. Distribution and complications of native arteriovenous fistulas in maintenance hemodialysis patients: a single-center study. J Nephrol. 2011 Jan 14;:2C9E7802–45E7. doi: 10.5301/JN.2011.6251. [DOI] [PubMed] [Google Scholar]
- 13.Davidson D, Louridas G, Guzman R, Tanner J, Weighell W, Spelay J, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003 Dec;46(6):408–12. [PMC free article] [PubMed] [Google Scholar]
- 14.Huber TS, Hirneise CM, Lee WA, Flynn TC, Seeger JM. Outcome after autogenous brachial-axillary translocated superficial femoropopliteal vein hemodialysis access. J Vasc Surg. 2004 Aug;40(2):311–8. doi: 10.1016/j.jvs.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Kusztal M, Weyde W, Letachowicz W, Porazko T, Krajewska M, Penar J, et al. Influence of autologous arteriovenous fistula on the blood supply to the hand in very elderly hemodialyzed patients. J Vasc Access. 2005 Apr;6(2):83–7. doi: 10.1177/112972980500600207. [DOI] [PubMed] [Google Scholar]
- 16.Valentine RJ, Bouch CW, Scott DJ, Li S, Jackson MR, Modrall JG, et al. Do preoperative finger pressures predict early arterial steal in hemodialysis access patients? A prospective analysis. J Vasc Surg. 2002 Aug;36(2):351–6. doi: 10.1067/mva.2002.125848. [DOI] [PubMed] [Google Scholar]
- 17.Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology. 2000 Oct;217(1):83–8. doi: 10.1148/radiology.217.1.r00oc2883. [DOI] [PubMed] [Google Scholar]
- 18.Silva MB, Jr., Hobson RW, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998 Feb;27(2):302–7. doi: 10.1016/s0741-5214(98)70360-x. [DOI] [PubMed] [Google Scholar]
- 19.Umphrey H, Abts CA, Robb Dialysis grafts and fistulae: planning and assessment. (6 ed.) 2011:477–90. [Google Scholar]
- 20.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002 Oct;225(1):59–64. doi: 10.1148/radiol.2251011367. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum P. A characterization of optimal designs for observational studies. Journal of the Royal Statistical Society. 1991;53:597–610. [Google Scholar]
- 22.Atkinson G, Batterham AM. The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med. 2013 Dec;18(6):354–65. doi: 10.1177/1358863X13508446. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Hertzmark ELMCL, editors. The SAS LGTPHCURV9 Macro. 2015 unpublished. [Google Scholar]
- 24.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, et al. Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016 Jan;63(1):163–70. doi: 10.1016/j.jvs.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheltinga MR, van HF, Bruijninckx CM. Time of onset in haemodialysis access-induced distal ischaemia (HAIDI) is related to the access type. Nephrol Dial Transplant. 2009 Apr 29; doi: 10.1093/ndt/gfp200. [DOI] [PubMed] [Google Scholar]
- 26.Rocha A, Silva F, Queiros J, Malheiro J, Cabrita A. Predictors of steal syndrome in hemodialysis patients. Hemodial Int. 2012 Oct;(4):539–44. doi: 10.1111/j.1542-4758.2012.00684.x. [DOI] [PubMed] [Google Scholar]
- 27.Whittaker L, Bakran A. Prevention better than cure. Avoiding steal syndrome with proximal radial or ulnar arteriovenous fistulae. J Vasc Access. 2011 Mar 31;:BADFD2EF–44D1. doi: 10.5301/JVA.2011.6502. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N, Yuo TH, Konig G, Dillavou E, Leers SA, Chaer RA, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011 Jan 26; doi: 10.1016/j.jvs.2010.10.134. [DOI] [PubMed] [Google Scholar]
- 29.Wilson Samuel E., editor. Vascular Access Principles and Practice. 3rd ed. Mosby; 1996. Physiology of the Arteriovenous Fistula. pp. 29–41. [Google Scholar]
- 30.Bavare CS, Bismuth J, El-Sayed HF, Huynh TT, Peden EK, Davies MG, et al. Volume Flow Measurements in Arteriovenous Dialysis Access in Patients with and without Steal Syndrome. Int J Vasc Med. 2013;2013:328601. doi: 10.1155/2013/328601. doi: 10.1155/2013/328601. Epub;%2013 Aug 27.:328601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopriva D, McCarville DJ, Jacob SM. Distal revascularization and interval ligation (DRIL) procedure requires a long bypass for optimal inflow. Can J Surg. 2014 Apr;57(2):112–5. doi: 10.1503/cjs.000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asif A, Gadalean FN, Merrill D, Cherla G, Cipleu CD, Epstein DL, et al. Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int. 2005 May;67(5):1986–92. doi: 10.1111/j.1523-1755.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 33.Kokkosis AA, Abramowitz SD, Schwitzer J, Nowakowski S, Teodorescu VJ, Schanzer H. Inflow stenosis as a contributing factor in the etiology of AV access-induced ischemic steal. J Vasc Access. 2014 Jul;15(4):286–90. doi: 10.5301/jva.5000205. [DOI] [PubMed] [Google Scholar]
- 34.Jennings W, Brown R, Blebea J, Taubman K, Messiner R. Prevention of vascular access hand ischemia using the axillary artery as inflow. J Vasc Surg. 2013 Nov;58(5):1305–9. doi: 10.1016/j.jvs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Vaes RH, Scheltinga MR. Side branch ligation for haemodialysis-access-induced distal ischaemia. Eur J Vasc Endovasc Surg. 2012 Oct;(4):452–6. doi: 10.1016/j.ejvs.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Anaya-Ayala JE, Pettigrew CD, Ismail N, Diez-De Sollano AL, Syed FA, Ahmed FG, et al. Management of dialysis access-associated “steal” syndrome with DRIL procedure: challenges and clinical outcomes. J Vasc Access. 2012 Jul;13(3):299–304. doi: 10.5301/jva.5000041. [DOI] [PubMed] [Google Scholar]
- 37.Huber TS, Brown MP, Seeger JM, Lee WA. Midterm outcome after the distal revascularization and interval ligation (DRIL) procedure. J Vasc Surg. 2008 Oct;48(4):926–32. doi: 10.1016/j.jvs.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. Journal of Computational and Graphical Statistics. 2006;15:597–610. [Google Scholar]