Abstract
Data from focal brain injury and functional neuroimaging studies implicate a distributed network of parieto-fronto-temporal areas in the human left cerebral hemisphere as playing distinct roles in the representation of meaningful actions (praxis). Because these data come primarily from right-handed individuals, the relationship between left cerebral specialization for praxis representation and hand dominance remains unclear. We used functional magnetic resonance imaging (fMRI) to evaluate the hypothesis that strongly left-handed (right hemisphere motor dominant) adults also exhibit this left cerebral specialization. Participants planned familiar actions for subsequent performance with the left or right hand in response to transitive (e.g., “pounding”) or intransitive (e.g. “waving”) action words. In linguistic control trials, cues denoted non-physical actions (e.g., “believing”). Action planning was associated with significant, exclusively left-lateralized and extensive increases of activity in the supramarginal gyrus (SMg), and more focal modulations in the left caudal middle temporal gyrus (cMTg). This activity was hand- and gesture-independent, i.e., unaffected by the hand involved in subsequent action performance, and the type of gesture (i.e., transitive or intransitive). Compared directly with right-handers, left-handers exhibited greater involvement of the right angular gyrus (ANg) and dorsal premotor cortex (dPMC), which is indicative of a less asymmetric functional architecture for praxis representation. We therefore conclude that the organization of mechanisms involved in planning familiar actions is influenced by one’s motor dominance. However, independent of hand dominance, the left SMg and cMTg are specialized for ideomotor transformations—the integration of conceptual knowledge and motor representations into meaningful actions. These findings support the view that higher-order praxis representation and lower-level motor dominance rely on dissociable mechanisms.
1. Introduction
Damage to the dominant left cerebral hemisphere has been long associated with ideomotor apraxia (hereafter “apraxia”), an acquired disorder in the representation of skilled actions that cannot be attributed to difficulties in linguistic, sensory or lower-level motor functions (Geschwind & Kaplan, 1962; Heilman & Rothi, 1997; Liepmann, 1900). A classic assessment of praxis at the bedside involves asking patients to pantomime familiar actions involving tools, or other manipulable objects, in response to verbal commands that provide little contextual information for retrieval (Liepmann, 1900). Apraxic patients may perform well with actual object use yet exhibit considerable difficulties with pantomime (Randerath, Goldenberg, Spijkers, Li, & Hermsdorfer, 2011), and sometimes also with intransitive gestures (e.g., waving hello or signaling thumbs-up) that do not involve objects (Cubelli, Marchetti, Boscolo, & Della Sala, 2000; Pazzaglia, Smania, Corato, & Aglioti, 2008; Stamenova, Roy, & Black, 2010). Critically, apraxia affects pantomime (and often imitation) regardless of the hand used, a phenomenon which indicates that it arises from disruptions of action representations at a hand-independent level (Leiguarda & Marsden, 2000). Because most apraxic patients have sustained injuries to the left cerebral hemisphere, testing typically involves the non-hemiplegic left hand, which also happens to be the non-dominant side for the vast majority of patients.
There is reasonable, though imperfect, degree of convergence between the neuropsychological literature on right-handed apraxics, and the results of functional neuroimaging studies of pantomime in healthy adults. Overall, neuroimaging investigations also find evidence that the motor dominant left hemisphere supports hand-independent representations of praxis skills (Bohlhalter et al., 2009; Choi et al., 2001; Fridman et al., 2006; Johnson-Frey, Newman-Norlund, & Grafton, 2005; Kroliczak & Frey, 2009; Moll et al., 2000; Ohgami, Matsuo, Uchida, & Nakai, 2004; Rumiati et al., 2004; Vingerhoets, Vandekerckhove, Honore, Vandemaele, & Achten, 2011). Despite using different paradigms – and in some cases both transitive pantomime and intransitive gestures (Fridman et al., 2006; Kroliczak & Frey, 2009; Bohlhalter et al., 2009) – these investigations consistently detect involvement of the left posterior parietal cortex, particularly the SMg and adjacent intraparietal sulcus (IPS). These data are therefore compatible with classic theories in neuropsychology that implicate the left SMg as playing a critical role in supporting hand-independent praxis representations (Heilman, Rothi, & Valenstein, 1982; Rothi, Heilman, & Watson, 1985).
Consistent with more recent neuropsychological findings pointing to a wider network of areas that are critical for praxis skills (e.g., Goldenberg, 2003b; including the middle frontal gyrus, MFg, e.g., Haaland, Harrington, & Knight, 2000, and left inferior frontal gyrus, with adjacent insular and ventral premotor cortices, Goldenberg, Hermsdorfer, Glindemann, Rorden, & Karnath, 2007), neuroimaging studies also report increased hand-independent activity in various regions that lie beyond the left posterior parietal cortex (for a recent neuroimaging meta-analysis see Niessen, Fink, & Weiss, 2014). These areas include the left MFg, supplementary motor (SMA) area, premotor, and/or the prefrontal cortices (Choi et al., 2001; Hermsdorfer, Terlinden, Muhlau, Goldenberg, & Wohlschlager, 2007; Johnson-Frey et al., 2005; Kroliczak & Frey, 2009; Moll et al., 2000; Ohgami et al., 2004; Rumiati et al., 2004). A notable subset of studies also finds greater engagement of the left caudal middle temporal gyrus (cMTg) (Choi et al., 2001; Hermsdorfer et al., 2007; Johnson-Frey et al., 2005; Kroliczak & Frey, 2009), an area implicated in the conceptual representation of familiar manipulable objects and associated actions (Beauchamp, Lee, Haxby, & Martin, 2002; Beauchamp & Martin, 2007; Chao & Martin, 2000; Martin, Wiggs, Ungerleider, & Haxby, 1996; Kellenbach, Brett, & Patterson, 2003; Mahon et al., 2007; Weisberg, van Turennout, & Martin, 2007), and/or the visual analysis of tool’s features (Vingerhoets, 2008). Indeed, damage in this vicinity impairs performances on tasks that require accessing such knowledge (Tranel, Damasio, & Damasio, 1997; Tranel, Kemmerer, Adolphs, Damasio, & Damasio, 2003). The cMTg and neighboring temporal regions are furthermore strongly interconnected with the SMg (Ruschel et al., 2014).
Together, these various sources of evidence are consistent with the hypothesis that a distributed parieto-fronto-temporal set of regions within the left hemisphere are critical nodes for ideomotor transformation, the integration of conceptual and motor representations in service of familiar, meaningful actions (Johnson-Frey, 2004). The question of whether this left cerebral asymmetry for hand-independent praxis representation depends on hand dominance, however, persists.
Due in part to the preponderance of dominant hand hemiplegia in apraxia, the relationship between cerebral dominance for sensorimotor control of the hand versus for the representation of praxis remains unclear. One account is that right-handedness is a direct reflection of the left-lateralized system for representing manual praxis (Geschwind & Galaburda, 1985; Heilman, 1997; Kimura & Archibald, 1974; Liepmann, 1908; for a discussion see Goldenberg, 2013b). Indeed, of the small number of left-handed cases of apraxia that have been investigated, some do show signs of apraxia following right hemisphere lesions (Dobato et al., 2001; Poeck & Kerschensteiner, 1971; Valenstein & Heilman, 1979). However, this can also be said for a minority of right-handed patients (Marchetti & Della Sala, 1997; Raymer et al., 1999), which is inconsistent with this speculation. Alternatively, praxis representation and hand dominance might depend on relatively independent mechanisms, with most left-handers also representing praxis skills in their left (motor non-dominant) hemispheres. Data from left-handed individuals that have undergone surgical transections of, or sustained injuries to, the corpus callosum support this view (Frey, Funnell, Gerry, & Gazzaniga, 2005; Lausberg, Gottert, Munssinger, Boegner, & Marx, 1999). Evidence for a potential dissociation between motor dominance and praxis mechanisms can also be found. A recent comprehensive report on 50 left-handed patients with unilateral brain injuries (Goldenberg, 2013a; for a discussion see also Goldenberg, 2013b) identified three cases with apraxia and aphasia following injuries to the left hemisphere, demonstrating clear dissociations between handedness and apraxia. Yet, three cases with apraxia and no aphasia subsequent to right (motor dominant) hemisphere injury have been also found, demonstrating at least the importance of some low level mechanisms linking handedness and praxis skills.
As these various sources indicate, resolving the relationship between mechanisms responsible for hand dominance and/or praxis representation on the basis of patient data alone has proven very challenging. Yet, apart from inferences based on these studies, remarkably little is known about the organization of praxis in healthy left-handed adults who constitute approximately 10% of the population (Coren & Porac, 1977; Porac & Coren, 1981; Willems, Van der Haegen, Fisher, & Francks, 2014). As a consequence of excluding left-handed participants, functional neuroimaging studies have done little to clarify the relationship between cerebral asymmetries for praxis and motor dominance. An exception is a report on strongly left-handed participants who pantomimed unilateral or bimanual actions in response to familiar visually presented objects (Vingerhoets et al., 2012). Yet, the absence of a control for linguistic functions (see Martin et al., 1996; Chao & Martin, 2000; Kroliczak & Frey, 2009), and the lack of a distinction between action planning vs. execution (Johnson-Frey et al., 2005) calls for additional studies on the relationships between the left hemisphere specialization for praxis representation and motor dominance in the healthy adult brain.
In our previous research on strongly left-handed individuals, we focused on the organization of language and praxis in selected regions of interest, and motor dominance was less of an issue (Kroliczak, Piper, & Frey, 2011). Here we revisit these same data using whole-brain statistical parametric mapping to test whether, similarly to right-handed adults (Kroliczak & Frey, 2009), these left-handers exhibit evidence for left lateralized parieto-fronto-temporal praxis representation network. Our primary focus is on hand-independent activity during gesture planning. As in our earlier work (Kroliczak & Frey, 2009), we did not expect to find evidence of dissociable mechanisms specialized for the representation of tool use pantomimes (transitive) vs. communicative (intransitive) gestures involving no objects. To the extent that the organization of praxis representation is independent of hand dominance, we predicted that lefthanders would also exhibit increases within a left-lateralized, parieto-fronto-temporal network when planning gestures for subsequent production with either hand. Finally, we expected that direct statistical comparisons with the data from right-handers (Kroliczak & Frey, 2009) would also fail to detect significant differences in activity related to hand dominance within the supramarginal gyrus and caudal middle temporal gyrus.
2. Methods
The local Ethics Committee for Research Involving Human Subjects at the University of Oregon approved the experimental protocols, which conformed to the WMA Declaration of Helsinki.
2.1 Participants
Healthy adult, self-identified left-handers (N = 51) were invited to visit the laboratory for additional screening. To verify left-handedness, participants 1) completed the revised Edinburgh inventory (Oldfield, 1971), 2) constructed a family tree based on which hand relatives used for writing, and 3) were assessed on gross and fine motor tasks. Fifteen English-speaking adults (8 females, 7 males, Mean = 24.75 years; SD = 8.42) with the strongest self-reported and behaviorally verified left hand dominance were selected for subsequent behavioral and neuroimaging tests. The majority of these participants (67%) had either a left-handed parent or grandparent. Edinburgh laterality indices in the selected sample were in the upper quartile of left-handedness, and ranged from −66 to −100 (Mean = −90; SD = 9.0), where a score of −100 indicates the use of the left hand on all activities listed in the test (e.g. writing, throwing, or using a spoon, etc.).
Rather than relying exclusively on self-report, we also sought to establish evidence of left hand dominance through two measures of performance. Grip strength was determined three times for each hand with a dynamometer (Stoelting, Wood Dale, IL). Twelve trials on a 9-Hole peg board test, based on Mathiowetz and colleagues (Mathiowetz, Weber, Kashman, & Volland, 1985; Oxford Grice et al., 2003), were completed: six trials per hand alternated between the dominant and non-dominant limb with the order of the starting hand counter-balanced across subjects. The duration to finish each trial was measured. Half the trials were executed in the standard fashion, i.e., using the fingers to place each peg in the hole and return it to the starting area. Participants also completed an additional 12 trials, six per limb, using a pair of forceps. (On three occasions, in different participants, the peg was dropped and the task was not completed; 1 time with the fingers, and two times with the forceps. In these cases the missing values were replaced with the average of the proceeding and the following trial.) SPSS version 18.0 (Chicago, IL) was used for all statistical analyses.
2.2 fMRI Testing
The fMRI tasks and methods are identical to those described in Kroliczak and Frey (2009). Familiarization. In order to ensure consistency in behavior during the fMRI, and to minimize head motion, subjects participated in an initial familiarization session, followed by practice within an MRI simulator. During initial familiarization, they watched and responded to instructions presented during a 16 min training video. The movie consisted of short clips preceded by a centrally presented instructional cues. These cues (presented in gerundive form, as verb-derived nouns) were of three types: 1) 14 transitive/tool use (e.g., “cutting”, “reeling”, or “writing”), 2) 14 intransitive (e.g., “beckoning”, “scolding”, or “wavering”), or 3) 14 nonphysical actions (e.g., “believing”, “evaluating”, or “thinking”). A full list of cues is given in the Appendix. Cues denoting transitive or intransitive actions were followed by a clip of an actor performing the associated unimanual tool use pantomimes or other meaningful gestures (henceforth “gesture”), one time with the right and one time with the left hand. The instructional cue was then presented again, and participants were asked to produce the associated gesture using their hands in the same order as the actor in the video. Thus, although there are some differences in motor cortex activity in response to the observation of actions performed with the left or right hand in right- and left-handed persons (as shown by Sartori, Begliomini, & Castiello, 2013), any effects from the pre-training phase should have little effect on later gesture planning activity. On trials with cues denoting nonphysical actions no gestures were presented and the task was to remain still and await the next cue.
Immediately after the training video, participants practiced at least two runs of the actual fMRI gesture task in a mock scanner. This allowed them to practice producing recognizable gesture responses without moving their heads. Trial order was randomized differently than fMRI testing. During mock and actual scanning, subjects were instructed that actions should be executed gently, with only the hand and forearm, and that head needed to remain still. Participants were informed that the gestures needed to be recognizable and would be video-recorded during later scanning.
fMRI Data Acquisition
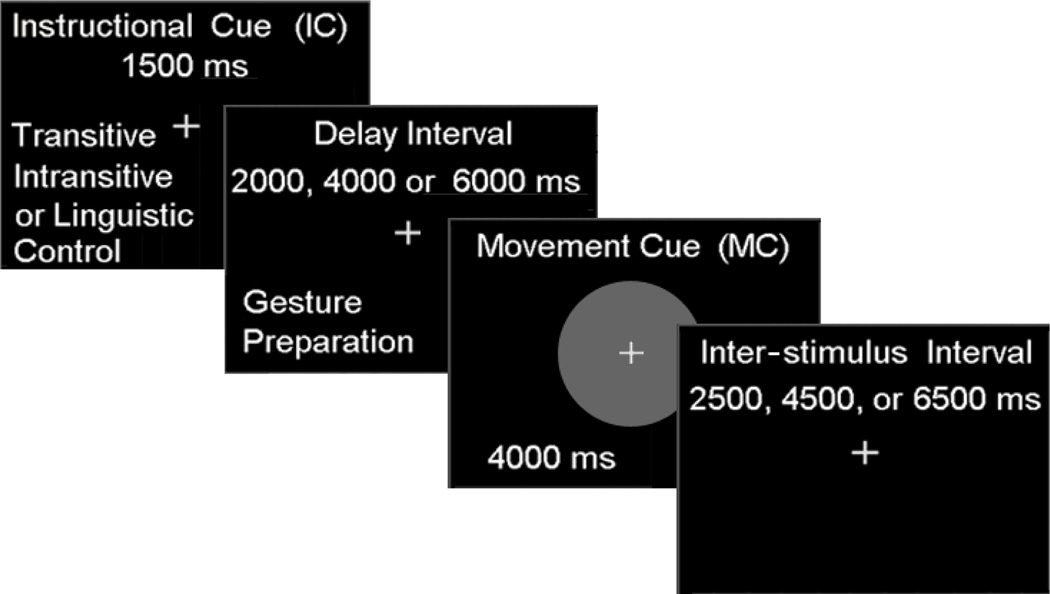
During actual scanning, all participants completed two separate experimental sessions on different days; one using the dominant left hand and the other using their non-dominant right hand. The order of the hand tested was counterbalanced across participants. Both fMRI sessions consisted of six functional runs of the gesture task, each lasting approximately 6 min. Every run involved the presentation of the three verb types (related to transitive, intransitive or non-physical actions) described above. As illustrated in Figure 1, trials of each condition consisted of a: 1) visually presented instructional cue (1,500 ms), 2) variable length delay interval (2,000, 4,000 or 6,000 ms), 3) visually presented movement cue (4,000 ms), and (4) a variable duration (2,500, 4,500 or 6,500 ms) inter-trial interval. For transitive and intransitive trials, participants prepared, i.e., planned, to gesture the actions identified by the instructional cue (IC) during the subsequent delay interval. Planning-related signal increases in each condition were modeled as the 3,500 ms period starting with the onset of IC (1,500 ms) and lasting through the offset of the shortest delay interval (of 2,000 ms). Production-related signal increases in the transitive and intransitive conditions, which began with a centrally presented movement cue (MC) in the form of green circle, were modeled as the 4,000 ms period during which the MC was visible. Cues indicating non-physical actions served as controls for linguistic stimulus processing. On these linguistic control (LC) trials, participants were instructed to relax and neither plan nor undertake any movements.
Figure 1. Structure and timing of the fMRI gesture task.
The instructional cue (1,500 ms) was followed by a variable delay interval (2,000, 4,000, or 6,000 ms) during which gestures were planned, a movement cue for gesture production (4,000 ms), and a variable inter-trial interval (2,500, 4,500, or 6,500 ms).
The stimuli were controlled with Presentation software (http://www.neurobs.com), back-projected on screen behind the scanner bore, and viewed via a mirror attached to the head coil. Throughout every functional run, participants were instructed to maintain fixation on the omnipresent central cross. Eye position was monitored with an MRI-compatible tracking system (http://www.a-s-l.com) to ensure subject adherence to task instructions. The resulting manual performances were also monitored continuously by the experimenter and digitally video recorded.
Neuroimaging was performed on a Siemens (Erlangen, Germany) 3-Tesla Allegra MRI scanner. Prior to the onset of the functional runs, Auto Align Scout and True FISP sequences were run to prescribe the position of slices. The blood-oxygenation level dependent (BOLD) echoplanar images were collected using a T2*-weighted gradient echo sequence: TR (time repetition) = 2,000 ms; TE (time echo) = 30 ms, FA (flip angle) = 80°; 64 × 64 matrix; FOV (field of view) = 200 mm; 33 contiguous axial slices, 3.0 mm isotropic voxels. High-resolution T1-weighted structural images were also acquired with an MP-RAGE pulse sequence with the following parameters: TR = 2,000 ms; TE = 4.38 ms; FA = 8.0°; 256 × 176 voxel matrix size; FOV = 256 mm; 176 contiguous axial slices; 1.0 mm isotropic voxels. Raw image data were reconstructed using two-dimensional fast Fourier transform with a distortion correction (in order to minimize artifacts attributable to magnetic field inhomogeneities) and then converted to NIFTI-1 format using MRI-Convert software (http://lcni.uoregon.edu/~jolinda/MRIConvert/). Data were preprocessed and modeled with FSL version 4.1.4 (http://www.fmrib.ox.ac.uk/fsl/).
2.3 fMRI Analyses
Prior to statistical analyses non-brain tissue was removed using BET (Smith, 2002). Subsequent pre-processing steps included motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), spatial smoothing with a Gaussian kernel of FWHM = 5 mm, mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering (50 s) to remove low frequency artifacts. For each participant, all fMRI runs were modeled separately at the first level. The degrees of freedom in the statistical model were estimated, and subsequently corrected for auto-correlation in the data, using the FSL pre-whitening technique (Woolrich, Ripley, Brady, & Smith, 2001). Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al., 2001). Registration to high-resolution and standard space images (Montreal Neurological Institute template) was implemented with FLIRT (Jenkinson & Smith, 2001). Inter-session (level 2) analyses were run using a fixed effects model. Inter-subject (level 3) and inter-experiment (level 4) random-effects components of mixed-effects variance were modeled and estimated using FLAME Stage 1 (Beckmann, Jenkinson, & Smith, 2003). Z (Gaussianized t/F) statistic images were obtained using clusters not smaller than those determined by Z > 2.3 and a corrected cluster significance threshold of p = 0.05 (Worsley, 2001). Region-of-interest (ROI) analyses employed Featquery, an FSL tool for computing mean percent signal change (PSC) associated with each of the three experimental conditions (i.e., transitive and intransitive gesture planning, as well as the linguistic control condition) relative to the baseline.
In order to compare responses between hands and to test for interactions with gesture types, a 2 (Hand: left, right) x 2 (Gesture: transitive, intransitive) ANOVA was run separately for gesture planning and production phases. To increase sensitivity, these analyses were conducted only across those voxels that earlier demonstrated significant increases in activity relative to resting baseline. Regions exhibiting significant increases regardless of the hand involved (i.e., hand-independent praxis planning), were identified by conjunction analysis using inclusive contrast masking (Nichols, Brett, Andersson, Wager, & Poline, 2005).
Anatomical localization was undertaken by manual comparison with an atlas (Duvernoy, 1991), and overlaying activation maps on the population, landmark and surface-based atlas (PALs) of Van Essen (Van Essen, 2005) using CARET software (Van Essen et al., 2001). The standard (i.e., MNI-152) volumetric group average data were projected onto the PALs surface using multifiducial mapping procedure in which the data are first mapped to a set of twelve individual brains, and then re-averaged to account for individual variations in cortical topography (Van Essen, 2005).
2.3.1 Region-of-Interest Analyses
Two a priori ROIs were defined by combining our group data and the maps from the Harvard-Oxford Atlas (Desikan et al., 2006). Based on our study in right-handers (Kroliczak & Frey, 2009), we focused only on the anterior division of the SMg (aSMG), and the temporo-occipital (i.e., caudal) part of the MTg (cMTg) of the left cerebral hemisphere.
Using FSL FEATQuery, mean percent signal change (PSC) values were computed from all voxels of significant activity versus baseline located within these ROIs, regardless of a condition and hand involved (for transitive and intransitive gesture planning, using the left and right hand). After the % signal change values (converted from parameter estimate [PE] values) were extracted for each task from each ROI, a 2 (Hand: left, right) × 2 (Gesture type: transitive, intransitive) repeated-measures ANOVA was run, and if necessary, the differences between the conditions were further assessed with a T-test, corrected for multiple comparisons using Bonferroni correction p-values [Bf-p].
2.3.2 Comparisons between left- and right-handers
A 2 (handedness: left dominant, right dominant) x 2 (hand: left, right) x 2 (gesture type: transitive, intransitive) repeated-measures ANOVA, with handedness as the between-subjects factor was run to perform direct statistical comparisons of the current left-handed data with data from our earlier study in right-handers (Kroliczak & Frey, 2009). This was done separately for the gesture planning and execution phases. Because no main effect of gesture type was observed, nor its interaction with any other factor, we then collapsed across the two gesture types and present the results of a 2 (handedness: left dominant, right dominant) x 2 (hand: left, right) repeated-measures ANOVA.
3. Results
3.1 Behavioral Results
Measures of hand performance complimented data from the Edinburgh Handedness Inventory, affirming the left hand dominance of our participants for strength and dexterity.
Maximal Grip Strength
Participants demonstrated stronger maximal voluntary contractions with their dominant left hands. A 2 (Hand) x 3 (Trial) repeated-measures ANOVA revealed a main effect of Hand for Grip Strength [F(1, 14) = 28.2, p < 0.001], which was significantly greater for the dominant left relative to the non-dominant right hand (Mean Left = 39.8 kg, SEM = 2.6 kg; Mean right = 35.4 kg, SEM = 2.2 kg). The effects of Trial (p = 0.3) and the Hand x Trial interaction (F < 1.0) were not significant.
Modified Pegboard Task
Manual dexterity was also greater for the dominant side. A 2 (Hand) x 6 (Trial) repeated-measures ANOVA revealed significantly shorter completion times when using the left (Mean Left = 15.5 s, SEM = 0.26 s) vs. right (Mean right = 17.6 s, SEM = 0.34 s) hand [F(1, 14) = 56.05, p < 0.001]. There was also a main effect of Trial [F(5, 70) = 25.26, p < 0.001], indicating that completion times decreased with practice. The Hand x Trial interaction was not significant (F < 1.0). Similar results were obtained when using forceps with participants requiring less time to complete the task with their dominant left (Mean Left = 29.4 s, SEM = 1.2) vs. non-dominant right (Mean right = 37.4 s, SEM = 1.7 s) hand [F(1, 14) = 71.2, p < 0.001]. Likewise, performance improved across trials [F(5, 70) = 14.6, p < 0.001]. The Hand x Trial interaction, again, was non-significant (F < 1.0).
3.2. fMRI Results
3.2.1 Planning Phase
Planning-related signal increases in each condition were modeled as the 3,500 ms period starting with the onset of the IC (1,500 ms) and lasting through the offset of the shortest delay interval (of 2,000 ms). A 2 (Hand: left, right) x two (Gesture type: transitive, intransitive) repeated-measures ANOVA was conducted on only those voxels demonstrating significant increases in activity in any of the four conditions relative to resting baseline. As with right-handers (Kroliczak & Frey, 2009), this analysis revealed a main effect of hand (detailed below), and no effect of gesture type, nor an interaction. Subsequent analyses therefore collapsed across the transitive and intransitive conditions.
Left Hand Planning vs. Linguistic Control
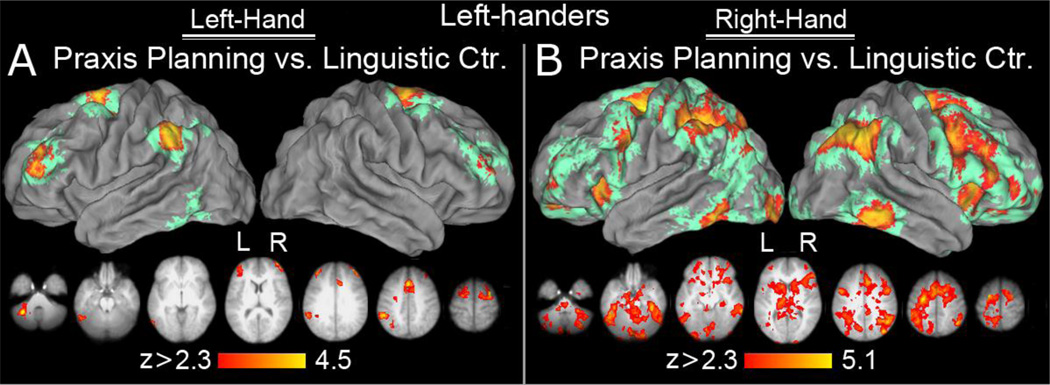
Significant left-lateralized increases in activity were found in the SMg, (extending into the IPS) and in the cMTg, when gesture planning was contrasted against the LC condition. Bilateral activity was detected in dorsal premotor (dPMC) cortex, extending medially into the pre-supplementary motor (pre-SMA) and the cingulate motor (CMA) areas. Bilateral, left asymmetrical clusters of increased activity were also detected in the rostral middle frontal gyrus (rMFG), and cerebellum (Figure 2A).
Figure 2. Areas activated during gesture planning for the left (panel A) and right (panel B) hand, controlled for linguistic processing.
The volumetric surface renderings in the upper panels show significant group effects displayed on the PALs atlas in Caret 5.6 using the multifiducial procedure (see Methods). Clusters that survived multifiducial re-mapping are shown in warm hues. Pale green represents areas that were activated in some individuals (as revealed by the re-mapping algorithm) but did not survive the multifiducial average threshold after accounting for intrasubject variability in gyri and sulci anatomy. Axial slices in the lower panels display significant group mean statistical parametric maps projected onto an average brain obtained from high resolution, T1-weighted anatomical scans of participants’ brains. Neurological convention is used in which right hemisphere is on the right side. The range of the Z statistic values is shown at the bottom and voxels activated at a given Z value are displayed according to the code on the color bar. (A) Praxis planning with the dominant left hand versus the linguistic control (LC) condition. In addition to bilateral signal increases in the dorsal premotor, pre-supplementary motor, cingulate motor area, and rostral middle frontal gyrus (with the latter being substantially more invoked on the left), the inferior parietal lobule was activated only on the left. The left cerebellum and caudal inferior temporal gyrus (cITG) were also involved. (B) Praxis planning with the non-dominant right hand vs. LC. The bilateral activation involved all the major structures typically observed in action planning and/or production, including the right angular, middle frontal, and inferior temporal gyri.
Right Hand Planning vs. Linguistic Control
As with the left hand, gesture planning was associated with the expected left-lateralized increase in SMg activity (extending along the IPS and into the SPL). Here, however, increases in cMTg were bilateral (and extended into caudal inferior temporal gyri). Bilateral increases were again detected in the dPMC, pre-SMA, CMA, rMFG, as well as in the insular cortices. In contrast to the left hand, vPMC, DLPFC, orbito-frontal cortex, inferior temporal gyrus, and the cerebellum also exhibited significant bilateral, and often widespread increases (Figure 2B).
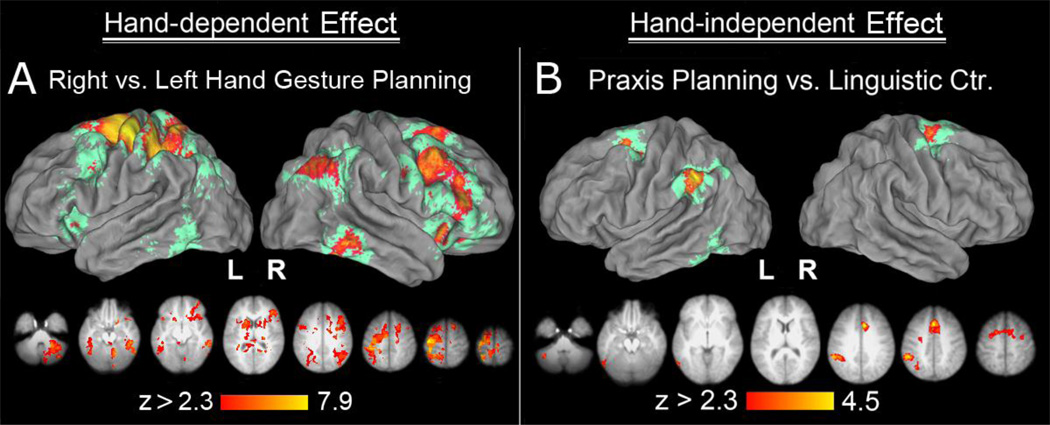
Hand-Dependent Planning
In order to evaluate the significance of the apparent hand-dependent differences described above, gesture planning phase activity was contrasted between left and right hand conditions. As it turned out, no region showed significantly greater modulation during gesture planning with the left vs. right hand. The opposite contrast, namely the comparison for the right vs. left hand planning, was however associated with significant and widespread signal changes. Contralateral left hemisphere increases were detected in the SPL (extending into the IPS), and in the post- and precentral gyri. Right-asymmetrical, bilateral increases were detected in the ANg, cMTg, vPMC, DLPFC, and MFg, as well as in the IFg and anterior insula. The right cerebellum also exhibited increased activity (Figure 3A). More widespread activity when planning gesture for subsequent production with the right hand was previously found in right-handers (Kroliczak & Frey, 2009). As will be discussed shortly, this therefore does not appear to be associated with the right hand’s non-dominant status.
Figure 3. Hand-dependent (panel A) and hand-independent (panel B) neural substrate of praxis planning.
(A) When left-handers plan gestures with their right hand, nearly all the areas revealed in an initial contrast of right-hand gesture planning vs. LC are activated more, and more extensively than for the left hand. The inverse contrast (of left vs. right hand) revealed no significant difference. (B) Areas engaged in praxis planning vs. the linguistic control (LC) across both hands. In addition to the dorsal premotor and pre-supplementary motor areas that were activated bilaterally, the left inferior parietal lobule, including the supramarginal gyrus and the ventro-lateral bank of the anterior intraparietal sulcus (SMg/aIPS) was activated only on the left. Additional small cluster of hand-independent activity was observed in cMTg, caudal inferior temporal gyrus (cITg), and the cerebellum on the left.
Hand-Independent Planning
Consistent with the hypothesis that the left hemisphere is involved in hand-independent representation of praxis independent of motor dominance (Lausberg et al., 1999; Frey et al., 2005; Frey, 2008), like right-handers, our strong left-handers exhibited left lateralized, hand-independent activity in the SMg (and adjacent IPS) and cMTg (extending into the inferior temporal gyrus). However, unlike right-handers, increases in dPMC, pre-SMA and CMA were bilateral rather than left lateralized (Figure 3B). Furthermore, left-handers failed to exhibit any significant hand-independent increases in rMFg activity. The effects of hand dominance on planning-related activity are considered further below, where we test statistically for differences between these data and a previously reported set from righthanders. First, we report tests conducted in several regions-of-interest.
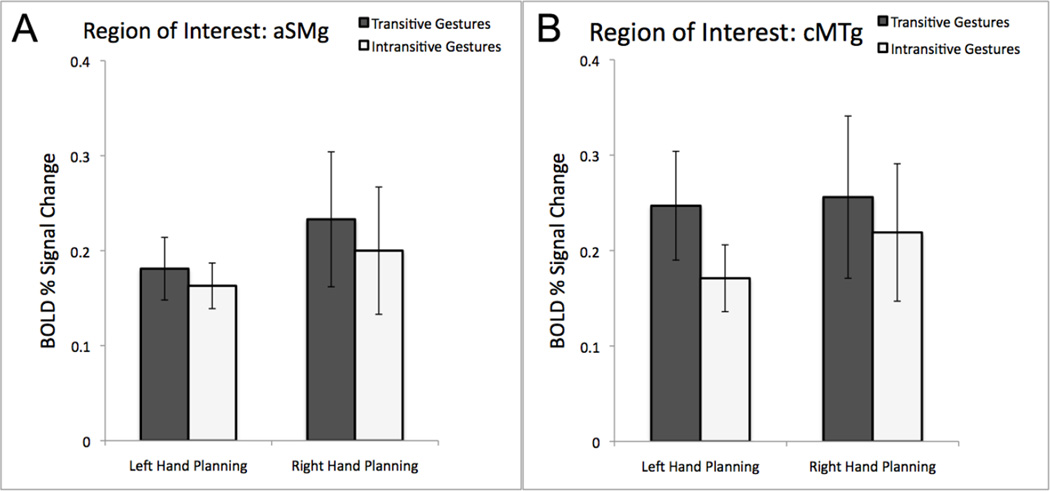
Region-of-Interest-Analyses
Consistent with the whole-brain analyses, we failed to detect any evidence of differences between responses to planning transitive vs. intransitive gestures, regardless of the hand involved, within the two critical ROIs. In the aSMg ROI, the lack of any effects were indisputable: the main effect of hand was not significant (F(1,14) = 0.37; p = 0.56); the main effect of gesture type was not significant (F(1,14) = 0.69; p = 0.42), and the interaction was not significant either (F(1,14) = 0.22; p = 0.65). In the cMTg ROI, again, no significant effects were observed. Yet, although, the lack of main effect of hand was clear-cut (F(1,14) = 0.17; p = 0.69), the main effect of gesture type showed a weak trend towards significance (F(1,14) = 2.79; p = 0.12). As before, the interaction was not significant (F(1,14) = 0.74; p = 0.40). Because the null effects are important for the arguments presented here, the results are shown in Figure 4.
Figure 4. Region-of-Interest analyses of gesture planning with the dominant left and the non-dominant right hand.
The average % signal change during the planning-phase is shown relative to the resting baseline for tool use (transitive) pantomimes and intransitive gestures within the independently defined left SMg (A) and cMTg (B). No significant effects were detected.
To enable more direct comparisons with the ROI analyses from our previous study (Kroliczak & Frey, 2009), further results are shown in Supplemental Fig. 1A-J
In contrast to the earlier results, a direct comparison of the two conditions in each ROI revealed that the difference between the mean percent signal change associated with the planning of the two gesture categories was not significantly different in any of the areas (Bf-p > 0.44). Given how these ROIs were defined (i.e., by contrasting both gesture planning tasks with processing of non-gesture related words followed by no planning), in each ROI responses in both conditions also exceeded those associated with the linguistic control (Bf-p < 0.05; in majority of comparisons Bf-p < 0.01).
To enable direct comparisons with the analyses of individual variation in posterior parietal involvement in gesture planning from the previous study (Kroliczak & Frey, 2009), and to help assess its typicality (or lack of thereof) in a given participant, anatomical landmarks were used to establish whether significant clusters of activity were located in SMg, Ag, and SPL (Johnson-Frey et al., 2005). Percentages in Supplemental Table 1 reflect proportions based on the total sample of 15 individuals. It turns out that 70% of our participants engage left SMg, and only 40% engage right SMg, for gesture planning regardless of the hand used.
3.2.2 Production Phase
Although our hypotheses concerned activity during the planning phase we nevertheless evaluated responses during the production phase, modeled from the appearance of the visually presented movement cue and continuing through the subsequent 4000 ms. A 2 (Hand: left, right) x two (Gesture type: transitive, intransitive) repeated-measures ANOVA was performed across all voxels that showed significant increases in activity relative to resting baseline in any of the four conditions (2 hands x 2 gesture types; Z > 3.1, p = .01). As in the planning phase, a number of regions exhibited a main effect of hand, while none showed a significant main effect of gesture type, nor a hand x gesture type interaction. Subsequent analyses therefore collapsed across transitive and intransitive conditions.
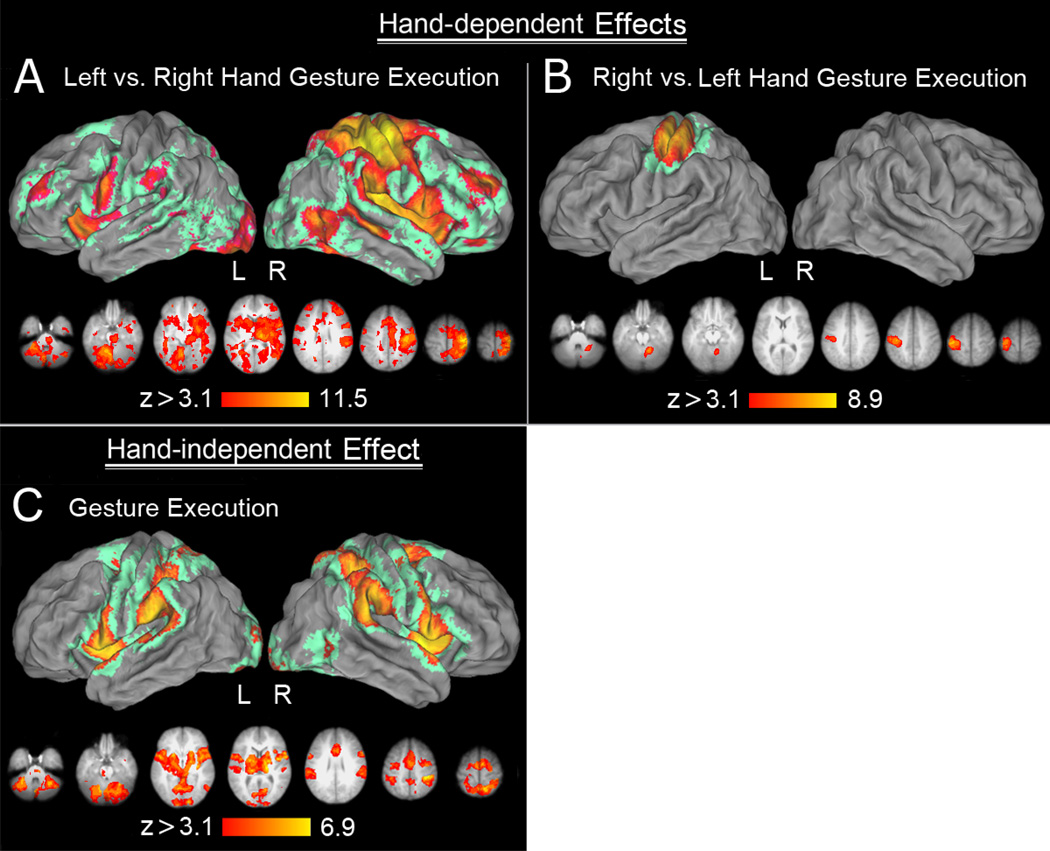
Hand-Dependent Production
Left vs. right hand production revealed increases in contralateral sensorimotor cortex extending caudally into the SPL and rostrally into the dPMC. Use of the left hand was also associated with distributed increased activity within bilateral SMg, cMTg, vPMC, MFg, DLPFC, insula, as well as basal ganglia and cerebellum (Figure 5A). These widespread increases for the left vs. right hand are similar to what we observed previously when right-handers performed these same actions with their (non-dominant) left hands (Kroliczak & Frey, 2009). The inverse contrast of right vs. left hand production revealed only significant activity in the left sensorimotor cortex and ipsilateral right cerebellum (Figure 5B).
Figure 5. Hand-dependent (panel A and B), and hand-independent effects (panel C) during gesture production.
(A) A main effect of hand revealing all voxels involved more in the control of the left (vs. right) hand. In addition the expected activity observed in the right hemisphere, including the sensorimotor cortex, posterior parietal, frontal and pre-frontal (MFG) activity, it is worth emphasizing that the left SMg, vPMC and MFg were also involved. (B) A main effect of hand revealing all voxels involved more in the control of the right (vs. left) hand. The activity was primarily limited to the left sensorimotor cortices and a small cluster in the right cerebellum. (C) Areas activated across both hands during gesture production. Except for the right caudal middle temporal cortex (cMTg), all the remaining parieto-frontal, the insular and temporal cortices, as well as subcortical regions were involved bilaterally.
Hand-Independent Production
Consistent with earlier findings in right-handers (Johnson-Frey et al., 2005), production with either hand increased activity bilaterally in both the ventral and dorsal parieto-premotor networks (including IPL, SPL, dPMC, vPMC,) and the cerebellum. Increases were also apparent in the anterior insula, pre-SMA, as well as the right cMTg (Figure 5C).
3.2.3 Hand Dominance Effects
As already mentioned, because the procedures in this study were identical to those of our earlier study in right-handers (Kroliczak & Frey, 2009), we performed direct statistical comparisons of the two data sets.
Planning Phase
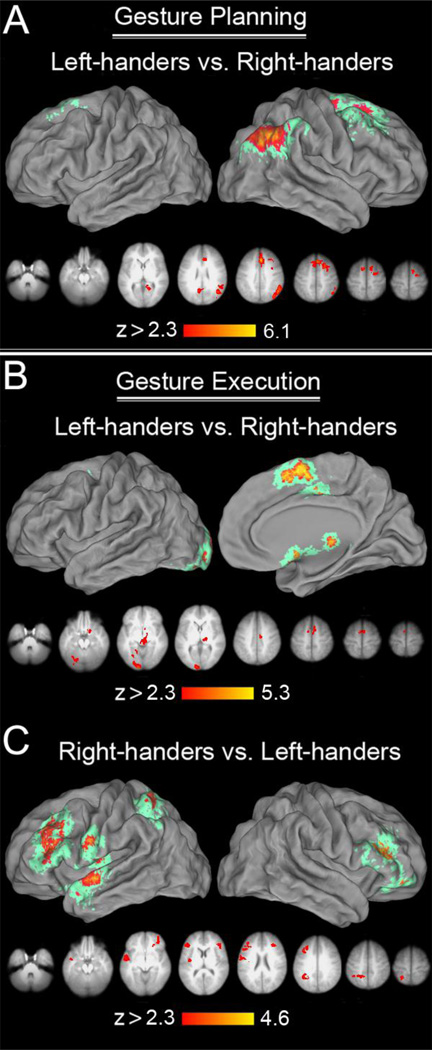
A 2 (handedness: left dominant, right dominant) x 2 (hand: left, right) repeated-measures ANOVA, with handedness as the between-subjects factor, revealed main effects of both hand and of hand dominance. There was no significant hand by hand dominance interaction. Relative to righthanders, left-handers showed greater involvement of the right ANg, dPMC, and DLPFC, and in bilateral retrosplenial cortex (RSC), and SMA/CMA (Figure 6A). In sharp contrast, we detected no regions that were more responsive for right- vs. left-handers during the planning phase. These differences are consistent with the general view that left handers exhibit less cerebral asymmetry of praxis functions than do right-handers (Vingerhoets et al., 2012), but, as will be discussed, these results differ from this earlier study in several details.
Figure 6. The effect of handedness on gesture planning, and production.
(A) Areas involved more in gesture planning in left-handers: Areas engaged more by left-handers were located either in the right hemisphere, i.e. right ANg, dPMC, or bilaterally (dorso-medial, and dorso-lateral prefrontal cortices). (B) Areas involved more in gesture production in left-handers: Again, except for the left-sided early visual and cerebellar contribution, areas engaged more by left-handers were in the dorso-medial prefrontal cortices. (C) Areas involved more in gesture production in right-handers: In addition to left SPL, dPMC, MFg, and some dPMC, and SMg/aIPS, there was also some noticeable involvement of the left anterior STg, and the nearby insular cortex. The right-hemisphere involvement was substantially less pronounced and included some lateral-ventral prefrontal contributions.
Both groups exhibited increased activity when planning gestures for the right vs. left hand, while neither showed any evidence for the opposite effect. Despite lack of significant interaction, because the areas displaying these greater right hand responses differed somewhat between groups, these effects are shown separately in Supplemental Figure 2. For left-handers, gesture planning with the right hand resulted in increased activity in left SPL, bilaterally in dPMC, MTg, and the insular cortex, as well as less symmetrically (with clear right-hemispheric advantage) in vPMC, and IFg, and finally with ANg and MFg engaged mainly on the right (Suppl. Figure 2A). In right-handers, these effects were nearly exclusive to the dominant left hemisphere and involved a partially different set of parieto-fronto-temporal areas (cMTg, dPMC, SMg and ANg, rMFg, SPL, (Suppl. Figure 2B). Greater engagement of cortical regions when planning gestures with the right hand in both groups is difficult to explain, but would seem to be unrelated to hand dominance.
Production Phase
A repeated-measures ANOVA on the production phase data also revealed significant main effects of hand and of handedness, but no significant interaction. Relative to right-handers, lefthanders exhibited increased activity primarily on the medial brain surfaces, in SMA and CMA. A left-lateralized occipital, and right thalamic increase was also present (Figure 6B). Compared to lefthanders, right-handers exhibited increased left-lateralized activity in the SPL, vPMC, and rostral MFg, as well as in right inferior frontal sulcus (IFs), extending into IFg, and orbito-frontal cortex (Figure 6C).
4. Discussion
Data from left-handed patients with unilateral brain injuries suggest that the mechanisms responsible for the left hemisphere specialization of praxis are dissociable from those that underlie hand dominance (Frey et al., 2005; Goldenberg, 2013a; Goldenberg, Hermsdorfer, & Spatt, 1996; Lausberg et al., 1999; see also Goldenberg, 2013b). Here, we used fMRI to test whether this holds for healthy adults. If so, then similarly to right-handers, strongly left-handed adults should exhibit left cerebral asymmetries in activity during the planning/retrieval of familiar meaningful gestures for subsequent production with either hand. More precisely, we expected to detect these asymmetries in the supramarginal gyrus and its vicinity (SMg/IPs) and caudal middle temporal regions (e.g., cMTg) even after controlling for linguistic stimulus processing demands. We found three sources of support for this hypothesis. First, whole-brain analyses detected a pronounced left cerebral asymmetry within both SMg/IPs and cMTg during the gesture planning phase, regardless of the hand involved in subsequent performance of the task. Second, more sensitive ROI analyses carried out within independently-defined left SMg and cMTg areas failed to detect significant differences in activity between planning for the left or right hands. Third, comparing the present data directly with a published set acquired from right-handers failed to reveal any significant differences in activity related to hand dominance within the SMg and cMTg. Together, these results support the view that regardless of motor dominance, the human left hemisphere (specifically the left supramarginal gyrus and caudal middle temporal gyrus) is specialized for ideomotor transformations— the integration of conceptual and motor representations in service of familiar, meaningful actions.
We also found evidence that compared to right-handers, left-handers more strongly engage a number of other cortical regions during hand-independent gesture planning. They showed greater involvement of the right ANg, dPMC, and DLPFC, and bilateral RSC, SMA/CMA. This is consistent with other findings in the literature suggesting that left-handed individuals tend to exhibit less cerebral asymmetry than right-handers for a variety of cognitive functions including language (Vingerhoets et al., 2012; Goldenberg, 2013a; Vingerhoets et al., 2013; see also Haberling & Corballis, 2015).
Finally, as with right-handers, we failed to detect any evidence for hand-independent differences in the representation of different gesture types (transitive vs. intransitive). This null result is in agreement with the hypothesis that these two types of gesture share common representation mechanisms, and that the reported dissociations in the neuropsychological literature may be attributable to other factors such as differences in familiarity or difficulty (Kroliczak & Frey, 2009; Carmo & Rumiati, 2009). Each of these sets of findings is discussed in further detail below.
Left supramarginal and caudal middle temporal gyri are the key regions involved in hand-independent gesture representation regardless of motor dominance
As introduced earlier, data from brain-injured patients and functional neuroimaging of healthy adults converge on the hypothesis that the left SMg and cMTg serve as critical nodes for the integration of conceptual knowledge and movement representations in service of meaningful actions in right-handed adults (Johnson-Frey, 2004). We find evidence that this specific network is left-lateralized even in strongly left-handed individuals whose right hemispheres are dominant for fine motor control. These data are inconsistent with the hypothesis that the right hand dominance exhibited by approximately 90% of humans is due to a left-lateralized praxis representation system (Geschwind & Galaburda, 1985; Kimura & Archibald, 1974; Liepmann, 1908). Instead, they support the view that praxis representation and motor dominance rely on dissociable mechanisms (Goldenberg et al., 1996; Frey et al., 2005; Goldenberg, 2013a).
Our observation that gesture planning activity is greater for the right hand – regardless of hand preference – is not only consistent with the notion that hemispheric specialization for skilled actions is independent of handedness but also with the idea that the right hand may have some privileged access to processing within areas involved in the control of praxis skills even in left-handed individuals (e.g., Gonzalez, Ganel, & Goodale, 2006). The additional engagement of right-hemisphere regions is consistent with earlier reports demonstrating right-sided contributions in pantomimed and off-line actions (Kroliczak, Cavina-Pratesi, Goodman, & Culham, 2007; Rossit et al., 2011). Notably, counter to the planning stage, this putative right-hand advantage is then reflected in more focused sensorimotor processing in the action execution phase (which is also consistent with behavioral observations by Gonzalez et al., 2006).
Our findings converge with evidence that the cerebral specialization for praxis is closely related to dominance for language, which is left-lateralized even in the vast majority of left-handed adults (Knecht et al., 2000; Meador et al., 1999; see also Haberling & Corballis, 2015). As reported previously (Kroliczak et al., 2011; see also Bidula & Kroliczak, 2015), this particular sample of strongly left-handed adults exhibits a left cerebral asymmetry in activity within Broca’s Area (probabilistically defined Brodmann Areas 44/45) during performance of a verbal fluency task. The strength of this asymmetry ranges across participants and correlates with the variations in asymmetry detected during hand-independent praxis planning in the supramarginal gyrus (probabilistically defined BA40). The reason(s) for this relationship are uncertain but include such possibilities as joint dependence on left hemisphere specializations for constructing symbolic representations (Duffy & Liles, 1979), representing motor sequences (Kimura & Archibald, 1974) and/or sequential hierarchies (Greenfield, 1991). Despite this association, it is important to appreciate that aphasia and apraxia can dissociate (Goodglass & Kaplan, 1963; Heilman, 1975), and that the assessments chosen to diagnose apraxia impact the observed relationship.
Goldenberg’s (2013) report of 50 left-handed patients with unilateral brain injuries revealed evidence for dissociations between motor dominance and apraxia, as well as apraxia and aphasia. Difficulties with pantomime, as opposed to imitation of meaningless hand or finger postures—a task that minimizes demands on symbolic representation—tended to co-occur with aphasia. This evidence suggests that the relationship between praxis and language representation is quite complex, and likely involves both shared and relatively independent functions. As recently summarized by Michael C. Corballis (Corballis, 2015; but see also Corballis, 2003; Buckingham & Christman, 2010), the existence of such a link might be a natural consequence of language evolving from dual-stream circuity that initially mediated grasping skills but were later tailored for the control of skilled manual actions (praxis) and gestural communication. On the other hand, the emergence of speech as the dominant mode of communication (Hickok & Poeppel, 2007) likely contributed to some encapsulation of the praxis and language systems. Moreover, hand preference (or lack of thereof at a certain stage of development) and other individual predispositions may in turn lead to reshuffling of some asymmetrically represented functions, resulting in their more random distribution in the brain (Goldenberg, 2013b).
Effects of Hand Dominance
We are aware of only one other neuroimaging study of tool use pantomime in left-handers (Vingerhoets et al., 2012). Yet, due to the nature of the design, the results of this investigation may be influenced by a number of factors loosely linked to praxis skills per se, including differences between experimental and control conditions in linguistic and/or sensorimotor processing demands, as well as responses associated with the mere visual perception of the familiar objects. Nevertheless, similarly to Vingerhoets and colleagues (2012) we also failed to detect differences between left- and right-handers in left parietal or temporal lobe activity during praxis planning. Yet, we found that left-handers exhibited greater planning-related involvement of several cortical regions in the motor dominant right hemisphere, including inferior parietal (primarily ANg), and frontal/prefrontal (dPMC, DLPFC) areas. Notably, no area showed greater activity in right-handers during praxis planning. The one-directional nature of these handedness-related differences is consistent with long-standing evidence that left-handers generally exhibit less cerebral asymmetry of various cognitive functions, including language (Hecaen, De Agostini, & Monzon-Montes, 1981; Hecaen & Sauguet, 1971; Levy & Reid, 1978; see also Michalowski & Kroliczak, 2015; Haberling & Corballis, 2015; Haberling, Steinemann, & Corballis, 2016). By contrast, during the production phase we detected substantial differences in brain activity between groups in both directions. Left-handers exhibited greater increases in activity primarily in the bilateral SMA/CMA, left thalamus and right inferior occipito-temporal cortices. Right-handers, on the contrary, exhibited greater left-lateralized engagement of structures typically linked to praxis skills (including the SPL, vPMC, MFg and IFg, middle insular and the nearby temporal cortex), as well as the right-sided advantage in inferior frontal regions (here IFs/IFg). Together these findings suggest that hand dominance may have more substantial and complex effects on the organization of mechanisms involved in praxis production.
As with the previous study by Vingerhoets and collaborators (2012), we elected to use individuals whose left-handedness was comparable in strength to that of typical right-handers. The inclusion of individuals covering the full range of left-handedness in future investigations may help to clarify the nature of the handedness-related differences in praxis representation. Understanding this natural variation is important for clinical reasons as well, where approximately one in ten patients will be left-handed. Indeed, the common practice of excluding left-handers from behavioral and neuroscience research samples significantly limits the generalizability of the obtained results to the population (Willems et al., 2014).
This project, nevertheless, has several limitations. We primarily used younger adults, and there is considerable evidence that cerebral asymmetries tend to increase in older adults, a phenomenon referred to as the so-called dedifferentiation (Bangert, Reuter-Lorenz, Walsh, Schachter, & Seidler, 2010; Fling, Chapekis, et al., 2011; Fling, Walsh, et al., 2011). Whereas left-handers tend to show more variation in hand dominance across tasks, our participants were all strongly left-handed. Similarly to the earlier study of Vingerhoets et al. (2012), this choice was made to achieve a degree of hand preference comparable to the one observed in right-handers, and allowed for a direct comparison with our 2009 work in the latter group (Kroliczak & Frey, 2009). However, future research should look at the relationship between variation in the strength of left-handedness and cerebral asymmetries within the brain regions dedicated to praxis representation. Finally, it should be mentioned that the use of abstract words as stimuli in the control condition substantially activated the right hemisphere. This unexpected contribution could have biased our outcomes in a direction that emphasizes left cerebral asymmetries in the supramarginal gyrus and caudal middle temporal gyrus. However, because these stimuli are identical to those employed in our earlier work with right-handers, the observed differences and similarities between groups cannot be attributed to this potentially biasing effect.
Conclusions
Liepmann’s observation more than a century ago that patients with left hemisphere injuries often exhibit difficulties while pantomiming familiar skills with their relatively unaffected ipsilesional limb is inarguably a watershed moment in the history of behavioral neurology (Goldenberg, 2003a). It provided a new insight into the complex interactions between perceptual, cognitive and motor functions that underlie goal-directed actions, identified what happens when these go awry, and inspired a vast amount of research. Our work in healthy adults presented here adds to a small but impactful body of evidence from the apraxia literature, suggesting that the relationship between hand dominance and praxis is more nuanced than previously appreciated. Greater inclusion of left-handers, who constitute a large minority of the population, will further provide an important window into potential variation in the functional architecture of human cognition.
Supplementary Material
Highlights.
Praxis laterality was assessed with fMRI in left-handed individuals
Gesture planning leads to hand-independent, left-lateralized activity in supramarginal gyrus
Left-handers exhibit a less asymmetric functional architecture for praxis representation
Praxis representation and motor dominance rely on dissociable mechanisms
Acknowledgments
This project was conceptualized by S.H.F. and B.J.P. Data was collected and analyzed by B.J.P. and G.K. The manuscript was written by S.H.F. and G.K. This work was supported by grant (#NS053962) from NIH/NINDS to S.H.F. During data acquisition G.K. was supported by the “Brain, Biology and Machine Initiative” (BBMI) Research Fellowship in the Department of Psychology at the University of Oregon. During later stages of data analyses and preparation of this manuscript G.K. was also supported by grant Maestro 2011/02/A/HS6/00174 from National Science Center (NCN) to G.K.
Appendix: 3 categories of cues (gerundive verb-derived nouns) in alphabetic order
Transitive Gesture Planning: Cutting, Dialing, Painting, Pounding, Pouring, Reeling, Scooping, Scrubbing, Sewing, Stabbing, Stirring, Typing, Unlocking, Writing.
Intransitive Gesture Planning: Beckoning, Conducting, Counting, Flicking, Hitchhiking, Pointing, Scolding, Shooing, Snapping, Stopping, Talking, Tickling, Wavering, Waving.
Linguistic Control (no manual action planning involved): Adapting, Being, Believing, Devising, Evaluating, Innovating, Interpreting, Knowing, Planning, Qualifying, Resolving, Solving, Thinking, Understanding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangert AS, Reuter-Lorenz PA, Walsh CM, Schachter AB, Seidler RD. Bimanual coordination and aging: neurobehavioral implications. Neuropsychologia. 2010;48(4):1165–1170. doi: 10.1016/j.neuropsychologia.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34(1):149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43(3):461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bidula SP, Kroliczak G. Structural asymmetry of the insula is linked to the lateralization of gesture and language. European Journal of Neuroscience. 2015;41(11):1438–1447. doi: 10.1111/ejn.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, Hallett M. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex. 2009;19(6):1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham HW, Christman SS. Charles Darwin and the evolution of human grammatical systems. J Hist Neurosci. 2010;19(2):121–139. doi: 10.1080/09647040903506455. [DOI] [PubMed] [Google Scholar]
- Carmo JC, Rumiati RI. Imitation of transitive and intransitive actions in healthy individuals. Brain Cogn. 2009;69(3):460–464. doi: 10.1016/j.bandc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12(4):478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Choi SH, Na DL, Kang E, Lee KM, Lee SW, Na DG. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp Brain Res. 2001;139(3):311–317. doi: 10.1007/s002210100777. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behavioral and Brain Sciences. 2003;26(2):199–208. doi: 10.1017/s0140525x03000062. discussion 208-160. [DOI] [PubMed] [Google Scholar]
- Corballis MC. What’s left in language? Beyond the classical model. Ann N Y Acad Sci. 2015;1359(1):14–29. doi: 10.1111/nyas.12761. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198(4317):631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- Cubelli R, Marchetti C, Boscolo G, Della Sala S. Cognition in action: testing a model of limb apraxia. Brain Cogn. 2000;44(2):144–165. doi: 10.1006/brcg.2000.1226. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dobato JL, Baron M, Barriga FJ, Pareja JA, Vela L, Sanchez Del Rio M. Apraxia cruzada secundaria a infarto parietal derecho. Revista de Neurologia. 2001;33(8):725–728. [PubMed] [Google Scholar]
- Duffy RJ, Liles BZ. A translation of Finkelnburg’s (1870) lecture on aphasia as “asymbolia” with commentary. J Speech Hear Disord. 1979;44(2):156–168. doi: 10.1044/jshd.4402.156. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: Surface, three-dimensional sectional anatomy and MRI. Vienna, Austria: Springer-Verlag; 1991. [Google Scholar]
- Fling BW, Chapekis M, Reuter-Lorenz PA, Anguera J, Bo J, Langan J, Seidler RD. Age differences in callosal contributions to cognitive processes. Neuropsychologia. 2011;49(9):2564–2569. doi: 10.1016/j.neuropsychologia.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential callosal contributions to bimanual control in young and older adults. Journal of Cognitive Neuroscience. 2011;23(9):2171–2185. doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1499):1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Funnell MG, Gerry VE, Gazzaniga MS. A dissociation between the representation of tool-use skills and hand dominance: insights from left- and right-handed callosotomy patients. J Cogn Neurosci. 2005;17(2):262–272. doi: 10.1162/0898929053124974. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Hallett M. The role of the dorsal stream for gesture production. Neuroimage. 2006;29(2):417–428. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology. 1985;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Kaplan E. A human cerebral deconnection syndrome. A preliminary report. Neurology. 1962;12:675–685. doi: 10.1212/wnl.12.10.675. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and beyond: life and work of Hugo Liepmann. Cortex. 2003a;39(3):509–524. doi: 10.1016/s0010-9452(08)70261-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Pantomime of object use: a challenge to cerebral localization of cognitive function. Neuroimage. 2003b;20(Suppl 1):S101–S106. doi: 10.1016/j.neuroimage.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia in left-handers. Brain. 2013a;136:2592–2601. doi: 10.1093/brain/awt181. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia: The cognitive side of motor control. Oxford: Oxfrod University Press; 2013b. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hermsdorfer J, Glindemann R, Rorden C, Karnath HO. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cerebral Cortex. 2007;17(12):2769–2776. doi: 10.1093/cercor/bhm004. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hermsdorfer J, Spatt J. Ideomotor apraxia and cerebral dominance for motor control. Brain Res Cogn Brain Res. 1996;3(2):95–100. doi: 10.1016/0926-6410(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Ganel T, Goodale MA. Hemispheric specialization for the visual control of action is independent of handedness. Journal of Neurophysiology. 2006;95(6):3496–3501. doi: 10.1152/jn.01187.2005. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Disturbance of gesture and pantomime in aphasia. Brain. 1963;86:703–720. doi: 10.1093/brain/86.4.703. [DOI] [PubMed] [Google Scholar]
- Greenfield PM. Language, tools and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behav Brain Sci. 1991;14(4):531–551. [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Haberling IS, Corballis MC. Cerebellar asymmetry, cortical asymmetry and handedness: Two independent networks. Laterality: Asymmetries of Body, Brain and Cognition. 2015;19:1–18. doi: 10.1080/1357650X.2015.1110161. [DOI] [PubMed] [Google Scholar]
- Haberling IS, Steinemann A, Corballis MC. Cerebral asymmetry for language: Comparing production with comprehension. Neuropsychologia. 2016;80:17–23. doi: 10.1016/j.neuropsychologia.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hecaen H, De Agostini M, Monzon-Montes A. Cerebral organization in left-handers. Brain and Language. 1981;12(2):261–284. doi: 10.1016/0093-934x(81)90018-3. [DOI] [PubMed] [Google Scholar]
- Hecaen H, Sauguet J. Cerebral dominance in left-handed subjects. Cortex. 1971;7(1):19–48. doi: 10.1016/s0010-9452(71)80020-5. [DOI] [PubMed] [Google Scholar]
- Heilman KM. A tapping test in apraxia. Cortex. 1975;11(3):259–263. doi: 10.1016/s0010-9452(75)80008-6. [DOI] [PubMed] [Google Scholar]
- Heilman KM. Handedness. In: Rothi LJG, Heilman KM, editors. Apraxia: The neuropsychology of action. Hove, England: Psychology Press/Erlbaum (UK) Taylor & Francis; 1997. pp. 19–28. [Google Scholar]
- Heilman KM, Rothi LJ. Limb apraxia: a look back. In: Rothi LJ, Heilman KM, editors. Apraxia. The neuropsychology of action. Hove (UK): Psychology Press; 1997. pp. 7–18. [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32(4):342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Terlinden G, Muhlau M, Goldenberg G, Wohlschlager AM. Neural representations of pantomimed and actual tool use: evidence from an event-related fMRI study. Neuroimage. 2007;36(Suppl 2):T109–T118. doi: 10.1016/j.neuroimage.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8(2):71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15(6):681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cogn Neurosci. 2003;15(1):30–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kimura D, Archibald Y. Motor functions of the left hemisphere. Brain. 1974;97(2):337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Cavina-Pratesi C, Goodman DA, Culham JC. What does the brain do when you fake it? An FMRI study of pantomimed and real grasping. Journal of Neurophysiology. 2007;97(3):2410–2422. doi: 10.1152/jn.00778.2006. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;19(10):2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, Piper BJ, Frey SH. Atypical lateralization of language predicts cerebral asymmetries in parietal gesture representations. Neuropsychologia. 2011;49(7):1698–1702. doi: 10.1016/j.neuropsychologia.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausberg H, Gottert R, Munssinger U, Boegner F, Marx P. Callosal disconnection syndrome in a left-handed patient due to infarction of the total length of the corpus callosum. Neuropsychologia. 1999;37(3):253–265. doi: 10.1016/s0028-3932(98)00079-7. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD. Limb apraxias: higher-order disorders of sensorimotor integration. Brain. 2000;123:860–879. doi: 10.1093/brain/123.5.860. [DOI] [PubMed] [Google Scholar]
- Levy J, Reid M. Variations in cerebral organization as a function of handedness, hand posture in writing, and sex. Journal of Experimental Psychology: General. 1978;107(2):119–144. doi: 10.1037//0096-3445.107.2.119. [DOI] [PubMed] [Google Scholar]
- Liepmann H. Das Krankheitshild der Apraxie (motorischen/Asymbolie) Monatschrift fur Psychiatry und Neurologie. 1900;8:15–44. 102-132, 182-197. [Google Scholar]
- Liepmann H. Drei aufsatze aus dem apraxiegebiet. Berlin: Karger; 1908. [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55(3):507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Della Sala S. On crossed apraxia. Description of a right-handed apraxic patient with right supplementary motor area damage. Cortex. 1997;33(2):341–354. doi: 10.1016/s0010-9452(08)70010-8. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for Nine Hole Peg Test of finger dexterity. Occupational Therapy Journal of Research. 1985;5:24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, Heilman KM. Cerebral lateralization: relationship of language and ideomotor praxis. Neurology. 1999;53(9):2028–2031. doi: 10.1212/wnl.53.9.2028. [DOI] [PubMed] [Google Scholar]
- Michalowski B, Kroliczak G. Sinistrals are rarely “right”: evidence from tool-affordance processing in visual half-field paradigms. Front Hum Neurosci. 2015;9:166. doi: 10.3389/fnhum.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Passman LJ, Cunha FC, Souza-Lima F, Andreiuolo PA. Functional MRI correlates of real and imagined tool-use pantomimes. Neurology. 2000;54(6):1331–1336. doi: 10.1212/wnl.54.6.1331. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niessen E, Fink GR, Weiss PH. Apraxia, pantomime and the parietal cortex. NeuroImage Clinical. 2014;5:42–52. doi: 10.1016/j.nicl.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami Y, Matsuo K, Uchida N, Nakai T. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15(12):1903–1906. doi: 10.1097/00001756-200408260-00014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther. 2003;57(5):570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Smania N, Corato E, Aglioti SM. Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci. 2008;28(12):3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck K, Kerschensteiner M. Ideomotor apraxia following right-sided cerebral lesion in a left-handed subject. Neuropsychologia. 1971;9(3):359–361. doi: 10.1016/0028-3932(71)90032-7. [DOI] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral Preferences and Human Behaviour. New York, NY: Springer-Verlag; 1981. [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdorfer J. From pantomime to actual use: how affordances can facilitate actual tool-use. Neuropsychologia. 2011;49(9):2410–2416. doi: 10.1016/j.neuropsychologia.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Raymer AM, Merians AS, Adair JC, Schwartz RL, Williamson DJ, Rothi LJ, Heilman KM. Crossed apraxia: implications for handedness. Cortex. 1999;35(2):183–199. doi: 10.1016/s0010-9452(08)70793-7. [DOI] [PubMed] [Google Scholar]
- Rossit S, Malhotra P, Muir K, Reeves I, Duncan G, Harvey M. The role of right temporal lobe structures in off-line action: evidence from lesion-behavior mapping in stroke patients. Cerebral Cortex. 2011;21(12):2751–2761. doi: 10.1093/cercor/bhr073. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Heilman KM, Watson RT. Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry. 1985;48(3):207–210. doi: 10.1136/jnnp.48.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21(4):1224–1231. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Ruschel M, Knosche TR, Friederici AD, Turner R, Geyer S, Anwander A. Connectivity architecture and subdivision of the human inferior parietal cortex revealed by diffusion MRI. Cerebral Cortex. 2014;24(9):2436–2448. doi: 10.1093/cercor/bht098. [DOI] [PubMed] [Google Scholar]
- Sartori L, Begliomini C, Castiello U. Motor resonance in left- and right-handers: evidence for effector-independent motor representations. Front Hum Neurosci. 2013;7:33. doi: 10.3389/fnhum.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenova V, Roy EA, Black SE. Associations and dissociations of transitive and intransitive gestures in left and right hemisphere stroke patients. Brain & Cognition. 2010;72(3):483–490. doi: 10.1016/j.bandc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio AR. Neural correlates of conceptual knowledge for actions. Cognitive Neuropsychology. 2003;20(3):409–432. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Heilman KM. Apraxic agraphia with neglect-induced paragraphia. Arch Neurol. 1979;36(8):506–508. doi: 10.1001/archneur.1979.00500440076016. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G. Knowing about tools: neural correlates of tool familiarity and experience. Neuroimage. 2008;40(3):1380–1391. doi: 10.1016/j.neuroimage.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Alderweireldt AS, Nys J, Vandemaele P, Achten E. Cerebral lateralization of praxis in right- and left-handedness: same pattern, different strength. Human Brain Mapping. 2012;33(4):763–777. doi: 10.1002/hbm.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G, Alderweireldt AS, Vandemaele P, Cai Q, Van der Haegen L, Brysbaert M, Achten E. Praxis and language are linked: evidence from co-lateralization in individuals with atypical language dominance. Cortex. 2013;49(1):172–183. doi: 10.1016/j.cortex.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Vandekerckhove E, Honore P, Vandemaele P, Achten E. Neural correlates of pantomiming familiar and unfamiliar tools: action semantics versus mechanical problem solving? Human Brain Mapping. 2011;32(6):905–918. doi: 10.1002/hbm.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg J, van Turennout M, Martin A. A neural system for learning about object function. Cereb Cortex. 2007;17(3):513–521. doi: 10.1093/cercor/bhj176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nature Reviews Neuroscience. 2014;15(3):193–201. doi: 10.1038/nrn3679. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. USA: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.