Abstract
The functional basis of cognitive and quality of life (QOL) change after liver transplant is unclear. We aimed to evaluate the neuro-metabolic and functional brain changes as modulators of cognition and QOL post-transplant in cirrhotics with/without pre-transplant cognitive impairment and hepatic encephalopathy (HE). Cirrhotics underwent detailed cognitive and QOL assessment at enrollment and six months post-transplant. A subset underwent brain MRI [Functional (fMRI), diffusion tensor imaging (DTI) and spectroscopy(MRS)] pre/post-transplant. Changes pre/post-transplant were analyzed in all patients and by dividing groups in those with/without pre-transplant cognitive impairment, or with/without pre-transplant HE. fMRI studied brain activation for correct lure inhibition on inhibitory control test, MRS evaluated ammonia-related metabolites and DTI studied white matter integrity. Sixty-six patients (MELD 21.8, 38 HE, 24 cognitively impaired) were enrolled. QOL was significantly worse in cognitively impaired and HE groups pre-transplant, which improved to a lesser extent in those with prior cognitive impairment. In the entire group post-transplant there was (i) fMRI: significantly lower brain activation needed for lure inhibition (ii) MRS: reversal of pre-transplant ammonia-associated changes and (iii) DTI: improved white matter integrity. Importantly, study findings suggest that pre-transplant cognitive impairment serves as a marker for clinical outcomes. Regardless of pre-transplant history of HE, it was the pre-transplant cognitive impairment that was predictive of both post-transplant cognitive and psychosocial outcomes. Therefore, when working with patients and their families a clinician may rely on the pre-transplant cognitive profile to develop expectations regarding post-transplant neurobehavioral recovery. We conclude that functional brain changes after liver transplant depend on pre-transplant cognitive impairment and ultimately are linked with post-transplant cognition and QOL in cirrhosis.
Keywords: Functional MRI, Spectroscopy, Hepatic Encephalopathy, Cirrhosis, Quality of Life
INTRODUCTION
Patients with cirrhosis and hepatic encephalopathy (HE) often have residual cognitive impairment before transplant as well as after liver transplant in some studies(1-6). There are also mixed results of incomplete recovery post-transplant using specific brain MRI techniques(4, 7). However, a comprehensive overview of functional brain imaging, neuro-metabolic profile and daily functional assessment in the setting of cognitive recovery is unclear. While most patients undergo cognitive evaluation before transplant, these tests need to be interpreted in order to set realistic post-transplant recovery expectations for patients and caregivers. It is assumed largely that patients with prior HE will have a worse recovery than those without prior HE. A robust strategy that combines these variables will help clinicians to counsel patients and family members prior to transplant regarding their post-transplant functioning(8).
Our aims were to (1) evaluate the extent and determinants of cognitive change and patient-reported outcomes pre and 6 months post-liver transplant and (2) define the impact of liver transplantation on multi-modal MRI analysis including functional MRI, MR spectroscopy and diffusion tensor imaging (DTI). We hypothesized that the extent of functional brain change after transplant parallels cognitive and psychosocial performance and is modulated by the presence of pre-transplant cognitive impairment and pre-transplant HE.
METHODS
We prospectively included cirrhotic patients listed for deceased solitary liver transplant at the VCU Medical Center from June 2011 to June 2015. Included patients were between 21 to 65 years of age, who were able to give informed consent and to answer questions regarding HRQOL. We excluded those with severe depression (Beck Depression Inventory >20)(9), who were not able to give informed consent, prior transplant , HIV infection or transplanted under Priority 1 status.
At the pre-transplant visit, the subjects were given the mini-mental status examination and were only allowed to proceed if it was ≥25. After this the following: (i) patient-reported outcomes analysis (ii) cognitive testing (iii) formal structured psychological interview were performed. We performed these assessments to gain a global view of the patient's issues and to evaluate their potential correlates.
Details of the evaluation were as follows. Patient reported questionnaires used were (a) Sickness Impact Profile (SIP)(10): 12 domains, 2 dimensions and total score; higher score suggest poor health-related quality of life (HRQOL), (b): PROMIS tools (Patient reported outcomes measurements system)(11) using the computerized adaptive system Cognitive tests used were the recommended PHES (psychometric hepatic encephalopathy score) and block design test (BDT) (12). The inhibitory control test (ICT) was administered during the fMRI scan(13).
We studied all patients pre and post-transplant as an entire group, and also divided them into groups using two methods (a) pre-transplant cognitively impaired vs. those who were unimpaired and (b) pre-transplant HE compared to those without history of HE pre-transplant. Cognitive impairment was defined as a PHES score <-4SD based on our published US-based norms(14), while prior HE was defined as at least one episode of HE that required treatment with lactulose and/or rifaximin(12). These different groupings were performed because cognitive impairment can occur independent of prior HE episodes and vice-versa(15).
A formal psychological evaluation was performed by JBW who had access to cognitive testing and the questionnaires. A subset that was eligible underwent multi-modal MRI on the day of this evaluation (details below and supplementary data).
Subjects were then followed till liver transplantation, death on the waiting list or delisting. For those who were transplanted, the details of the liver transplant surgery, immune-suppression regimen, hospitalizations, rejection episodes, duration of hospital stay, need for relisting and continued extra-hepatic organ support (dialysis) was recorded. The post-transplant visit was performed at 6 (±3) months for patients who were alive and willing, and had not undergone a second transplant or were listed for another transplant (including renal transplant). We chose this post-transplant timeframe to avoid immediate and transient post-operative complications(16). At six months, all procedures performed at the baseline visit, including brain MRI, if the patients had undergone this pre-transplant, were repeated.
Multi-modal MRI methods
ICT task
During this task the subject was asked to respond to targets and withhold responses to lures, while in the scanner(17). The ICT is well suited to the scanner environment in which subjects view a simple visual stimulus and respond with a single button press. A brief training session was performed prior to the MRI scan. Each subject underwent six runs of the ICT inside the scanner (supplementary information). Since the outcome for subjects during the ICT is to successfully inhibit a lure, the change in activation required to perform this task from the pre to the post-transplant stage was assessed.
MR Imaging Methods and analyses (supplementary information)
All images were acquired on a 1.5T Siemens Avanto (Erlangen, Germany) scanner using a quadrature birdcage RF head coil. We performed high-resolution structural brain imaging, functional MR imaging (fMRI) using ICT task, Diffusion Tensor imaging (DTI) and single voxel 1H MR Spectroscopy (MRS).
fMRI data analysis
fMRI data analysis was carried out using FEAT (FMRI Expert Analysis Tool) v 5.98 part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) (18-20). After standard preprocessing, a time-series statistical analysis was carried out with local autocorrelation correction using the General Linear Model (GLM). The model had correct inhibition to lures (CIL) as contrast of interest. Incorrect response to lure (IncRL), random responses and six motion parameters were added as contrasts of no interest. Group average statistical maps were created in MNI standard space for CIL trials. Also, Pre>Post-transplant and Post>Pre-transplant statistical maps were generated and thresholded using cluster-based thresholding(21). Using the locations of the peak z-scores in these maps, spherical ROIs with a 7 voxel radius were constructed for ROI analysis. Mean contrasts of parameter estimates (COPE), which is related to the BOLD (blood oxygenation level dependent) signal, were extracted from each patient in order to study the effect of pre-transplant cognitive performance status and to test the correlation between the change in brain activation during inhibition with change in cognitive test scores.
DTI analysis
Maps of fractional anisotropy (FA), mean diffusivity (MD), longitudinal diffusivity (LD) maps were computed using the diffusion Toolbox in FSL. Twelve a priori ROIs for major white matter tracts were created using the DTI-based probabilistic white matter atlases. Mean values from within each ROI were extracted from these diffusion maps for each subject(22, 23).
Spectroscopy analysis
The choline (Cho), creatine (Cr), myo-inositol (mI) and glutamate+glutamine (Glx) complex peak areas were computed using a quantitative assessment of the metabolite concentration by means of LCModel software (24, 25). These metabolites were chosen based on prior HE research showing cirrhosis related changes in these metabolites(26).
Statistical analysis
All statistical analyses were performed using the SPSS (version 12, SPSS Inc., Chicago, IL, USA) with appropriate tests. Laboratory tests, neuropsychological tests, measures of daily functioning, MRS, Diffusion indices were compared before and after transplant by the two-tailed paired t-test. These were performed for the entire group, as well as in groups divided on the basis of pre-transplant cognitive testing into impaired and unimpaired separately. Multiple comparison correction was done wherever applicable using Bonferroni method. The relationships between fMRI activation, MRS, regional diffusivity indices and neuropsychological tests were investigated using Pearson's partial correlation analysis. The study protocol was approved by the VCU and Richmond VA Institutional Review Boards.
RESULTS
We enrolled 98 patients for this study, of whom 16 died or were withdrawn from the waiting list prior to transplant, 9 were less than 6 months post-transplant, 5 were lost to follow-up or did not want to return and two patients died before the post-transplant visit. Ultimately we included 66 patients with a mean listing MELD score of 21.8±8.6. The average listing age was 56±7 years with mostly men (n=48) with a race/ethnicity breakdown towards Caucasians (n=48) with the remaining being African-American (n=12) and Latino (n=6). The etiology of cirrhosis was HCV only in 37%, HCV+alcohol in 16%, Alcohol only in 10%, NAFLD in 13% and other etiologies in the remainder. Hepato-cellular cancer (HCC) was the reason for listing in 20 subjects, while synthetic failure was the reason in the rest.
Prior HE
Thirty-eight patients had history of HE, all of whom were on lactulose and rifaximin, and were adherent on these medications through corroboration with relatives and direct questioning. The median number of HE episodes were 3 (range 2-5) over a median of 5 months (range 2-17 months) prior to enrollment. Biological MELD score for HE patients was significantly higher than no-HE patients (22.2±8.8 vs. 15.5±5.7, p=0.01) at time of testing. The cirrhosis etiology distribution in HE and no-HE patients was similar. None of the patients had low-grade HE at the time of testing pre or post-transplant.
Cognitive impairment
Based on PHES cut-offs, we found that 67% of patients were cognitively impaired. These patients had a higher biological MELD (21.5±8 vs. 16.7±6.9, p=0.018) and likelihood of prior HE (74% vs. 42%, p=0.009), compared to cognitively unimpaired patients. The cirrhosis etiology distribution was similar between groups.
Peri and post-transplant course
Transplant was carried out in all patients a median of 4 months (2-11 months IQR) post-listing. During this interval, none of the patients were hospitalized with HE but ten were hospitalized with complications related to liver disease (six for ascites-related, two for infections and two for other anasarca-related conditions). None of the no-HE patients developed HE during the interim. Patients stayed in the hospital after transplant for a median 8 (4-15 days IQR) with ICU stay lasting a median of 4 (2-7 days IQR). None of the patients had primary graft non-function or had to be relisted in the period before the second visit. The immunosuppression regimen used at VCU is steroids tapered over one month with mycophenolate mofetil initiated in the peri-operative period. Tacrolimus is initiated at day 3 to maintain levels between 8-12 ng/ml for the first three months and between 5-10 ng/ml after that period depending on the liver disease etiology.
Of the 66 patients, fifteen required medical intervention prior to the post-transplant visit. Four subjects had evidence of acute cellular rejection that required hospitalization and treatment with steroids in all and anti-thymocyte globulin in two patients. An additional three patients experienced delirium a median of 35 (IQR 14-42) days post-transplant that responded to steroid withdrawal in one patient and changing the primary immunosuppression from tacrolimus to cyclosporine in the remaining two. Three patients had biliary issues (one with a bile leak, two patients had required stenting of their duct-duct anastomosis because of cholestasis without cholangitis) that responded to this intervention. Three additional patients required treatment for infections (C. difficile colitis, vancomycin-resistant Enterococcus bacteremia and groin hidradenitis) while two patients had an elective repair of their umbilical hernias. Regular tacrolimus trough levels were monitored throughout and all patients were within the proscribed trough range (10.3±4.3 ng/ml average tacrolimus level prior to 6-month visit) regardless of cognitive recovery.
None of the HCV patients had SVR pre-transplant and did not receive any anti-HCV therapy before the post-transplant testing. No new anti-depressant or anti-anxiety medications were initiated post-transplant. The patients were retested 6±2 months post-transplant. As expected, there was a significant improvement in laboratory parameters, apart from creatinine post-transplant (Table 1). None of the patients had asterixis, other tremors or focal neurological deficits and all subjects had MMSE scores >25 at the time of post-transplant testing
Table 1.
Changes in laboratory values and cognitive performance after liver transplant
| All patients (n=66) | Cognitive Impairment (n=42) | Cognitively unimpaired (n=24) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Hemoglobin (g/dL) | 10.5±2.2 | 11.6±2.2** | 10.1±1.9†† | 11.5±2.5** | 11.8±2.1 | 11.9±2.1 |
| WBC Count (/mm3) | 5.5±2.9 | 3.8±2.1** | 4.9±2.4 | 4.3±2.3 | 5.9±2.8 | 3.4±1.5*** |
| INR | 1.7±0.5 | 1.1±0.3*** | 1.9±0.7† | 1.1±0.3*** | 1.5±0.6 | 1.1±0.3** |
| Total Bilirubin (mg/dL) | 6.9±10.9 | 0.8±0.9*** | 5.3±8.6 | 0.8±1.0** | 6.1±9.1 | 0.7±0.3** |
| Serum albumin (g/dL) | 3.0±0.6 | 3.8±0.5*** | 2.9±0.6 | 3.8±0.6*** | 3.0±0.7 | 3.9±0.5*** |
| Serum creatinine (mg/dL) | 1.6±1.3 | 1.7±1.3 | 1.8±1.5† | 2.1±1.6 | 1.1±0.6 | 1.6±1.5 |
| Serum sodium (meq/L) | 134.9±5.1 | 140.6±2.7*** | 135.9±4.2 | 140.3±3.0*** | 135.5±6.2 | 140.8±2.7*** |
| Venous ammonia | 60.2±33.9 | 28.7±14.1*** | 62.1±26.3 | 28.8±11.9*** | 58.4±39.0 | 28.1±14.9* |
| Cognitive tests | ||||||
| Number connection-A (seconds) | 51.6±40.9 | 34.4±17.5** | 63.9±39.8††† | 42.9±23.6**†† | 31.5±5.9 | 26.9±8.0** |
| Number connection-B (seconds) | 139.6±92.0 | 93.6±72.5** | 195.1±92.0††† | 117.3±82.4***†† | 80.6±26.2 | 68.0±21.8** |
| Block design (raw score)* | 24.6±12.5 | 31.8±13.2*** | 19.0±11.5††† | 29.2±12.0*** | 31.4±11.3 | 36.4±13.9* |
| Digit symbol (raw score)* | 45.6±15.6 | 56.9±18.7*** | 35.4±11.5††† | 47.8±13.1***††† | 56.9±11.5 | 65.8±17.2*** |
| Serial dotting test (seconds) | 85.3±38.9 | 67.8±31.2** | 102.7±38.7††† | 72.7±31.2***† | 61.8±14.3 | 54.8±14.0** |
| Line tracing time (seconds) | 117.5±54.4 | 91.4±31.2*** | 149.4±72.5††† | 96.5±31.2*** | 86.8±24.1 | 82.7±25.0 |
| Line tracing errors | 44.0±37.8 | 38.5±31.0 | 45.1±35.1 | 39.1±29.9 | 33.0±30.0 | 35.5±27.4 |
| PHES score (median IQR) | −4 (−2, −10) | −1 (0, −4) | −9 (−5, −11) | −3 (−1, −5) | −1 (0.5, −2) | 0 (−1, 1) |
All data presented as mean±SD unless mentioned otherwise.
p<0.05
p<0.01
p<0.001 pre vs. post-transplant
p<0.05
p<0.01
p<0.001 Cognitively impaired vs. Cognitively unimpaired groups.
A significant improvement in most cognitive tests and laboratory values was seen in all patients. A high score on block design and digit symbol test indicates good performance; the reverse is true for other cognitive tests.
Neuropsychological testing and patient-reported outcomes change
The proportion who had cognitive impairment significantly reduced from 67% pre-transplant to 21% post-transplant (p<0.0001). There were significant improvements in cognitive function that was largely uniform across patients with and without cognitive impairment and patients with and without prior HE (Table 1, Supplementary table 2). However patients with pre-transplant cognitive impairment remained significantly worse post-transplant on almost all tests apart from Block design and line tracing tests.
There was a significant improvement on the Beck Depression Inventory in all patients regardless of cognitive impairment or prior HE (Table 2, supplementary table 3). Interestingly, significant improvements in SIP were mostly seen in patients with cognitive impairment and prior HE, which was corroborated by changes in PROMIS scores to a large extent. The cognitively unimpaired group and no-HE group had a comparatively smaller extent of change post-transplant on indices of daily function and HRQOL.
Table 2.
Changes in patient-reported outcomes after liver transplant
| All patients (n=66) | Cognitive Impairment (n=42) | Cognitively unimpaired (n=24) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Beck Depression Inventory | 14.7±9.4 | 10.0±8.8*** | 15.7±9.9 | 11.3±9.8** | 12.8±8.2 | 8.2±6.8** |
| Total SIP | 25.2±15.4 | 17.9±17.3* | 29.7±15.3†† | 21.5±19.4** | 17.6±12.3 | 12.6±12.4 |
| Psychosocial SIP | 22.4±18.5 | 15.4±20.7* | 26.1±19.3† | 19.1±24.8 | 16.0±15.4 | 10.0±11.2 |
| Physical SIP | 20.8±16.8 | 14.1±17.2* | 26.0±16.6†† | 18.1±18.6*† | 12.1±13.6 | 8.3±13.3 |
| PROMIS tools | ||||||
| Anger | 49.7±29.4 | 41.9±29.8 | 48.7±31.1 | 44.0±32.7 | 51.0±27.8 | 38.9±25.8* |
| Anxiety | 64.7±24.1 | 56.4±26.2** | 67.4±23.7 | 59.4±27.0* | 61.2±24.8 | 52.3±25.3 |
| Depression | 52.6±24.5 | 44.5±26.4* | 54.0±25.6 | 46.4±29.0 | 50.8±23.5 | 41.7±22.8* |
| Fatigue | 66.9±24.6 | 52.1±27.4** | 64.4±28.1 | 51.9±28.1* | 70.4±19.2 | 52.5±27.2** |
| Physical function‡ | 22.1±19.0 | 34.3±21.7** | 21.4±18.5 | 31.5±19.9* | 23.0±20.0 | 38.3±24.0** |
| Pain-related behavior | 61.5±27.4 | 51.1±28.7* | 68.3±23.3† | 54.0±29.0* | 52.3±30.3 | 47.1±28.6 |
| Pain-related interference | 62.8±35.0 | 51.3±31.5* | 70.9±32.7 | 53.6±31.7* | 51.8±35.9 | 48.1±31.8 |
| Social impairment‡ | 30.9±23.7 | 38.5±27.3* | 25.9±21.3 | 35.5±26.9 | 37.6±25.5 | 42.8±28.1 |
| Social role‡ | 19.0±16.8 | 45.7±26.2*** | 18.1±17.4 | 41.1±25.4*** | 20.3±16.3 | 52.1±26.6*** |
| Sleep disturbance | 70.7±27.2 | 54.7±30.2** | 70.9±27.7 | 54.2±29.9* | 70.4±27.1 | 55.4±31.6* |
| Sleep-related impairment | 72.3±25.0 | 55.0±30.9*** | 74.5±26.2 | 56.5±31.4** | 69.4±23.6 | 52.9±31.0** |
All data presented as mean±SD.
p<0.05
p<0.01
p<0.001 pre vs. post-transplant
p<0.05
p<0.01
†††:p<0.001 Cognitively impaired vs. Cognitively unimpaired groups.
Low score in these PROMIS tools indicates poor function compared to US-norms; a higher score indicates poor function in other domains.
PROMIS: Patient-reported outcomes measurement information system, SIP: sickness impact profile.
MRI results
Of the 66 enrolled patients, 12 patients did not agree to an MRI, while 11 had contra-indications. Forty-three patients underwent MRI at the pre-transplant stage; two were not able to complete the MRI because of claustrophobia, three did not return for their post-transplant MRI and three had poor image quality. Ultimately 35 patients underwent MRI before and after transplant (14 no-HE and 21 HE patients). The subset that underwent MRI analysis was not significantly different from the rest of the group (Supplementary Table 1).
Structural MRI did not show any cortical hyperintensity or changes suggestive of calcineurin toxicity post-transplant(27).
fMRI
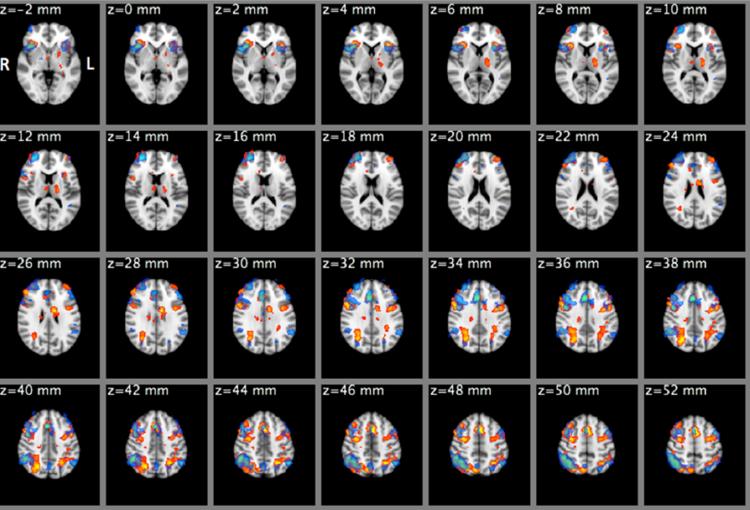
There was a significant improvement in the number of correct inhibition to lures (CIL) after transplant in all subjects (84.6±12.6% vs. 91.7±11.2%, p=0.008), in those with pre-transplant cognitive impairment (77.8±12.3% vs. 85.8±9.4%, p=0.001) and in those without cognitive impairment pre-transplant (82.5±12.3% vs. 90.3±10.4%, p=0.01). As expected, the group average contrast for CIL revealed robust activation in areas within the dorsolateral prefrontal cortex, anterior cingulate cortex, pre-cuneous cortex, posterior parietal cortical regions and supplementary motor cortex (Figure 1).
Figure 1.
Mixed-effects mean activation maps during correct inhibition to lures (CIL) conditions for the entire group before (Red-Yellow) and after (Blue-LightBlue) liver transplant. In both conditions, activation was observed in the dorsolateral prefrontal and posterior parietal cortex, orbitofrontal cortex, insular cortex, anterior cingulate cortex etc. Cluster-forming threshold z=2.3, p<0.05 (corrected)
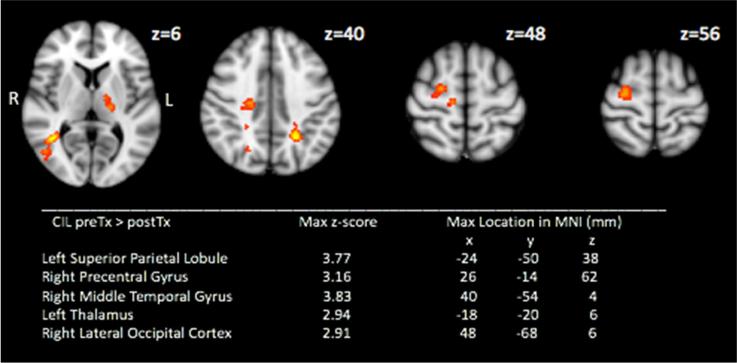
Significant clusters were also found in the pre-transplant>post-transplant contrast for CIL trials in the left superior parietal lobule, right middle temporal gyrus, right precentral gyrus and left thalamus. (Figure 2) i.e. lower neural recruitment may be required to achieve correct lure inhibition post-transplant compared to the pre-transplant stage
Figure 2.
Mixed-effects pre-transplant > post-transplant activation map during correct inhibition to lures (CIL) (Red-Yellow. Cluster-forming threshold z=2.3, p<0.05 (corrected)
There were no significant clusters found in post-transplant >pre-transplant CIL contrast.
Using ROI analysis on cluster peaks, we found that before transplant, patients with pre-transplant cognitive impairment required higher activation for inhibitory control than patients without it. These patients also showed greater change in activation after transplant(Table 3). Similar changes were found in patients who had experienced prior HE, in that a significantly higher improvement was seen in them post-transplant (Supplementary table 4).
Table 3.
Activation of brain regions during correct lure inhibition on Functional MRI
| Left superior parietal lobule | |||
|---|---|---|---|
| All patients | Cognitive Impairment | Cognitively unimpaired | |
| pre-Tx | 78.46 ± 15.9 | 94.92 ± 25.3 | 65.00 ± 20.5 |
| post-Tx | −20.44 ± 14.5 | −16.22 ± 24.9 | −23.90 ± 18.0 |
| pre vs. post | <0.001 | 0.026 | 0.003 |
| Right precentral gyrus | |||
|---|---|---|---|
| All patients | Cognitive Impairment | Cognitively unimpaired | |
| pre-Tx | 70.16 ± 12.3 | 58.06 ± 17.2 | 75.86 ± 14.6 |
| post-Tx | −16.81 ± 14.5 | −7.76 ± 18.9 | −21.68 ± 21.1 |
| pre vs. post | 0.001 | 0.037 | 0.011 |
| Left Thalamus | |||
|---|---|---|---|
| All patients | Cognitive Impairment | Cognitively unimpaired | |
| pre-Tx | 69.51 ± 19.2 | 104.26 ± 24.7 | 46.08 ± 21.1 |
| post-Tx | −9.02 ± 17.7 | −22.29 ± 22.2 | 1.38 ± 21.6 |
| pre vs. post | 0.013 | 0.005 | 0.204 |
| Right middle temporal gyrus | |||
|---|---|---|---|
| All patients | Cognitive Impairment | Cognitively unimpaired | |
| pre-Tx | 54.12 ± 17.1 | 61.81 ± 26.5 | 37.94 ± 15.2 |
| post-Tx | −33.33 ± 16.0 | −24.46 ± 19.0 | −57.84 ± 22.8 |
| pre vs. post | <0.001 | 0.002 | 0.005 |
Data presented as mean±SD, Values in bold are below the bonferroni corrected threshold p-value = 0.013. The positive number pre-transplant changing to a negative number post-transplant indicates that a higher brain volume, i.e. greater brain resources, in these regions were required to achieve the similar lure inhibition before transplant.
MR Spectroscopy
We used metabolite concentrations from only those spectra with an autoshim linewidth of <10Hz, effective water suppression, a stable baseline and Cramer-Rao lower bounds < 20%. Firstly, we tested whether the absolute concentration of creatine (Cr), an often used reference metabolite, changed before and after transplant. We found that the pre vs. post-transplant change in Cr was non-significant in RPWM (p=0.256), PGM (p=0.351) and ACC (p=0.740). Therefore, we used Cr concentration as reference to compute ratios for other metabolites. We found significant changes in metabolite ratios after transplant in RPWM, PGM and ACC in the entire group (Table 4). Choline and myoInositol increased whereas glutamate+glutamine decreased significantly after transplant. We also found that there was significant reduction in glutamine post- transplant. N-acetylaspartate, a neuronal marker and glutamate did not change significantly after transplant. Similar results were found when we separated the patients based on prior cognitive impairment status, specifically in the RPWM and PGM. Interestingly, prior HE patients also had higher improvement in metabolites in the ACC, along with PGM and RPWM (Supplementary table 5)
Table 4.
Proton MR Spectroscopy: Metabolite ratios changed significantly after transplant
| RPWM | Choline | Glutamate+Glutamine | ||||||
| All | CI | CU | All | CI | CU | |||
| pre-Tx | 0.238 ± 0.04 | 0.243 ± 0.04 | 0.236 ± 0.04 | pre-Tx | 2.768 ± 0.84 | 3.082 ± 1.01 | 2.480 ± 0.53 | |
| post-Tx | 0.278 ± 0.06 | 0.291 ± 0.05 | 0.267 ± 0.07 | post-Tx | 2.045 ± 0.37 | 2.083 ± 0.37 | 2.013 ± 0.37 | |
| pre vs. post | 0.008 | 0.017 | 0.074 | pre vs. post | 0.002 | 0.006 | 0.004 | |
| Myoinositol | Glutamine | |||||||
| pre-Tx | 0.309 ± 0.28 | 0.242 ± 0.21 | 0.372 ± 0.32 | pre-Tx | 1.341 ± 0.62 | 1.556 ± 0.73 | 1.144 ± 0.43 | |
| post-Tx | 0.882 ± 0.21 | 0.900 ± 0.24 | 0.866 ± 0.17 | post-Tx | 0.694 ± 0.28 | 0.718 ± 0.36 | 0.673 ± 0.19 | |
| pre vs. post | <0.001 | <0.001 | <0.001 | pre vs. post | <0.001 | 0.005 | <0.001 | |
| PGM | Choline | Glutamate+Glutamine | ||||||
| All | CI | CU | All | CI | CU | |||
| pre-Tx | 0.178 ± 0.05 | 0.184 ± 0.05 | 0.172 ± 0.04 | pre-Tx | 2.956 ± 0.67 | 3.043 ± 0.69 | 2.875 ± 0.67 | |
| post-Tx | 0.225 ± 0.05 | 0.229 ± 0.06 | 0.221 ± 0.05 | post-Tx | 2.313 ± 0.40 | 2.471 ± 0.35 | 2.186 ± 0.40 | |
| pre vs. post | 0.010 | 0.122 | 0.033 | pre vs. post | <0.001 | 0.025 | 0.008 | |
| Myoinositol | Glutamine | |||||||
| pre-Tx | 0.375 ± 0.22 | 0.361 ± 0.22 | 0.388±0.23 | pre-Tx | 1.367 ± 0.53 | 1.440 ± 0.56 | 1.300 ± 0.50 | |
| post-Tx | 0.838 ± 0.18 | 0.864 ± 0.23 | 0.817 ± 0.13 | post-Tx | 0.741 ± 0.32 | 0.874 ± 0.28 | 0.635 ± 0.33 | |
| pre vs. post | <0.001 | <0.001 | <0.001 | pre vs. post | <0.001 | 0.015 | 0.001 | |
| ACC | Choline | Glutamate+Glutamine | ||||||
| All | CI | CU | All | CI | CU | |||
| pre-Tx | 0.228 ± 0.04 | 0.233 ± 0.03 | 0.225 ± 0.04 | pre-Tx | 3.404 ± 0.64 | 3.554 ± 0.50 | 3.323 ± 0.72 | |
| post-Tx | 0.269 ± 0.04 | 0.281 ± 0.03 | 0.261 ± 0.05 | post-Tx | 2.748 ± 0.49 | 2.694 ± 0.63 | 2.781 ± 0.40 | |
| pre vs. post | 0.014 | 0.215 | 0.046 | pre vs. post | 0.022 | 0.230 | 0.063 | |
| Myoinositol | Glutamine | |||||||
| pre-Tx | 0.346 ± 0.19 | 0.322 ± 0.10 | 0.359 ± 0.23 | pre-Tx | 1.655 ± 0.51 | 1.735 ± 0.39 | 1.735 ± 0.39 | |
| post-Tx | 0.832 ± 0.24 | 0.855 ± 0.23 | 0.818 ± 0.26 | post-Tx | 1.034 ± 0.40 | 1.011 ± 0.54 | 1.011 ± 0.54 | |
| pre vs. post | <0.001 | 0.022 | <0.001 | pre vs. post | 0.004 | 0.209 | 0.209 | |
RPWM: right parietal white matter, PGM: posterior gray matter, ACC: anterior cingulate cortex, CI: Cognitively impaired, CU: Cognitively unimpaired; Data presented as mean±SD, P values in bold are below the Bonferroni corrected threshold p-value = 0.017. There was a significant improvement in ammonia-related metabolites in
Diffusion Tensor imaging
We found no significant change in fractional anisotropy in the ROIs. Mean diffusivity and longitudinal diffusivity increased in the posterior internal capsule after transplant. Splenium of the corpus callosum also showed an increase in longitudinal diffusivity. These changes were more pronounced in patients with pre-transplant cognitive impairment and in those with prior HE(Table 5/supplementary table 6).
Table 5.
Pre- and post-transplant changes in Diffusion Tensor Imaging
| MEAN DIFFUSIVITY × 10−3 mm2/s | |||
|---|---|---|---|
| Left posterior internal capsule | All | CI | CU |
| pre-Tx | 0.690 ± 0.02 | 0.691 ± 0.03 | 0.689 ± 0.02 |
| post-Tx | 0.707 ± 0.02 | 0.709 ± 0.02 | 0.706 ± 0.02 |
| pre vs. post | <0.001 | 0.002 | 0.004 |
| Right posterior internal capsule | All | CI | CU |
| pre-Tx | 0.695 ± 0.02 | 0.695 ± 0.02 | 0.696 ± 0.01 |
| post-Tx | 0.710 ± 0.02 | 0.712 ± 0.02 | 0.708 ± 0.01 |
| pre vs. post | <0.001 | 0.002 | 0.004 |
| LONGITUDINAL DIFFUSIVITY × 10−3 mm2/s | |||
| Corpus Callosum Splenium | All | CI | CU |
| pre-Tx | 1.570 ± 0.05 | 1.554 ± 0.04 | 1.583 ± 0.04 |
| post-Tx | 1.590 ± 0.05 | 1.581 ± 0.04 | 1.598 ± 0.05 |
| pre vs. post | <0.001 | 0.001 | 0.040 |
| Left posterior internal capsule | All | CI | CU |
| pre-Tx | 1.327 ± 0.04 | 1.319 ± 0.04 | 1.333 ± 0.03 |
| post-Tx | 1.360 ± 0.04 | 1.359 ± 0.05 | 1.360 ± 0.04 |
| pre vs. post | <0.001 | <0.001 | 0.001 |
| Right posterior internal capsule | All | CI | CU |
| pre-Tx | 1.336 ± 0.04 | 1.328 ± 0.05 | 1.343 ± 0.03 |
| post-Tx | 1.364 ± 0.05 | 1.364 ± 0.06 | 1.364 ± 0.04 |
| pre vs. post | <0.001 | <0.001 | 0.001 |
Values in bold are below the Bonferroni corrected threshold p-value = 0.0022
CI: Cognitively impaired, CU: Cognitively unimpaired, Mean diffusivity (MD), Longitudinal diffusivity (LD). No significant changes in Fractional anisotropy (FA) were seen.
There were significant correlations between cognitive tests and fMRI and MRS variables (supplementary data)
DISCUSSION
The study results show a significant improvement from their pre-transplant baseline in most patients from a neuropsychological, functional and multi-modal MR perspective.
The cognitive recovery of cirrhotic patients after transplant has been a source of debate (8, 28). Pre-transplant factors that would predict an incomplete recovery are important to adequately counsel patients and caregivers. Our results confirm prior results that pre-transplant cognition in HE patients is worse than those without HE, but also extend them by studying the concomitant functional MRI and neuro-metabolic status of the brain pre and post-transplant (3, 29). Since residual cognitive deficit does not always coincide with prior HE episodes, we decided to separately analyze the impact of impaired pre-transplant cognition as well (30). Not surprisingly, most patients with poor post-transplant cognition had prior HE, but almost half of the patients with cognitive impairment did not. Therefore it was important to separately analyze this group. While patients with prior HE showed a remarkable cognitive recovery, the situation when patients were divided on the basis of pre-transplant cognitive impairment was more nuanced. Overall, while cognitively impaired patients improved significantly after transplant, they remained more impaired compared to those who did not have pre-transplant cognitive impairment (3). These findings suggest a subject's pre-transplant cognitive status is a critical factor in determining post-transplant neurobehavioral outcome. The severity of pre-transplant cognitive impairment may indicate the extent of structural and/or metabolic pathology associated with liver disease as well as the cognitive reserve (31). Pre-transplant cognitive impairment was not only predictive of post-transplant cognitive function, but also fMRI, MR spectroscopy, diffusion tensor imaging findings and psychosocial outcome. In support of the relationship between pre-transplant cognitive function and brain reserve, improvement in cognition following transplant was associated with a significantly better HRQOL as demonstrated by the SIP and PROMIS tools. There was evidence of improvement of a greater magnitude with respect to baseline in patients with prior HE and cognitive impairment. In contrast, subjects with pre-transplant cognitive impairment had worse physical SIP dimension post-transplant. This finding reinforces the notion that once a decline in brain reserve, as depicted by pre-transplant cognitive impairment, has taken place it may not be fully reversible.
The mechanisms behind changes in cognition and daily function after transplant are unclear. We sought to investigate the potential mechanisms behind this post-transplant improvement using multi-modal MRI that evaluates the functionality, connectivity, edema and metabolic function of several brain regions. Our results for the first time have shown that the neuropsychological benefits after liver transplant are associated with specific cerebral alterations assessed by functional MRI. For example, attenuated activation in visuo-spatial processing areas post-transplant was associated with improved behavioral inhibition (i.e., ICT Lure accuracy). Increased activation while performing cognitive tasks pre-transplant may reflect a generalized response to neural disruption (32). This indicates that as the brain's metabolic state improves a subject can successfully perform cognitive tasks with less regional brain activation, or becomes more efficient. Post-transplant, the prior HE patients showed significant improvement on fMRI (i.e., decreased regional activation associated with improved cognitive performance), which again, like QOL was more nuanced in those with pre-transplant cognitive impairment. Consistent with the hypothesis presented previously in this paper regarding cognitive impairment as a marker for brain reserve, pre-transplant subjects with cognitive impairment manifested the highest brain regional activation and poorest ICT performance. This again points towards the usefulness of relying on pre-transplant cognitive function in helping the clinician formulate outcome expectations for the patient and their family.
On MRS, the entire group showed an improvement due to ammonia-related consequences. Significant reductions in glutamine, glutamate+glutamine and increased choline and myoinositol(33) in all three regions of interest (e.g., RPWM, ACC, and PGM) were associated with improved cognitive function on the vast majority of the administered neuropsychological measures. This suggests bilateral, and diffuse, metabolic benefit following liver transplant. This extends prior studies that have either studied smaller voxel numbers, have a lower sample size and did not sub-divide patients (4, 33).
While DTI revealed no change in FA, when HE/no-HE groups or groups with/without cognitive impairment were compared, MD and LD changed in both after transplant. An increase in LD is related to axonal recovery and improved white matter integrity(34), while the apparent increase in MD is likely due to the concomitant increase in longitudinal diffusivity. Specifically, improved axonal integrity post-transplant involved the internal capsule and splenium of the corpus callosum. These structures represent large collections of white matter fiber with vast projections that transmit information across the hemispheres and connect visual to associative areas. Changes in neural network connectivity may account for improved cognitive function in study subjects. In summary, our multi-modal MRI findings reveal that post-transplant, the brain functions more efficiently due to improved white matter integrity, decrease in generalized ammonia-associated astrocytic swelling and a more efficient energy utilization (i.e., reflected by decreased regional activation) of cortical regions necessary for visuo-association and psychomotor function.
The implications of these results are that the pre-transplant cognitive profile, regardless of prior HE status, was an important determinant of post-transplant HRQOL and brain function. This could be due to the notion that cognitive function is a marker for the extent of neuropathological processes underlying the liver disease. Importantly, study findings suggest that this pattern of pre-transplant cognitive impairment, rather than simply pre-transplant HE, is associated with poorer recovery following liver transplantation. Recognizing this relationship between pre-transplant cognitive impairment and treatment response may serve to provide realistic expectations regarding higher mental function recovery following transplantation for patients and family members after physical and functional recovery have occurred.
Our study results are limited by the 6 month follow-up; cognitive recovery could continue with further follow-up. Also like in prior LT studies, the use of potentially neurotoxic CNIs can impact cognition(27). However, patients did not have any physical signs, symptoms or MRI changes of CNI-associated toxicity. It is however difficult to separate out the impact of CNI towards this cognitive impairment since all patients were on these medications. Reflecting the realities of transplant listing, only 10% of our patients had alcoholic liver disease as the sole cirrhosis etiology. Therefore results may not be generalizable to centers with significantly higher proportion of alcoholic patients.
We conclude that while neuro-metabolic and functional brain changes occurring after liver transplant foster improved cognition and daily function, pre-transplant cognitive performance and a history of prior hepatic encephalopathy modulate the post-transplant global functioning. .
Supplementary Material
Acknowledgements
The authors would like to thank Jean Snow, RT-R in the Department of Radiology at VCU Medical Center.
Grants and Financial Support:
NIH RO1DK087913 and VA Merit Review CX10076. The funders had no role in the study design, protocol development, analysis of results and decision to publish.
Abbreviations
- HRQOL
health-related quality of life
- HE
hepatic encephalopathy
- MMSE
mini-mental status exam
- PHES
Psychometric hepatic encephalopathy score
- BOLD
blood oxygen level dependent
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- LD
longitudinal diffusivity
- Cho
choline
- Cr
creatine
- mI
myo-inositol
- Glx
glutamate+glutamine
- ROI
regions of interest
- COPE
contrasts of parameter estimates
Footnotes
Conflicts of Interest:
None for any author
Presentation: Portions of this study were presented as an oral presentation at the Liver Transplant Parallel Session at the Liver Meeting in San Francisco in 2015.
REFERENCES
- 1.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggio O, Ridola L, Pasquale C, Nardelli S, Pentassuglio I, Moscucci F, Merli M. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9:181–183. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Campagna F, Montagnese S, Schiff S, Biancardi A, Mapelli D, Angeli P, Poci C, et al. Cognitive impairment and electroencephalographic alterations before and after liver transplantation: what is reversible? Liver Transpl. 2014;20:977–986. doi: 10.1002/lt.23909. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simon-Talero M, Chavarria L, Vargas V, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17:38–46. doi: 10.1002/lt.22197. [DOI] [PubMed] [Google Scholar]
- 5.Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl. 2009;15:184–192. doi: 10.1002/lt.21593. [DOI] [PubMed] [Google Scholar]
- 6.Mattarozzi K, Cretella L, Guarino M, Stracciari A. Minimal hepatic encephalopathy: follow-up 10 years after successful liver transplantation. Transplantation. 2012;93:639–643. doi: 10.1097/TP.0b013e318244f734. [DOI] [PubMed] [Google Scholar]
- 7.Lin WC, Chou KH, Chen CL, Chen HL, Lu CH, Li SH, Huang CC, et al. Longitudinal brain white matter alterations in minimal hepatic encephalopathy before and after liver transplantation. PLoS One. 2014;9:e105887. doi: 10.1371/journal.pone.0105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allampati S, Mullen KD. Does overt hepatic encephalopathy cause persistent cognitive defects even after successful liver transplantation? Liver Transpl. 2014;20:874–875. doi: 10.1002/lt.23938. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 10.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Thacker LR, Wade JB, Sanyal AJ, Heuman DM, Sterling RK, Gibson DP, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34:1123–1132. doi: 10.1111/j.1365-2036.2011.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. e1591. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol. 2016;111:78–86. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 15.Umapathy S, Dhiman RK, Grover S, Duseja A, Chawla YK. Persistence of Cognitive Impairment After Resolution of Overt Hepatic Encephalopathy. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.107. [DOI] [PubMed] [Google Scholar]
- 16.Pillai AA, Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol. 2009;15:4225–4233. doi: 10.3748/wjg.15.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 21.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 22.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 26.Sarma MK, Huda A, Nagarajan R, Hinkin CH, Wilson N, Gupta RK, Frias-Martinez E, et al. Multi-dimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. Metab Brain Dis. 2011;26:173–184. doi: 10.1007/s11011-011-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijdicks EF, Wiesner RH. Acquired (non-Wilsonian) hepatocerebral degeneration: complex management decisions. Liver Transpl. 2003;9:993–994. doi: 10.1053/jlts.2003.50107. [DOI] [PubMed] [Google Scholar]
- 28.Campagna F, Biancardi A, Cillo U, Gatta A, Amodio P. Neurocognitive-neurological complications of liver transplantation: a review. Metab Brain Dis. 2010;25:115–124. doi: 10.1007/s11011-010-9183-0. [DOI] [PubMed] [Google Scholar]
- 29.Tryc AB, Pflugrad H, Goldbecker A, Barg-Hock H, Strassburg CP, Hecker H, Weissenborn K. New-onset cognitive dysfunction impairs the quality of life in patients after liver transplantation. Liver Transpl. 2014;20:807–814. doi: 10.1002/lt.23887. [DOI] [PubMed] [Google Scholar]
- 30.Umapathy S, Dhiman RK, Grover S, Duseja A, Chawla YK. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Am J Gastroenterol. 2014;109:1011–1019. doi: 10.1038/ajg.2014.107. [DOI] [PubMed] [Google Scholar]
- 31.Patel AV, Wade JB, Thacker LR, Sterling RK, Siddiqui MS, Stravitz RT, Sanyal AJ, et al. Cognitive reserve is a determinant of health-related quality of life in patients with cirrhosis, independent of covert hepatic encephalopathy and model for end-stage liver disease score. Clin Gastroenterol Hepatol. 2015;13:987–991. doi: 10.1016/j.cgh.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillary FG, Roman CA, Venkatesan U, Rajtmajer SM, Bajo R, Castellanos ND. Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology. 2015;29:59–75. doi: 10.1037/neu0000110. [DOI] [PubMed] [Google Scholar]
- 33.Cordoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, Vargas V, et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35:598–604. doi: 10.1016/s0168-8278(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 34.Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.