Abstract
Purinergic P2X4 receptors (P2X4Rs) belong to the P2X superfamily of ion channels regulated by ATP. We recently demonstrated that P2X4R knockout (KO) mice exhibit deficits in sensorimotor gating, social interaction and ethanol drinking behavior. Dopamine (DA) dysfunction may underlie these behavioral changes, but there is no direct evidence for P2X4Rs' role in DA neurotransmission. To test this hypothesis, we measured markers of DA function and dependent behaviors in P2X4R KO mice. P2X4R KO mice exhibited altered density of presynaptic markers including tyrosine hydroxylase, dopamine transporter; postsynaptic markers including dopamine receptors and phosphorylation of downstream targets including dopamine and cyclic-AMP regulated phosphoprotein of 32kDa (DARPP-32) and cyclic-AMP response element binding protein (CREB) in different parts of the striatum. Ivermectin, an allosteric modulator of P2X4Rs, significantly affected DARPP-32 and ERK 1/2 phosphorylation in the striatum. Sensorimotor gating deficits in P2X4R KO mice were rescued by DA antagonists. Using the 6-Hydroxydopamine (6-OHDA) model of DA depletion, P2X4R KO mice exhibited an attenuated levodopa (L-DOPA) induced motor behavior, whereas IVM enhanced this behavior. Collectively, these findings identified an important role for P2X4Rs in maintaining DA homeostasis and illustrate how this association is important for CNS functions including motor control and sensorimotor gating.
Keywords: P2X4 receptors, ivermectin, dopamine receptors, 6-OHDA, schizophrenia, Parkinson's disease
Introduction
Purinergic P2X receptors are cation permeable ion channels gated by ATP. They form homo- and heterotrimeric channels from seven subunits namely, P2X1-P2X7 (North 2002, Khakh & North 2012). Amongst them, P2X4 receptors (P2X4Rs) are abundantly expressed on neurons and glial (microglia and astrocytes) cells across the CNS as well as the PNS (Le et al. 1998). Gene knockout and pharmacological strategies have implicated P2X4R mediated transmission in hippocampal synaptic plasticity, inflammatory processes in the spinal cord and neuroendocrine functions (Sim et al. 2006, Ulmann et al. 2008, Zemkova et al. 2010). Despite this growing body of evidence, there remains a paucity of information regarding the functional significance of P2X4Rs in the CNS.
We recently reported that mice deficient in the p2rx4 gene [i.e. P2X4R knockout (KO)] exhibited deficits in sensorimotor gating, social behavior and ethanol drinking behavior (Wyatt et al. 2013, Wyatt et al. 2014). However, we did not identify any molecular mechanism that could explain these behavioral deficits. One plausible mechanism could be a result of P2X4Rs modulating major neurotransmitter systems including the glutamate and GABA systems. For instance, P2X4Rs are suggested to regulate postsynaptic currents mediated by NMDA receptors, AMPA receptors and GABAA receptors as well as presynaptic release of glutamate and GABA (Baxter et al. 2011, Andries et al. 2007, Jo et al. 2011, Gu & MacDermott 1997, Hugel & Schlichter 2002). Moreover, P2X4R KO mice exhibited altered subunit expression of multiple glutamatergic and GABAA receptors across multiple brain regions. This latter finding suggests that P2X4R deficiency disrupts homeostasis of postsynaptic ionotropic receptors (Wyatt et al. 2013, Wyatt et al. 2014). Notably, disruption of glutamatergic and GABAergic function has been linked to deficits in sensorimotor gating, social interaction and ethanol drinking behavior (Duncan et al. 2004, Du et al. 2012, Blednov et al. 2003). Together, these findings support the hypothesis that P2X4Rs can interact with other ionotropic receptors in regulation of multiple CNS functions.
In contrast to the building evidence supporting a role for P2X4Rs in glutamatergic and GABAergic function, little is known regarding the interaction of P2X4Rs with dopamine (DA) neurotransmission. Early evidence suggests that P2X4Rs are indirectly involved in DA neurotransmission (Krugel et al. 2001, Krügel et al. 2003, Xiao et al. 2008), but the direct role for P2X4Rs in regulating DA homeostasis has not been demonstrated. Considering that P2X4Rs are expressed on DA neurons and GABAergic medium spiny neurons (MSNs) of the basal ganglia (Heine et al. 2007, Amadio et al. 2007) and the behavioral deficits exhibited by P2X4R KO mice may represent DA dysfunction (Gendreau et al. 2000, Rodriguiz et al. 2004, Zhou et al. 1995, Ralph et al. 2001), we hypothesized that P2X4Rs control DA signaling with a relevant impact on DA associated behaviors.
In the present study, we utilized a P2X4R KO mouse model as a genetic approach and ivermectin (IVM), a positive allosteric modulator of P2X4Rs (Priel & Silberberg 2004, Khakh et al. 1999, Jelinkova et al. 2008, Jelinkova et al. 2006, Hattori & Gouaux 2012), as a pharmacological approach to test the aforementioned hypothesis. We measured protein densities of different markers of DA neurotransmission including tyrosine hydroxylase (TH), dopamine transporter (DAT), dopamine D1 and D2 receptors (D1Rs and D2Rs) and downstream targets integral to DA signaling including dopamine and cyclic-AMP regulated phosphoprotein of 32 kDa (DARPP-32), extracellular regulated kinase-1/2 (ERK 1/2) and cyclic-AMP response element binding protein (CREB) in different regions of the striatum of P2X4R KO and wildtype (WT) male mice. We also measured the degree of phosphorylation of DARPP-32, ERK 1/2 and CREB isolated from different striatal regions of WT and P2X4R KO mice in the presence and/or absence of IVM. The interaction between P2X4Rs and DA system in the regulation of CNS functions was addressed by employing behavioral pharmacology paradigms. The 6-Hydroxydopamine model (6-OHDA) of DA depletion was used to link P2X4R function with DA neurotransmission in modulation of motor control. Finally, using the prepulse inhibition (PPI) of acoustic startle reflex coupled with DA antagonists, we evaluated the effects of DA dysregulation as it is pertained to sensorimotor gating deficits. Overall, the findings support the hypothesis that P2X4R function plays a role in maintaining DA signaling with an impact on DA associated behaviors such as motor control and sensorimotor gating.
Experimental procedures
Animals
Experimentally naïve male WT and P2X4R KO mice were obtained from our breeding colony at the University of Southern California. The breeding colony was established from a previous P2X4R KO colony that was maintained on a C57BL/6 background (Sim et al. 2006). Our overall breeding scheme for generation of P2X4R KO mice and genotyping has been described previously (Wyatt et al. 2013, Wyatt et al. 2014). Mice were housed in groups of 5 per cage in rooms maintained at 22°C with 12 h/12 h light: dark cycle and ad libitum access to food and water. All experiments were undertaken in compliance to guidelines established by National Institute of Health (NIH) and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Southern California.
2-3 month old P2X4R KO and WT mice were used for biochemical assays. 2-4 month old P2X4R KO and WT mice were used for the motor behavior studies. 4-6 month old mice were used for the PPI of acoustic startle reflex study with DA antagonists.
Materials
Levodopa (L-DOPA) (Sinemet; 100 mg L-DOPA, 25 mg Carbidopa per pill) was diluted in 0.9% saline solution to achieve a concentration of 0.75 mg/ml. IVM (Norbrook, Lenexa, KS) was diluted in 0.9% saline solution, to achieve a concentration of 0.5 mg/ml and injected at a volume of 0.01 ml/g of body weight. Propylene glycol (Sigma Aldrich, St.Louis, MO) was used as the vehicle control for IVM. SCH-23390 HCl and raclopride tartrate (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline at concentrations of 0.2 mg/ml and 0.6 mg/ml respectively. Both drugs were injected at a volume of 0.005 ml/g of body weight.
Western immunoblotting
Tissue preparation
The dorsal and ventral striata were dissected from P2X4R KO and WT mice following euthanasia with CO2 asphyxiation. For the experiment that tested the effects of IVM (5 mg/kg, i.p.) on dopaminergic signaling, the dorsal and ventral striatal regions were dissected out 8 h post drug administration. The dorsal and ventral striata were dissected out as per the neuroanatomical landmarks described in the mouse brain atlas (Franklin & Paxinos 2007). The brain tissues were homogenized in a buffer containing 50 mM Tris-HCl pH (8.0), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1/100 dilution proteinases inhibitor cocktail (Millipore,Temecula,CA) and protein content was determined using BCA assay kit (Thermo Scientific, Rockford, IL). Homogenates were treated with a cocktail of phosphatase inhibitors (1 mM sodium pyrophosphate, 10 mM sodium fluoride, 0.5 mM sodium orthovandate, 10 mM β-glycerol phosphate, 1 μM microcysteine LR) (Sigma-Aldrich, St. Louis, MO) for detection of phosphoproteins.
Immunoblotting procedure
Protein samples of 50 μg ran on 10% SDS PAGE gels and transferred onto polyvinylidine diflouride membranes using semi-dry transfer method (Trans turbo blot; BioRad Laboratories, Hercules, CA). Striatal samples from WT and P2X4R KO mice were made to run on the same gel and transferred onto the same membrane. Non specific binding was blocked by incubation in 5% non fat dry milk, followed by incubation with primary antibodies overnight at 4°C. The antibodies used were rabbit anti- TH (1:5000), mouse anti-DAT (1:1000), rabbit anti-D2 receptor (1:1000), rabbit anti- DARPP-32 (1:1000) (Millipore, Temecula, CA), rabbit anti -D1 receptor (1:500; SantaCruz biotechnology, Santacruz, CA), mouse anti-total ERK 1/2 (1:1000), rabbit anti- total CREB (1:1000) (Cell Signaling Technology, Beverly,MA), mouse anti-β-actin (1:20,000; Sigma Aldrich, St. Louis, MO), mouse anti-α-tubulin (1:10,000; Millipore, Temecula, CA). The antibodies for phospho proteins included rabbit anti-phospho-Thr34- DARPP-32 (1:400; Millipore, Temecula, CA), rabbit anti-phospho-Ser133- CREB (1:500), rabbit anti-diphospho- Thr202/Tyr204- ERK 1/2 (1:500) (Cell Signaling Technology, Beverly, MA). Secondary antibodies included goat anti-mouse and goat anti-rabbit antibodies (1:10,000; BioRad Laboratories, Hercules, CA). Bands were visualized using chemiluminescence method (Clarity western plus ECL substrate; BioRad Laboratories, Hercules, CA) followed by exposure to HyBlot autoradiography films (Denville Scientific, Metuchen, NJ). Protein quantification was carried out by optical densitometry using ImageJ software (NIH, Bethesda, MD). Protein densities were normalized to β-actin or α-tubulin levels.
HPLC assay
Brain tissue was homogenized with 0.5 M perchloric acid, centrifuged at 16,873 × g for 12 mins at 4°C and protein was resuspended in 0.5 M NaOH. Protein content was detected by BCA assay. DA concentrations were determined using electrochemical detection method consisting of a ESA model Coularray 5600A coupled with a four channel analytical cell at -175, 50, 220, 300 mV. Samples were injected with ESA autosampler (Chelmsford, MA) and DA was separated by a 150 × 3.2 mm reverse phase 3 μm diameter C-18 column regulated at 28°C. The mobile phase MD-TM (ESA) consisted of acetylnitrile in phosphate buffer and a non ion-pairing reagent delivered at a rate of 0.6 ml/min. The HPLC was integrated with a Dell GX 280 computer with analytical programs including ESA Coularray for Windows software.
Acoustic Startle Reflex and PPI of Startle
Apparatus
Acoustic startle reflex and PPI were tested as previously described (Wyatt et al. 2013). The apparatus used for detection of startle reflex (San Diego Instruments, San Diego, CA) consisted of a standard cage placed in sound attenuated chambers with fan ventilation. Each cage consisted of a Plexiglass cylinder of 3 cm diameter, mounted on piezoelectric accelerometric platform connected to an analog-digital converter. Background noise and acoustic bursts were conveyed by two separate speakers, each one oriented appropriately so as to produce a variation of sound within 1 dB across the startle cage. Both speakers and startle cages were connected to the main PC, which detected and analyzed all chamber variables with Startle software (San Diego Instruments, San Diego, CA). Before each testing session, acoustic stimuli were calibrated via a digital sound level meter.
Startle and PPI session
During the baseline session, mice were exposed to background noise of 70 dB and after an acclimatization period of 5 mins, were presented with 12 40 ms trials of 115 dB interposed with 3 trials of a 82 dB prestimulus preceding the 115 dB by 100 ms. Subsequently, treatment groups were established so that the average startle response and %PPI were equivalent within the WT and P2X4R KO groups. On the testing day, each mouse was placed in the cage and exposed to a 5 mins acclimatization period with a 70 dB white noise background, which continued for remainder of the session. Each session consisted of three consecutive sequences of trials. Unlike the first and third session, wherein the mice were exposed to five alone pulse trials of 115 dB, the second period consisted of a pseudorandom sequence of 50 trials, including 12 pulse alone trials, 30 trials of pulse preceded by 73 dB, 76 dB or 82 dB prepulse (respectively, defined as PPI 3, PPI 6 and PPI 12; 10 for each level of prepulse loudness) and 8 no stimulus trials, wherein the mice were presented with background noise without any prepulse or pulse stimuli. Inter trial intervals were randomly chosen between 10 and 15 seconds. Delta PPI (ΔPPI) was calculated as mean startle amplitude for pulse alone trials – (mean startle amplitude for prepulse trial). DA antagonists, SCH-23390 (1 mg/kg, i.p) and raclopride (3 mg/kg, i.p) were administered 10 mins prior to testing session.
6-OHDA lesioning in mice and motor behavior testing
6-OHDA lesioning surgery
Mice were treated with desipramine hydrochloride (25 mg/kg, i.p.) (Sigma-Aldrich, St. Louis, MO) 30 mins prior to surgery to prevent concurrent damage of noradrenergic pathways by 6-OHDA. Mice were anesthetized with avertin (25 mg/kg, i.p.) and placed in the stereotaxic apparatus. 2 μl of freshly prepared 6-OHDA bromide salt (4 mg/ml in 0.2% ascorbic acid and 0.9% saline; Sigma-Aldrich, St. Louis, MO) was unilaterally infused into left median forebrain bundle (from Bregma point: 1.2 mm posterior, 1.1 mm lateral, 5 mm ventral) (Franklin & Paxinos 2007) at a rate of 0.5 μl/min using a 10 μl Hamilton syringe and a microlitre syringe pump. The injection cannula was left in place for 3-5 mins to prevent reflux and ensure complete absorption. Postoperative procedures involved daily subcutaneous (s.c.) injections with sucrose (5% w/v in saline) and warming on a heating pad for 2 weeks.
Motor behavior testing
Mice were subjected to behavioral testing 3 weeks after 6-OHDA lesioning surgery. Mice were placed in a plastic cylinder (318 mm in diameter) with a video camera mounted above it. Baseline rotational behavior was established by saline injections which causes the mouse to rotate towards the lesioned side with lesser DA activity (ipsilateral rotations). L-DOPA (5 mg/kg, s.c.) was administered 5 mins prior to behavioral testing and the number of contralateral and ipsilateral rotations were counted in every ten minute interval for a total period of 90 mins. After monitoring the behavioral activity with L-DOPA alone, the same cohort of WT and P2X4R KO mice received a combination of IVM (5 mg/kg, i.p.) and L-DOPA (5 mg/kg, s.c.) to study the modulatory effect of IVM on L-DOPA induced motor behavior. IVM and L-DOPA were administered 8 h and 5 mins respectively prior to behavioral testing. To monitor effect of IVM alone on rotational behavior, a separate cohort of 6-OHDA lesioned WT mice received IVM (5 mg/kg, i.p) 8 h prior to behavioral testing and motor activity was monitored for 2 h. The 8 h time point was selected since IVM has been shown to achieve maximal concentration in brain and plasma post 8 h (Yardley et al. 2012).
Peroxidase based immunohistochemistry
Collection and processing of brain tissues
Transcardial perfusion was performed using 0.9 % NaCl/ 4% phosphate buffered paraformaldehyde. Perfused brains were post fixed overnight followed by storage in 20% sucrose for 48 h and quickly frozen with 4-Methylbutane on dry ice. Perfused brains were cut coronally at 25 μm thickness in a cryostat and stored in a cryoprotective solution containing 30% sucrose in PBS at 4°C until further use.
TH staining using peroxidase method of immunohistochemistry in ventral mesencephelon
Brain sections were heated in 10 mM sodium citrate buffer (pH 8.5) for 30 mins at 80°C followed by permeabilization in TBS + 0.2%Triton-X for 30 mins. Slices were then quenched in solution containing 10% methanol and 3% H2O2 in TBS for 10 mins and subsequently blocked in 5% non fat dry milk and in 5% normal goat serum (each step for 30 mins). Sections were incubated overnight with rabbit anti-TH (1:5000) (Millipore, Temecula, CA) diluted in TBS +0.2% Triton-X containing 1% normal goat serum. The avidin-biotin complex method of detection was used, wherein slices were incubated with biotinylated goat anti-rabbit antibody (1:500) for 1 h and then with 1% avidin linked peroxidase complex for 45 mins (Vector Laboratories, Burlingame, CA). This was followed by treatment with solution containing 0.05% 3,3-diaminobenzidine and 0.015% H2O2 in PBS for 5 mins, dehydration of slides in a dilution series of ethanol, clearance in xylene and evaluation under light microscope.
Statistical analyses
For the Western blotting analysis, the average of densitometry values of WT striatal samples was used to arbitrarily normalize WT samples to 1 and the P2X4R KO striatal samples were normalized by dividing each densitometry value by the average of WT samples. Normalization of the two genotypes was done within the same membrane and presented as fold change of P2X4R KO versus WT in that membrane. For D1, D2, DARPP-32, ERK1/2 and CREB immunoblotting; WT and P2X4R KO mice were generated in separate cohorts. The normalization of P2X4R KO to WT samples was done within the same cohort followed by combining data from two cohorts for analyses. The same method of normalization was used for the study that tested the effects of IVM on dopaminergic signaling in the dorsal and ventral striatum of WT and P2X4R KO mice. The average of vehicle treated WT mice was used to arbitrarily normalize the WT control samples to 1 and the IVM treated WT, vehicle and IVM treated P2X4R KO mice were normalized by dividing each value by the average of vehicle treated WT mice. Normalized density of proteins were expressed as mean ± SEM. Phosphorylation was calculated as ratio of normalized values of phosphorylated form to total form of protein. DA levels were expressed as ng/mg of protein content. Unpaired Student t-test was used for analyzing the differences in protein densities and DA levels between WT and P2X4R KO groups. Two-way ANOVA with Bonferroni post hoc test was used to evaluate the effect of IVM on phosphorylation of various signaling molecules between WT and P2X4R KO. Pharmacological studies for motor behavior and sensorimotor gating were analyzed by two way repeated measures ANOVA with time/PPI intensity and genotype/treatment as within and between subjects variability respectively followed by Bonferroni post hoc test for multiple comparisons. Significance was set at P<0.05. All data was analyzed using GraphPad Prism software (San Diego, CA).
Results
P2X4R KO mice exhibit alterations in expression of presynaptic DA markers in striatum
To investigate changes in presynaptic markers of DA neurotransmission, we compared TH and DAT protein density between P2X4R KO and WT mice using Western immunoblotting. P2X4R KO mice exhibited a significant increase in TH protein density in the dorsal striatum by 64% (p<0.01) but no change in protein density in the ventral striatum [Fig 1A &1B (i)]. P2X4R KO mice exhibited significant increases in DAT protein density by 106% (p<0.05) and by 98% (p<0.01) in the dorsal and ventral striatum, respectively [Fig 1A &1B (ii)].
Figure 1.
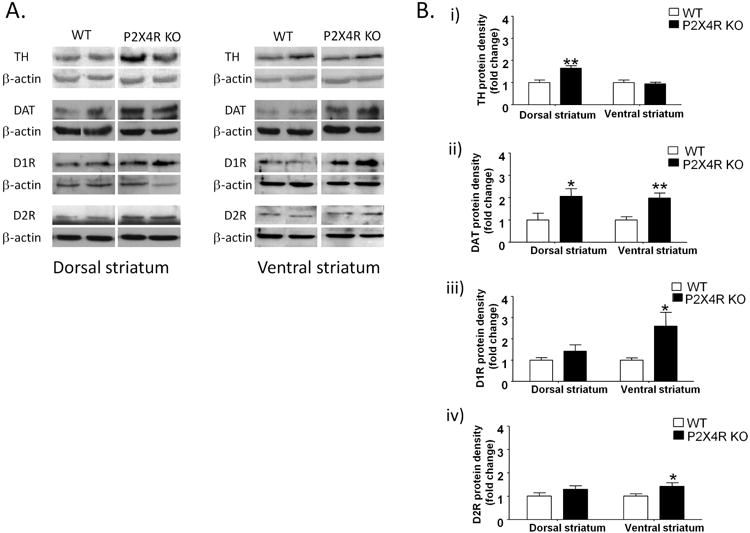
P2X4R KO mice exhibited significant increases in TH protein density in the dorsal striatum, but no changes in the ventral striatum [A & B (i)]; increased DAT protein density in both parts of the striatum [A & B (ii)]; increased D1R [A &B (iii)] and D2R [A&B (iv)] protein densities in the ventral, but no change in the dorsal striatum. The protein levels of DA markers were normalized to β-actin and expressed as arbitrary units (AU). The average of densitometry value of WT samples was arbitrarily normalized to 1. P2X4R KO samples were normalized by dividing each value by the average of WT samples and presented as fold change of P2X4R KO versus WT in that membrane. Values represent mean ± SEM from 5-8 WT, 7-8 P2X4R KO mice for TH, DAT analyses and 11-12 WT, 12-13 P2X4R KO for D1Rs and D2Rs analyses. 2 representative bands from each genotype from the same membrane are shown. *P <0.05, ** P <0.01 versus WT controls. Unpaired Student's t-test
The significant increase in TH protein density in the dorsal striatum did not appear to be associated with an increase in DA levels as there were no significant changes in DA levels in the dorsal striatum (Fig S1A). Moreover, there were no changes in DA levels in the ventral striatum (Fig S1B) between P2X4R KO and WT mice.
P2X4R KO mice exhibit significant alterations in expression of DA receptors and downstream targets
Based on the significant changes in TH and DAT protein densities, we posit that changes within presynaptic DA environment could impact the densities of DA receptors and downstream targets. Among the DA receptor subtypes, D1Rs belong to excitatory Gαs/olf family of G-coupled protein receptors (GPCRs), whereas D2Rs belong to inhibitory Gαi/olf family of GPCRs (Girault 2012). To test this hypothesis, we measured protein densities of D1Rs, D2Rs and phosphorylation states of major downstream targets in striatum. P2X4R KO mice exhibited significant increases in D1R protein density by 159% [p<0.05; Fig 1A &1B (iii)] and in D2R protein density by 42% [p<0.05; Fig 1A &1B (iv)] in the ventral striatum as compared to WT mice. There were no changes in either of protein densities for DA receptors in the dorsal striatum of P2X4R KO mice.
To investigate downstream pathways regulated by DA receptors, we measured total and phosphorylated form of DARPP-32, ERK 1/2 and CREB in the dorsal and ventral striatum using Western immunoblotting. P2X4R KO mice exhibited a significant increase in DARPP-32 phosphorylation (by 164%, p<0.05) without any changes in total DARPP-32 protein density in the dorsal striatum as compared to WT counterparts [Fig 2A & 2B (i)]. In the ventral striatum, P2X4R KO mice exhibited a significant decrease in DARPP-32 phosphorylation (by 48%, p<0.05) without any changes in protein density of total DARPP-32 [Fig 2A & 2B (i)]. P2X4R KO mice did not exhibit any significant changes in total ERK 1/2 protein density or ERK 1/2 phosphorylation in the dorsal and ventral striatum compared to WT mice [Fig 2A & 2B (ii)]. There were no significant changes in protein density of total CREB or CREB phosphorylation in the dorsal striatum of P2X4R KO mice [Fig 2A & 2B (iii)]. On the other hand, P2X4R KO mice did exhibit a significant decrease in total CREB density (by 36%, p<0.01) and a corresponding increase in CREB phosphorylation (by 141%, p<0.001) in the ventral striatum compared to WT mice [Fig 2A & 2B (iii)].
Figure 2.
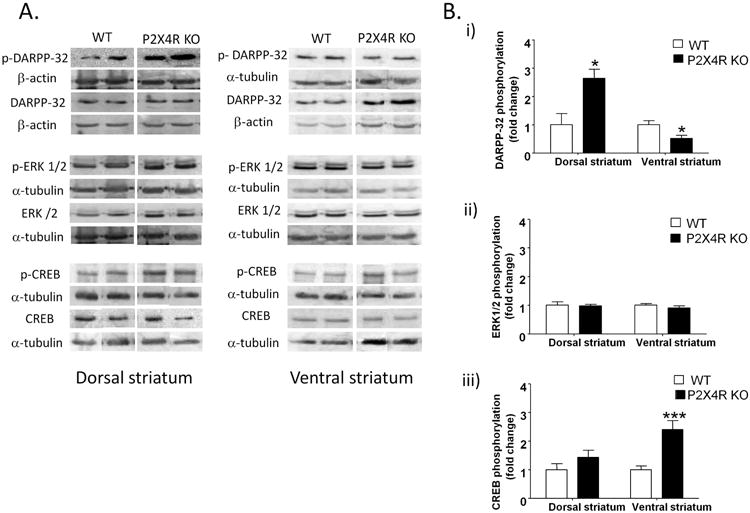
P2X4R KO mice exhibited increased DARPP-32 phosphorylation in the dorsal striatum, but a decrease in the ventral striatum [A & B (i)]; no changes in ERK 1/2 phosphorylation in dorsal or ventral striatum of P2X4R KO mice [A & B(ii)]; increased phosphorylation of CREB in the ventral, but not in the dorsal striatum [A & B(iii)].. Details of normalization and analyses are presented in Figure 1. Values represent mean ± SEM from 3-6 WT and 4-8 P2X4R KO mice for DARPP-32 analysis; 6-8 WT & P2X4R KO for ERK 1/2 and CREB analyses. 2 representative bands from each genotype from the same membrane are shown. *P <0.05, ***P<0.001 versus WT counterparts. Unpaired Student's t-test.
IVM significantly affected DARPP-32 and ERK 1/2 phosphorylation, but not CREB phosphorylation in the dorsal striatum
IVM was used to gain pharmacological insights into the role of P2X4Rs in dopaminergic signaling in the dorsal striatum. There was no significant effect of IVM treatment or genotype on total DARPP-32 levels or phosphorylation but the drug treatment × genotype interaction was significant for both the total DARPP-32 levels [F(1,18)=5.61, p<0.05] and DARPP-32 phosphorylation [F(1,18)=5.48, p<0.05]. Bonferroni post hoc test confirmed significant increase in DARPP-32 phosphorylation upon IVM treatment in WT (t=2.834, p<0.05), but not in P2X4R KO mice (t=0.5829, p>0.05) [Fig 3A & 3B (i)]. There was no significant effect of IVM treatment or genotype on total ERK 1/2 levels, but the drug treatment × genotype interaction was significant [F(1,18)=10.00, p<0.01]. There was a significant effect of IVM treatment [F(1,18)=, p<0.05] but not genotype or drug treatment × genotype interaction for ERK 1/2 phosphorylation. There was no significant effect of IVM treatment, genotype or drug treatment × genotype interaction for total CREB levels. There was no significant effect of IVM treatment or drug treatment × genotype interaction, but the effect of genotype trended towards significance for CREB phosphorylation [F(1,16)=4.37, p=0.0529] [Fig 3A & 3B (iii)].
Figure 3.
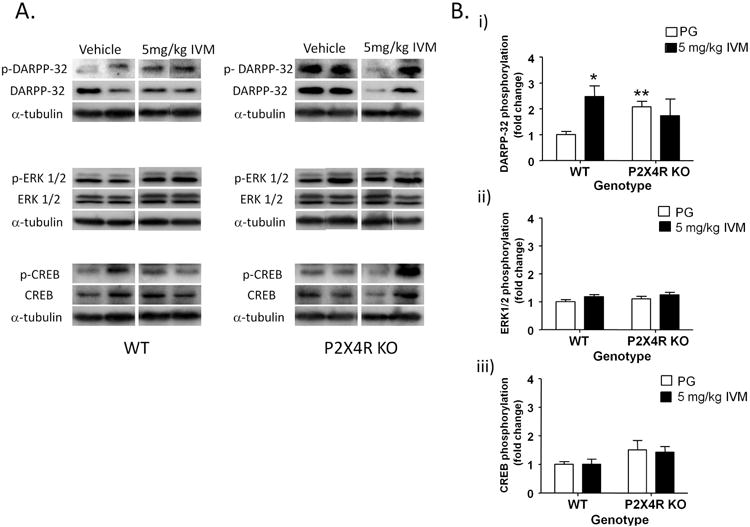
IVM (5 mg/kg) significantly upregulated DARPP-32 phosphorylation [A & B (i)] via P2X4R potentiation in the dorsal striatum. IVM increased ERK 1/2 phosphorylation independent of P2X4R function [A& B (ii)]. No effect of IVM on CREB phosphorylation [A& B (iii)]. The average of densitometry value of vehicle treated WT samples was arbitrarily normalized to 1. The IVM treated WT mice, vehicle and IVM treated P2X4R KO were normalized by dividing each value by the average of the vehicle treated WT samples. The data is presented as fold change of IVM treated WT, P2X4R KO and vehicle treated P2X4R KO samples versus vehicle treated WT samples in that membrane. Values represent mean ± SEM from 4-6 WT and P2X4R KO mice per treatment group. 2 representative bands from each treatment group are shown. *P<0.05 versus vehicle treated WT group, Bonferroni post hoc test.
IVM significantly affected DARPP-32 phosphorylation, but not ERK 1/2 or CREB phosphorylation in the ventral striatum
Similar to dorsal striatum, we investigated the effect of IVM on phosphorylation of the same signaling molecules in the ventral striatum. There was no significant effect of IVM treatment or genotype on total DARPP-32 levels or DARPP-32 phosphorylation but the drug treatment × genotype interaction was significant for both total DARPP-32 levels [F(1,16)=6.40, p<0.05] and DARPP-32 phosphorylation [F(1,16)=6.20, p<0.05] [Fig 4A & 4B (i)]. There was no significant effect of IVM treatment, genotype or drug treatment × genotype interaction for total ERK 1/2 levels or ERK 1/2 phosphorylation [Fig 4A & 4B (ii)]. There was no significant effect of IVM treatment, genotype or drug treatment × genotype interaction for total CREB levels. Similarly, there was no significant effect of IVM treatment or genotype on CREB phosphorylation. However, the drug treatment × genotype interaction trended towards significance for CREB phosphorylation [F(1,15)=4.43, p=0.0527] [Fig 4A& 4B (iii)].
Figure 4.
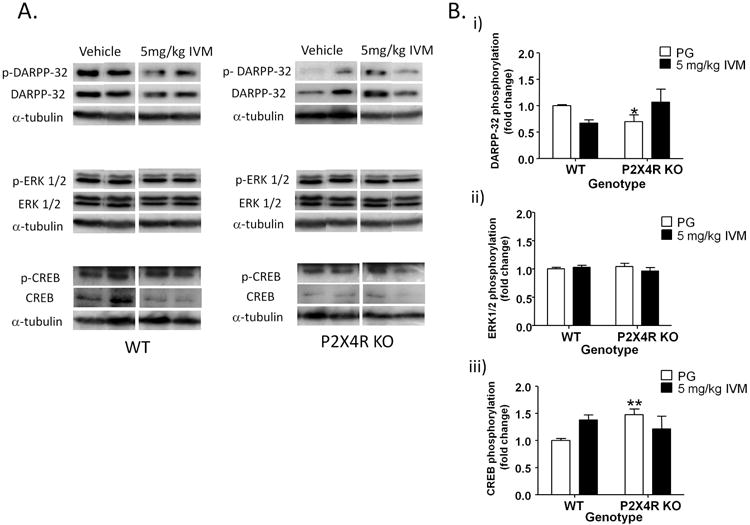
IVM (5 mg/kg) significantly affected DARPP-32 [A & B (i)] but not, ERK 1/2 [A & B (ii)] or CREB phosphorylation [A& B (iii)], via P2X4R potentiation in the ventral striatum. Details of normalization and analyses are presented in Figure 3. Values represent mean ± SEM from 4-6 WT and P2X4R KO mice per treatment group. 2 representative bands from each treatment group are shown. *P<0.05 versus vehicle treated WT group, Bonferroni post hoc test.
Pharmacological inhibition of D1Rs and D2Rs significantly enhanced prepulse inhibition (PPI) of acoustic startle reflex in P2X4R KO mice
We investigated the response of P2X4R KO mice to selective antagonists for D1Rs (SCH-23390; 1 mg/kg) and D2Rs (raclopride; 3 mg/kg) on PPI startle response, to link imbalances in DA homeostasis to PPI deficits in P2X4R KO mice. The doses tested were chosen based on previous studies that investigated PPI function in C57BL/6 mice (Doherty et al. 2008, Ralph-Williams et al. 2003, Ralph et al. 2001). We found a significant effect of genotype on PPI function [F(1,26)= 5.50, p<0.05] (Fig 4A) which supports our previous findings (Wyatt et al. 2013). SCH-23390 significantly increased PPI in P2X4R KO mice [F(1,28)=9.33, p<0.01] with Bonferroni post hoc test identifying a significant increase at PPI 6 (t=3.294, p<0.01) and PPI 12 (t=3.240, p<0.01) (Fig 4A). Similarly, raclopride significantly enhanced PPI function in P2X4R KO mice [F(1,28)=11.98, p<0.01] as compared to saline treated P2X4R KO mice with Bonferroni post hoc test identifying a significant increase at PPI 6 (t=3.748, p<0.001) and PPI 12 (t=3.507, p<0.01) (Fig 4A). There were no significant changes in PPI function in WT mice upon treatment with SCH-23390 or raclopride (Fig 4A). Moreover, neither SCH- 23390 nor raclopride induced any significant change in startle amplitude in P2X4R KO mice (Fig 4B).
Pharmacological or genetic manipulation of P2X4R function significantly influenced L-DOPA induced motor behavior in the 6-OHDA model of DA depletion
Using the 6-OHDA model of DA depletion, we investigated the role of P2X4Rs in regulation of motor behavior (Schwarting & Huston 1996). As presented, ablation of DA neurons in ventral mesencephelon in both genotypes (Fig S2) resulted in reduced TH expression by 95.7% and 96.3% in the striatum of WT (p<0.001) and P2X4R KO mice (p<0.01) respectively (Fig S2). In the presence of L-DOPA treatment (5 mg/kg), both genotypes exhibited contralateral rotations, since L-DOPA metabolizes into DA in the synapses, followed by activation of the supersensitive postsynaptic DA receptors on lesioned striatum (Ungerstedt 1971) (Fig 5A). In the presence of L-DOPA, we found that P2X4R KO mice exhibited significantly fewer contralateral turns as compared to WT mice (Fig 5A). There was a significant effect of genotype [F(1,20)=4.39, p<0.05] and time [F(8,160)=58.46, p<0.001] on L-DOPA induced rotational behavior in P2X4R KO. There was no significant time × genotype interaction for L-DOPA induced motor behavior. Bonferroni post hoc test confirmed significant reduction in L-DOPA induced motor behavior in P2X4R KO mice during the 45-55 mins interval (t=3.154, p<0.05) (Fig 5A).
Figure 5.
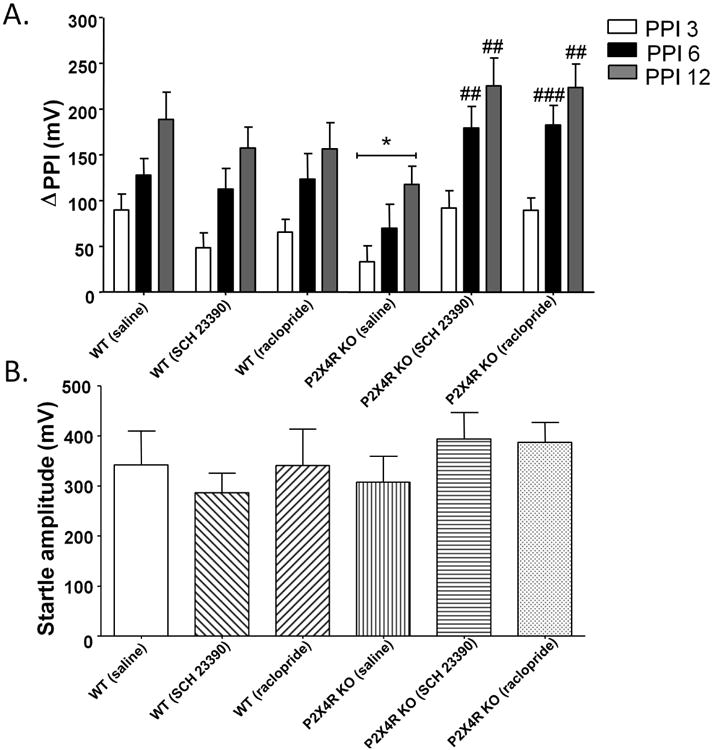
SCH-23390 (1 mg/kg) and raclopride (3 mg/kg) significantly increased prepulse inhibition of acoustic startle reflex in P2X4R KO mice (A) without any changes in startle amplitude (B). There were no changes in PPI in WT mice upon treatment with both antagonists (A). Values represent the mean of ΔPPI and mean startle amplitude ± SEM from 14 WT (saline), 15 WT (SCH 23390 and raclopride), 14 P2X4R KO (saline) and 16 P2X4R KO (SCH-23390 and raclopride). *P <0.05 versus saline treated WT mice, ## P<0.01, ### P<0.001 versus saline treated P2X4R KO mice, Bonferroni post hoc test.
We reasoned that if genetic disruption of P2X4Rs reduces L-DOPA response on motor behavior, then pharmacological potentiation of P2X4Rs should enhance motor behavior. In support of this hypothesis, we found that IVM (5 mg/kg) significantly increased the number of L-DOPA induced rotations in WT mice. There was a significant effect of time [F(8,208)=95.32, p<0.001] and IVM treatment [F(1,26)=33.80, p<0.001] on L-DOPA induced motor behavior. There was also a significant time × drug treatment interaction [F(8,208)=10.99, p<0.001] with Bonferroni post hoc test identifying a significant increase in contralateral rotations upon IVM treatment during 5-15 mins (t=5.509, p<0.001), 15-25 min (t=4.161, p<0.001), 25-35 min (t=5.491, p<0.001), 35-45 min (t=6.254, p<0.001), 45-55 min (t=6.484, p<0.001) and 55-65 min (t=4.878, p<0.001) intervals (Fig 5A). IVM alone induced an average of 12 rotations ± 2 rotations (SEM) for period of 2 hours post drug treatment, although it was still statistically significant compared to sham controls [F(1,9)=26.64, p<0.001] (Fig S3).
There was a significant effect of time [F(8,112)=36.93, p<0.001] and IVM treatment [F(1,14)=4.80,p<0.05] on L-DOPA induced motor behavior in P2X4R KO mice (Fig 5A). There was also a significant time × drug treatment interaction [F(8,112)=2.88, p<0.01] with Bonferroni post hoc test confirming a significant increase in L-DOPA induced motor behavior during the 5-15 mins interval (t=3.481, p<0.01). Taking into consideration the lower L-DOPA baseline in P2X4R KO mice, we analyzed the difference in the number of contralateral rotations between L-DOPA alone and IVM + L-DOPA in WT and P2X4R KO mice. There was a significant effect of time [F(8,160)=11.33, p<0.001] and a non-significant trend of effect of genotype [F(1,20)=3.17, p=0.0903] on contralateral rotations between L-DOPA and L-DOPA + IVM (Fig 5B). There was a significant time × genotype interaction [F(8,160)=2.07, p<0.05], suggesting that IVM's ability to increase L-DOPA induced motor behavior differed between WT and P2X4R KO mice. Bonferroni post hoc test identified significant differences in L-DOPA induced motor behavior upon IVM treatment between WT and P2X4R KO mice at 55-65 mins (t=2.948, p<0.05) (Fig 5B).
Discussion
Our current study investigated the role of P2X4Rs in the dopaminergic system and its impact on DA dependent behaviors. Impairments in DA neurotransmission were observed with respect to changes in protein densities of pre- and postsynaptic markers. There was increased TH protein density in the dorsal, but not the ventral, striatum of P2X4R KO mice. DAT was significantly increased in both parts of the striatum in P2X4R KO mice, which is indicative of higher presynaptic reuptake of DA. In addition to changes in presynaptic markers of DA activity, we also identified significant changes in protein densities of DA receptors in the ventral, but not the dorsal, striatum of P2X4R KO mice. The increased DA receptors' density in the ventral striatum could be an adaptive response to the altered synaptic DA availability due to increased DAT expression in the same brain region of P2X4R KO mice. This interpretation is based on previous studies that have postulated a positive correlation between DAT and DA receptor density (Fauchey et al. 2000, Ghisi et al. 2009). The increased DAT and DA receptor density levels suggest that P2X4R KO mice may have alterations in DA neurotransmission. However, there were no significant changes in DA levels in both parts of the striatum of P2X4R KO mice despite significant alterations in TH density levels in the dorsal striatum of P2X4R KO mice. There are several other factors that control DA levels such as storage, release, reuptake, and catabolism (Eells 2003). Based on our finding of increased density of DAT and DA receptors, future studies will be necessary using in vivo microdialysis or fast scan cyclic voltammetry to help to identify any differences in the extracellular DA levels between WT and P2X4R KO mice and elucidate the dopaminergic tone in P2X4R KO mice. Overall, the findings suggest that p2rx4 deficiency affects DA synthesis and transport that could impact normal DA neuron function.
In addition to DA receptors, we identified dysregulation of signaling molecules (i.e. DARPP-32 and CREB) that can be regulated through DA receptors in different striatal regions of P2X4R KO mice. D1R stimulation on striatonigral MSNs phosphorylates DARPP-32 at Thr34 via protein kinase A (PKA) that inhibits PP-1 activity and allows phosphorylation of ERK1/2 and CREB. D2R stimulation produces the opposite effects in the striatopallidal MSNs (Girault 2012, Bertran-Gonzalez et al. 2008). Despite the lack of significant changes in D1Rs protein density, P2X4R KO mice exhibited a significant increase in DARPP-32 phosphorylation in the dorsal striatum, which is typically indicative of upregulated D1R mediated signaling function. On the other hand, we saw a significant decrease in DARPP-32 phosphorylation in the ventral striatum of P2X4R KO mice that correlates well with the increased D2R protein density in the same brain region. But, we saw significant increases in D1R density and CREB phosphorylation in the same brain region of P2X4R KO mice that did not corroborate with the decreased DARPP-32 phosphorylation. One possible explanation for these neurochemical differences identified in the P2X4R KO mice is that there are multiple interactions or involvement of various neurotransmitter systems besides DA in regulating DARPP-32 phosphorylation such as glutamate, GABA and serotonin (Svenningsson et al. 2004). Thus, the alterations in signaling molecules including DARPP-32 and CREB in P2X4R KO mice suggest a complex compensatory change other than that of DA receptors. Taken together; the increased density of pre- and postsynaptic markers suggests dysregulation of DA system in P2X4R KO mice which may partially underlie the behavioral deficits previously reported in P2X4R KO mice.
In addition to the genetic approach, we used a pharmacological approach to explore a role for P2X4Rs in regulating DA receptor associated signaling pathways. Since, there are limited specific antagonists to test P2X4R related signaling in vivo, we used the P2X4R allosteric modulator, IVM, to investigate a link between P2X4R function and DA receptor associated signaling molecules in the dorsal and ventral striatum. As presented above, there was a significant increase in DARPP-32 phosphorylation upon IVM treatment in the WT, but not in P2X4R KO mice, suggesting a role for P2X4Rs in regulating DARPP-32 phosphorylation in the dorsal striatum. The changes in DARPP-32 phosphorylation upon P2X4R potentiation by IVM did not fully agree with the result from genetic deletion of P2X4Rs in the dorsal striatum. This difference in finding may be linked to neurodevelopmental changes in P2X4R KO mice. This hypothesis is supported by several lines of evidence: first, IVM did not increase DARPP-32 phosphorylation in P2X4R KO mice; second, P2X4Rs have been reported to be expressed from postnatal day 1 (Cheung et al. 2005); third, the P2X4R KO mice exhibit communication deficits during their pre-adult period (Wyatt et al. 2013). Since, phospho-Thr34-DARPP-32 can indirectly increase ERK 1/2 phosphorylation in the striatum (Girault 2012), it was not surprising to see a significant effect of IVM treatment on ERK 1/2 phosphorylation. However, increase in ERK 1/2 phosphorylation was seen in both the genotypes, indicating that IVM's ability to modulate ERK 1/2 phosphorylation is independent of P2X4R function. Interestingly, there was a significant interaction between IVM treatment and genotype for total ERK 1/2 levels, indicating that effect of IVM on total ERK 1/2 expression was dependent upon P2X4R function. Hence, IVM might be increasing ERK 1/2 phosphorylation in the WT mice by regulating total expression of the protein upon P2X4R potentiation. Although previous investigations have used IVM as a pharmacological tool for studying P2X4R function in vitro and in vivo (Bortolato et al. 2013, Sim et al. 2006, Popova et al. 2013, Asatryan et al. 2010), IVM does have other protein targets in the CNS including GABAA receptors (Dawson et al. 2000), nicotinic acetylcholine receptors (Krause et al. 1998) and glycine receptors (Shan et al. 2001). Hence, the mechanism by which IVM increases ERK 1/2 phosphorylation in P2X4R KO mice needs further investigation. Similar to the dorsal striatum, IVM modulated DARPP-32 phosphorylation via P2X4R potentiation in the ventral striatum. Moreover, IVM had a tendency to differentially modulate CREB phosphorylation via P2X4R activity in the same brain region. Taken together, using genetic and pharmacological approaches, the data suggests that there is a link between P2X4R activity and DARPP-32 phosphorylation in the striatum and P2X4Rs may have a role in regulating DA neurotransmission in GABAergic MSNs of the striatum via modulating DARPP-32 activity.
The significant increases in DA receptor protein density in the ventral striatum reported herein may underlie the PPI deficits in P2X4R KO mice. PPI measures reduction in startle reflex that occurs when the eliciting acoustic burst is immediately preceded by a weak stimulus and is highly reliable index for measuring sensorimotor gating (Ison & Hoffman 1983). Multiple findings have reported a critical role for DA receptors in regulation of PPI function, of which D2Rs have received considerable attention on basis of findings from pharmacological studies in rats and patient population (Swerdlow et al. 1991, Abduljawad et al. 1998, Volter et al. 2012, Kumari et al. 1998). However, gene knockout and pharmacological studies in mice have implicated both D1Rs and D2Rs (Ralph-Williams et al. 2003, Ralph-Williams et al. 2002, Doherty et al. 2008). In the context of findings from the literature, we used both D1R (SCH-23390) and D2R (raclopride) antagonists to identify potential contribution of DA receptors to PPI functioning in P2X4R KO mice. We found that the PPI deficits in P2X4R KO mice were significantly ameliorated by SCH-23390 and raclopride, indicating D1Rs and D2Rs as important modulators of PPI function in mice. The increased density of DA receptors in the ventral striatum, integral to corticolimbic-striato-pallidal circuitry of PPI (Swerdlow et al. 2008), of P2X4R KO mice may contribute to PPI dysfunction and that blocking these receptors can reverse the deficit. The pharmacological studies provide insights into the functional consequences of altered DA receptor protein densities on behaviors such as sensorimotor gating in the P2X4R KO mice. Moreover, we reported IVM mediated PPI disruption in WT C57BL/6J mice and its attenuated response in P2X4R KO mice, which further supports a role for P2X4Rs in sensorimotor gating (Bortolato et al. 2013). Overall, these studies identify potential interactions between P2X4R function and DA neurotransmission in regulating sensorimotor gating.
The neurochemical and behavioral alterations in P2X4R KO mice could be relevant to multiple psychiatric disorders such as schizophrenia, attentional deficit hyperactivity disorder, Obsessive-Compulsive disorder and bipolar depression. For example, post mortem studies have reported an increase in D2R and DAT expression in psychotic and non-psychotic disorders (Krause et al. 2000, Brunswick et al. 2003, Pearlson et al. 1995, Perez et al. 2003). In addition, increased TH expression and presynaptic DA synthesis has been reported in neuroleptic naïve psychotic patients (Hietala et al. 1995, Hietala et al. 1999). We observed increased density in D2Rs in the ventral striatum, DAT in both striatal regions and TH in the dorsal striatum in P2X4R KO mice. Perhaps, one of the most notable findings of our study was the identification of increased D2Rs in the striatum, since D2Rs are considered as an important genetic marker for susceptibility to neuropsychiatric diseases (Seeman 2013a, Seeman 2013b, Zhan et al. 2011). Additionally, P2X4R KO mice exhibited PPI deficits, an important behavioral biomarker of neuropsychiatric diseases (Seeman et al. 2006, Feifel et al. 2009, Perry et al. 2001, Braff 1993). Interestingly, the upregulation of D2Rs, altered sensitivity to DA receptor acting drugs and PPI deficits in P2X4R KO mice correlate with findings from mouse models linked to psychiatric disorders (Wolinsky et al. 2007, Lipina et al. 2010, Ralph et al. 2001, Kinkead et al. 2005). Taken together, the altered DA homeostasis and resultant behavioral deficits induced upon p2rx4 deficiency suggests a role for this receptor in disorders characterized by DA dysfunction such as schizophrenia, bipolar disorder and attention deficit hyperactivity.
Our findings also suggest that P2X4Rs are involved in other DA dependent functions of the basal ganglia including motor behavior. To test this, we used the 6-OHDA animal model in combination with IVM and P2X4R KO mice, which is a well established model for elucidating DA interactions with other neurotransmitter systems in motor activity (Fox & Brotchie 2000, Xiao et al. 2011). Also, this model is used for understanding the pathogenesis of movement disorders including Parkinson's disease and screening of novel therapeutics (Deumens et al. 2002, Schwarting & Huston 1996). We found that the L-DOPA induced motor behavior was significantly decreased in 6-OHDA lesioned P2X4R KO mice, indicating that disruption of P2X4R function significantly affected L-DOPA induced behavioral response. This attenuated response in P2X4R KO mice may be due to alterations in DA system in striatonigral circuitry of the basal ganglia. Alternatively, L-DOPA attenuated response could be linked to its faster clearance or metabolism in P2X4R KO mice. This hypothesis will be explored in future studies. Conversely, we demonstrated that pharmacological modulation of P2X4R activity by IVM significantly enhanced L-DOPA induced rotational behavior. A plausible mechanism underlying L-DOPA + IVM response is a synergy between P2X4Rs and D1Rs on MSNs in disinhibiton of neurons projecting from the substantia nigra pars reticulata (SNR) to the thalamus, superior colliculus and pendenculopontine nucleus and thereby, producing contralateral rotations. Notably, increased expression of P2X4Rs has been reported in the MSNs of SNR of 6-OHDA treated rats (Amadio et al. 2007) and so, these compensatory changes may partially explain the augmented L-DOPA dependent motor response in presence of IVM. IVM did not influence motor behavior independently, suggesting that activation of P2X4Rs alone is not sufficient enough to cause disinhibiton of SNR to induce such a response. Unlike D1Rs that are present exclusively on postsynaptic MSNs, P2X4Rs are present both on presynaptic DA neurons and postsynaptic MSNs. The simultaneous activation of P2X4Rs at the presynapses and postsynapses would counteract each other, thus preventing the mice from turning to either side. The lack of effect with IVM alone supports the notion that IVM has a modulatory effect on L-DOPA's motor behavior. In addition to increased behavioral response in WT mice, IVM also enhanced L-DOPA's motor behavior in P2X4R KO mice. However, while comparing the absolute increase in L-DOPA's motor behavior in presence of IVM, we saw a significant interaction between time and genotype, suggesting an altered effect of IVM between WT and P2X4R KO mice. This finding supports our previous finding that IVM mediates its behavioral effects partially via action on P2X4Rs (Wyatt et al. 2014, Bortolato et al. 2013). Nevertheless, the increase in L-DOPA response in P2X4R KO mice suggests that IVM may be modulating L-DOPA response through a complex network of receptor systems. Overall, the findings suggest that P2X4Rs have a synergistic role in DA modulation of motor control and can alter behavioral responses to dopaminergic drugs. As such, P2X4R allosteric modulators may represent potential adjuvant pharmacotherapies for Parkinson's disease.
In conclusion, the present investigation supports the hypothesis that there are signaling interactions between P2X4Rs and DA neurotransmission in regulation of multiple CNS functions in the basal ganglia. Finally, though at nascent stage, these findings implicate P2X4Rs in neurobiological mechanisms of multiple neurological disorders. There is growing interest in P2XRs as novel drug targets for therapeutic development in psychiatry disorders (Ortiz et al. 2015). In support of this hypothesis, others report that the gene for P2X4Rs is located in chromosome 12q24 (Gu et al. 2010) which contains several loci that can alter susceptibility to schizophrenia, bipolar disorder and attention deficit hyperactivity (Dawson et al. 1995, Jones et al. 2002, Bailer et al. 2000). Non-synonymous single nucleotide polymorphisms in p2rx4 gene have been linked to high pulse pressure and age related macular degeneration (Caseley et al. 2014) but further investigation is needed before definite conclusions can be drawn regarding P2X4Rs and psychiatric diseases.
Supplementary Material
Figure 6.
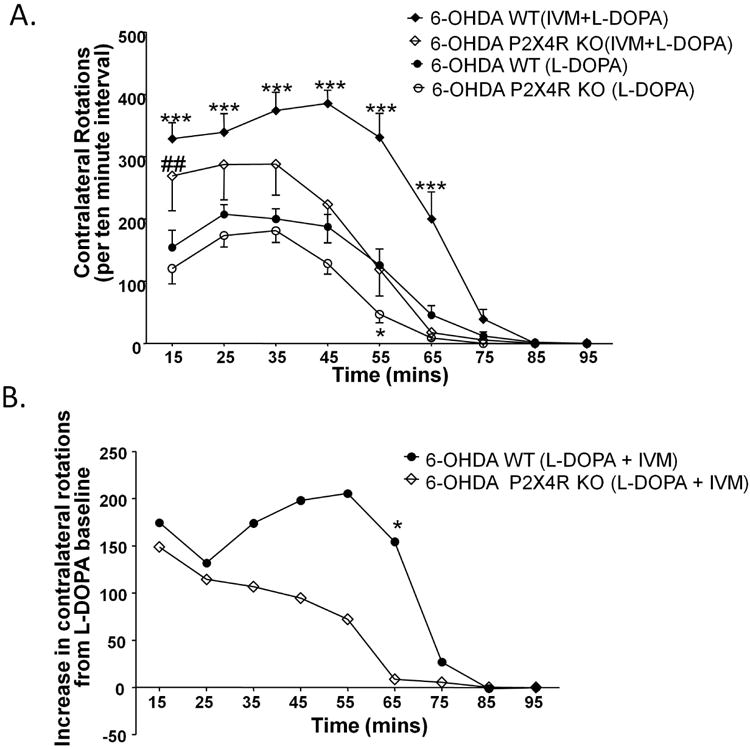
L-DOPA (5 mg/kg) induced rotational behavior is significantly attenuated in P2X4R KO mice. IVM (5 mg/kg) significantly potentiated L-DOPA's effect on the number of contralateral turns in WT and P2X4R KO mice (A). IVM' ability to enhance L-DOPA induced motor behavior was significantly altered in P2X4R KO mice (B). Values on the y-axis represent the mean of number of contralateral turns per 10 minute interval ± SEM from 14 WT, 8 P2X4R KO. *P<0.05, *** P <0.001 versus L-DOPA treated WT mice, ## P<0.01 versus L-DOPA treated P2X4R KO mice for (A), *P<0.05 versus WT mice for (B), Bonferroni post hoc test.
Acknowledgments
We would like to thank Nhat Huynh, Jamie Thuy for their technical assistance. This work was conducted in partial fulfillment for PhD degree in Molecular Pharmacology and Toxicology, University of Southern California (S.K.). This work was supported by National Institute of Alcohol Abuse and Alcoholism (NIAAA) Grant R01 AA022448 (D.L.D) and USC School of Pharmacy. D.L.D. and L.A. are inventors on a patent for the use of IVM for treatment of alcohol use disorders.
List of abbreviations
- P2X4Rs
P2X4 receptors
- P2X4 KO
P2X4 knockout
- DA
dopamine
- DAT
dopamine transporter
- D1Rs
dopamine D1 receptors
- D2Rs
dopamine D2 receptors
- DARPP-32
dopamine and cyclic AMP regulated phosphoprotein of 32kDa
- GPCRs
G-coupled protein receptors
- TH
tyrosine hydroxylase
- ERK 1/2
extracellular regulated kinase1/2
- CREB
cyclic-AMP response element binding protein
- PP-1
protein phosphatase-1
- PKA
protein kinase A
- 6-OHDA
6-Hydroxydopamine
- SNR
substantia nigra pars reticulata
- MSNs
medium spiny neurons
- PPI
prepulse inhibition
- WT
wildtype
- L-DOPA
levodopa
- IVM
ivermectin
Footnotes
Conflict of Interest Disclosure: Authors have no other conflict of interest to declare.
References
- Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of bromocriptine and haloperidol on prepulse inhibition of the acoustic startle response in man. J Psychopharmacol. 1998;12:239–245. doi: 10.1177/026988119801200302. [DOI] [PubMed] [Google Scholar]
- Amadio S, Montilli C, Picconi B, Calabrei P, Volont C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries M, Van Damme P, Robberecht W, Van Den Bosch L. Ivermectin inhibits AMPA receptor-mediated excitotoxicity in cultured motor neurons and extends the life span of a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;25:8–16. doi: 10.1016/j.nbd.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. J Pharmacol Exp Ther. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer U, Leisch F, Meszaros K, et al. Genome scan for susceptibility loci for schizophrenia. Neuropsychobiology. 2000;42:175–182. doi: 10.1159/000026690. [DOI] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34:213–220. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–863. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Yardley M, Khoja S, Godar SC, Asatryan L, Finn DA, Alkana RL, Louie SG, Davies DL. Pharmacological insights into the role of P2X4 receptors in behavioral regulation: lessons from ivermectin. Int J Neuropsychopharmacol. 2013;16:1059–1070. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Brunswick DJ, Amsterdam JD, Mozley PD, Newberg A. Greater availability of brain dopamine transporters in major depression shown by [99m Tc]TRODAT-1 SPECT imaging. Am J Psychiatry. 2003;160:1836–1841. doi: 10.1176/appi.ajp.160.10.1836. [DOI] [PubMed] [Google Scholar]
- Caseley EA, Muench SP, Roger S, Mao HJ, Baldwin SA, Jiang LH. Non-synonymous single nucleotide polymorphisms in the P2X receptor genes: association with diseases, impact on receptor functions and potential use as diagnosis biomarkers. Int J Mol Sci. 2014;15:13344–13371. doi: 10.3390/ijms150813344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Dawson E, Parfitt E, Roberts Q, et al. Linkage studies of bipolar disorder in the region of the Darier's disease gene on chromosome 12q23-24.1. Am J Med Genet. 1995;60:94–102. doi: 10.1002/ajmg.1320600203. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid A receptor. J Pharmacol Exp Ther. 2000;295:1051–1060. [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–2656. doi: 10.1038/sj.npp.1301657. [DOI] [PubMed] [Google Scholar]
- Du X, Elberger AJ, Matthews DB, Hamre KM. Heterozygous deletion of NR1 subunit of the NMDA receptor alters ethanol-related behaviors and regional expression of NR2 subunits in the brain. Neurotoxicol Teratol. 2012;34:177–186. doi: 10.1016/j.ntt.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Eells JB. The control of dopamine neuron development, function and survival: insights from transgenic mice and the relevance to human disease. Curr Med Chem. 2003;10:857–870. doi: 10.2174/0929867033457700. [DOI] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. J Psychiatr Res. 2009;43:484–489. doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. 5-HT(2C) receptor antagonists enhance the behavioural response to dopamine D(1) receptor agonists in the 6-hydroxydopamine-lesioned rat. Eur J Pharmacol. 2000;398:59–64. doi: 10.1016/s0014-2999(00)00238-7. [DOI] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; 2007. [Google Scholar]
- Gendreau PL, Petitto JM, Petrova A, Gariepy J, Lewis MH. D(3) and D(2) dopamine receptor agonists differentially modulate isolation-induced social-emotional reactivity in mice. Behav Brain Res. 2000;114:107–117. doi: 10.1016/s0166-4328(00)00193-5. [DOI] [PubMed] [Google Scholar]
- Ghisi V, Ramsey AJ, Masri B, Gainetdinov RR, Caron MG, Salahpour A. Reduced D2-mediated signaling activity and trans-synaptic upregulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal. 2009;21:87–94. doi: 10.1016/j.cellsig.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA. Integrating neurotransmission in striatal medium spiny neurons. Adv Exp Med Biol. 2012;970:407–429. doi: 10.1007/978-3-7091-0932-8_18. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Sun C, Valova VA, Skarratt KK, Wiley JS. Identification of the promoter region of the P2RX4 gene. Mol Biol Rep. 2010;37:3369–3376. doi: 10.1007/s11033-009-9924-5. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vilkman H, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2002;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychol Bull. 1983;94:3–17. [PubMed] [Google Scholar]
- Jelinkova I, Vavra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemkova H. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun. 2006;349:619–625. doi: 10.1016/j.bbrc.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Bio Chem. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I, Jacobsen N, Green EK, Elvidge GP, Owen MJ, Craddock N. Evidence for familial cosegregation of major affective disorder and genetic markers flanking the gene for Darier's disease. Mol Psychiatry. 2002;7:424–427. doi: 10.1038/sj.mp.4000989. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neuroscience. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J Pharmacol Exp Ther. 2005;315:256–264. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: A positive allosteric effector of the alpha 7 meuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krugel U, Kittner H, Franke H, Illes P. Stimulation of P2 receptors in the ventral tegmental area enhances dopaminergic mechanisms in vivo. Neuropharmacology. 2001;40:1084–1093. doi: 10.1016/s0028-3908(01)00033-8. [DOI] [PubMed] [Google Scholar]
- Krügel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–142. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- Kumari V, Mulligan OF, Cotter PA, Poon L, Toone BK, Checkley SA, Gray JA. Effects of single oral administrations of haloperidol and d-amphetamine on prepulse inhibition of the acoustic startle reflex in healthy male volunteers. Behav Pharmacol. 1998;9:567–576. doi: 10.1097/00008877-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Le KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Séguéla P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Niwa M, Jaaro-Peled H, Fletcher PJ, Seeman P, Sawa A, Roder JC. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav. 2010;9:777–789. doi: 10.1111/j.1601-183X.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Ulrich H, Zarate CA, Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:117–131. doi: 10.1016/j.pnpbp.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52:471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- Perez V, Catafau AM, Corripio I, Martin JC, Alvarez E. Preliminary evidence of striatal D2 receptor density as a possible biological marker of prognosis in naive schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:767–770. doi: 10.1016/s0278-5846(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Popova M, Trudell J, Li K, Alkana R, Davies D, Asatryan L. Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic Signal. 2013 doi: 10.1007/s11302-013-9373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of Ivermectin Facilitation of Human P2X4 Receptor Channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Seeman P. Are dopamine D2 receptors out of control in psychosis? Prog Neuropsychopharmacol Biol Psychiatry. 2013a;46:146–152. doi: 10.1016/j.pnpbp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Seeman P. Schizophrenia and dopamine receptors. Eur Neuropsychopharmacol. 2013b;23:999–1009. doi: 10.1016/j.euroneuro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neuroscience. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J Pharmacol Exp Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Volter C, Riedel M, Wostmann N, et al. Sensorimotor gating and D2 receptor signalling: evidence from a molecular genetic approach. Intl J Neuropsychopharmacol. 2012;15:1427–1440. doi: 10.1017/S1461145711001787. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, Davies DL. Contribution of P2X4 Receptors to Ethanol Intake in Male C57BL/6 Mice. Neurochem Res. 2014;39:1127–1139. doi: 10.1007/s11064-014-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, Bortolato M, Davies DL. Sociocommunicative and Sensorimotor Impairments in Male P2X4-Deficient Mice. Neuropsychopharmacology. 2013;38:1993–2002. doi: 10.1038/npp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Cassin JJ, Healy B, Burdett TC, Chen JF, Fredholm BB, Schwarzschild MA. Deletion of adenosine A(1) or A((2)A) receptors reduces L-3,4-dihydroxyphenylalanine-induced dyskinesia in a model of Parkinson's disease. Brain Res. 2011;1367:310–318. doi: 10.1016/j.brainres.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley M, Wyatt L, Khoja S, et al. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Kerr JR, Lafuente MJ, Maclean A, Chibalina MV, Liu B, Burke B, Bevan S, Nasir J. Altered expression and coregulation of dopamine signalling genes in schizophrenia and bipolar disorder. Neuropathol Appl Neurobiol. 2011;37:206–219. doi: 10.1111/j.1365-2990.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Zhang JK, Lumeng L, Li TK. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


