Abstract
Objective
Pro-inflammatory cytokines have been implicated in the pathophysiology and maintenance of depression. This study investigated the effects of a brief mindfulness intervention on salivary pro-inflammatory correlates of depression (IL-6, TNF-α) and self-reported symptoms of depression in college women.
Methods
Sixty-four females with a cut score of ≥ 16 on the Center for Epidemiological Studies for Depression Scale (CES-D) were assigned to a 4-week mindfulness-based intervention (MBI; N=31) or a contact-control group (N=33). For both groups, salivary cytokines and depressive symptoms were assessed at baseline and post-treatment. For the mindfulness group only, salivary cytokines were also assessed at a 3-month follow-up.
Results
Both groups showed similar reductions in depression. However, MBI (vs. control) predicted greater reductions in IL-6 and TNF-α; changes in IL-6 were sustained at 3-month follow-up. Higher baseline depressive symptoms predicted greater reductions in inflammation in the mindfulness group.
Conclusion
MBIs may reduce inflammatory immune markers commonly implicated in depression. Individuals with greater depressive symptoms may benefit more from mindfulness training. Although reductions in salivary cytokines in the mindfulness condition were not attributable to changes in depressive symptoms, future work should examine the possibility that such reductions are protective against the development of future depressive episodes.
Public Health Significance
Pro-inflammatory cytokines, which have been implicated in the pathophysiology of depression, may be reduced following brief mindfulness training among healthy young women with depressive symptomatology, particularly for those with higher baseline symptoms. These findings may highlight an important treatment target for reducing risk of depressive disorders via mindfulness-based interventions.
Keywords: Mindfulness, Cytokines, Inflammation, Depression, Intervention
Depression is a common and serious psychiatric disorder associated with negative psychosocial and physical outcomes (American Psychiatric Association, 2013; Evans et al., 2005). A majority of adults meeting criteria for major depression experienced symptom onset in childhood, adolescence, or young adulthood (Kessler, Chiu, Demler, Merikangas, & Walters, 2005); even mild depressive symptoms in adolescence predict heightened risk for major depression and suicide later in life (Fergusson, Horwood, Ridder, & Beautrais, 2005). Prevention efforts in women may be particularly important, since women in the United States are 70% more likely than men to experience lifetime depression (Kessler et al., 2003). Therefore, reduction of major depression and suicide risk requires the development of prevention and intervention strategies targeting early depressive symptoms in female children, adolescents, and young adults.
Dysregulation of inflammatory immune responses, which are implicated in the pathophysiology and maintenance of depression, represents a promising target for the prevention of depression. Immune profiles characterized by elevated pro-inflammatory cytokines (e.g., interleukin-6 or IL-6, tumor necrosis factor α or TNF-α) promote “sickness behavior”, a set of vegetative, somatic, and psychological responses that mimic depression (Dantzer, O'Connor, Freund, Johnson, & Kelley, 2008; Raison, Capuron, & Miller, 2006). In line with this evidence, low-grade baseline inflammation has been shown to prospectively predict onset and persistence of depression across the lifespan (Zalli, Jovanova, Hoogendijk, Tiemeier, & Carvalho, 2015; Wichers et al., 2006). Sickness behaviors such as social withdrawal and psychomotor slowing are thought to have evolved to conserve and redirect energy to the immune system in order to effectively fight infection. In spite of this evolutionary hypothesis, the link between pro-inflammatory cytokines and depressive behaviors appears bidirectional; inflammation promotes depressive behavior, and depressive behavior enhances inflammation (Dantzer, 2012; Howren, Lamkin, & Suls, 2009). A recent meta-analysis suggests that these associations are particularly robust for IL-6 and TNF-α (Dowlati et al., 2010). Therefore, prevention and intervention programs that target psychosocial, behavioral, and immunological characteristics of depression may be particularly promising.
Mindfulness based interventions (MBIs) have demonstrated the capacity to improve both psychosocial and immunological aspects of depression. Mindfulness training promotes nonjudgmental, non-attached awareness of experiences in the present moment (Baer, 2003). Cultivation of such states enhances attentional regulation, disengagement from rumination, and tolerance of aversive experiences (Baer, 2003). MBIs have gained empirical support for the treatment of diverse disorders (Keng, Smoski, & Robins, 2011), including reduced symptoms in acutely depressed individuals and the prevention of depressive relapse among those at-risk (Chiesa & Serretti, 2011; Strauss, Cavanagh, Oliver, & Pettman, 2014). Additionally, several studies show that MBIs reduce depression and other psychological symptoms among children, adolescents, and young adults (Caldwell, Harrison, Adams, Quin, & Greeson; Deckro et al., 2002; Greeson, Juberg, Maytan, James, & Rogers, 2014; Semple, Lee, Rosa, & Miller, 2010). Finally, recent evidence suggests that MBIs may be of particular benefit to individuals with greater baseline depressive symptom severity (Arch & Ayers, 2013; Greeson et al., 2015). Though mechanisms of this association are still being actively investigated, it is hypothesized that MBIs promote the development of skills that target cognitive-emotional processes relevant to chronic or severe depression (e.g., rumination), which may mitigate the physiological stress reactivity that promotes chronic inflammation (e.g., Creswell & Lindsay, 2014).
In addition to their psychosocial effects, MBIs have been shown to alter pro-inflammatory immune profiles, including reduced inflammatory cytokines and c-reactive protein (Carlson, Speca, Faris, & Patel, 2007; Carlson, Speca, Patel, & Goodey, 2003; Malarkey, Jarjoura, & Klatt, 2013; Witek-Janusek et al., 2008) and down-regulated expression of inflammation-related genes (Creswell et al., 2012). However, existing studies have largely examined populations with chronic disease (e.g., cancer), making it difficult to assess whether changes in immune function are a result of improvement in illness, psychological health, or both. Therefore, it is unknown whether findings are generalizable to younger and otherwise healthy individuals with depressive symptoms. Finally, many studies have failed to include control conditions or investigate the long-term benefits of MBIs on inflammatory function.
The current study evaluated whether a brief MBI reduces pro-inflammatory salivary cytokines in young women with depressive symptomatology. Healthy women with mild to moderate self-reported depressive symptoms were assigned to either the MBI or a contact-control condition. Participants completed three assessments: (a) before treatment initiation (baseline), (b) immediately following treatment (6-weeks post-MBI), and (c) 3 months after treatment (MBI only). Depressive symptoms and cytokines (IL-6, TNF-α) were assessed at each timepoint. There were three hypotheses:
-
1)
Participation in a brief MBI, compared to contact-control condition, would reduce both depressive symptoms and inflammation at immediate post-intervention assessment, and that these changes would be sustained at a 3-month follow-up in the mindfulness group.
-
2)
Given recent evidence suggesting that greater baseline depression severity predicts a stronger beneficial effect of MBIs on psychological symptoms (e.g., Greeson et al., 2015), we predicted that individuals with greater baseline depressive symptoms would show greater reductions in self-reported depression and inflammation (controlling for one's baseline levels of depression and inflammation).
-
3)
The impact of mindfulness training on inflammation would be mediated by changes in depressive symptoms.
Methods
CONSORT Guidelines
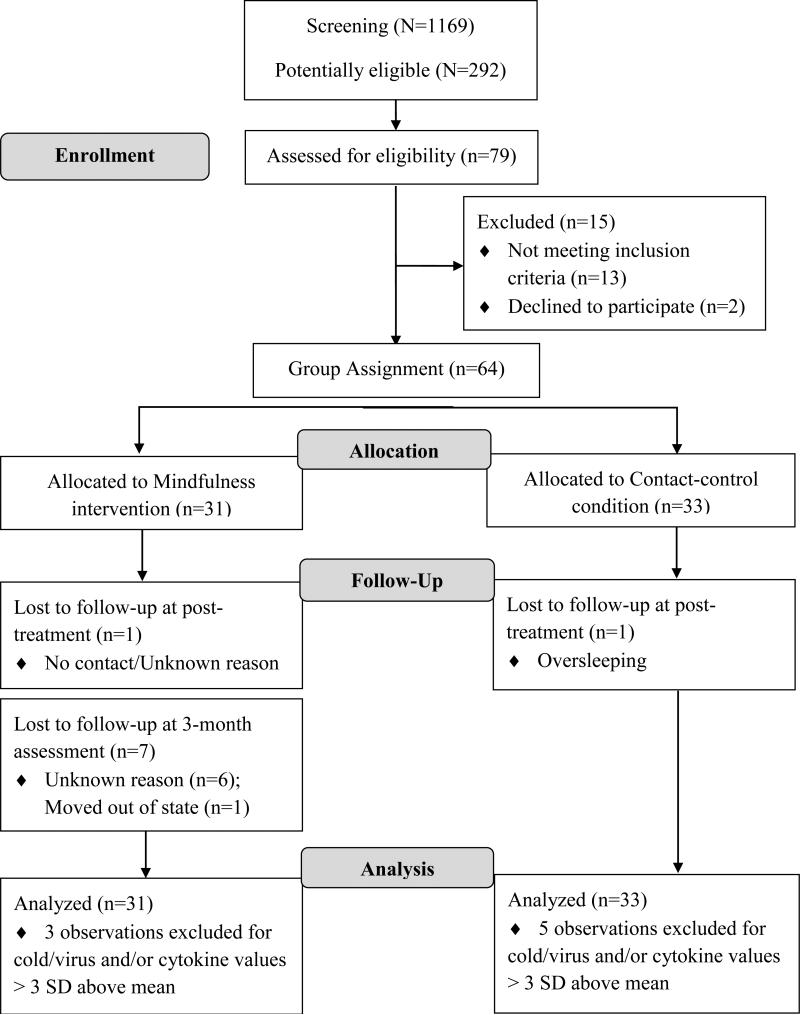
Information on participant flow is presented in the Results section (based on consolidated standards of reporting trials [CONSORT] recommendations) and can be viewed in Figure 1.
Figure 1.
Flowchart for participant progress (based on the consolidated standards of reporting trials [CONSORT] recommendations)
Participant Recruitment and Allocation
Screening Process
Students in introductory psychology courses were pre-screened for depressive symptoms using the Center for Epidemiological Depression Scale (CES-D; Radloff, 1977). Women endorsing mild to moderate depressive symptoms (scores ranging from 16-24) were invited to complete a phone screening. Eligibility criteria included: (a) endorsement of depressive symptoms based upon the CES-D, (b) ages 18-25 years, (c) female, (d) absence of active suicidal ideation, (d) absence of specific psychiatric disturbance (i.e., psychosis, mania, PTSD, substance dependence), (e) absence of immune disease or disorder, (f) absence of pregnancy, (g) not currently taking psychotropic agents, statins, beta-blockers, anti-hypertensives, and nonsteroidal anti-inflammatory drugs, (h) willingness to be randomly assigned to either mindfulness or contact-control condition, and (i) fluency in English.
Group Assignment
Due to scheduling constraints (e.g., course conflicts), unrestricted randomization for all participants was not possible. Therefore, group allocation procedures were as follows: a) If a participant could participate in any of the available group times, she was randomized to a group; b) If a participant could only participate in a restricted number of groups, she was randomized to a group that fit within her schedule; and c) If a participant could only participate in one group, she was assigned to that particular group. To further minimize selection bias, the PI and participants were blind to condition until all eligible participants were enrolled. After enrollment was complete, condition (i.e., mindfulness training or contact-control) was randomized for each group. Participants learned their condition status at the baseline visit. Groups (N=8), ranging from 5-16 participants, took place in the early mornings (8am or 9am). The same master's level clinical psychologist served as group leader for both conditions. Participants provided informed consent and procedures were approved by the Institutional Review Board. Multilevel models revealed negligible intraclass correlations (ICCs) between group membership and outcomes within both the MBI condition and contact-control condition (ICCs < .05); therefore, outcome data were treated as independent rather than group-nested.
Study Procedures
Baseline Assessment
Participants received an overview of the study and completed self-report measures and a saliva sample for assessment of cytokines. Participants were asked to abstain from caffeine, nicotine, exercise, and alcohol/drugs at least 12 hours prior to the session.
Mindfulness Training Sessions
Mindfulness training sessions were completed in weeks 2-5 following baseline assessment. Participants met in groups with the group leader once per week to practice a specific mindfulness exercise. Each session included 35 minutes of mindfulness practice and 15 minutes discussion (e.g., discussion of weekly experience, applicability of practice to daily life). Digital recordings of each mindfulness practice and tracking logs were given to participants to monitor home practice. However, due to the low frequency of at-home practice during the study, this variable was not included in any analyses.
Body Scan (Week 2): The group leader led participants in an exercise asking them to non-judgmentally observe their body. Emphasis was placed on examining the sensations present in each location. Practice is described in Segal, Williams, & Teasdale (2012).
Sitting Meditation (Weeks 3 & 5): The group leader led participants through an exercise asking them to non-judgmentally observe: 1) the sensations of breathing, 2) the sensations present in the body, 3) the content of thoughts, 4) the presence of emotions, and 5) the sounds in the environment. Practice is described in Segal et al. (2012). Because sitting meditation receives considerable attention in MBIs, two sessions were devoted to this exercise.
Yoga (Week 4): The group leader led participants through light stretching exercises described in Kabat-Zinn (1990). Participants were asked to focus nonjudgmentally on the particular sensations associated with bodily movement.
Contact-control Sessions
During weeks 2-5, participants met in groups with the group leader for 50 minutes once per week to complete questionnaires unrelated to the study.
Post-treatment Assessment
Participants completed assessment measures equivalent to those collected during the baseline session. At this assessment, participants in the contact-control groups were invited to take part in a brief mindfulness course the following semester.
3-month Follow-up Assessment (mindfulness training ONLY)
Participants met with the group leader in small groups or individually to complete self-report and salivary assessments. These sessions were held approximately three months following completion of the post-treatment assessment and lasted 45 minutes. Twenty-one participants attended this session and provided complete follow-up data. An additional three participants who moved out of state completed self-report questionnaires only and returned by mail within two weeks of receipt.
Individuals in the contact-control group were not evaluated at the 3-month follow-up. Based upon IRB requirements, participants in the contact-control group were offered the described mindfulness intervention immediately following the post-treatment assessment. Because we could not predict whether participants would engage in treatment at the start of the following semester, and thereby confound results, we chose not to assess them at this time point.
Compensation
Participants received course credit and up to $60 for participation. All participants received course credit and $20 for participation, and a $20 bonus for completing all sessions. MBI participants received an additional $20 for the 3-month follow-up visit.
Psychological Measures
Depressive Symptoms
The Center for Epidemiologic Depression Scale (CES-D) is a widely used 20-item inventory of depressive symptoms (Radloff, 1977). A score of 16 or higher is extensively used as a cut point for significant depressive symptoms (Radloff, 1977). For the current study, scores ranging from 16-24 were used to screen for mild to moderate symptoms of depression (Greden & Schwenk, 1997). The CES-D was also administered at baseline and follow-up assessments to monitor changes in depression severity. CES-D items were scored such that higher total scores were indicative of greater depressive symptoms. Internal consistencies were as follows: Baseline (α = .80), post-treatment (α = .79), 3-month follow-up (α = .65).
Structured Clinical Interview for DSM-IV-TR Axis-I Disorders (SCID-I)
The SCID-I is a semi-structured interview used to diagnose the major Axis I DSM-IV-TR disorders (First, Spitzer, Gibbon, & Williams, 1997). During phone screening, the following modules were used to exclude participants based on the presence of particular psychiatric features or disorders: active suicidality, Substance Use Disorders (current or within one year), Post-Traumatic Stress Disorder (current), and lifetime history of mania and/or psychosis.
Biological Measures
Salivary cytokine samples
IL-6 and TNF-α were measured in whole unstimulated saliva (e.g., Ebersole et al., 2013; Izawa, Miki, Liu, & Ogawa, 2013; Pezelj-Ribaric et al., 2004). Saliva was collected via passive drool method. Participants were asked to abstain from caffeine, tobacco, alcohol, illicit drugs, and exercise for 12 hours prior to saliva sample collection. To collect saliva, participants rinsed their mouths with bottled water for 30 seconds, waited 10 minutes, then filled a tube with 2-5ml of their saliva (Sarstedt AG & CO). Saliva was initially stored for 1-4 weeks at −25°C before being transferred to −80°C before assay.
Immunoassays were performed according to established protocols for salivary measurement of IL-6 and TNF-α (e.g., Ebersole et al., 2013). Salivary concentrations of IL-6 and TNF-α were measured according to manufacturer specifications using the High Sensitivity Human Cytokine Millipore™ MILLIPLEX® MAP kit (Millipore, Billerica, MA). Assays were performed in duplicate to maximize reliability. Intra- and inter-assay coefficients of variance were within respective specifications (IL-6: 3.51% and 4.48%; TNF-α: 3.49% and 3.78%).
Potential Covariates
General health questionnaire
Demographic information (age, race/ethnicity, marital status, grade, religious affiliation), history of psychotherapy and mindfulness practice were collected at the baseline assessment. At all assessments, participants reported on health-related information: height and weight (to calculate BMI); health history (e.g., current illness, allergies); oral health (e.g., gingivitis, mouth sores); medications (e.g., hormonal contraception use); menstrual cycle day; caffeine, alcohol, and drug use; sleep; and physical activity. These variables were assessed as potential controls in cytokine analyses. The only a priori covariate was hormonal contraception use. For the remaining variables, only those that significantly correlated with salivary cytokines were included as covariates in analyses (see Segerstrom, 2009).
Data Screening
None of our dependent variables met the assumption of normality (i.e., the skewness for a given dependent variable was more than three times the standard error of its skewness). In the case of the CES-D, a linear square root transformation resulted in a normal distribution. IL-6 and TNF-α, however, could not be corrected to normality using linear transformations. Further inspection of the cytokine variables suggested that they could be best characterized by either an overdispersed Poisson or negative binomial distribution; comparison of model fit for regressions described in the following paragraph revealed that an overdispersed Poisson regression provided the best model fit when examining cytokine outcomes (Gardner, Mulvey, & Shaw, 1995). Between-person covariates (e.g., BMI) were grand-mean centered for use as predictors.
Statistical Analyses
Because some visits were missing at random in the 6-week follow-up (1 participant in Contact Control, 1 participant in MBI) and at 3-month follow-up in the MBI condition (7 participants), missing values were imputed using multiple imputation in SAS PROC MI. To test the prediction that mindfulness training would predict greater reductions in depressive symptoms, regression models were fit in SAS PROC REG with baseline depressive symptoms in step 1, and condition (MBI = 1, Contact-control = 0) in step 2. To test the prediction that mindfulness training would predict greater reductions in cytokines, overdispersed Poisson regression models were fit in SAS PROC GENMOD with baseline levels of the relevant cytokine in step 1, and condition in step 2 (Gardner et al., 1995). Where significant effects of condition were not found, paired sample t-tests (standard paired samples t-tests for depression, Wilcoxon signed-rank tests for cytokines) were carried out to determine if the outcome variable changed pre-post intervention in the whole sample. All analyses were carried out in 20 datasets imputed using SAS PROC MI; results were combined using SAS PROC MIANALYZE.
To test the prediction that improvements in cytokine levels and depressive symptoms would remain significant at 3-month follow-up for those in the mindfulness intervention, paired samples t-tests (standard paired samples t-tests for depressive symptoms, Wilcoxon signed-rank tests for cytokines) tested for differences between baseline and 3-month follow-up within the mindfulness condition. Finally, we also tested the hypothesis that baseline depressive symptoms as measured using the CES-D Total Score would moderate the impact of condition on treatment outcomes at time 2 by testing overdispersed Poisson regression models predicting time 2 outcomes from baseline depressive symptoms, baseline levels of the outcome, condition, and the interaction of condition and baseline depressive symptoms.
Results
Participant Flow
Figure 1 provides information on accrual and retention of study participants. Out of 1,169 female students screened, 292 met the cut score criterion for mild to moderate depressive symptoms (~25% of the pool) and were invited to participate in the study. 79 students were assessed for eligibility, of which 64 met eligibility criteria and were assigned to a mindfulness training group (n=31) or a contact-control group (n=33). At the post-treatment assessment, 62 participants completed the study (MBI=30; contact-control=32). Twenty-four participants in the mindfulness group completed the 3-month follow-up assessment (retention=77%). Participants who completed the 3-month follow-up were similar to those who did not complete this visit with respect to baseline depressive symptoms and demographic variables (all p's > .10).
Baseline Data
Demographic information by group can be found in Table 1. There were no significant differences in age, race/ethnicity, current grade, or religious affiliation between the two conditions (age: t(62) = −.18, p = .86; race: X2(2) = 2.19, p = .33, current grade: X2(2) = 1.44, p = .49, religious affiliation: X2(2) = 1.24, p = .74). No significant associations were found between demographic variables and baseline IL-6 or TNF-α. Descriptives for outcome variables at baseline and post-treatment for the full sample and by condition can be found in Table 2.
Table 1.
Descriptives by Condition (N = 64)
| Variable | Mindfulness Condition (n = 31) | Contact-Control Condition (n = 33) |
|---|---|---|
| Age: M (SD) | 19.15 (.17) | 19.11 (.16) |
| Body Mass Index (BMI) | 22.76 (3.03) | 22.22 (2.72) |
| Grade Level: N (%) | ||
| Freshman | 22 (70%) | 27 (82%) |
| Sophomore | 6 (20%) | 3 (9%) |
| Junior | 3 (10%) | 3 (9%) |
| Race: N (%) | ||
| Caucasian | 25 (81%) | 30 (91%) |
| African-American | 4 (13%) | 1 (3%) |
| Asian | 2 (6%) | 2 (6% |
| Religious Affiliation: N (%) | ||
| Christian | 26 (84%) | 25 (76%) |
| Hindu | 1 (4%) | 1 (3%) |
| No Religious Affiliation | 2 (6%) | 5 (15%) |
| No Answer | 2 (6%) | 2 (6%) |
Note. Standard Deviations and Within-group Percentages in Parentheses.
Table 2.
Means and Standard Deviations for Outcome Variables at Baseline and Post-treatment by Condition
| Variable | Full Sample (N = 64) |
Mindfulness Condition (n = 31) |
Contact-Control Condition (n = 33) |
||||
|---|---|---|---|---|---|---|---|
| PRE M (SD) | POST M (SD) | PRE M (SD) | POST M (SD) | 3-month M (SD) | PRE M (SD) | POST M (SD) | |
| IL-6 (pg/ml) | 3.58 (4.55) | 3.14 (5.36) | 2.73a (3.01) | 2.20b (2.23) | 1.75b (2.25) | 3.66 (4.01) | 2.95 (4.19) |
| TNF-α (pg/ml) | 2.21 (2.95) | 1.64 (1.68) | 1.52a (1.66) | 1.22b (1.21) | 1.43 (1.53) | 2.29 (1.93) | 1.93 (2.29) |
| CES-D total | 12.53a (6.25) | 9.44b (5.64) | 13.26a (5.67) | 10.30b (5.89) | 8.54b (3.90) | 11.85a (6.76) | 8.63b (5.36) |
Note. Differing superscripts indicate significant differences (as indicated by paired samples t-tests) between baseline and post-treatment values (p < .05).
Treatment Effects
Condition Effects on Salivary Cytokines and Depression
Hormonal contraceptive (HC) use was controlled for a priori. Upon inspection of correlations between IL-6, TNF-α, and variables measured in the general health questionnaire, only one significant correlation emerged: baseline BMI was correlated with TNF-α at baseline (r(60) = .25, p < .0001) and at post-treatment (r(59) = .35, p < .0001). Of note, correlations between BMI and IL-6 measures were not significant at baseline (r(60) = .21, p = .19) or at post-treatment (r(60) = .15, p = .20). Therefore, HC use was controlled in the model predicting IL-6, and both HC use and BMI (standardized) were controlled for in the model predicting TNF-α.
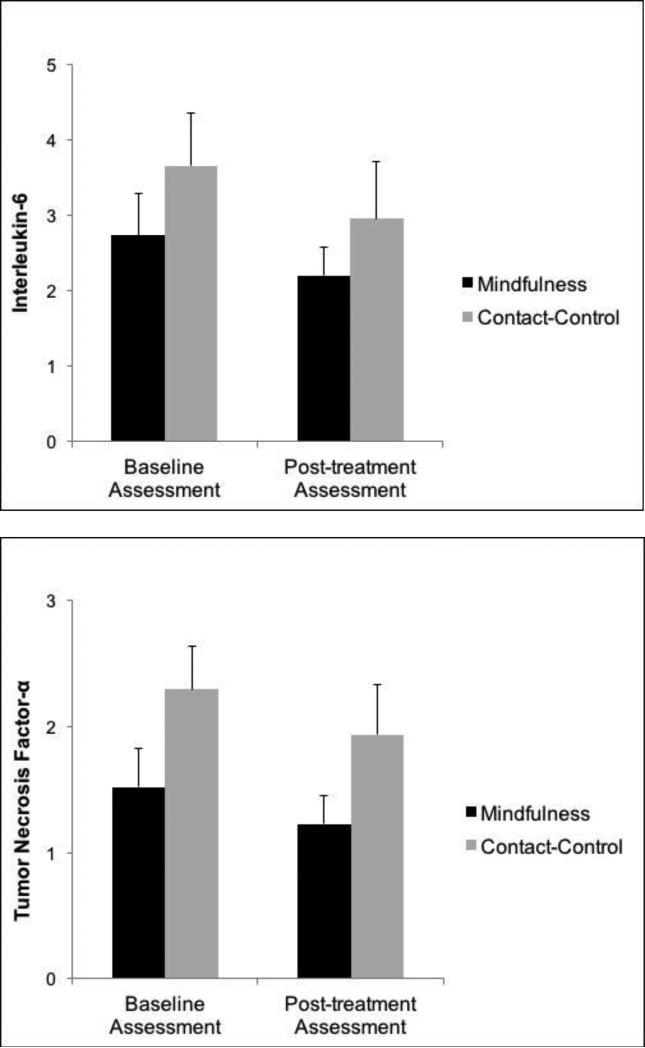
Controlling for baseline IL-6, mindfulness training predicted lower IL-6 at post-treatment (BCONDITION = −.57, SE = .16, 95% CI: −.91 to −.25, p = .0006; ExpB = .54, 95% CI: .38 to .75); see Figure 2). Likewise, controlling for baseline TNF-α, mindfulness training predicted lower TNF-α post-treatment (BCONDITION = −.52, SE = .21, 95% CI: −.94 to −.10, p = .013; ExpB = .69, 95% CI: .49 to .96; see Figure 2). In contrast, controlling for baseline depression, participation in mindfulness training did not predict depression at post-treatment (CES-D: BCONDITION = 1.06, SE = 1.22, 95% CI: .67 to 1.56, p = .39). However, depression decreased from baseline to post-treatment regardless of condition (CES-D: Mean Difference: −3.10, 95% CI = −4.59 to −1.61, t = − 4.08, p < .0001; standardized B = −.16; 95% CI: −.08 to −.23)1.
Figure 2.
Mean levels of cytokines at baseline and post-treatment assessment by condition, illustrating condition effect on change in cytokines pre- to post-treatment.
Longitudinal Outcomes within the Mindfulness Training Condition
Additional within-group analyses tested whether changes within the mindfulness condition were significant from pre- to post-treatment and whether they persisted at 3-month follow-up. Within the mindfulness condition, IL-6 decreased significantly from baseline at both post-treatment and 3-month follow-up (Mean Difference from baseline to post-treatment = −.58, 95% CI = −.27 to −.98; z = −45.6, p = .012 ; d = −.38; Mean Difference from baseline to 3-month follow-up = −.75, 95% CI = −.45 to −1.87, t = −1.98, p = .045; d = −.29; z = −48.5, p = .022; d = −.29). Although TNF-α decreased significantly from baseline to post-treatment, the decrease from baseline to the 3-month follow-up assessment was not significant (Mean Difference from baseline to post-treatment = −.53, 95% CI = −1.05 to −.41, z = −49.3, p = .01; d = −.49; Mean Difference from baseline to 3-month follow-up = −.45, 95% CI = −1.48 to .72, z = −39.2, p = .10; d = −.25). CES-D decreased significantly from baseline to post-treatment (Mean Difference from baseline to post-treatment = 2−2.71, 95% CI = −3.46 to −1.83, t = −2.35, p = .018; d = −.48) and from baseline to the 3-month follow-up (Mean Difference from baseline to 3-month follow-up = −2.76, 95% CI = −5.19 to −.32, t = −2.25, p = .026; d = −.45).
Baseline Depression as a Moderator of Condition Effects on Depression and Inflammation
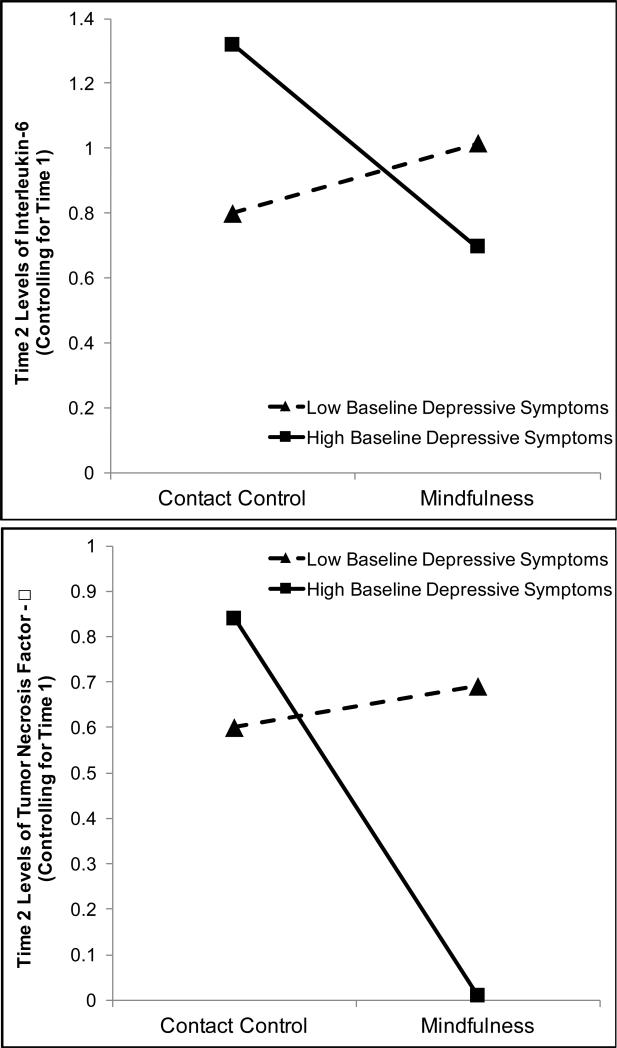
Next, we tested the hypothesis that those with higher depressive symptoms at time 1 (i.e., higher scores on the CES-D) would benefit more from mindfulness training; see Table 3 for model information. Contrary to prediction, the interaction of baseline CES-D and condition did not predict post-treatment depressive symptoms over and above baseline scores (BCONDITION*BLDEP = 1.34, SE = 1.31, 95% CI: −1.81 to 2.34, p = .16). Consistent with predictions, baseline depressive symptoms interacted with condition to predict post-treatment IL-6 (standardized BCONDITION*BLDEP = −.42, SE = .16, 95% CI: −.73 to −.10, p = .009; see Figure 3). At 1 standard deviation above the mean of baseline CES-D, mindfulness training predicted lower IL-6 at post-treatment controlling for baseline (BCONDITION = −.95, SE = .26, 95% CI: −1.48 to −.58, p < .0001; ExpB = .37, 95% CI: .21 to .64); however, condition had no effect at 1 standard deviation below the mean of baseline CES-D (BCONDITION = −.06, SE = .36, 95% CI: -.78 to .61, p = .95).
Table 3.
Poisson Regression Estimates Predicting Cytokines at Post-Treatment
| Variable | Estimate | SE | p value | 95% CI for Estimate |
|---|---|---|---|---|
| Interleukin-6 (IL-6) | ||||
| Hormonal Contraceptive Use | −.42 | .20 | .045 | −.83 to −.0084 |
| Baseline IL-6 | .27 | .075 | .0004 | .12 to .42 |
| Baseline Depression | .22 | .12 | .081 | −.03 to .48 |
| MBI Condition | −.46 | .21 | .14 | −.82 to .12 |
| Baseline Depression X MBI Condition | −.42 | .16 | .006 | −.84 to −.12 |
| Tumor Necrosis Factor-α (TNF-α) | ||||
| Hormonal Contraceptive Use | −.73 | .26 | .006 | −1.25 to −.20 |
| Body Mass Index | .046 | .036 | .19 | −.024 to .11 |
| Baseline TNF-α | .37 | .086 | <.0001 | .20 to .54 |
| Baseline Depression | .12 | .13 | .13 | −.15 to .39 |
| MBI Condition | −.45 | .25 | .071 | −.94 to .041 |
| Baseline Depression X MBI Condition | −.54 | .20 | .006 | −.98 to −.091 |
Note. BMI was not significantly associated with IL-6, and inclusion of BMI in the model predicting IL-6 did not affect the results.
Figure 3.
Depiction of the interaction between baseline scores on the CES-D and condition predicting Time 2 cytokines controlling for baseline cytokines.
The result for TNF-α was similar: the model predicting post-treatment TNF-α controlling for baseline revealed an interaction of baseline depressive symptoms and condition (BCONDITION*BLDEP = −.44, SE = .21, 95% CI: −.85 to −.026, p = .037; see Figure 3). Again, the effect of mindfulness training was significant at 1 standard deviation above the mean of baseline CES-D (BCONDITION = −1.04, SE = .38, 95% CI: −1.81 to −.31, p = .005; ExpB = .31, 95% CI: .20 to .51), but was not significant at 1 standard deviation below the mean of baseline CES-D (BCONDITION = .03, SE = .48, 95% CI: −.76 to .83, p = .85). Thus, women with higher baseline depressive symptoms who participated in mindfulness training did not benefit more than their less depressed counterparts in terms of self-reported depression; however, they did appear to show greater reductions in pro-inflammatory salivary cytokines.
Discussion
Summary and Integration with Existing Evidence
The present study examined whether a brief mindfulness-based intervention (MBI) could reduce self-reported depression and associated salivary inflammatory markers in young women with depressive symptomatology. As predicted, mindfulness training predicted greater reductions in pro-inflammatory immune correlates of depression (IL-6, TNF-α) than a contact control group pre- to post-assessment. In the mindfulness condition, reductions in IL-6 were sustained at a 3-month follow-up assessment. Contrary to the prediction, however, there were no significant group differences in self-reported depression changes; both groups showed significant, similar reductions in depressive symptoms pre- to post-assessment.
Results for IL-6 and TNF-α are consistent with other investigations examining the effects of MBIs on inflammatory responses. For example, significant reductions in pro-inflammatory cytokines have been observed in cancer patients in 8-week mindfulness courses (Carlson et al., 2003; Witek-Janusek et al., 2008) and sustained at a one-year follow-up (Carlson et al., 2007). Additionally, Creswell et al. (2012) reported that compared to a wait-list control group, Mindfulness-Based Stress Reduction (MBSR) down-regulated pro-inflammatory gene expression in healthy older adults. Similarly, we observed reductions in pro-inflammatory salivary cytokines pre-post treatment for the mindfulness group only and found that improvements in IL-6 were maintained after 3 months. This investigation extends previous findings by demonstrating similar effects (1) in a young, immunocompetent sample with depressive symptomatology, (2) in response to a brief mindfulness intervention, and (3) includes both a control group and a long-term follow-up assessment.
Mindfulness-Related Cytokine Reductions and Baseline Depressive Symptoms
In the present study, higher baseline depressive symptoms moderated the effect of mindfulness training; women with higher depressive symptoms at baseline showed the strongest cytokine reductions in response to mindfulness training. Furthermore, baseline levels of cytokines were not significantly correlated with baseline depressive symptoms (IL-6: r(59) = .22, p = .09; TNF-α: r(60) = −.053, p = .68), suggesting those with greater depressive symptoms did not merely have higher levels of inflammation at baseline. Although baseline depression did not moderate improvement in self-reported depressive symptoms following MBI, this finding is still consistent with recent literature showing MBIs may be a particularly effective treatment for those with greater baseline depressive symptom severity (Arch & Ayers, 2013; Greeson et al., 2015). While some evidence supports that MBIs are efficacious in reducing inflammatory markers (e.g., Carlson et al., 2007), a comprehensive review on this topic highlights that these effects are not always replicated (Black & Slavich, 2016), suggesting the presence of critical moderators. Baseline depression is predictive of impaired physiological recovery from stress (Burke, Davis, Otte, & Mohr, 2005; Gold, Zakowski, Valdimarsdottir, & Bovbjerg, 2004), which may lead to chronic or excessive inflammation and ultimately increased risk for disease (Slavich & Irwin, 2014). Thus, baseline depression may represent an important individual difference in stress responding and recovery, and serve as a proxy for a variety of other person-level risk factors, such as early life adversity, experiential avoidance, or negative cognitive appraisals, any of which would be expected to respond favorably to mindfulness practice (Barnhofer, Brennan, Crane, Duggan, & Williams, 2014; Brown & Jones, 2010; Williams et al., 2014). Further work testing these other moderators is necessary.
Mechanisms of Mindfulness Training on Inflammation
Compared to a contact control group, mindfulness training predicted greater reductions in salivary inflammatory cytokines. Unexpectedly, depressive symptoms did not mediate this relation. Therefore, it is important to consider other mediators of this treatment effect. Self-conscious affect such as that elicited by social-evaluative threat plays a central role in inflammatory stress responses (e.g., Rohleder, Chen, Wolf, & Miller, 2008), suggesting that feelings of shame or social threat may be a potential mediator. Rumination is another likely candidate given it is a proximal prospective risk factor for onset of depressive episodes (Spasojević & Alloy, 2001) and is associated with sustained inflammatory responses (Zoccola, Figueroa, Rabideau, Woody, & Benencia, 2014). Though not assessed in the current study, self-conscious affect or rumination may be plausible mediators for the effects seen here and should be investigated in future studies. Such mediators may be tied to other person-level risk factors (e.g., baseline depression), thus providing greater insight into not only for whom MBIs may be particularly beneficial, but identifying how MBIs exert salutary health effects.
Treatment Implications
Mindfulness training may influence inflammatory immune responses that contribute to psychological health. The clinical significance of these findings is difficult to quantify given a lack of norms for salivary cytokines in healthy young women with depressive symptomatology. However, the present study provides some normative information in this population that may be used in future work. Baseline cytokines have been found to prospectively predict incidence and persistence of depressive symptoms (Zalli et al., 2015; Vogelzangs et al., 2014), even among individuals without psychiatric disorders (Wichers et al., 2006). The results of the present study suggest that, even if depression scores do not change significantly as a result of treatment, participation in MBIs may be associated with lower prospective physiological risk for depressive episodes. Moreover, behavioral interventions may represent promising alternatives for individuals with greater inflammatory dysregulation, who show a poorer response to antidepressant medications (Vogelzangs et al., 2014). Finally, future work should examine the possibility that MBIs may lower risk of physical disorders with high depression comorbidity (e.g.,cardiovascular disease; Joynt, Whellan, & O'Connor, 2003) by reducing inflammation.
Limitations and Future Directions
The present study has several limitations. First, although participants were recruited for mild to moderate depressive symptoms (scores of 16-24 on CES-D), mean depression scores had fallen to 12.53 (SD: 6.25; range: 2-32) by the first session. This is likely due to some baseline scores being artificially elevated due to life stress. While the standard deviation and range reflect that some participants were still experiencing significant depressive symptoms at this time, others were not. Because this decrease may have limited our ability to find group differences on this measure, higher cut scores (Lawrence et al., 2006) or standardized interviews for depression screening may be necessary. Second, generalizability is limited to young, primarily Caucasian, college females. However, gender bias in depression emerges in mid-to-late adolescence (e.g., ages 14-18) and rates of depression do not differ between students and non-students (Hankin et al., 1998; Rutter, Caspi, & Moffitt, 2003). While findings may not be generalizable to specific clinical populations, they may be generalizable to the population as a whole, particularly during this developmental period of heightened vulnerability to depression. Additional studies are needed to replicate these findings in populations with greater depressive symptomatology.
Third, the study measured cytokines via whole unstimulated saliva rather than blood, and some studies report low correlations between the two (e.g., Riis et al., 2014). Thus, salivary cytokines may reflect oral immune processes rather than systemic inflammation (Izawa, Sugaya, et al., 2013). However, salivary cytokines correlate with psychosocial factors, including depression (Cloak, Alicata, Ernst, & Chang, 2015; Keller, El-Sheikh, Vaughn, & Granger, 2010; Sjögren et al., 2006), and reliably increase during stress (Slavish, Graham-Engeland, Smyth, & Engeland, 2015). The oral-pharyngeal cavity is a route through which most foreign pathogens access the body's internal tissues and cells and is therefore critical in immune surveillance. Local inflammatory signaling from tissues in the mouth may be transmitted to the brain via the trigeminal nerve (Navarro, Iyomasa, Leite-Panissi, Almeida, & Branco, 2006). Salivary cytokines have been shown to correlate with brain activity in regions strongly implicated in depression, suggesting that inflammation within the oral cavity may communicate with the brain and alter motivational state or emotional processing (O'Connor, Irwin, & Wellisch, 2009). Future research should continue to examine associations between salivary inflammation, systemic inflammation, and health (Slavish et al., 2015). Even if salivary cytokines do not reliably correspond to blood-based measures, salivary inflammation may still be strongly predictive of disease and well-being.
Fourth, cytokines in the contact-control group were not assessed at 3-month follow-up due to an ethical obligation to offer treatment to control participants following the post-treatment assessment. Future studies should assess both intervention and control groups at longer-term follow-up assessments to determine the effects of time and dose (Carmody & Baer, 2009). Furthermore, although the contact-control condition accounted for the influence of the passage of time, it did not account for the impact of expectancy, demand, social support, or other nonspecific factors of a group intervention.
Finally, the current study did not offer a full-length, empirically supported mindfulness training program, such MBSR or MBCT. These programs, which typically include 8 weekly 2.5-hour sessions, have significant therapeutic effects (Keng et al., 2011; Chiesa & Serretti, 2011; Piet & Hougaard, 2011). It is possible that components not offered in the current study are necessary for increasing awareness of emotional and cognitive processes and would have differentially impacted depressive symptoms.
Conclusion and Implications
The current study supports the notion that brief mindfulness training can have salutary effects on inflammation among relatively healthy, immunocompetent individuals with depressive symptomatology. Notably, pro-inflammatory cytokines may play a role in the development or persistence of depression (e.g, Vogelzangs et al., 2014; Wichers et al., 2006). Therefore, the finding that mindfulness training is associated with reduced inflammation supports further investigation of the capacity of mindfulness training to prevent onset and relapse of depressive disorders via reduced inflammation. Reducing this risk factor may be a meaningful step towards depression prevention.
Acknowledgments
Sources of Funding. This research was supported by the Mind and Life Institute (2009-1-017), National Institute on Drug Abuse (P50DA05312), National Center for Complementary and Integrative Health (T32AT003378), National Institute of Mental Health (T32MH093315), and the University of Kentucky Clinical Research Development and Operations Center.
Footnotes
Conflicts of Interest. The authors have no conflicts of interest to report.
Because there were no effects of mindfulness training on depression, hypothesized meditational models were not considered. However, several post-hoc analyses ruled out mediation by changes in health-related variables such as oral health, physical activity, and caffeine or alcohol consumption (all p's > .85).
Contributor Information
Erin Walsh, University of North Carolina-Chapel Hill, Department of Physical Medicine & Rehabilitation, Program on Integrative Medicine; University of Kentucky, Department of Psychology.
Tory Eisenlohr-Moul, University of North Carolina-Chapel Hill, Department of Psychiatry; University of Kentucky, Department of Psychology
Ruth Baer, University of Kentucky, Department of Psychology
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Washington, D.C.: 2013. http://doi.org/doi:10.1176/appi.books.9780890425596.744053. [Google Scholar]
- Arch JJ, Ayers CR. Which treatment worked better for whom? Moderators of group cognitive behavioral therapy versus adapted mindfulness based stress reduction for anxiety disorders. Behaviour Research and Therapy. 2013;51(8):434–442. doi: 10.1016/j.brat.2013.04.004. http://doi.org/10.1016/j.brat.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clinical Psychology: Science and Practice. 2003;10(2):125–143. http://doi.org/10.1093/clipsy.bpg015. [Google Scholar]
- Barnhofer T, Brennan K, Crane C, Duggan D, Williams JMG. A comparison of vulnerability factors in patients with persistent and remitting lifetime symptom course of depression. Journal of Affective Disorders. 2014;152-154:155–61. doi: 10.1016/j.jad.2013.09.001. http://doi.org/10.1016/j.jad.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Annals of the New York Academy of Sciences. 2016 doi: 10.1111/nyas.12998. http://doi.org/10.1111/nyas.12998. [DOI] [PMC free article] [PubMed]
- Brown CA, Jones AKP. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150(3):428–38. doi: 10.1016/j.pain.2010.04.017. http://doi.org/10.1016/j.pain.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56. doi: 10.1016/j.psyneuen.2005.02.010. http://doi.org/10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Caldwell K, Harrison M, Adams M, Quin RH, Greeson J. Developing mindfulness in college students through movement-based courses: effects on self-regulatory self-efficacy, mood, stress, and sleep quality. Journal of American College Health : J of ACH. 58(5):433–42. doi: 10.1080/07448480903540481. http://doi.org/10.1080/07448480903540481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity. 2007;21(8):1038–49. doi: 10.1016/j.bbi.2007.04.002. http://doi.org/10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-Based Stress Reduction in Relation to Quality of Life, Mood, Symptoms of Stress, and Immune Parameters in Breast and Prostate Cancer Outpatients. Psychosomatic Medicine. 2003;65(4):571–581. doi: 10.1097/01.psy.0000074003.35911.41. http://doi.org/10.1097/01.PSY.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA. How long does a mindfulness-based stress reduction program need to be? A review of class contact hours and effect sizes for psychological distress. Journal of Clinical Psychology. 2009;65(6):627–38. doi: 10.1002/jclp.20555. http://doi.org/10.1002/jclp.20555. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Research. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. http://doi.org/10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Alicata D, Ernst TM, Chang L. Psychiatric Symptoms, Salivary Cortisol and Cytokine Levels in Young Marijuana Users. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on NeuroImmune Pharmacology. 2015;10(2):380–90. doi: 10.1007/s11481-015-9606-0. http://doi.org/10.1007/s11481-015-9606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JMG, Ma J, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain, Behavior, and Immunity. 2012;26(7):1095–101. doi: 10.1016/j.bbi.2012.07.006. http://doi.org/10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Lindsay EK. How Does Mindfulness Training Affect Health? A Mindfulness Stress Buffering Account. Current Directions in Psychological Science. 2014;23(6):401–407. http://doi.org/10.1177/0963721414547415. [Google Scholar]
- Dantzer R. Depression and inflammation: an intricate relationship. Biological Psychiatry. 2012;71(1):4–5. doi: 10.1016/j.biopsych.2011.10.025. http://doi.org/10.1016/j.biopsych.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews. Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. http://doi.org/10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckro GR, Ballinger KM, Hoyt M, Wilcher M, Dusek J, Myers P, Benson H. The evaluation of a mind/body intervention to reduce psychological distress and perceived stress in college students. Journal of American College Health : J of ACH. 2002;50(6):281–7. doi: 10.1080/07448480209603446. http://doi.org/10.1080/07448480209603446. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. http://doi.org/10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Schuster JL, Stevens J, Dawson D, Kryscio RJ, Lin Y, Miller CS. Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. Journal of Clinical Immunology. 2013;33(1):271–9. doi: 10.1007/s10875-012-9771-3. http://doi.org/10.1007/s10875-012-9771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biological Psychiatry. 2005;58(3):175–89. doi: 10.1016/j.biopsych.2005.05.001. http://doi.org/10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry. 2005;62(1):66–72. doi: 10.1001/archpsyc.62.1.66. http://doi.org/10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). for DSMIV. 1997. et.
- Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychological Bulletin. 1995;118(3):392–404. doi: 10.1037/0033-2909.118.3.392. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7501743. [DOI] [PubMed] [Google Scholar]
- Gold SM, Zakowski SG, Valdimarsdottir HB, Bovbjerg DH. Higher Beck depression scores predict delayed epinephrine recovery after acute psychological stress independent of baseline levels of stress and mood. Biological Psychology. 2004;67(3):261–73. doi: 10.1016/j.biopsycho.2003.12.001. http://doi.org/10.1016/j.biopsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Greden JF, Schwenk TL. Major Mood Disorders. In: Knesper D, Riba M, Schwenk TL, editors. Primary Care Psychiatry. WB Saunders Co.; Philadelphia, PA: 1997. p. 129. [Google Scholar]
- Greeson JM, Juberg MK, Maytan M, James K, Rogers H. A randomized controlled trial of Koru: a mindfulness program for college students and other emerging adults. Journal of American College Health : J of ACH. 2014;62(4):222–33. doi: 10.1080/07448481.2014.887571. http://doi.org/10.1080/07448481.2014.887571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeson JM, Smoski MJ, Suarez EC, Brantley JG, Ekblad AG, Lynch TR, Wolever RQ. Decreased Symptoms of Depression After Mindfulness-Based Stress Reduction: Potential Moderating Effects of Religiosity, Spirituality, Trait Mindfulness, Sex, and Age. Journal of Alternative and Complementary Medicine. 2015;21(3):166–74. doi: 10.1089/acm.2014.0285. http://doi.org/10.1089/acm.2014.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva P. a, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. http://doi.org/10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. http://doi.org/10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain, Behavior, and Immunity. 2013;27(1):38–41. doi: 10.1016/j.bbi.2012.07.001. http://doi.org/10.1016/j.bbi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Kimura K, Ogawa N, Yamada KC, Shirotsuki K, Nomura S. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biological Psychology. 2013;94(2):249–54. doi: 10.1016/j.biopsycho.2013.06.006. http://doi.org/10.1016/j.biopsycho.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biological Psychiatry. 2003;54(3):248–261. doi: 10.1016/s0006-3223(03)00568-7. http://doi.org/10.1016/S0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your mind and body to face stress, pain, and illness. Delacorte; New York: 1990. [Google Scholar]
- Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children's mental health: The role of child sex. Physiology & Behavior. 2010;101(5):705–712. doi: 10.1016/j.physbeh.2010.08.012. http://doi.org/10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng S-L, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review. 2011;31(6):1041–56. doi: 10.1016/j.cpr.2011.04.006. http://doi.org/10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. http://doi.org/10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, McKeown RE. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics. 2006;117(4):1348–58. doi: 10.1542/peds.2005-1398. http://doi.org/10.1542/peds.2005-1398. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain, Behavior, and Immunity. 2013;27(1):145–54. doi: 10.1016/j.bbi.2012.10.009. http://doi.org/10.1016/j.bbi.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VP, Iyomasa MM, Leite-Panissi CRA, Almeida MC, Branco LGS. New role of the trigeminal nerve as a neuronal pathway signaling brain in acute periodontitis: participation of local prostaglandins. Pflügers Archiv : European Journal of Physiology. 2006;453(1):73–82. doi: 10.1007/s00424-006-0113-2. http://doi.org/10.1007/s00424-006-0113-2. [DOI] [PubMed] [Google Scholar]
- O'Connor M-F, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. NeuroImage. 2009;47(3):891–6. doi: 10.1016/j.neuroimage.2009.05.049. http://doi.org/10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezelj-Ribaric S, Prso IB, Abram M, Glazar I, Brumini G, Simunovic-Soskic M. Salivary levels of tumor necrosis factor-alpha in oral lichen planus. Mediators of Inflammation. 2004;13(2):131–3. doi: 10.1080/09629350410001688530. http://doi.org/10.1080/09629350410001688530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clinical Psychology Review. 2011;31(6):1032–40. doi: 10.1016/j.cpr.2011.05.002. http://doi.org/10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. http://doi.org/10.1177/014662167700100306. [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. http://doi.org/10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, Pabst S, Granger DA. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Developmental Psychobiology. 2014;56(4):797–811. doi: 10.1002/dev.21149. http://doi.org/10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Chen E, Wolf JM, Miller GE. The psychobiology of trait shame in young women: extending the social self preservation theory. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 2008;27(5):523–32. doi: 10.1037/0278-6133.27.5.523. http://doi.org/10.1037/0278-6133.27.5.523. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. http://doi.org/10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. Second Edition. Guilford Press: 2012. Retrieved from https://books.google.com/books?hl=en&lr=&id=1_NcsDZ17icC&pgis=1. [Google Scholar]
- Segerstrom SC. Biobehavioral controls: threats to psychoneuroimmunology research? Brain, Behavior, and Immunity. 2009;23(7):885–6. doi: 10.1016/j.bbi.2009.05.053. http://doi.org/10.1016/j.bbi.2009.05.053. [DOI] [PubMed] [Google Scholar]
- Semple RJ, Lee J, Rosa D, Miller LF. A Randomized Trial of Mindfulness-Based Cognitive Therapy for Children: Promoting Mindful Attention to Enhance Social-Emotional Resiliency in Children. Journal of Child and Family Studies. 2010;19(2):218–229. Retrieved from http://eric.ed.gov/?id=EJ924264. [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain, Behavior, and Immunity. 2006;20(3):270–8. doi: 10.1016/j.bbi.2005.08.001. http://doi.org/10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin. 2014;140(3):774–815. doi: 10.1037/a0035302. http://doi.org/10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity. 2015;44C:253–269. doi: 10.1016/j.bbi.2014.08.008. http://doi.org/10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion (Washington, D.C.) 2001;1(1):25–37. doi: 10.1037/1528-3542.1.1.25. http://doi.org/10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Strauss C, Cavanagh K, Oliver A, Pettman D. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: A meta-analysis of randomised controlled trials. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0096110. http://doi.org/10.1371/journal.pone.0096110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, van Reedt Dortland AK, Schoevers RA, Giltay EJ, de Jonge P, Penninx BW. Inflammatory and Metabolic Dysregulation and the 2-Year Course of Depressive Disorders in Antidepressant Users. Neuropsychopharmacology. 2014;39(7):1624–1634. doi: 10.1038/npp.2014.9. http://doi.org/10.1038/npp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biological Psychiatry. 2006;60(1):77–9. doi: 10.1016/j.biopsych.2005.11.024. http://doi.org/10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJV, Russell IT. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. Journal of Consulting and Clinical Psychology. 2014;82:275–86. doi: 10.1037/a0035036. http://doi.org/10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain, Behavior, and Immunity. 2008;22(6):969–81. doi: 10.1016/j.bbi.2008.01.012. http://doi.org/10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJG, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3919-9. http://doi.org/10.1007/s00213-015-3919-9. [DOI] [PMC free article] [PubMed]
- Zoccola PM, Figueroa WS, Rabideau EM, Woody A, Benencia F. Differential effects of poststressor rumination and distraction on cortisol and C-reactive protein. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 2014;33(12):1606–9. doi: 10.1037/hea0000019. http://doi.org/10.1037/hea0000019. [DOI] [PubMed] [Google Scholar]