Abstract
Background
Research has shown that previous experiences during development, especially if stressful, can alter an organism’s response to opioids later in life. Given the previous literature on opioid modulation of cocaine self-administration, the current study raised rats in either an enriched condition (EC) or isolated condition (IC) and employed behavioral economics to study the effects of naltrexone and morphine on cocaine self-administration.
Methods
EC and IC rats were trained to lever press for cocaine using a within-session demand procedure. This procedure measured cocaine consumption under changing cocaine price by decreasing the dose of cocaine earned throughout a session. Rats were able to self-administer cocaine on a FR1; every 10 min the cocaine dose was systematically decreased (0.75 – 0.003 mg/kg/infusion cocaine). After reaching stability on this procedure, rats were randomly pretreated with 0, 0.3, 1, or 3 mg/kg naltrexone once every 3 days, followed by random pretreatments of 0, 0.3, 1, or 3 mg/kg morphine once every 3 days. Economic demand functions were fit to each rat’s cocaine consumption from each pretreatment, and appropriate mathematical parameters were extracted and analyzed.
Results
Naltrexone decreased the essential value of cocaine in IC rats only. However, morphine decreased the essential value of cocaine and the consumption of cocaine at zero price in both EC and IC rats.
Conclusion
These results indicate that environmental experiences during development should be considered when determining the efficacy of opioid drugs, especially for the treatment of substance abuse.
Keywords: Naltrexone, morphine, opioid, environmental enrichment, social isolation, behavioral economics
1. INTRODUCTION
Multiple lines of evidence suggest that endogenous opioids affect reward processing, but the exact mechanism of their modulatory effect is debated (Koob and Le Moal, 1997; Laurent et al., 2015; Peciña and Berridge, 2005). Exposure to a reinforcer causes release of endogenous opioids in nucleus accumbens (Olive et al., 2001; Roth-Deri et al., 2003). This reinforcer-induced opioid release is important for reward; administration of mu receptor antagonists, such as naltrexone, have been shown to decrease the rewarding properties of some drugs of abuse, including alcohol (O’Malley et al., 2015; Ripley et al., 2015; Williams and Woods, 1998) and cocaine (Giuliano et al., 2013; Kiyatkin and Brown, 2003; Ramsey et al., 1999). In contrast, mu receptor agonists, such as morphine, are reinforcing by themselves (Devine and Wise, 1994) and have been shown to enhance consumption of natural rewards (Zhang and Kelley, 2002).
However, some general discrepancies on the effectiveness of naltrexone and morphine in the human literature exist, which may be attributed to individual differences in components of the endogenous opioid system. In humans, genetic factors have been implicated in patients’ response to opioids. Individuals possessing the less common mu receptor polymorphism (A118G) may be more responsive to high dose naltrexone for the treatment of alcohol dependence (Anton et al., 2008), and these individuals require higher average morphine doses for pain management after surgery (Hwang et al., 2014). Mouse models of this polymorphism yield similar results; mice homozygous for the less common allele are more sensitive to naltrexone’s efficacy in reducing brain stimulation reward (Bilbao et al., 2015) and demonstrate reduced sensitivity to morphine’s antinociceptive properties (Mague et al., 2009).
Individual differences in an organism’s response to opioids might extend beyond genetics; evidence suggests that experience can affect an individual’s response to opioids. Episodes of chronic pain (Corder et al., 2013) and social defeat (Chaijale et al., 2013) result in long-lasting changes to opioid efficacy and endogenous opioid signaling in rodents. These studies suggest that stressful states might alter opioid efficacy in nociceptive processing. Since opioids also regulate reward, it is possible that stressful states, such as isolation rearing (Bowers et al., 2008; Djordjevic et al., 2012), might affect drug reinforcement through alteration of endogenous opioid signaling. Given that opioid antagonists are sometimes prescribed for the treatment of alcohol use disorder (O’Malley et al., 2015; Oslin et al., 2015) and opioid abuse (Goonoo et al., 2014), understanding how life experience alters opioid efficacy is crucial to improving patient outcomes.
Many studies examining the role of opioids in reward have measured their modulation of alcohol and food intake. While other studies have examined the effects of opioid ligands on cocaine self-administration, these have yielded mixed results. The majority of studies in rodents and monkeys suggest that both opioid agonists (Gerak et al., 2009; Lynch et al., 1998; Negus and Mello, 2002) and antagonists (Corrigall and Coen, 1991; Mello et al., 1990; Ramsey and van Ree, 1991) decrease cocaine self-administration. However, one study found no effect (Ettenberg et al., 1982) and other studies found dose-dependent potentiation of cocaine self-administration (Carroll et al., 1986; Corrigall et al., 1999) after administration of these compounds systemically (Carroll et al., 1986) or into the ventral tegmental area (VTA; Corrigall et al., 1999).
There are several important methodological discrepancies in this literature. The biggest differences among these studies include cocaine dose and schedule of reinforcement. Naltrexone has been effective in decreasing medium- to low-dose cocaine self-administration, but not higher-dose cocaine self-administration (Corrigall and Coen, 1991). Additionally, opioid antagonists are not consistently effective on single schedules of reinforcement in rodents (Ettenberg et al., 1982; Giuliano et al., 2013; Ward et al., 2003), but have been successful at attenuating cocaine self-administration using second-order schedules (Giuliano et al., 2013) and decreasing breakpoints using progressive ratio (Ward et al., 2003; Wee et al., 2009). The literature on opioid agonists also differs by cocaine dose, with the mu selective agonist DAMGO increasing cocaine responding at low cocaine doses, but decreasing it at high cocaine doses (Corrigall et al., 1999), suggesting that opioids affect cocaine self-administration, but only under specific schedules of reinforcement and at certain cocaine doses.
Since the ability of opioids to modulate cocaine self-administration appears to depend, at least in part, on the cocaine unit dose tested, this suggests that opioids are affecting sensitivity to changes in cocaine-associated reinforcement cost; when framed in behavioral economic terms, these previous studies measured drug consumption at various prices of cocaine. Conceptually, price of cocaine is the response effort an animal exerts to obtain an infusion of cocaine, expressed as response requirement per unit dose. Thus, in the case of a fixed ratio 5 (FR5) for a 1 mg/kg/infusion cocaine unit dose, unit price would be described as 5/1. Clearly, higher fixed ratio requirements raise unit price. Additionally, because unit price is a ratio, lower cocaine doses can also produce equivalent changes in the price per unit. An animal would have to obtain more infusions of a low unit dose to earn a specific amount of drug (e.g., 10 infusions of a 0.1 mg/kg/infusion dose to earn 1 mg/kg) compared to fewer infusions of a high unit dose to receive the same amount of drug (e.g., 1 infusion of a 1 mg/kg/infusion dose), making the lower unit dose higher in price. Thus, the experimenter can manipulate unit price by keeping dose constant and raising the ratio requirement (increasing values in the numerator), or by keeping the ratio requirement constant and decreasing the unit dose available (decreasing values in the denominator). When these two things are combined, unit prices are increased in a multiplicative fashion.
Behavioral economics and analysis of demand borrow from economic theory in which consumption of some goods is often inversely related to price. Unlike the standard self-administration dose-effect curve analysis, an economic approach can help to separate hedonic set-point (how much of a good an animal would consume if it were free) and essential value (how willing an animal is to work for a reinforcer as it gets more costly to obtain; Oleson et al., 2011). When applied to animal operant behavior, this paradigm can help address some of the methodological inconsistencies present in previous studies of this kind (i.e., schedule of reinforcement and cocaine dose). This approach has been proposed to help identify potential treatments for drug abuse (Hursh and Winger, 1995), and has recently been employed to this end in rodent self-administration studies (Bentzley et al., 2014; Porter-Stransky et al., 2015).
To assess the effect of early life experiences on the ability of opioids to modulate the essential value of cocaine, the current study measured cocaine self-administration in two different groups of rats; one group was raised in an enriched condition (EC) during adolescence and the other group was raised in an isolated condition (IC). Each group was then pretreated in young adulthood with various doses of naltrexone or morphine before cocaine self-administration sessions. Self-administration sessions utilized a within-session threshold procedure that assessed drug intake at various cocaine doses within a single session (Oleson et al., 2011). By standardizing cocaine consumption across doses and converting cocaine dose to unit price, such that higher cocaine doses are easier to obtain and are lower in price, an economic demand function was fit to rats’ cocaine intake (Hursh and Silberberg, 2008). Both hedonic set-point (Qo) and essential value (α) after pretreatment with opioid ligands were compared between EC and IC rats.
2. MATERIALS AND METHODS
2.1. Subjects and housing
Male Sprague-Dawley rats arrived to the colony on PND 21. Upon arrival, they were randomly separated into one of two housing conditions: either EC (enriched condition, n = 5) or IC (isolated condition, n = 8). EC rats were housed 5–12 per cage in large, stainless steel cages (122 × 61 × 45.5 cm) with ample bedding. Fourteen objects were placed throughout the cage and were rearranged daily and completely replaced every other day. IC rats were housed singly in small stainless steel cages (17 × 24 × 20 cm) with wire mesh bottoms and no objects. Rats remained in their respective conditions for the entire study. Rats were kept in a temperature and humidity controlled colony room on a 12h light:dark cycle (lights on at 7:00am). All animal procedures were approved by the University of Kentucky’s Institutional Animal Care and Use Committee and conformed to NIH standards.
2.2. Surgical procedures
Between PND 55 and 60, all rats received jugular catheter implantation surgery. Briefly, rats were anesthetized with a ketamine (Butler Schein, Dublin OH) /xylazine (Akorn, Inc., Decatur IL) /acepromazine (Boehringer Ingelheim, St. Joseph MO) cocktail (75/7.5/0.75 mg/kg; 0.15ml/100g body weight; i.p.). A jugular catheter was implanted into the right jugular vein, threaded under the skin, and exited the body through an incision on the scalp. The catheter port was attached to the skull using four jeweler’s screws and dental acrylic. Rats were allowed to recover for one week before starting self-administration.
2.3. Cocaine self-administration training
All operant procedures occurred in standard 2-lever operant conditioning chambers (28 × 24 × 21 cm; ENV-008CT; MED Associates, St. Albans VT) equipped with syringe pumps for drug delivery (PHM-100; MED Associates). Ten days before surgery, all rats were trained to lever press for food pellets (45 mg Dustless Precision Pellets, Bio-Serv, Frenchtown NJ) on a FR1 schedule for 60 min as described previously (Hofford et al., 2015). Rats received 15g of food at the end of their session for the first five days of training, but were returned to free feed for the remainder of the experiment. One week after surgery, rats were returned to the operant boxes and connected to the syringe pump via silastic leashes. Rats began cocaine self-administration training where they received 0.75 mg/kg/infusion cocaine (i.v., 0.1 ml/infusion, 5.9 sec duration) on a FR1 schedule with a time out of 20 sec. Sessions were 60 min in duration and occurred once daily until stability.
2.4. Within-session threshold procedure
Once rats achieved stable cocaine responding at 0.75 mg/kg/infusion (< 20% variability over 3 consecutive days), they were trained on a threshold, within-session demand procedure (Oleson et al., 2011). Sessions were 60 min in duration and rats started each session earning 0.75 mg/kg/infusion. However, every ten minutes the dose of cocaine decreased (0.75, 0.27, 0.08, 0.03, 0.01, 0.003 mg/kg/infusion) by shortening the length of infusion. The response requirement remained the same (FR1) throughout the session.
2.5. Opioid drug pretreatment
Rats continued performing the within-session demand procedure once daily until they reached stability (defined as all rats having no significant change in active lever presses on the first 2 doses of cocaine over 3 consecutive days), then they were pretreated with 0, 0.3, 1 or 3 mg/kg naltrexone (s.c., 1 ml/kg) 5 min before their session. Every rat received each dose in random order and two maintenance sessions (no pretreatment) intervened between each pretreatment. Rats were then pretreated with 0, 0.3, 1 or 3 mg/kg morphine (s.c., 1 ml/kg) 5 min before their session (random order, counterbalanced across rats, two maintenance sessions between pretreatments as above).
2.6. Drugs
Cocaine hydrochloride, morphine sulfate, and naltrexone hydrochloride were gifts from the National Institute of Drug Abuse (Bethesda, MD) and all were dissolved in isotonic saline.
2.7. Statistical analysis
Cocaine consumption was measured for each rat at each pretreatment session and was calculated as: (infusions earned at each cocaine dose) × (cocaine dose). This generated 6 values for each rat at each pretreatment. Cocaine consumption was analyzed as a function of unit price (the number of responses necessary to receive 1 mg/kg cocaine). Exponential demand functions (Hursh and Silberberg, 2008) of the form: log Q = log(Qo)+k*(e(−αQoC) −1), where Q equals consumption, Qo equals consumption at zero cost (intercept of function), C equals unit price, k equals a scalar constant for consumption range, and α equals essential value (slope of function), were fit to cocaine consumption at each pretreatment for each individual rat. The demand function was fit to the data via nonlinear mixed effects modeling (NLME; Beckmann and Chow, 2015; Pinheiro et al., 2007; Young et al., 2009) using the NLME tool in the R statistical software package (Pinheiro et al., 2007), with Qo and α as free parameters and k as a global constant. The NLME models defined unit price as a fixed, continuous within-subject factor, drug dose as a fixed, nominal within-subject factor, housing condition as a fixed, nominal between-subject factor, and subject defined as a random factor. Identical NLME models were used to analyze morphine and naltrexone treatments.
Akin to linear mixed effects modeling (Gelman and Hill, 2006), NLME is a hierarchical, multi-level modeling technique that utilizes maximum likelihood estimation (Myung, 2003) to determine parameter estimates of a predefined non-linear function fit to data over different experimental conditions (incorporating model fits from each individual), providing metrics of goodness of fit and determining statistical significance of parameter estimates across levels of experimental conditions. Like linear mixed effects, NLME analysis is superior to traditional ANOVA, as it significantly increases power, reduces Type I error rates, and through the use of defined functions aids in interpretation by bringing the researcher closer to specific underlying relationships in the data (Beckmann and Chow, 2015; Young et al., 2009). All p values less than 0.05 were deemed statistically significant. Significant interactions were further probed using Bonferroni correction.
3. RESULTS
3.1. Effect of naltrexone pretreatment on α and Qo
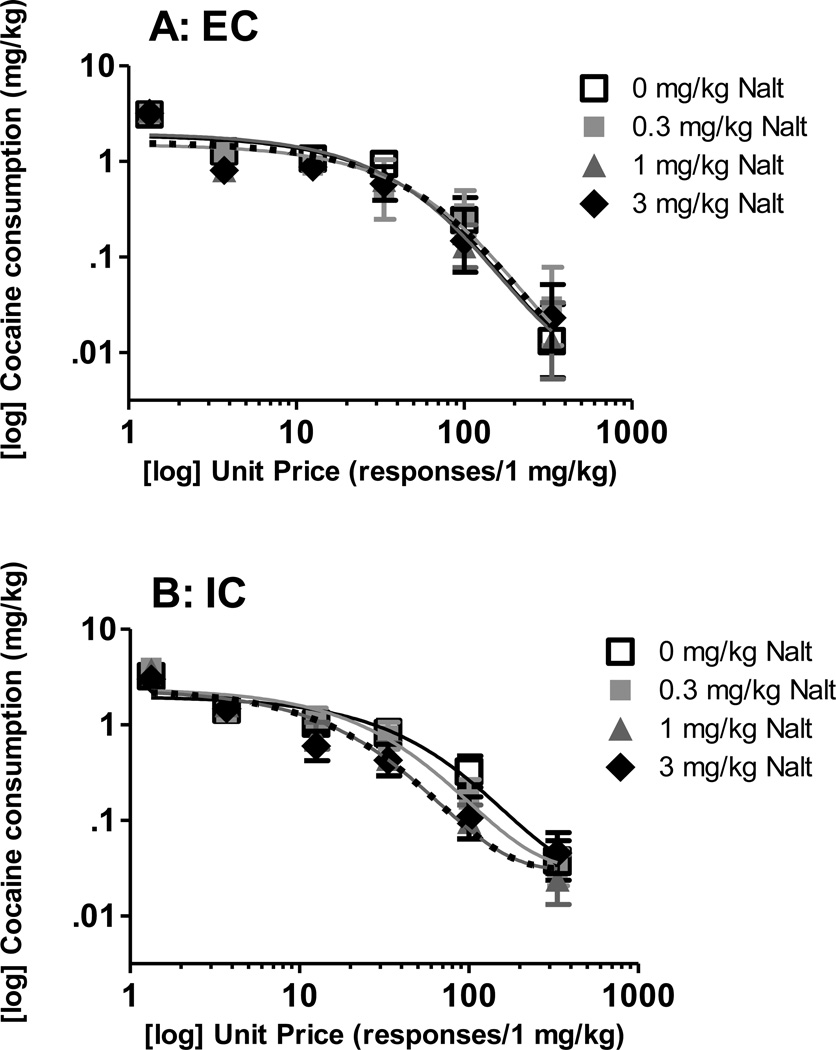
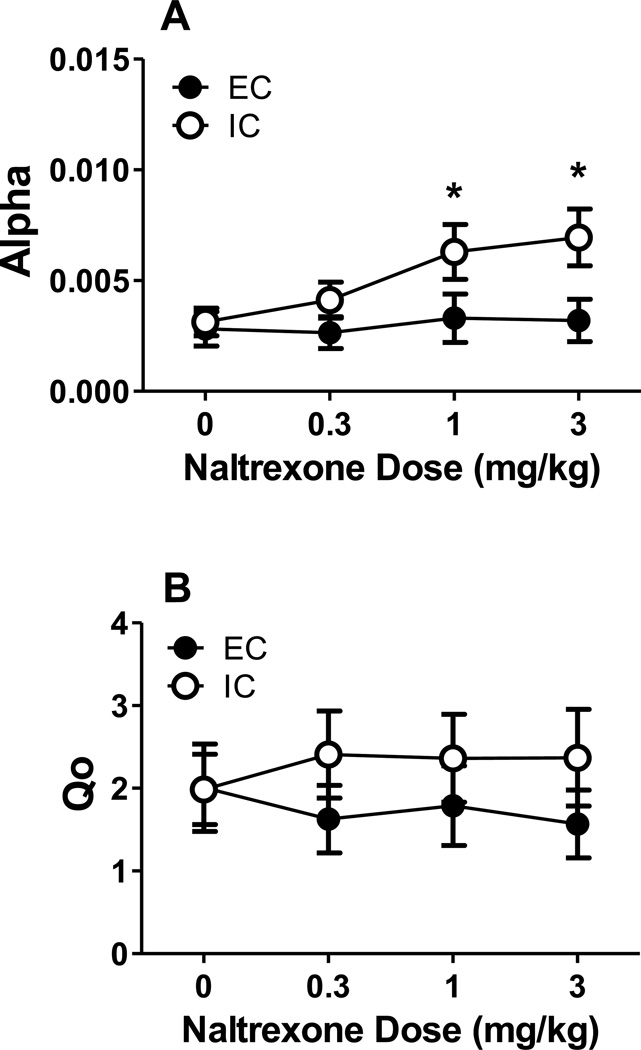
The demand curves from EC and IC rats pretreated with naltrexone are illustrated in Figure 1. Using best-fit functions with a k of 2.17, NLME analysis revealed no main effects or interaction on Qo. However, NLME analysis indicated a significant effect of naltrexone dose [F(3, 258) = 6.58, p < .05] and a significant environment×naltrexone dose interaction on α [F(3, 258) = 4.71, p < .05]. Post hoc analysis revealed significant differences between EC and IC α at 1 mg/kg and 3 mg/kg naltrexone (Figure 2).
Figure 1. Cocaine consumption after naltrexone pretreatment.
Log mean (±SEM) cocaine consumption at each unit price of cocaine in EC (A) and IC (B) rats. Mean consumption denoted by symbols, best fit curves denoted by color-matching line (best fit curve 0 mg/kg naltrexone: black solid line; 0.3 mg/kg naltrexone: light gray solid line; 1 mg/kg naltrexone: dark gray solid line; 3 mg/kg naltrexone: black dotted line).
Figure 2. Parameter values after naltrexone pretreatment.
(A) Mean (±SEM) α values after pretreatment at 0, 0.3, 1, and 3 mg/kg naltrexone in EC (●) and IC (○) rats. (B) Mean (±SEM) Qo values after pretreatment at 0, 0.3, 1, and 3 mg/kg naltrexone in EC (●) and IC (○) rats.
3.2. Effect of morphine pretreatment on α and Qo
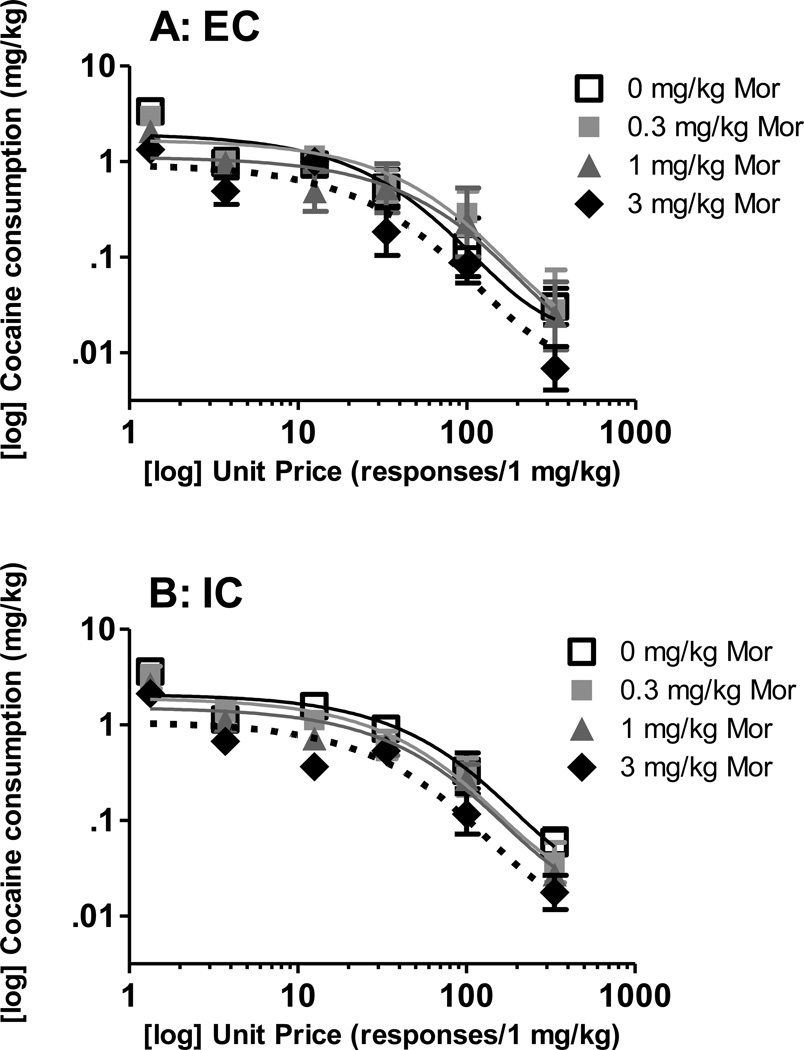
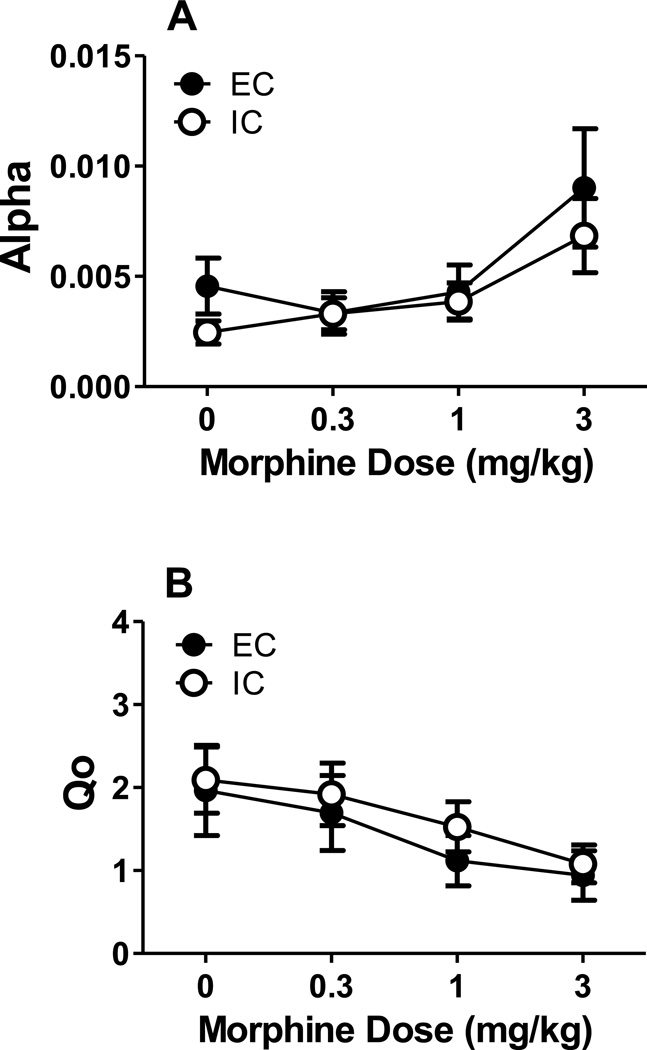
The demand curves from EC and IC rats pretreated with morphine are illustrated in Figure 3. Using best fit functions with a k of 2.24, NLME analysis revealed that morphine dose-dependently decreased Qo [F(3, 258) = 9.26, p < 0.05], but there was no significant effect of environment. NLME analysis also identified a significant effect of morphine dose [F(3, 258) = 5.97, p< 0.05] on α estimates, but no other significant effects (Figure 4).
Figure 3. Cocaine consumption after morphine pretreatment.
Log mean (±SEM) cocaine consumption at each unit price of cocaine in EC (A) and IC (B) rats. Actual consumption denoted by symbols, best fit curves denoted by color-matching line (best fit curve 0 mg/kg morphine: black solid line; 0.3 mg/kg morphine: light gray solid line; 1 mg/kg morphine: dark gray solid line; 3 mg/kg morphine: black dotted line).
Figure 4. Parameter values after morphine pretreatment.
(A) Mean (±SEM) α values after pretreatment at 0, 0.3, 1, and 3 mg/kg morphine in EC (●) and IC (○) rats. (B) Mean (±SEM) Qo values after pretreatment at 0, 0.3, 1, and 3 mg/kg morphine in EC (●) and IC (○) rats.
4. DISCUSSION
The main finding of the current study is that rearing environment influences the ability of an opioid antagonist to decrease cocaine reinforcement, with IC rats more sensitive than EC rats to the naltrexone-induced decrease in essential value of cocaine. While no studies have directly examined how opioid systems may differentially contribute to cocaine reinforcement in EC and IC rats, several studies have examined environment-induced differences in the sensitivity to opioid agonists in other behavioral preparations. For example, similar to the current study, EC and IC rats did not differ in their sensitivity to morphine reward as measured by conditioned place preference (CPP) and did not differ on their sensitivity to morphine-induced antinociception (Smith et al., 2005). In contrast to these results in rats, however, EC mice showed reduced heroin-induced CPP compared to standard housed mice (El Rawas et al., 2009) and group-housed mice had decreased sensitivity to morphine’s antinociceptive effects compared to IC mice (Coudereau et al., 1997). It is not clear if the inability of morphine to modulate sensitivity to cocaine observed here is specific to rats.
Although EC and IC rats did not differ in cocaine self-administration following pretreatment with morphine, IC rats showed a greater change in essential value (α parameter) than EC rats following naltrexone. It is unlikely that this change in essential value is related directly to environment-induced changes in opioid receptor densities, as autoradiographic evidence indicates that EC and IC rats do not differ in mu opioid receptor binding in rewardrelevant brain regions (Bardo et al., 1997). Alternatively, changes in essential value after naltrexone in EC and IC rats could be due to underlying differences in cocaine-induced endogenous opioid release. While cocaine is known to evoke opioid release via D2 dopamine receptors (Soderman and Unterwald, 2009), it is unclear if this release differs between EC and IC rats. However, it is possible that cocaine-induced opioid release is greater in IC rats compared to EC rats, especially since it is known that isolation rearing increases stimulant-induced dopamine release in nucleus accumbens (Jones et al., 1992). Thus, if IC rats have greater release of endogenous opioids in response to cocaine, this could explain why naltrexone had a greater effect on IC rats compared to EC rats when there was more receptor occupation at higher naltrexone doses.
Regardless of rearing environment, naltrexone increased α but had no effect on Qo, indicating that naltrexone decreased the essential value of cocaine. Conceptually, this is consistent with previous work showing that naltrexone decreased low-dose, but not high-dose, cocaine self-administration (Corrigall and Coen, 1991). This effect of naltrexone is also consistent with previous literature demonstrating that administration of the mu antagonist β-funaltrexamine into VTA or nucleus accumbens decreased breakpoints on a progressive ratio task, but did not affect fixed ratio responding for cocaine (Ward et al., 2003), since increases in α also reflect decreased motivation to work for a reinforcer.
Morphine also dose-dependently increased α values similar to naltrexone. While it may be surprising that both an antagonist (naltrexone) and an agonist (morphine) would both attenuate cocaine self-administration, this finding is supported by the literature (Lynch et al., 1998; Negus and Mello, 2002). Additionally, the nature of these drug effects on cocaine self-administration is made evident using the economic demand function. That is, while naltrexone affected only the α parameter, morphine dose-dependently altered both α and Qo. When comparing consumption of two reinforcers using an economic demand function, their relative positions to each other can reveal their relationship. For instance, a decrease in Qo after exposure to an alternative reinforcer suggests that the alternative reinforcer may substitute for the first (Bickel et al., 2010). In the current study, this suggests that morphine (a known reinforcer) given before the start of the session partially substituted for cocaine. Importantly, the free reinforcer (morphine) decreased consumption of the reinforcer requiring effort output (cocaine). Stated another way, by giving morphine for free, it decreased self-administration of cocaine at zero price. Consistent with this interpretation, human studies comparing theoretical purchase of cocaine under varying prices of heroin suggest that these drugs can substitute for one another despite their different mechanisms of action (Petry and Bickel, 1998).
One noteworthy limitation in interpreting morphine’s effects on parameter estimates is that the order of naltrexone and morphine were not counterbalanced (naltrexone pretreatments always occurred before morphine pretreatments). This raises the possibility that assessment of the naltrexone demand curves affected parameter estimates of the morphine demand curves due to naltrexone-induced opioid receptor up-regulation. However, previous demonstrations of naltrexone-induced up-regulation have used long-term exposure to naltrexone delivered via pellet implantation or continuous infusion (Lesscher et al., 2003; Sirohi et al., 2007). In contrast to that work, the current study exposed rats to naltrexone using a randomized dose order across rats and administered naltrexone acutely only once every three days, thus making it unlikely that the morphine demand curve assessment was subject to receptor supersensitivity.
This study identified a difference in opioid sensitivity between EC and IC rats by the ability of naltrexone and morphine to alter cocaine consumption. While previous studies have shown differential behavioral sensitivity to opioids in EC and IC rats, this study is the first to measure opioid modulation of the reinforcing efficacy of cocaine in these groups using the within-session threshold procedure. This provides an advantage over previous studies that did not control for schedule of reinforcement or cocaine dose. Naltrexone is sometimes prescribed as a treatment for alcohol use disorder (O’Malley et al., 2015; Oslin et al., 2015) and opioid abuse (Goonoo et al., 2014). This study suggests that environmental history can influence measures of its efficacy.
Highlights.
-
*
Behavioral economics was used to measure cocaine reinforcement in EC and IC rats.
-
*
EC and IC rats were pretreated with naltrexone or morphine before their session.
-
*
Naltrexone and morphine caused rats to decrease cocaine consumption at high prices.
-
*
IC rats demonstrated greater changes in cocaine essential value after naltrexone.
Acknowledgments
Role of Funding Source
This work was financially supported by the National Institute on Drug Abuse branch of the National Institutes of Health (grants R01 DA012964, T32 DA016176, T32 DA035200, F32 DA036291, and R00 DA033373). The NIH had no role in the design, preparation, experimentation, or decision to publish any portion of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RSH conceptualized, helped design, and conducted the experiment. Additionally, RSH aided in the statistical analysis and wrote the manuscript. JSB helped design the experiment, conducted most of the statistical analysis, and edited the manuscript. MTB helped design the experiment and edited the manuscript. All authors have approved the final article.
Conflicts of interest
none.
REFERENCES
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An Evaluation of μ-Opioid Receptor (OPRM1) as a Predictor of Naltrexone Response in the Treatment of Alcohol Dependence: Results From the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Arch. Gen. Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Robinet PM, Hammer RF., Jr Effect of differential rearing environments on morphine-induced behaviors, opioid receptors and dopamine synthesis. Neuropharmacology. 1997;36:251–259. doi: 10.1016/s0028-3908(96)00139-6. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn. Mem. 2015;22:116–127. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc. Natl. Acad. Sci. U.S.A. 2014;111:11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Mueller ET, Jones BA, Christensen DR. The behavioral economics of drug dependence: towards the consilience of economics and behavioral neuroscience. In: Self WD, Staley Gottschalk KJ, editors. Behavioral Neuroscience of Drug Addiction. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010. pp. 319–341. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, Thorsell A. A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol. Psychiatry. 2015;77:850–858. doi: 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav. Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Walker MJ, Kragh R, Newman T. Effects of naltrexone on intravenous cocaine self-administration in rats during food satiation and deprivation. J. Pharmacol. Exp. Thera. 1986;238:1–7. [PubMed] [Google Scholar]
- Chaijale NN, Curtis AL, Wood SK, Zhang X-Y, Bhatnagar S, Reyes BAS, Van Bockstaele EJ, Valentino RJ. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38:1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall W, Coen K. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology. 1991;104:167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BLC. The mu opioid agonist DAMGO alters the intravenous self-administration of cocaine in rats: mechanisms in the ventral tegmental area. Psychopharmacology. 1999;141:428–435. doi: 10.1007/s002130050853. [DOI] [PubMed] [Google Scholar]
- Coudereau J-P, Monier C, Bourre J-M, Frances H. Effect of isolation on pain threshold and on different effects of morphine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1997;21:997–1018. doi: 10.1016/s0278-5846(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J. Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Djordjevic A, Adzic M, Radojcic MB. Effects of chronic social isolation on wistar rat behavior and brain plasticity markers. Neuropsychobiology. 2012;66:112–119. doi: 10.1159/000338605. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology. 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit H, Bloom F, Koob G. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression And Multilevel/Hierarchical Models. New York: Cambridge University Press; 2006. [Google Scholar]
- Gerak L, Galici R, France C. Self administration of heroin and cocaine in morphine-dependent and morphine-withdrawn rhesus monkeys. Psychopharmacology. 2009;204:403–411. doi: 10.1007/s00213-009-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Robbins T, Wille D, Bullmore E, Everitt B. Attenuation of cocaine and heroin seeking by μ-opioid receptor antagonism. Psychopharmacology. 2013;227:137–147. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonoo N, Bhaw-Luximon A, Ujoodha R, Jhugroo A, Hulse GK, Jhurry D. Naltrexone: a review of existing sustained drug delivery systems and emerging nano-based systems. J. Control. Release. 2014;183:154–166. doi: 10.1016/j.jconrel.2014.03.046. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Prendergast MA, Bardo MT. Pharmacological manipulation of glucocorticoid receptors differentially affects cocaine self-administration in environmentally enriched and isolated rats. Behav. Brain Res. 2015;283:196–202. doi: 10.1016/j.bbr.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol. Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J. Exp. Anal. Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IC, Park JY, Myung SK, Ahn HY, Fukuda K, Liao Q. OPRM1 A118G gene variant and postoperative opioid requirement: a systematic review and meta-analysis. Anesthesiology. 2014;121:825–834. doi: 10.1097/ALN.0000000000000405. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol. Biochem. Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Naloxone depresses cocaine self-administration and delays its initiation on the following day. Neuroreport. 2003;14:251–255. doi: 10.1097/00001756-200302100-00019. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Laurent V, Morse AK, Balleine BW. The role of opioid processes in reward and decision-making. Br. J. Pharmacol. 2015;172:449–459. doi: 10.1111/bph.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HMB, Bailey A, Burbach JPH, Van Ree JM, Kitchen I, Gerrits MAFM. Receptor-selective changes in μ-, δ- and κ-opioid receptors after chronic naltrexone treatment in mice. Eur. J. Neurosci. 2003;17:1006–1012. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Heaser WA, Carroll ME. Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp. Clin. Psychopharmacol. 1998;6:255–263. doi: 10.1037//1064-1297.6.3.255. [DOI] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen L-Y, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J. Pharmacol. Exp. Thera. 1990;254:926–939. [PubMed] [Google Scholar]
- Myung IJ. Tutorial on maximum likelihood estimation. J. Math. Pyschol. 2003;47:90–100. [Google Scholar]
- Negus SS, Mello NK. Effects of μ-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of μ-agonist efficacy. J. Pharmacol. Exp. Thera. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, Romano DM, Wu R, Toll BA, Sher KJ, Gueorguieva R, Kranzler HR. Naltrexone reduces alcohol drinking in young adults: a double-blind, randomized clinical trial of efficacy and safety. J. Clin. Psychiatry. 2015;76:e207–e213. doi: 10.4088/JCP.13m08934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson E, Richardson J, Roberts DS. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J. Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Leong SH, Lynch KG, Berrettini W, O'Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial. JAMA Psychiatry. 2015;72:430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids cause increased hedonic impact of sweetness? J. Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Polydrug abuse in heroin addicts: a behavioral economic analysis. Addiction. 1998;93:321–335. doi: 10.1046/j.1360-0443.1998.9333212.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. 2007 [Google Scholar]
- Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict. Biol. 2015 doi: 10.1111/adb.12323. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Gerrits MA, Van Ree JM. Naltrexone affects cocaine self-administration in naive rats through the ventral tegmental area rather than dopaminergic target regions. Eur. Neuropsychopharmacol. 1999;9:93–99. doi: 10.1016/s0924-977x(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol. Biochem. Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- Ripley T, Sanchez-Roige S, Bullmore E, Mugnaini M, Maltby K, Miller S, Wille D, Nathan P, Stephens D. The novel mu-opioid antagonist, GSK1521498, reduces ethanol consumption in C57BL/6J mice. Psychopharmacology. 2015;232:3431–3441. doi: 10.1007/s00213-015-3995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular β-endorphin levels in the nucleus accumbens. J. Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Kumar P, Yoburn BC. Mu-opioid receptor up-regulation and functional supersensitivity are independent of antagonist efficacy. J. Pharmacol and Exp. Thera. 2007;323:701–707. doi: 10.1124/jpet.107.127019. [DOI] [PubMed] [Google Scholar]
- Smith M, Chisholm K, Bryant P, Greene J, McClean J, Stoops W, Yancey D. Social and environmental influences on opioid sensitivity in rats: importance of an opioid’s relative efficacy at the mu-receptor. Psychopharmacology. 2005;181:27–37. doi: 10.1007/s00213-005-2218-2. [DOI] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine-induced mu opioid receptor occupancy within the striatum is mediated by dopamine D2 receptors. Brain Res. 2009;1296:63–71. doi: 10.1016/j.brainres.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Martin TJ, Roberts DCS. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacol. Biochem. Behav. 2003;75:301–307. doi: 10.1016/s0091-3057(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the μ-,κ-, or δ-receptor. Alcohol. Clin. Exp. Res. 1998;22:1634–1639. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: advantages and cautionary notes. Learn. Motiv. 2009;40:160–177. [Google Scholar]
- Zhang M, Kelley A. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]