Abstract
Purpose
The goals of this study were to measure normal characteristics of ambient and tympanometric wideband acoustic reflectance, which was parameterized by absorbance and group delay, in newborns cared for in well-baby and Neonatal Intensive Care Unit (NICU) nurseries, and to characterize the normal development of reflectance over the first year after birth in a group of infants with clinically normal hearing status followed longitudinally from birth to one year of age.
Methods
Infants were recruited from a well-baby and NICU nursery, passed newborn otoacoustic emissions (OAE) and automated auditory brainstem response (ABR) tests as well as follow-up diagnostic ABR and audiometry. They were tested longitudinally for up to one year using a wideband middle ear acoustic test battery consisting of tympanometry and ambient-pressure tests. Results were analyzed for ambient reflectance across frequency and tympanometric reflectance across frequency and pressure.
Results
Wideband absorbance and group delay showed large effects of age in the first 6 months. Immature absorbance and group delay patterns were apparent in the low frequencies at birth and one month, but changed substantially to a more adult-like pattern by age 6 months for both ambient and tympanometric variables. Area and length of the ear canal estimated acoustically increased up to age 1 year. Effects of race (African American and others compared to Caucasian) were found in combination with age effects. Mean and confidence intervals are provided for use as a normative longitudinal database for newborns and infants up to one year of age, for both well-baby and NICU infants.
Keywords: Development, reflectance, tympanometry, newborn screening, middle ear
1. INTRODUCTION
Acoustic ear-canal measurements in the human external ear canal can be used to describe normal function and development of the middle ear, as well as abnormal function. The ear canal and middle ear are immature at birth and undergo substantial and complex anatomical and physiologic development, especially during the first several months after birth. Anatomical development alters acoustic immittance measurements across the frequency range, due to concomitant changes in mechanical properties of mass, stiffness and resistance (Holte, Margolis & Cavanaugh, 1991). Major anatomical and physical post-natal development of the ear canal includes increased diameter and length, altered orientation (Ikui, Sando, Sudo, & Fujita, 1997) and increased rigidity due to gradual ossification of the medial bony portion (Eby and Nadol, 1986). The TM thins as mesenchymal tissue is resorbed (Ruah, Schachern, Zelterman, Paparella, & Yoon, 1991). The middle ear cavity increases after birth in aeration and size, and the mastoid air cells pneumatize; these factors increase both the physical volume of the middle-ear cavity as well as the acoustical compliance (Cinamon, 2009). After birth, the middle ear is not immediately aerated, and may contain amniotic fluid and other debris (Palva, Northrup, & Ramsay, 1999). By the end of the first 24 hours after birth, approximately 50% of ears retain middle-ear effusion, decreasing to 27% after 48 hours of age and 13% after 2 weeks of age (Roberts et al., 1992). The ossicles become less dense over the first six 6 months after birth as mesenchyme dissipates and ossification occurs, and the ossicular joints stiffen (Saunders, Kaltenbach, & Relkin, 1983; Eby & Nadol, 1986).
These anatomic changes are associated with physiological changes in the function of the infant middle ear compared to the adult ear. Accordingly, tympanometry measures reveal lower static admittance, broader tympanometric width, an appearance of notching at 0.226 kHz, and less energy transmission in frequencies above 1 kHz in newborns that appear related to physical ear canal flaccidity (Holte, Cavanaugh and Margolis, 1990; Keefe & Levi, 1996). In neonate ears with middle-ear fluid, 0.226-kHz tympanograms are not reliably different from those obtained from normal ears (Paradise, Smith & Bluestone, 1976; Marchant, McMillan, Shurin, Johnson, Turczyk, Feinstei, & Panek, 1986). Higher frequencies from 0.66 - 1 kHz are better able to detect middle ear fluid than at the standard 0.226 kHz probe (Hunter & Margolis, 1992; Baldwin, 2006; Zhiqi, Kun and Zhiwu, 2010). Variability in conventional tympanometry features in young infants has led to conflicting interpretations of what tympanometric criteria define a normal infant middle ear, and cast doubt on the accuracy and validity of tympanometry for newborn ears.
Wideband acoustic immittance (WAI) provides a means to study physiologic development. WAI is a family of middle ear acoustical measures that use click stimuli, analyzed across a broad frequency range (0.2 to 8 kHz), and may also include reflectance variables and acoustic stapedial reflex (ASR) responses in addition to admittance or impedance variables (Keefe, Ling, & Bulen, 1992; Feeney, Hunter, Kei, Lilly, Margolis, Nakajima & Voss, 2013; Rosowski, Stenfelt, & Lilly, 2013).
For an incident sound propagating in the ear canal in a forward direction towards the eardrum, the pressure reflectance at the probe microphone is the complex ratio (i.e., magnitude and phase) of the reverse-directed pressure at the microphone to its forward-directed sound pressure at the microphone. In this one-dimensional representation of sound transmission in the ear canal, this reverse-directed sound is generated by the reflection of the forward wave from the eardrum. The energy reflectance is the corresponding ratio of reflected sound energy to incident sound energy, and is equal to the squared magnitude of the pressure reflectance. Assuming that the sound energy in the ear canal is predominantly absorbed by the middle ear at the eardrum, the absorbance is the ratio of absorbed sound energy to incident energy, and is equal to one minus the energy reflectance. Although either quantity may be used, absorbance is used in the present study rather than energy reflectance inasmuch as absorbance is a measure of how much energy is absorbed by the middle ear. The latter energy is an upper bound to the energy that is ultimately delivered to the cochlea. The (reflectance) group delay is defined as the negative gradient of the pressure reflectance phase with respect to the frequency of sound (divided by a factor of 2π).
Wideband absorbance can be measured across frequency at ambient pressure in the ear canal and also as a function of varying air pressure in the form of a wideband absorbance tympanogram (Keefe & Levi, 1996; Margolis, Saly & Keefe, 1999). A corresponding wideband group delay may also be measured under ambient and tympanometric conditions. Because middle-ear pressure has a large influence on absorbance at different frequencies, normal and abnormal characteristics of wideband tympanometry are needed across the age range.
Most reports of WAI in infants have used magnitude measures (energy reflectance or absorbance) obtained at ambient pressure, and have been cross-sectional studies, without confirmation of clinically normal hearing status. The largest changes in ambient energy reflectance in infants relative to adults occur below 0.5 kHz and above 2 kHz between 1 and 6 months of age (Aithal, Kei, & Driscoll, 2014; Keefe, Bulen, Arehart, & Burns, 1993; Shahnaz, Cai and Qi, 2014). There is up to a 30% change in mean energy reflectance and admittance magnitude from 0.25 - 0.75 kHz between 4 and 24 weeks of age (Sanford and Feeney, 2008). Changes are less apparent from 0.75 - 2 kHz, while developmental changes from 2 - 6 kHz are complex (Sanford and Feeney, 2008). Since the rate of post-natal maturation of the ear canal and middle ear is substantial during the first few months of life and ambient aural acoustic measures do not directly account for middle ear middle ear pressure effects, more information is needed to understand longitudinal developmental effects on pressurized WAI measures (Keefe, Folsom, Gorga, Vohr, Bulen, & Norton, 2000). Infants cared for in well-baby nurseries who did not pass newborn hearing screening show significantly greater energy reflectance (or less absorbance) in the frequency region from 1 - 4 kHz compared to infants who pass newborn screening (Sanford, Keefe, Liu, Fitzpatrick, McCreery, Lewis, & Gorga, 2009; Hunter, Feeney, Lapsley Miller, Jeng, & Bohning, 2010; Aithal, Kei, Driscoll, Khan, & Swanston, 2015). Similar results have been reported in infants tested in a neonatal intensive care unit (NICU) (Shahnaz, 2008). Wideband absorbance or energy reflectance is able to predict middle ear dysfunction (Keefe, Gorga, Neely, Zhao, & Vohr, 2003b) and conductive hearing loss in infants (Prieve, Vander Werff, Preston, Georgantas, 2013).
The age range from birth to one year is a critically important developmental period that has clinical implications for detection of middle-ear dysfunction relative to newborn hearing screening. There have been no reported measurements of group delay in infants, thus data are needed to evaluate whether group delay measurements are feasible in young infants, and whether group delay conveys clinically relevant information. Measures of absorbance and group delay may help to better understand the functional changes with age that bear upon clinical diagnosis of sensorineural compared to conductive hearing loss.
The goals of this study were: (1) to measure the normal characteristics of ambient and tympanometric reflectance, which was parameterized in terms of absorbance and group delay, in newborns cared for in well-baby and NICU nurseries, and (2) to characterize normal development of wideband ambient and tympanometric reflectance over the first year after birth in a group of infants with clinically normal hearing status from birth to one year of age.
2. MATERIAL AND METHODS
2.1 Subject enrollment
This study was part of a prospective, longitudinal project on translational wideband acoustic tests developed for identification of middle-ear, cochlear and neural hearing loss in infants, children and adults. Infants were enrolled after they received Newborn Hearing Screening (NHS) tests in normal and NICU nurseries at two urban hospitals in Cincinnati, Ohio: Good Samaritan Hospital (GSH) and Cincinnati Children’s Hospital (CCHMC). The NHS protocol for the normal newborn nursery consisted of screening of all infants using Transient Evoked Otoacoustic Emissions (TEOAE, clicks at 80 dB SPL). If the infant did not pass TEOAE in either ear, Automated Auditory Brainstem Response (ABR, clicks at 35 dB nHL) was completed before hospital discharge.
A WAI test battery was composed of ambient and tympanometric wideband absorbance and group delay, and wideband acoustic stapedial reflexes. The WAI tests were measured after enrollment, on the same or the next day after the NHS exam, and over the first year after birth at follow-up study visits (1, 6, 9, 12 mo.) in which other tests were also completed. These other tests included threshold Tone-Burst ABR (TB-ABR), Distortion Product OAE (DPOAE) and Visual Reinforced Audiometry (VRA). The protocol used in this study was approved by the Institutional Review Boards of Cincinnati Children’s Hospital Medical Center (CCHMC) and Good Samaritan Hospital (GSH), and informed consent was obtained from the parent(s) of all infants. Data described in this report were analyzed in a subset of 184 infants who passed hearing screening in the hospital, and returned for follow-up with diagnostic test results in the normal range at each visit completed.
2.2 Screening and Follow-up Diagnostic Tests
In the newborn nurseries, screening TEOAEs or ABR (both from Natus Medical, Inc.) were performed first, followed by a WAI battery of tests described below. In total, 591 visits were included in the analysis, with 129 visits at birth, 138 at about 1 month (.25-4 months), 95 at about 6 months (4-8 months), 136 at 9 months (8-11 months) and 93 at 12 months (11-15 months). Follow-up diagnostic threshold toneburst ABR was scheduled at age 1 month. Otoscopy (Welch Allyn pneumatic otoscope) and DPOAE testing using the Vivosonic Integrity system (Version 5.2) were performed prior to the diagnostic threshold ABR. For DPOAE, primary tone levels were set at SPLs of 65 dB (L1) and 55 dB (L2), and primary tone frequencies f1 and f2 were set with an f2/f1 ratio equal to 1.22. Pass criteria were SNR of 6 dB or greater at 3 of 5 test (f2) frequencies (2000, 3000, 4000, 5500, and 8000 Hz). In addition, DP levels were required to be at or above 0 dB SPL (Gorga, Dierking, Johnson, Beauchaine, Garner, & Neely, 2005).
Diagnostic ABR at 1-2 months was conducted within a shielded double-walled sound-attenuated booth, using the Vivosonic Integrity system (Version 5.2). Stimuli for AC were presented via insert earphones (Etymotic Research ER-3A) using pediatric ear foam tips, and stimuli for bone conduction were presented via a B-71 bone oscillator, hand-held at the temporal bone above the pinna. Recording methods and analysis were described by Elsayed, Hunter, Keefe, Feeney, Brown, Meinzen-Derr, Baroch, Sullivan-Mahoney, Francis & Schaid (2015). VRA was completed using the Intelligent Hearing Systems Smart VRA device with insert earphones. The minimal protocol to retain data included speech awareness threshold and pure tone air conduction results at 1 kHz and 4 kHz. The criterion for a normal minimum response level was 25 dB HL or better, with no air bone gaps exceeding 10 dB.
2.1 Wideband acoustic measures
Wideband reflectance data were acquired using a Titan ear-canal probe and modified AT-235 tympanometry hardware (both manufactured by Interacoustics Wideband Tympanometry research system, Copenhagen, DK). The Titan probe was similar to that described by Liu, Sanford, Ellison, Fitzpatrick, Gorga, & Keefe, 2008. Soft plastic disposable probe tips were fitted to the probe to accommodate a variety of infant ear sizes with hermetic sealing needed for tympanometry. Data were recorded using custom software running on a personal computer with a two-channel CardDeluxe sound card (22.05 kHz sample rate, 24 bit converters) and RS-232 serial port using custom software. Detailed methods used to calibrate and measure wideband reflectance tympanograms are described in Keefe, Hunter, Feeney & Fitzpatrick (2015). Briefly, the probe was inserted into one end of each of a pair of cylindrical rigid-walled tubes with a cross-sectional area of 0.1781 cm2. The tubes were closed at their far ends with nominal lengths of 236,5 cm and 5.9 cm. A brief acoustic click was presented at a fixed voltage level to the sound source within the probe and the pressure response was measured by the probe microphone. The reflectance calibration procedure was based on calculating the source pressure reflectance of the probe and the incident pressure spectrum of the click for frequencies between 0.2 and 8 kHz. The source reflectance and incident pressure were calculated using the acoustic measurements in the pair of tubes and by fitting the tube data to a model of viscothermal sound transmission in each cylindrical tube. Once this calibration was completed, the electrical stimulus level of the click was maintained constant in all ear tests, and was the same for tests at ambient pressure and in the tympanometric sweeps. Wideband acoustic reflectance tests were performed over a frequency range from 0.25 to 8 kHz. Three-dimensional WB pressure-reflectance tympanograms were measured with absorbance and group delay as a function of induced ear-canal air pressure and frequency.
Energy reflectance has a major advantage at high frequencies in that it is insensitive to probe location within the ear canal under the assumptions that the ear-canal wall is immobile and wall losses are small (Stinson, Shaw & Lawton, 1982). A complication in reflectance testing of infant ears is that the ear-canal wall is highly compliant and lossy below 1 kHz, and especially so in the first five months of life (Keefe et al., 1993). The group delay measurements in the present study provided new data relevant to how wall motion in infants may influence the sound field in the infant ear canal.
WB tympanograms were obtained with pressure swept from +200 daPa to −300 daPa, in both descending and ascending directions, with an ambient absorbance group-delay measure taken in between the tympanogram measures. Preliminary testing in infants showed that starting the test battery with the down-swept test had advantages of confirming a hermetic seal and minimizing effects of ear-canal collapse on the ambient reflectance. The acoustic response to a train of clicks, with an inter-click interval of 46 ms, was measured during the pressure sweep in each direction. The typical total SPL of the click train used to measure the WAI responses was 69 dB in both ambient and tympanometric tests. This SPL was similar across the age range of infants tested. The acoustic absorbance and group delay were measured for each click response as joint functions of air pressure and frequencies from 0.25 to 8 kHz, and were analyzed as three-dimensional tympanograms.
The tympanometric information in the joint function of frequency and pressure was reduced to pairs of tympanograms that varied across frequency at fixed tympanometric pressures, and pairs of tympanograms that varied across pressure after first averaging over a low-frequency and a high-frequency interval. This data reduction to a much smaller set of responses was helpful in analyzing wideband tympanograms in groups of test ears.
Because of the maturation of middle-ear response from infancy to adulthood, it has proven to be convenient to define low-frequency and high-frequency bandwidths differently at different ages. For infants younger than six months, which included the newborn and 1-month ages in the present study, the low-frequency bandwidth was 0.71 to 1.414 kHz, i.e., a one-octave band centered at 1 kHz. For infants of age six months and older in the present study (and older children and adults), the low-frequency bandwidth was 0.354 kHz up to 1.414, i.e., the full two octaves with center frequencies of 0.5 and 1 kHz. For all infants, the high-frequency bandwidth was 1.41 to 8 kHz. The difference in definitions is that the low-frequency bandwidth extends down to 0.354 kHz for older infants, and down to 0.71 kHz for newborns and one-month-olds. The latter controls for additional noise and immaturities in ear-canal wall mobility below 0.7 kHz. This is somewhat inconvenient for interpreting the subset of tympanometric variables that use these low- and high-frequency averages, because the low-frequency bandwidth varies with age. This is offset by the increased ability to interpret wideband tympanograms in younger infants. Other tympanometric variables analyzed in the present study (as described below) do not rely upon these averaging bandwidths, e.g., the absorbance and group delay responses versus frequency at the tympanometric peak pressure (TPP), and at each of the positive and negative tail pressures.
The reflectance measurement system was calibrated daily using a procedure based on responses to the click stimulus (Keefe et al., 2015). Based on click recordings in the ear canal of each test subject and the results of this calibration, the reflectance was measured under ambient-pressure conditions in the ear canal and in a tympanometric pressure sweep. This ear reflectance was calculated based on an acoustic estimate of the ear-canal area at the probe tip.
3.1 ANALYSIS AND STATISTICS
Descriptive statistics were used to summarize sample demographics and outcome measurements. The outcomes variables were grouped into 15 sets as summarized in Table 2. These sets consisted of absorbance and group delay at half-octave frequencies, with each of these measured at ambient ear-canal pressure in the ambient measurement, and at positive and negative tail pressures as well as at the TPP in the tympanometric measurement. The operational definition of TPP in the present study was the pressure at which the low-frequency averaged absorbance tympanogram attained its maximum value. The acoustically estimated ear-canal area and length were obtained from the ambient measurement. Other quantities estimated from the reflectance tympanogram included the tympanometric width and height at the positive and negative tail values relative to the height at TPP, as well as the half-width at the positive and negative tympanometric tail values. The half-width was the pressure difference over which the low-frequency-averaged absorbance decreased to a value halfway between the absorbance at TPP and the absorbance at the positive or negative tail pressure. The positive and negative half-widths allowed an assessment of the asymmetry of the absorbance tympanogram on the positive- and negative-tail side of the TPP.
Table 2.
Statistical results of linear mixed repeated measures model for corrected age at testing, gestational age at birth, nursery group, and race on wideband ambient and tympanometry variables. Significance levels found in analysis: 1p <0.05, 2p <0.01, 3p <0.001, 4p <0.0001. If only one notation is given for a group of variables, they all reached the same level of significance.
| Outcomes | Corrected Age | Gestational Age | NICU | Race | Corrected Age * Race |
|---|---|---|---|---|---|
|
Ambient
Absorbance |
40.25, 0.35, 0.5, 0.7, 1, 1.4, 2,3,4,6,8 kHz |
14,36,48 kHz | 24,6 kHz | ||
|
| |||||
|
Ambient Group
Delay |
40.25,0.35 ,0.5,0.7, 11, 1.4,2,33, 44,6,8 kHz |
10.35,1.4,2 kHz | 26 kHz | 26 kHz | 16 kHz |
|
| |||||
|
Downswept
Absorbance TPP |
40.25, 0.35, 0.5, 0.7, 1, 1.4, 2,3,4,6,8 kHz |
10.25, 20.35,10 .5 kHz |
26,8 kHz |
26 kHz | |
|
| |||||
|
Downswept
Absorbance +200 daPa |
40.25, 0.35, 0.5, 0.7, 1, 1.4, 2,3,4,6,8 kHz |
11.4,3 kHz | 24,46,28 kHz | ||
|
| |||||
|
Downswept
Absorbance −300 daPa |
40.25, 0.35, 0.5, 0.7, 1, 1.4, 2,3,4,6,8 kHz |
40.25,11.4 kHz |
14,26,8 kHz |
14,26,18 kHz |
24,6 kHz |
|
| |||||
|
Downswept
Group Delay TPP |
40.25, 20.35, 0.5, 0.7, 41, 1.4, 2,3,4 kHz |
32,13 kHz |
12,3,26, 8 kHz |
||
|
| |||||
|
Downswept
Group Delay +200 daPa |
40.25,0.5, 0.7,4,26, 48 kHz |
20.35 kHz |
18 kHz | ||
|
| |||||
|
Downswept
Group Delay −300 daPa |
40.25, 0.35, 30.5, 0.7, 21, 42, 3,14,46 kHz |
21,11,4,3 kHz | 11 kHz | ||
|
| |||||
|
Upswept
Absorbance TPP |
40.25,0.35 ,0.5,0.7,1, 1.4, 2,3,4,6, 8 kHz |
18 kHz |
11,4,26,3 8 kHz |
||
|
| |||||
|
Upswept
Absorbance +200 daPa |
40.25,0.35 ,0.5,0.7,1, 1.4, 2,3,4,6, 8 kHz |
13 kHz |
24,36,28 kHz |
||
|
| |||||
|
Upswept
Absorbance −300 daPa |
40.25,0.35 ,0.5,0.7,1, 1.4,2,3,4, 6, 8 kHz |
11,1.4,8 kHz |
14,26, 18 kHz |
14,6,8 kHz |
24,6 kHz |
|
| |||||
|
Upswept Group
Delay TPP |
40.25,0.35 ,0.5, 10.7, 43, 24, 16, 8 kHz |
10.25, 2,4 kHz |
10.7,1,8 kHz |
||
|
| |||||
|
Upswept Group
Delay +200 daPa |
40.25,10.3 5,40.5,0.7, 31,43, 4, 6, 8 kHz |
20.35 kHz | |||
|
| |||||
|
Upswept Group
Delay −300 daPa |
40.25,0.35 ,0.5,0.7,1, 1.4,22,33,4 4,26, 48 kHz |
21,18 kHz |
21,16,28 kHz |
||
|
| |||||
|
Tympanometric
Peak Pressure (TPP) |
4average, d, u |
2average, d, 1u | |||
|
| |||||
|
Tympanometric
Width (TW) |
4full, p, n | 2p, 1n | 1n | ||
|
| |||||
| Δ Absorbance Low Freq. |
4p, n | 1n | |||
|
| |||||
| Δ Absorbance High Freq. |
4p, n | ||||
|
| |||||
| Δ Group Delay Low Freq. |
3p, 4n | ||||
|
| |||||
| Area | 4 | 2 | |||
|
| |||||
| Length | 4 | 1 | 1 | ||
Δ Absorbance is the difference in the absorbance at TPP and the absorbance at the positive (p) or negative (n) tail pressure, +200 and −300 daPa, respectively. The d denotes the downswept tympanogram, and the u denotes the upswept tympanogram. The high freq. bandwidth extends from 2 to 8 kHz; the low freq. bandwidth extends from 0.8 to 2 kHz. With the same conventions, Δ Group Delay is the difference in the group delay at TPP and the group delay at the positive (p) or negative (n) tail pressure.
Analysis of the ambient and tympanometric variables was conducted within a longitudinal repeated-measure design using a linear mixed model with frequency as the repeated measure to study changes with age. All analyses controlled for gestational age at birth (prematurity), birth group (NICU or well-baby nursery) and race (Caucasian or not Caucasian). The following results describe the overall significant age-related effects based on least square (LS) means and 95% confidence intervals (CIs) provided in the figures and tables. Interaction terms for significant main effects were explored where appropriate. Data were analyzed employing SAS statistical software, version 9.3 (SAS Institute, Cary, N.C.). A two-sided significance level was set at 0.05.
4.1 RESULTS
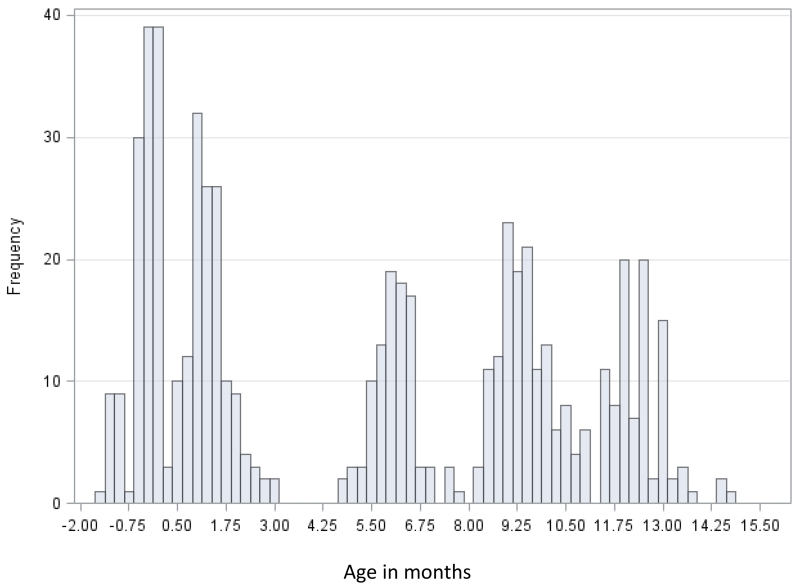
Table 1 details the demographic characteristics of the 182 infants included in the longitudinal study. The sample was comprised of 54% males, with race classified as Caucasian (60%), African-American (30%), and Other (10%). Because the WAI data for ears were highly correlated in preliminary analysis, one ear with the most complete longitudinal data was selected from each infant for the normative analysis, and the group data were equally balanced between right and left ears. Overall, about 1/3 of infants had one or more risk factors for hearing loss, primarily family history and NICU stay. The distribution of ages at the time of initial assessment, corrected for premature birth, is shown in Figure 1. Correction was done according to the number of weeks post-menstrual, with 38 weeks considered to be full term.
Table 1.
Demographics of study sample. Summary statistics are provided in terms of the mean and standard deviation (SD).
| Variable | |
|---|---|
|
| |
| Gestational age (weeks): Mean(SD), Range | 37.5 (3.3), 26-41 |
|
| |
| Gender: n (%) | |
| Male | 99 (54.4) |
| Female | 83 (45.6) |
|
| |
| Race: n (%) | |
| Black or African American | 54 (29.7) |
| White or Caucasian | 109 (59.9) |
| Other | 19 (10.4) |
|
| |
| Ethnicity: n (%) | |
| Hispanic/Latino | 3 (1.7) |
| Non-Hispanic/Latino | 179 (98.4) |
|
| |
| Birth weight (gram): Mean (SD), Range | 3053.9 (809.8), 760-4340 |
|
| |
| Group: n (%) | |
| NICU | 37 (20.3) |
| Normal infants | 145 (79.7) |
|
| |
| Risk factor: n (%) | |
| Family history | 14 (7.7) |
| Stigmata | 0 (0) |
| IU infection | 2 (1.1) |
| Ototoxic drugs | 28 (15.4) |
| Hyperbilirubinemia | 25 (13.7) |
| Low birth weight | 14 (7.7) |
|
| |
| Have any risk factors: n (%) | 54 (29.7) |
|
| |
| Ear: n (%) | |
| Left | 295 (49.9) |
| Right | 321 (50.1) |
Figure 1.
Distribution of age in months corrected for prematurity in the longitudinal sample.
4.1.1 Ambient group measurements
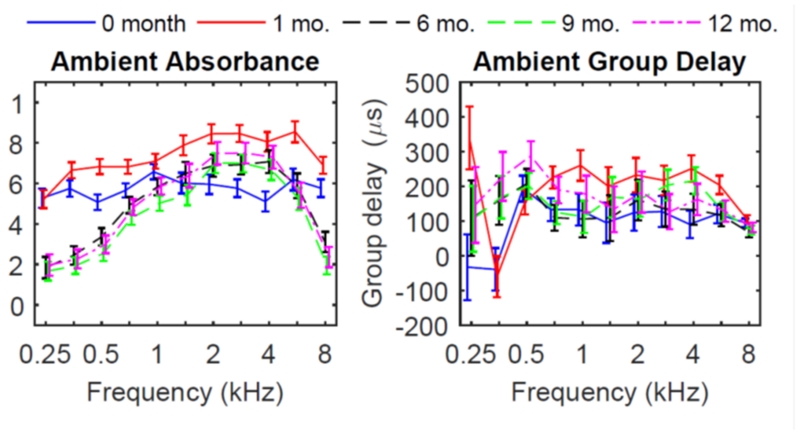
The mean ambient absorbance across the frequency range changed substantially from birth (screening) to 12 months of age, as shown in Figure 2 (left panel), and summarized in Table 2. The mean absorbance at all frequencies showed a highly significant age effect from birth to 1 year. Mean absorbance in newborns was relatively flat across all frequencies, and was more variable compared to older ages. Maximal mean absorbance was obtained at 1 kHz, and declined slightly in the high frequencies. At one year of age, the mean absorbance showed a smoothly increasing-decreasing pattern, with relatively less absorbance below 1 kHz, maximal absorbance from 2 - 4 kHz, and less absorbance above 4 kHz, relative to newborns and younger ages. Thus, the newborn ear had relatively more absorbance in low frequencies and less at high frequencies compared to 1 year-old ears. A large increase in absorbance occurred for the 1 month-olds relative to newborns, particularly from 1 - 8 kHz. A smaller increase in absorbance from birth to one month was observed below 1 kHz. The largest change in absorbance occurred between 1 and 6 months, with a large decrease in absorbance at older ages below 1 kHz, and smaller decreases above 1 kHz.
Figure 2.
Ambient absorbance (left) and group delay (right) for each age group, with model estimated mean and 95% confidence intervals.
In addition to the age effect, a significant effect of race was found from 4-8 kHz, in which the mean ambient absorbance was lower in non-Caucasians. There was no significant effect of NICU compared to the well-baby group for the mean ambient absorbance at any frequency. Because age and race were both significant, interaction between these variables was explored. Results showed an interaction between age and race from 6 to 8 kHz, indicating that the effect of race was due to an age effect.
Ambient group delay revealed complex changes across frequencies and age (Figure 2, right panel). Group delay was lower at 0.25-0.35 kHz at birth relative to older ages, and showed resonant properties with delays less than 0 μsec. Group delay increased with age in these low frequencies. At age 1 month, group delay was high at 0.25 kHz (325 μsec) relative to all other ages, then decreased to below 0 μsec at 0.35 kHz, and then increased to about 150 μsec at .5 kHz. A notable feature in the low frequencies was a high degree of variability in group delay, especially below 0.5 kHz. This variability may be related to increased noise at low frequencies, and to individual differences in compliant ear canal wall effects in infants at this age. In addition, the mean group delay at 0.35 kHz was much smaller than at 0.25 kHz or 0.5 kHz when compared to their respective CIs. This may be related to the resonant wall effect theory described in Keefe et al. (2015) for two ear tests in one-month-olds. For all ages except 1 month olds, the mean group delay was larger at 0.5 kHz than at higher frequencies. In addition to the age effects, a significant effect of race was found for group delay at 6 kHz, with a significant interaction between race and age. There was a significant effect of NICU compared to the well-baby group only at 6 kHz.
4.1.2 Tympanometric measurements
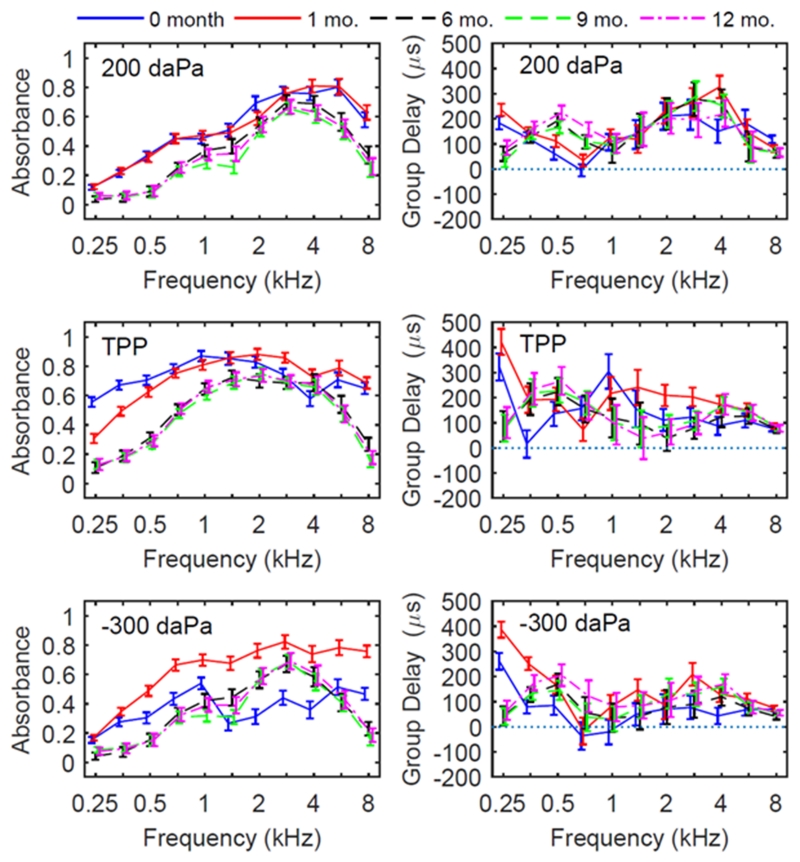
Downswept tympanometric absorbance was analyzed at the two extrema of the pressure sweep (200 daPa and −300 daPa), as well as at TPP. At the positive pressure point (200 daPa), absorbance across frequency showed significant age effects at all frequencies (Figure 3, top left panel, and Table 2). However, markedly different patterns were found in the tympanometric absorbance (Fig. 3, top left) at +200 daPa compared to ambient absorbance, TPP and −300 daPa (Fig. 2, left panels), in that developmental changes were smaller for absorbance across frequency, absorbance had a similar shape across ages and was more adult-like, with a smoothly increasing peak at 2-4 kHz. That is, with positive pressurization of the ear canal wall, less absorbance was apparent in the younger age groups below 1 kHz. At −300 daPa, developmental effects were accentuated in that the newborn group showed much less absorbance at all frequencies compared to the 1 month and older age groups. A significant effect of race was found for the mean absorbance at the positive tail at 6 and 8 kHz, but no significant interactions between age and race were found (Table 2).
Figure 3.
Downswept tympanometric absorbance (left panels) and group delay (right panels) for each age group, with model estimated mean and 95% CIs. Measures were taken from the ear-canal induced pressure, as noted in the top left of each panel. The direction of pressure change for tympanometry was from positive to negative pressures (downswept).
At TPP (Fig. 3, middle left), the mean absorbance patterns were qualitatively similar to the mean absorbance at ambient pressure (Fig. 2). However, for the newborns the mean absorbance at TPP was slightly higher overall than for the ambient condition. This was likely due to the dynamic effects of opening the ear canal and avoiding collapse of the canal at the initial pressurization to +200 daPa. At the negative ear-canal pressure extrema (-300 daPa), larger age differences between newborns and age 1 month were observed than at the +200 daPa or TPP conditions, and effects were more complex across frequencies for the newborns (Fig. 3, bottom left). Newborn ears had a marked decrease in absorbance at high frequencies relative to older ages. At age one month, the mean absorbance was increased across all frequencies relative to other age groups. At 6 months and older, patterns at 200 daPa (Fig. 3, top left) were similar to +200patterns at −300 daPa, indicating that the effects of pressurization were more symmetrical than at younger ages.
For the downswept tympanograms at +200 daPa, the mean group delay for newborns and 1-month olds was larger at 0.25 kHz and smaller at 0.5 and 0.71 kHz than for older infants (Fig. 3, top right). In the frequency range from 2 - 6 kHz, developmental effects on group delay were not systematic, in that newborns had less group delay, 1 month-olds had higher group delay, and older infants were in between these extremes. For mean group delay at TPP in the downswept tympanogram (Fig. 3, middle right), there were significant age effects at all frequencies except 6 and 8 kHz (see Table 2). For mean group delay at −300 daPa, age was significant at all frequencies except 8 kHz (Table 2). However, the overall patterns for mean group delay across age were complex and typically non-systematic across frequency (Fig. 2, bottom right panel). In general, the mean group delay was larger below 0.5 kHz in the negative-tail response compared to the positive-tail response.
In Table 2, the fact that gestational age was significant for mean group delay and mean absorbance at some frequencies at the negative tail pressure but not at the positive tail pressure shows that group delay is sensitive to age-dependent pressurization effects. It is notable that the mean group delay at 8 kHz in both the ambient response and the downswept tympanometric responses at all three pressures was just below 100 μs with relatively small CIs indicating the lack of pressurization effects at this frequency.
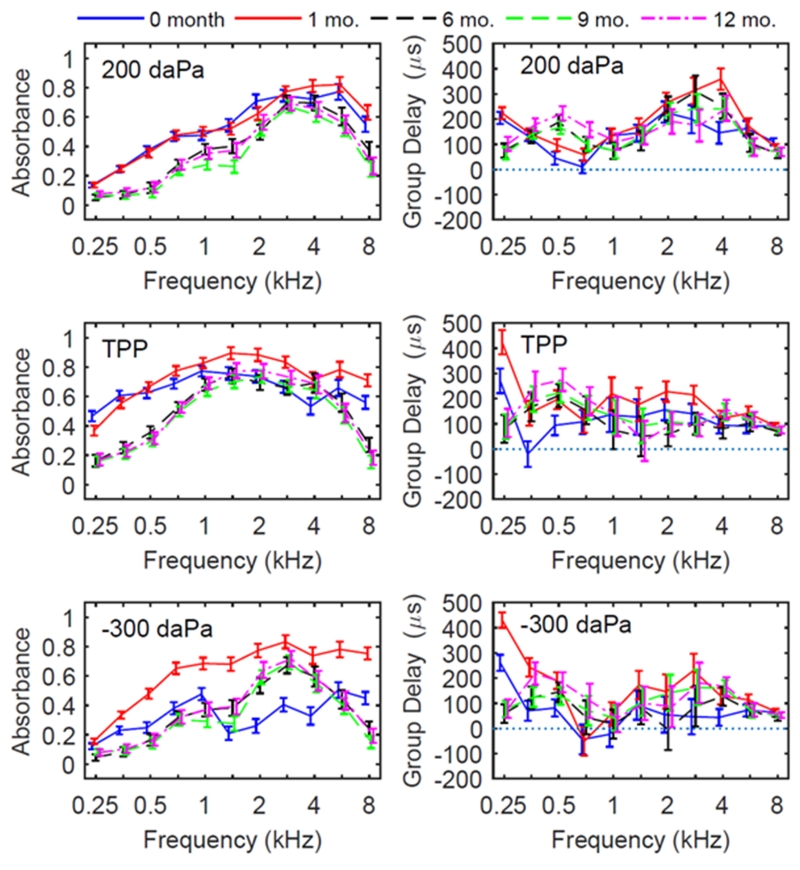
Absorbance and group delay were analyzed for upswept tympanograms in the same manner as for downswept tympanograms at the TPP and two extremal sweep pressures. For mean absorbance at the positive pressure tail of the upswept tympanogram, the mean absorbance varied across frequency and age groups in a generally similar manner as for the downswept tympanogram (Fig. 4, left column compared to Fig. 3, left column).. Highly significant age effects were present at all frequencies (see Table 2). Upswept group delay patterns for TPP, positive and negative tails were also qualitatively similar to the corresponding patterns in downswept tympanometry. One exception to this general summary was that the upswept group delay was larger at 0.25 kHz for newborns and 1-month olds, and smaller from 0.5-1 kHz relative to older infants (Fig. 4, right column compared to Fig. 3, right column).
Figure 4.
Upswept tympanometric absorbance (left panels) and group delay (right panels) for each age group, with model estimated mean and 95% CIs. Measures were taken from ear canal induced pressure, as noted in the top left of each panel. The direction of pressure change for tympanometry was from negative to positive pressures (upswept).
Elementary tympanometric variables were analyzed for upswept and downswept tympanograms. These features derived from the tympanograms included the TPP, tympanometric width, and the height difference of the tympanogram at the TPP relative to either the positive or negative tails. The elementary features from the ambient test included estimates of the ear canal area at the probe and the length between the probe tip and a mid-point region of the TM. This mid-point region was assumed to be 4 mm back from the most interior point of the ear canal. The mean estimates and 95% CIs for these additional tympanometric variables are provided in Table 3.
Table 3.
Other tympanometric variables analyzed by age group, including Tympanometric Peak Pressure (TPP). All group delays measured in μs. Δ: Change in values relative to baseline as specified.
| Outcome | 95% CI | |||
|---|---|---|---|---|
|
| ||||
| LS mean | Lower | Upper | ||
|
| ||||
| Downswept TPP (daPa) |
Age Group | |||
| Birth | −104 | −124 | −85 | |
| 1 month | −146 | −165 | −127 | |
| 6 months | −46 | −69 | −24 | |
| 9 months | −65 | −85 | −46 | |
| 12 months | −71 | −93 | −48 | |
|
| ||||
| Upswept TPP (daPa) |
Age Group | |||
| Birth | 1 | −19 | 21 | |
| 1 month | −114 | −133 | −95 | |
| 6 months | −25 | −48 | −2 | |
| 9 months | −39 | −59 | −20 | |
| 12 months | −41 | −64 | −18 | |
|
| ||||
| TW (daPa) | Age Group | |||
| Birth | 259 | 241 | 277 | |
| 1 month | 277 | 260 | 295 | |
| 6 months | 212 | 192 | 232 | |
| 9 months | 198 | 180 | 216 | |
| 12 months | 206 | 186 | 226 | |
|
| ||||
| TW half-width (TPP to +200 daPa) |
Age Group | |||
| Birth | 135 | 121 | 148 | |
| 1 month | 126 | 113 | 139 | |
| 6 months | 82 | 67 | 97 | |
| 9 months | 74 | 61 | 88 | |
| 12 months | 81 | 66 | 96 | |
|
| ||||
| TW half-width (TPP to −300 daPa) |
Age Group | |||
| Birth | 76 | 67 | 85 | |
| 1 month | 51 | 42 | 59 | |
| 6 months | 85 | 75 | 95 | |
| 9 months | 82 | 73 | 90 | |
| 12 months | 84 | 74 | 94 | |
|
| ||||
| Δ Low Freq Absorbance (+200 daPa to TPP) |
Age Group | |||
| Birth | 0.40 | 0.37 | 0.43 | |
| 1 month | 0.34 | 0.31 | 0.37 | |
| 6 months | 0.27 | 0.24 | 0.31 | |
| 9 months | 0.32 | 0.29 | 0.35 | |
| 12 months | 0.30 | 0.27 | 0.34 | |
|
| ||||
| Δ Low Freq Absorbance (−300 daPa to TPP) |
Age Group | |||
| Birth | 0.37 | 0.34 | 0.41 | |
| 1 month | 0.12 | 0.09 | 0.16 | |
| 6 months | 0.22 | 0.18 | 0.26 | |
| 9 months | 0.27 | 0.23 | 0.31 | |
| 12 months | 0.26 | 0.21 | 0.30 | |
|
| ||||
| Δ High Freq Absorbance (+200 daPa to TPP) |
Age Group | |||
| Birth | −0.03 | −0.06 | −0.01 | |
| 1 month | 0.05 | 0.02 | 0.07 | |
| 6 months | 0.01 | −0.03 | 0.04 | |
| 9 months | 0.03 | −0.01 | 0.05 | |
| 12 months | 0.01 | −0.02 | 0.04 | |
|
| ||||
| Δ High Freq Absorbance (−300 daPa to TPP) |
Age Group | |||
| Birth | 0.30 | 0.27 | 0.33 | |
| 1 month | 0.02 | −0.01 | 0.05 | |
| 6 months | 0.10 | 0.06 | 0.13 | |
| 9 months | 0.07 | 0.04 | 0.10 | |
| 12 months | 0.06 | 0.02 | 0.09 | |
|
| ||||
| Δ Low Freq Group Delay (μs; +200 daPa to TPP) |
Age Group | |||
| Birth | 125 | 70 | 181 | |
| 1 month | 87 | 34 | 140 | |
| 6 months | 20 | −43 | 83 | |
| 9 months | 23 | −32 | 78 | |
| 12 months | −15 | −78 | 49 | |
| Birth Group | ||||
| NICU | 57 | 29 | 85 | |
| Normal | 40 | −33 | 113 | |
|
| ||||
| Δ Low Freq Group Delay (μs; −300 daPa to TPP) |
Age Group | |||
| Birth | 235 | 170 | 300 | |
| 1 month | 94 | 32 | 156 | |
| 6 months | 69 | −4 | 143 | |
| 9 months | 85 | 20 | 149 | |
| 12 months | 20 | −54 | 95 | |
| Birth Group | ||||
| NICU | 107 | 75 | 140 | |
| Normal | 94 | 9 | 179 | |
|
| ||||
| Δ High Freq Group Delay (μs; +200 daPa to TPP) |
Corrected Age | |||
| Birth | −71 | −105 | −36 | |
| 1 month | −34 | −67 | −1 | |
| 6 months | −67 | −106 | −28 | |
| 9 months | −42 | −76 | −8 | |
| 12 months | −27 | −66 | 13 | |
| Birth Group | ||||
| ICU | −43 | −61 | −26 | |
| Normal | −53 | −99 | −7 | |
|
| ||||
| Δ High Freq Group Delay (μs; −300 daPa to TPP) |
Age Group | |||
| Birth | 36 | 18 | 54 | |
| 1 month | 37 | 20 | 55 | |
| 6 months | 22 | 1 | 43 | |
| 9 months | 16 | −2 | 34 | |
| 12 months | 10 | −11 | 31 | |
|
| ||||
| Ear Canal Area (cm2) |
Age Group | |||
| Birth | 0.06 | 0.05 | 0.08 | |
| 1 month | 0.10 | 0.08 | 0.11 | |
| 6 months | 0.24 | 0.22 | 0.25 | |
| 9 months | 0.25 | 0.23 | 0.26 | |
| 12 months | 0.22 | 0.20 | 0.24 | |
|
| ||||
| Ear Canal Length (cm) |
Age Group | |||
| Birth | 1.36 | 1.14 | 1.59 | |
| 1 month | 1.99 | 1.78 | 2.21 | |
| 6 months | 0.97 | 0.71 | 1.23 | |
| 9 months | 1.13 | 0.90 | 1.36 | |
| 12 months | 1.26 | 1.00 | 1.51 | |
Statistical results for WAI variables on corrected age at test, gestational age at birth, NICU status, and race are shown in Tables 2 and 3. Nearly all of the tympanometric absorbance variables showed a highly significant age effect. TPP showed a significant overall age effect for downswept and upswept tympanograms, and the difference in TPP between these pressure directions as also significant. The downswept tympanogram tended to have a more negative TPP value than the upswept tympanogram at all ages. TPP was the most negative at 1 month of age for both pressure directions, and was less negative for older ages. Tympanometric full width showed a significant age effect, and tended to decrease with age, as did the tympanometric half-widths on the positive side of the tympanogram. There was an overall age effect for the tympanometric half-width on the negative side of the tympanogram, but it was not systematic. Low-frequency averaged absorbance relative to the positive tail value changed with age, primarily from birth to six months. Low frequency averaged absorbance relative to the negative-tail value, and high frequency averaged absorbance relative to the positive- and negative-tail values changed with age, primarily from birth to one month. In Table 3, the ear-canal area estimates at the probe tip increased systematically from 0.06 cm2 at birth to 0.22 cm2 at 12 months. The CIs at birth and at age 1 month did not overlap with one another, and were well separated from the CIs at ages of 6 months and older. The CIs at 6, 9 and 12 months overlapped one another, which suggests that the maturation of ear-canal area rapidly changes between birth and 6 months. Ear-canal area also changed significantly with corrected age (Table 2), and there was a significant interaction of corrected age and race.
In older infants, the ear-canal length estimates from the probe tip to the mid-TM region increased systematically from 0.97 cm at 6 months to 1.26 cm at 12 months with partially overlapping CIs. The ear-canal length changed significantly with corrected age (Table 2). Estimates of ear canal length at birth and 1 month were larger than at the older ages, which is the opposite result of the expected trend that the ear canal lengthens with increasing age. The acoustic estimate of length is influenced by the presence of ear-canal wall mobility, which is particularly important in the newborn and 1-month age groups (Keefe et al., 2015). Another factor in the maturation of the ear canal is that the relative angle of the TM increases with increasing age in the first year of life, and this also affects the definition of the location of the mid-TM location. A main effect for race and an interaction of age and race was significant for the estimated ear-canal length.
The internal consistency or reliability for absorbance and group delay variables across the seven half-octave frequencies was estimated using the Cronbach α statistic (Cronbach, 1951). Cronbach’s alpha is a measure of internal consistency, that is, how closely related are a set of items as a group. For comparing groups, as in the age groups for this study, Cronbach α values of 0.7 to 0.8 are regarded as satisfactory. For clinical application, higher values than 0.8 are recommended. The Cronbach α was large (α>0.87, Table 4) for ambient absorbance, and for downswept and upswept tympanometric absorbance at TPP and positive and negative tail pressures. The reliability of group delay across the seven half-octave frequencies was smaller, in the range of α from 0.51 to 0.70 for ambient group delay, downswept tympanometric group delay (at TPP and the positive and negative tail pressures), and upswept tympanometric group delay (at TPP and the positive and negative tail pressures). Thus, the absorbance measurements were more internally reliable than the group delay measurements. It is not surprising that the absorbance variables are more internally consistent, since they are less affected by probe position, while group delay could vary across frequencies due to probe position in the ear canal.
Table 4.
Reliability coefficients among the outcome groups.
| Outcome groups | Cronbach Alpha |
|---|---|
| Ambient Absorbance | 0.91 |
| Ambient Group Delay | 0.59 |
| Down Swept Tymp Absorbance at TPP | 0.89 |
| Down Swept Tymp Absorbance at 200 daPa | 0.87 |
| Down Swept Tymp Absorbance at −300 daPa | 0.90 |
| Down Swept Tymp Group Delay TPP | 0.51 |
| Down Swept Tymp Group Delay at 200 daPa | 0.60 |
| Down Swept Tymp Group Delay at −300 daPa | 0.70 |
| Upswept Tymp Absorbance at TPP | 0.89 |
| Upswept Tymp Absorbance at 200 daPa | 0.88 |
| Upswept Tymp Absorbance at −300 daPa | 0.90 |
| Upswept Tymp Group Delay at TPP | 0.58 |
| Upswept Tymp Group Delay at 200 daPa | 0.59 |
| Upswept Tymp Group Delay at −300 daPa | 0.59 |
5.1 DISCUSSION
The timeline of infant ear-canal development from birth to 1 year has not previously been described in infants confirmed to have normal hearing status. This is important, since mild conductive impairments are common in newborns and infants, and could affect normative values. The infants in the present study were diverse in terms of racial characteristics and there was a substantial proportion of infants who were premature and cared for in the NICU. The large sample size, balance between right and left ears, inclusion of infants cared for in the NICU, diversity in races and as well as longitudinal measurement over the first year after birth give a more complete picture of development. The addition of group delay measures, not previously reported in infants with either wideband ambient or tympanometric tests, also provide more insight into the mechanism of developmental changes in wideband reflectance.
Several studies have reported ambient energy reflectance or absorbance in infants of different ages in cross-sectional designs (Keefe et al., 1993; Sanford and Feeney, 2008; Shahnaz, 2008; Shahnaz, Cai & Qi 2014; Sanford, Keefe, Liu, Fitzpatrick, McCreery, Lewis, & Gorga 2009; Hunter et al., 2010; Aithal et al., 2015). Overall, these studies are in agreement with the present report in regard to decreased absorbance as age increases below 1 kHz and above 4 kHz, combined with increased absorbance from 1-4 kHz as age increases. This developmental trend results in a more defined pattern with a maximum around 2 kHz that more closely follows the adult absorbance pattern at age 6 months and older. In the present study, one month-old infants showed higher absorbance across the frequency range compared to both younger (at birth) and older infants. Absorbance increased from newborn to one-month of age, but then decreased thereafter with changes in the frequency shape. The frequency shape changed from relatively flat across frequencies at birth, to a peaked pattern more similar to adult ears by 6 months of age. The finding of a shift from lower absorbance at birth to greater absorbance at 1 month, and then a reversal by 6 months agrees with findings in Keefe and Abdala (2007) for ambient energy reflectance at frequencies between 0.3 and 0.8 kHz. However, their study tested a much smaller number of infants, did not specifically describe whether the study design was purely longitudinal, and did not include tympanometric measurements. Shahnaz, Miranda & Polka (2014) studied infants longitudinally between birth and 6 months, and did not show such a reversal. However, that study also tested a much smaller number of infants, and measured only ambient measurements with a different system (Mimosa Acoustics). The present study found no reversal in tympanometric absorbance at TPP at low frequencies up to 0.5 kHz (Fig. 3, left middle). There was substantial overlap in groups CIs at most higher frequencies.
These age-related shifts in absorbance were accompanied by a shift in group delay from less delay at birth, to greater delays at 1 month in the frequency region from 0.5 - 6 kHz. The most likely explanation, with complementary temporal bone evidence, is that the newborn ear has remnants of amniotic fluid that resorbs by 1 month of age. Amniotic fluid would be expected to behave similarly to middle ear fluid, which can cause a flattened absorbance curve as seen in these infant ears at birth, despite having passed newborn hearing screening.
Group delay results have not previously been reported in a developmental study. Complex patterns in aural acoustic responses in infants below 4 months of age have been reported across a number of measures, including energy reflectance, absorbance, admittance tympanometry, and admittance phase angle. These patterns are likely related to ear canal wall flaccidity, as the more mass-like tissue in newborn ears may result in resonant notches at low frequencies, in contrast to the stiffness-controlled ear canal of adults.
Such complex response patterns, including the presence evidence of ear-canal wall compliance effects, were present in two reflectance measurements in one-month-old infants. These patterns were explained using a model in which the ear-canal admittance at the probe is the parallel combination of the admittance of the ear-canal wall mobility and the admittance directed into the ear canal towards the TM (Keefe et al., 2015). This model appears consistent with the present results showing a low group delay at 0.35 kHz in newborn and one-month-old infants. This was predicted in the form of a low-frequency resonance in the admittance related to ear-canal wall compliance (and related losses). Because this resonance frequency would vary across individual ears of young infants, the group averages in the present study would produce a smoothed group-delay response.
These results are consistent with the known developmental course of ossification of the ear canal structures including the tympanic ring and the ossicular chain (Eby & Nadol, 1986; Saunders, Kaltenbach, & Relkin, 1983). The mobility of the ear-canal wall is diminished as the ear-canal wall ossifies. These results also provide a physiologic mechanism for the otherwise paradoxical finding that newborns with middle ear effusion show normal tympanometric admittance at low probe tone frequencies (Paradise, et al., 1976; Marchant et al., 1986). Significant developmental changes are apparent through at least 6 months of age, consistent with reports from multifrequency tympanometry (Holte et al., 1991); ambient wideband energy reflectance (Keefe et al., 1993; Hunter, Bagger-Sjoback, & Lundberg, 2008; Shahnaz, Cai and Qi, 2015) and tympanometric energy reflectance (Sanford and Feeney, 2008).
Absorbance measured at ambient pressure was distinctly different in these ears with normal hearing function from the absorbance measured in the tympanometric tests at TPP, across all ages from birth to 12 months. The absorbance curves in the ambient condition were flatter for newborns and 1-month-olds, while measures at TPP were more rounded, with maximal absorbance from 1-4 kHz. In contrast, absorbance for ambient compared to TPP conditions was similar for 6-12 month olds. The direction of pressure change (downswept versus upswept tympanometry) had a relatively minor effect on group absorbance data for the positive and negative tails. At TPP, absorbance was higher in the low frequencies than at ambient pressure, particularly for the newborns. This indicates an effect of ear canal flaccidity in that the absorbance may be decreased due to closing of the ear canal with initial pressurization to negative air pressure values in the upswept direction. The mean group delays were similar for downswept and upswept pressure directions. Differences between ambient and TPP absorbance reflect greater energy absorbance by the middle ear under pressurized conditions that equilibrate middle-ear pressure, at least in older infants and adults, even in the presence of normal hearing and middle-ear function. Thus, wideband absorbance measures are expected to differ for ambient pressure and TPP measures.
The newborn infant ear has the additional complication that a positive air pressure within the ear canal would tend to open the ear canal wider due to its extremely compliant walls, and a negative air pressure would tend to close the ear canal. Thus, the TPP estimate in the newborn ear may be confounded by ear-canal volume changes, or ear-canal wall collapse. To that extent, more research is needed to interpret the TPP in newborn ears relative to the task of estimating the air pressure in the tiny middle-ear cavity on the opposite side of the TM. Tympanometric width (measured halfway down from the peak of the tympanogram) and the height of the absorbance for both positive and negative tail pressures showed developmental effects as well. Thus, age-related normative values are recommended for clinical applications of these measures.
Reliability among absorbance variables was high across frequencies, indicating that absorbance is an internally consistent test measure. Test-retest reliability of wideband energy reflectance has also been found to be high in infants (Vander Werff, Prieve, & Georgantas, 2007). Thus, wideband absorbance demonstrates both internal consistency as well as test-retest reliability. These factors are important for clinical application.
Significant effects of race for Caucasians compared to a non-Caucasian group (mainly composed of African-American infants) were found primarily in the high frequencies for both absorbance and group delay. There were also significant interactions between age and race. In general, absorbance was lower in the high frequencies for non-Caucasians. The mean area of the ear canal was larger in each age group for Caucasians, indicating that non-Caucasian infants had smaller ear canals. Because an age effect could be mainly responsible for the effect of race, a post-hoc analysis was performed to examine age for Caucasians and non-Caucasians at each of the follow-up visits. At each of 5 time points, from birth (screening) through the four follow-up visits, t-tests were done for age compared to race. There were no significant differences in age at any of the five visits for Caucasians compared to non-Caucasians. Thus, the race effect and race by age interactions found were not confounded by differences in ages at follow-up.
In summary, highly significant changes in absorbance and group delay over the first year were apparent from birth to 6 months across nearly all wideband tympanometry variables. These changes were largest below 1 kHz and above 4 kHz, but were significant at all frequencies. Overall, these results provide additional evidence of developmental effects in wideband absorbance that are consistent with previous studies and evidence based reviews (Kei, Sanford, Prieve, & Hunter, 2013; Hunter, Prieve, Kei, & Sanford, 2013; Sanford, Hunter, Feeney, & Nakajima, 2013).
6. 1 CONCLUSIONS
Due to the highly significant age effects on both absorbance and group delay, separate normative references are recommended for clinical application for birth, 1 month, and 6-15 months. Because differences were small and CIs were overlapping between 6-15 months for absorbance, group delay, tympanometric width and height, normative data may reasonably be collapsed for those ages. Tympanometric measures revealed differences from ambient pressure measures at all age groups, indicating that middle-ear pressure or ear-canal collapse affects ambient pressure measurements, and needs to be accounted for clinically. Differences between age groups for group delay illuminate the developmental changes in absorbance. The group-delay data show effects of canal wall mobility in newborns and one-month-olds, and fit the anatomical development pattern of the ear canal and middle ear in infants at age six months and older. These normative data are expected to be useful in comparisons with similar measurements in infant ears with confirmed conductive and sensorineural hearing loss.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). Co-author Keefe is involved in commercializing devices to assess middle-ear function in infants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the United States Government.
List of Abbreviations
- ABR
auditory brainstem response
- CI
confidence interval
- DPOAE
distortion product otoacoustic emission
- HP
high pass
- LP
low pass
- LS
least squares
- NHS
newborn hearing screening
- NICU
neonatal intensive care unit
- SNR
signal to noise ratio
- TEOAE
Transient evoked otoacoustic emissions
- TM
tympanic membrane
- TPP
tympanometric peak pressure
- TW
tympanometric width
- VRA
visual reinforcement audiometry
- WAI
Wideband acoustic immittance
REFERENCES
- 1.Aithal S, Kei J, Driscoll C. Wideband absorbance in young infants (0-6 months): a cross-sectional study. Journal of the American Academy of Audiology. 2014;25:471–481. doi: 10.3766/jaaa.25.5.6. doi: 10.3766/jaaa.25.5.6. [DOI] [PubMed] [Google Scholar]
- 2.Aithal S, Kei J, Driscoll C, Khan A, Swanston A. Wideband Absorbance Outcomes in Newborns: A Comparison With High-Frequency Tympanometry, Automated Brainstem Response, and Transient Evoked and Distortion Product Otoacoustic Emissions. Ear and Hearing. 2015;36:237–250. doi: 10.1097/AUD.0000000000000175. doi: 10.1097/aud.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin M. Choice of probe tone and classification of trace patterns in tympanometry undertaken in early infancy. International Journal of Audiology. 2006;45:417–427. doi: 10.1080/14992020600690951. doi: N7780J87482990GN [pii]10.1080/14992020600690951. [DOI] [PubMed] [Google Scholar]
- 4.Cinamon U. The growth rate and size of the mastoid air cell system and mastoid bone: a review and reference. European Archives of Otorhinolaryngology. 2009;266:781–786. doi: 10.1007/s00405-009-0941-8. doi: 10.1007/s00405-009-0941-8. [DOI] [PubMed] [Google Scholar]
- 5.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–333. [Google Scholar]
- 6.Eby TL, Nadol JB., Jr. Postnatal growth of the human temporal bone. Implications for cochlear implants in children. Annals of Otology, Rhinology and Laryngology. 1986;95:356–364. doi: 10.1177/000348948609500407. http://dx.doi.org/10.1177/000348948609500407. [DOI] [PubMed] [Google Scholar]
- 7.Elsayed AM, Hunter LL, Keefe DH, Feeney MP, Brown DK, Meinzen-Derr JK, Baroch K, Sullivan-Mahoney M, Francis K, Schaid LG. Air and Bone Conduction Click and Tone-Burst Auditory Brainstem Thresholds Using Kalman Adaptive Processing in Nonsedated Normal-Hearing Infants. Ear and Hearing. 2015;36:471–81. doi: 10.1097/AUD.0000000000000155. doi: 10.1097/AUD.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feeney MP, Hunter LL, Kei J, Lilly DJ, Margolis RH, Nakajima HH, Voss SE. Consensus statement: Eriksholm workshop on wideband absorbance measures of the middle ear. Ear and Hearing. 2013;34(Suppl 1):78S–79S. doi: 10.1097/AUD.0b013e31829c726b. doi: 10.1097/AUD.0b013e31829c726b. [DOI] [PubMed] [Google Scholar]
- 9.Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinical application of multivariate analysis of distortion product otoacoustic emission data. Ear and Hearing. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. http://dx.doi.org/10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holte L, Cavanaugh RM, Jr., Margolis RH. Ear canal wall mobility and tympanometric shape in young infants. Journal of Pediatrics. 1990;117:77–80. doi: 10.1016/s0022-3476(05)82448-5. http://dx.doi.org/10.1016/s0022-3476(05)82448-5. [DOI] [PubMed] [Google Scholar]
- 11.Holte L, Margolis RH, Cavanaugh RM., Jr. Developmental changes in multifrequency tympanograms. Audiology. 1991;30:1–24. doi: 10.3109/00206099109072866. http://dx.doi.org/10.3109/00206099109072866. [DOI] [PubMed] [Google Scholar]
- 12.Hunter LL, Margolis RH. Multifrequency tympanometry, current clinical application. American Journal of Audiology. 1992;1:33–43. doi: 10.1044/1059-0889.0103.33. http://dx.doi.org/10.1044/1059-0889.0103.33. [DOI] [PubMed] [Google Scholar]
- 13.Hunter LL, Bagger-Sjoback D, Lundberg M. Wideband reflectance associated with otitis media in infants and children with cleft palate. International Journal of Audiology. 2008;47(Suppl 1):S57–61. doi: 10.1080/14992020802294057. doi: 902366002 [pii] 10.1080/14992020802294057. [DOI] [PubMed] [Google Scholar]
- 14.Hunter LL, Feeney MP, Lapsley Miller JA, Jeng PS, Bohning S. Wideband reflectance in newborns: normative regions and relationship to hearing-screening results. Ear and Hearing. 2010;31:599–610. doi: 10.1097/AUD.0b013e3181e40ca7. doi: 10.1097/AUD.0b013e3181e40ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter LL, Prieve BA, Kei J, Sanford CA. Pediatric applications of wideband acoustic immittance measures. Ear and Hearing. 2013;34(Suppl 1):36S–42S. doi: 10.1097/AUD.0b013e31829d5158. doi: 10.1097/AUD.0b013e31829d5158. [DOI] [PubMed] [Google Scholar]
- 16.Ikui A, Sando I, Sudo M, Fujita S. Postnatal change in angle between the tympanic annulus and surrounding structures. Computer-aided three-dimensional reconstruction study. Annals of Otology, Rhinology and Laryngology. 1997;106:33–36. doi: 10.1177/000348949710600106. http://dx.doi.org/10.1177/000348949710600106. [DOI] [PubMed] [Google Scholar]
- 17.Keefe DH, Ling R, Bulen JC. Method to measure acoustic impedance and reflection coefficient. Journal of the Acoustical Society of America. 1992;91:470–485. doi: 10.1121/1.402733. http://dx.doi.org/10.1121/1.402733. [DOI] [PubMed] [Google Scholar]
- 18.Keefe DH, Bulen JC, Arehart KH, Burns EM. Ear-canal impedance and reflection coefficient in human infants and adults. Journal of the Acoustical Society of America. 1993;94:2617–2638. doi: 10.1121/1.407347. http://dx.doi.org/10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- 19.Keefe DH, Levi E. Maturation of the middle and external ears: acoustic power-based responses and reflectance tympanometry. Ear and Hearing. 1996;17:361–373. doi: 10.1097/00003446-199610000-00002. http://dx.doi.org/10.1097/00003446-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Keefe DH, Folsom RC, Gorga MP, Vohr BR, Bulen JC, Norton SJ. Identification of neonatal hearing impairment: ear-canal measurements of acoustic admittance and reflectance in neonates. Ear and Hearing. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. http://dx.doi.org/10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Keefe DH, Gorga MP, Neely ST, Zhao F, Vohr BR. Ear-canal acoustic admittance and reflectance measurements in human neonates. II. Predictions of middle ear in dysfunction and sensorineural hearing loss. Journal of the Acoustical Society of America. 2003b;113:407–422. doi: 10.1121/1.1523388. http://dx.doi.org/10.1121/1.1523388. [DOI] [PubMed] [Google Scholar]
- 22.Keefe DH, Abdala C. Theory of forward and reverse middle ear transmission applied to otoacoustic emissions in infant and adult ears. Journal of the Acoustical Society of America. 2007;121:978–993. doi: 10.1121/1.2427128. http://dx.doi.org/10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keefe DH, Hunter LL, Feeney MP, Fitzpatrick DF. Aural acoustic reflectance and admittance procedures to test human infants and adults. Accepted for publication, Journal of the Acoustical Society of America. 2015 Nov 15; doi: 10.1121/1.4936946. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kei J, Sanford CA, Prieve BA, Hunter LL. Wideband acoustic immittance measures: developmental characteristics (0 to 12 months) Ear and Hearing. 2013;34(Suppl 1):17S–26S. doi: 10.1097/AUD.0b013e31829db914. doi: 10.1097/AUD.0b013e31829db914. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y-W, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, Keefe DH. Wideband absorbance tympanometry using pressure sweeps: System development and results on adults with normal hearing. Journal of the Acoustical Society of America. 2008;124:3708–3719. doi: 10.1121/1.3001712. http://dx.doi.org/10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchant CD, McMillan PM, Shurin PA, Johnson CE, Turczyk VA, Feinstein JC, Panek DM. Objective diagnosis of otitis media in early infancy by tympanometry and ipsilateral acoustic reflex thresholds. Journal of Pediatrics. 1986;109:590–595. doi: 10.1016/s0022-3476(86)80218-9. http://dx.doi.org/10.1016/s0022-3476(86)80218-9. [DOI] [PubMed] [Google Scholar]
- 27.Palva T, Northrop C, Ramsay H. Spread of amniotic fluid cellular content within the neonate middle ear. International Journal of Pediatric Otorhinolaryngology. 1999;48:143–153. doi: 10.1016/s0165-5876(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 28.Paradise JL, Smith CG, Bluestone CD. Tympanometric detection of middle ear effusion in infants and young children. Pediatrics. 1976;58:198–210. http://dx.doi.org/10.1542/peds.2005-1879. [PubMed] [Google Scholar]
- 29.Prieve BA, Vander Werff KR, Preston JL, Georgantas L. Identification of conductive hearing loss in young infants using tympanometry and wideband reflectance. Ear and Hearing. 2013;34(2):168–178. doi: 10.1097/AUD.0b013e31826fe611. doi: 10.1097/AUD.0b013e31826fe611. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DG, Johnson CE, Carlin SA, Turczyk V, Karnuta MA, Yaffee K. Resolution of middle ear effusion in newborns. Archives of Pediatric and Adolescent Medicine. 1995;149:873–877. doi: 10.1001/archpedi.1995.02170210047008. http://dx.doi.org/10.1001/archpedi.1995.02170210047008. [DOI] [PubMed] [Google Scholar]
- 31.Rosowski JJ, Stenfelt S, Lilly D. An overview of wideband immittance measurements techniques and terminology: you say absorbance, I say reflectance. Ear and Hearing. 2013;34(Suppl 1):9S–16S. doi: 10.1097/AUD.0b013e31829d5a14. doi: 10.1097/AUD.0b013e31829d5a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruah CB, Schachern PA, Zelterman D, Paparella MM, Yoon TH. Age-related morphologic changes in the human tympanic membrane. A light and electron microscopic study. Archives of Otolaryngology Head and Neck Surgery. 1991;117:627–634. doi: 10.1001/archotol.1991.01870180063013. http://dx.doi.org/10.1001/archotol.1991.01870180063013. [DOI] [PubMed] [Google Scholar]
- 33.Sanford CA, Feeney MP. Effects of maturation on tympanometric wideband acoustic transfer functions in human infants. Journal of the Acoustical Society of America. 2008;124(4):2106–2122. doi: 10.1121/1.2967864. doi: 10.1121/1.2967864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanford CA, Keefe DH, Liu YW, Fitzpatrick D, McCreery RW, Lewis DE, Gorga MP. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear and Hearing. 2009;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanford CA, Hunter LL, Feeney MP, Nakajima HH. Wideband acoustic immittance: tympanometric measures. Ear and Hearing. 2013;34(Suppl 1):65S–71S. doi: 10.1097/AUD.0b013e31829c7250. doi: 10.1097/AUD.0b013e31829c7250. [DOI] [PubMed] [Google Scholar]
- 36.Saunders JC, Kaltenback JA, Relkin EM. The structural and functional development of the outer and middle ear. In: Romand R, Romand MR, editors. Development of auditory and vestibular systems. Academic Press; New York, NY: 1983. pp. 3–25. http://dx.doi.org/10.1016/b978-0-12-594450-2.50006-x. [Google Scholar]
- 37.Shahnaz N. Wideband reflectance in neonatal intensive care units., 2008. Journal of the American Academy of Audiology. 19:419–29. doi: 10.3766/jaaa.19.5.4. http://dx.doi.org/10.3766/jaaa.19.5.4. [DOI] [PubMed] [Google Scholar]
- 38.Shahnaz N, Miranda T, Polka L. Multifrequency Tympanometry in Neonatal Intensive Care Unit and Well Babies. Journal of the American Academy of Audiology. 2008;19:392–418. doi: 10.3766/jaaa.19.5.3. http://dx.doi.org/10.3766/jaaa.19.5.3. [DOI] [PubMed] [Google Scholar]
- 39.Shahnaz N, Cai A, Qi L. Understanding the developmental course of the acoustic properties of the human outer and middle ear over the first 6 months of life by using a longitudinal analysis of power reflectance at ambient pressure. Journal of the American Academy of Audiology. 2014;25:495–511. doi: 10.3766/jaaa.25.5.8. http://dx.doi.org/10.3766/jaaa.25.5.8. [DOI] [PubMed] [Google Scholar]
- 40.Stinson MR, Shaw EA, Lawton BW. Estimation of acoustical energy reflectance at the eardrum from measurements of pressure distribution in the human ear canal. Journal of the Acoustical Society of America. 1982;72:766–73. doi: 10.1121/1.388257. http://dx.doi.org/10.1121/1.388257. [DOI] [PubMed] [Google Scholar]
- 41.Vander Werff KR, Prieve BA, Georgantas LM. Test-retest reliability of wideband reflectance measures in infants under screening and diagnostic test conditions. Ear and Hearing. 2007;28:669–681. doi: 10.1097/AUD.0b013e31812f71b1. doi: 10.1097/AUD.0b013e31812f71b1. [DOI] [PubMed] [Google Scholar]
- 42.Zhiqi L, Kun Y, Zhiwu H. Tympanometry in infants with middle ear effusion having been identified using spiral computerized tomography. American Journal of Otolaryngology. 2010;31:96–103. doi: 10.1016/j.amjoto.2008.11.008. http://dx.doi.org/10.1016/j.amjoto.2008.11.008. [DOI] [PubMed] [Google Scholar]