Abstract
Objective
To investigate the relationships of cardiac structure and function with body composition and cardiorespiratory fitness (CRF) among adolescents with type 2 diabetes (T2D) in the TODAY study.
Study design
Cross-sectional evaluation of 233 participants [median age 18.3 (min-max 12.4–24.2) years, 63% females, median HbA1c 6.8%] who had echocardiography measurements of left ventricular (LV) mass, ejection fraction, left atrial (LA) dimensions, LV diastolic function (E/Em ratio from tissue Doppler imaging), and right ventricular (RV) function [tricuspid annular plane systolic excursion (TAPSE)]; body composition (DXA) and CRF [cycle ergometry determination of physical work capacity at heart rate of 170 beats/minute (PWC-170)].
Results
LV mass correlated positively with CRF (r=0.5, p<0.0001), lean body mass (LBM) (r=0.7, p<0.0001) and fat mass (FM) (r=0.2, p=0.00047); LV ejection fraction did not. E/Em was positively related to FM (r=0.14, p=0.03) and % body fat (r=0.18, p=0.007), and LA internal dimension (LAID) correlated with FM (r=0.4, p<0.0001), LBM (r=0.3, p<0.001) and CRF (r=0.2, p=0.0033). TAPSE weakly correlated with CRF (r=0.2, p=0.0014) and LBM (r=0.13, p<0.05) but not with FM. In multivariable regression analyses, LBM (β=2.13, p<0.0001) and CRF (β=0.023, p=0.008) were related to LV mass independent of race, sex, age, HbA1c, hypertension, smoking, and diabetes medications. CRF (β=0.0002, p=0.0187) and HbA1c (β=−0.022, p=0.0142) were associated with TAPSE.
Conclusions
In youth with T2D, LV size is related to physical fitness. LV ejection fraction is within normal limits. LV diastolic function is inversely related to FM. Higher fitness may counteract adverse effects of poor glycemic control on RV function.
Keywords: echocardiography, left ventricular function, right ventricular function, fitness, body composition, type 2 diabetes
The findings from the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) (1) indicated a high rate of dyslipidemia, microalbuminuria, and hypertension in T2D youth at baseline, with progression of these cardiovascular risk factors over time (2, 3). Almost a third of participants met criteria for the diagnosis of hypertension over an average period of 3.9 years of follow-up. Echocardiography performed in the last year of the study at a median of 4½ years from diagnosis of T2D, and at an average age of 18 years, demonstrated high left ventricular (LV) mass associated with higher BMI, higher blood pressure (BP), male sex, and African American race/ethnicity (4). Physical activity measurements and physical fitness testing highlighted the sedentary nature and low overall fitness levels of this group of adolescents in comparison with data on obese youth from the National Health and Nutrition Examination Survey (NHANES) and other clinical studies (5).
We sought to better understand echocardiographic determinants of cardiac structure and function and their relationship to fitness in T2D youth from TODAY. In healthy children, lean body mass (LBM) is a stronger determinant of LV mass than fat mass (FM) (6). In a group of lean and overweight 13-year-old children, a positive correlation was found between LV mass index and both lean mass and FM (7). Exercise training at high intensity increases LV mass, but data on the relationship between fitness and heart size in obese youth with T2D are lacking.
We hypothesized that body composition and cardio-respiratory fitness (CRF) are associated with cardiac structure and function in obese adolescents with T2D. We, therefore, evaluated the relationships of measures of LV structure, LV systolic and diastolic function, as well as right ventricular (RV) function, with body composition and cardio-respiratory fitness in TODAY participants, while adjusting for diabetes treatment, glycemic control, and cardiovascular disease risk factors including race, sex, BP, and smoking.
METHODS
The TODAY study population consisted of 699 youth ≥85th percentile for body mass index (BMI), aged 10–17 years, diagnosed with T2D ≤2 years, and negative for pancreatic autoantibodies (ClinicalTrials.gov: NCT00081328). Participants were randomized to one of 3 treatment arms: metformin alone (M), metformin plus rosiglitazone (M+R), and metformin plus an intensive lifestyle program (M+L). The primary outcome was defined as failure to maintain glycemic control (HbA1c<8%) on randomized treatment. At this point, insulin therapy was initiated and rosiglitazone was discontinued. Treatment with M+R was superior to M in preventing need for chronic insulin therapy; M+L was not different from M or M+R(1). The protocol was approved by the Institutional Review Boards for the Protection of Human Subjects of each participating institution. All participants provided informed consent/assent according to local guidelines.
Cardiovascular risk assessment and treatment
BMI was calculated from height and weight (weight in kg divided by height in m2). BP was taken using a CAS 740 monitor with standardized oscillometric cuff sizes. Participants with hypertension (defined as BP≥95th percentile for age, sex, and height or≥130/80 mm Hg, whichever was lower) received dietary counseling on a low salt diet. If values remained elevated, study-supplied Lisinopril was initiated and titrated to achieve target goals according to a predetermined algorithm. (2).
Echocardiography
Echocardiograms were performed in the last year of the study in 542 participants (of the 699 total randomized subjects) at a median of~4½ years from diagnosis of T2D at an average age of 18 years, 2–6 years after randomization in TODAY as previously reported (4). The current cross-sectional analysis presents data for those participants who had body composition and CRF measured within 6 months of the echocardiogram (n=233), with a median duration between the echocardiogram measures and body composition/PWC-170 of 56 (0, 96) days. Briefly, M-Mode and 2D measurements of LV and LA dimensions were performed and interpreted according to American Society of Echocardiography guidelines at a core laboratory using strict quality control procedures similar to those of the CARDIA study (8, 9). LV mass (0.8 (1.04 ((Interventricular septum diameter + LV posterior wall diameter + LV internal diameter)3 −LV internal diameter3) + 0.6 g; all measurements in diastole), LV ejection fraction ((LV end diastolic volume- LV end systolic volume)/LV end diastolic volume; volumes calculated by Simpson’s rule), and LV relative wall thickness ((LV posterior wall thickness in diastole x 2)/LV end diastolic diameter) were calculated from 2D directed MMode images of the left ventricle according to American Society of Echocardiography recommendations (9). Tissue Doppler imaging analysis of the lateral mitral valve annulus during diastole was performed and values from sequential beats were averaged; diastolic function was defined as the ratio of E/Em (10). RV function was assessed by tricuspid annular plane systolic excursion (TAPSE) (4).
Body composition
Dual energy X-ray absorptiometry (DXA) scans were performed at each clinical center according to study-specific guidelines for subject positioning standardized across the different DXA systems, as reported previously (11).
Cardiorespiratory fitness (CRF)
CRF was assessed by a submaximal test using cycle ergometry (818E bike, Quinton Monark, Seattle, WA). Observed workload and heart rate (HR) were recorded up to 4 times at 60 rpm at 3 minute intervals. Workload at a HR of 170 bpm was estimated according to a best-fit equation. Physical Work Capacity at a HR of 170 bpm in Kg*m/min (PWC-170) was calculated (5). Prior studies show that the PWC-170 is a valid indicator for predicting maximal oxygen uptake (VO2 max), the reference measure for aerobic fitness (12, 13).
Statistical analyses
Descriptive statistics presented are percent or median, minimum, and maximum. Analysis of variance was used to compare participants’ characteristics across the three treatment groups. Correlation analyses were used to evaluate bivariate relationships. Regression models were used to assess relationships among echocardiography outcomes and independent predictors including age, sex, race-ethnicity, HbA1c, CRF, body composition measures, study treatment group, time on assigned treatment, and cardiovascular risk factors (diagnosis of hypertension or BP medication use, smoking). We compared unindexed LV mass to body composition measures to be able to examine the individual association of body compartments with total LV mass. Serum lipids did not have an impact on cardiac structure and function in our previous analyses of the larger cohort (4) and were therefore not included in the regression models. All analyses are considered exploratory with statistical significance defined as p<0.05 and no adjustment for multiple testing; the study was powered for the primary outcome only. All analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Participant characteristics, body composition, and CRF data are shown in Table I by treatment group. Subjects had a median age of 18.3 (min-max 12.4–24.2) years, 63 % were females, with a median HbA1c of 6.8%. At the time of the echocardiogram, 109 of the 233(~46%) participants reached primary outcome and needed chronic insulin treatment. Hypertension was diagnosed in 30.3 % based on BP values or a history of taking BP medications. Comparison of the analysis sample with the remaining 465 TODAY participants showed no significant differences for sex, race-ethnicity, HbA1c, treatment group assignment, or study outcome. However, the current study’s cohort was about a year younger [median age at randomization of 13 (min-max 10–17) years vs. 14 (10–18) years], and had overall lower BMI at end of study [33.4 (22.9–53.1) vs. 36.7 (21.1–66.4) kg/m2] which may be a reflection of the fact that DXA studies were not technically possible in larger individuals.
Table 1.
Participant Characteristics at Time of Echocardiography: Anthropometrics, Body Composition, Cardiorespiratory Fitness, and Echocardiography Measures.
| Metformin Only (n=79, 61% F) | Metformin+Rosiglitazone (n=80, 69% F) | Metformin+Lifestyle (n=74, 61% F) | Adjusted P-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 18.8 (13.7–23.1) | 18.0 (12.4–22.7) | 18.2 (13.9–24.2) | -- |
|
| ||||
| Race | 0.8362 | |||
| Hispanic | 28 (35%) | 41 (51%) | 35 (47%) | |
| NHB | 28 (35%) | 21 (26%) | 18 (24%) | |
| NHW | 19 (24%) | 13 (16%) | 15 (20%) | |
| Other | 4 (5%) | 5 (6%) | 5 (7%) | |
|
| ||||
| HbA1c (%) | 8.2 (4.8–14.1) | 6.1 (4.3–14.7) | 7.2 (4.6–15.1) | 0.6126 |
|
| ||||
| Time in study (years) | 4.3 (2.0–7.2) | 4.5 (2.1–6.7) | 5.1 (2.1–7.2) | -- |
|
| ||||
| Reached PO | 44 (56%) | 29 (36%) | 36 (49%) | N/Ad |
|
| ||||
| BMI (kg/m2) | 33.5 (23.4–47.0) | 34.7 (23.8–53.1) | 31.2 (22.9–47.6) | 0.0147 a |
|
| ||||
| Fat mass (FM) (kg) | 35.3 (16.1–62.6) | 37.9 (17.8–70.7) | 31.7 (10.8–59.1) | 0.0032 a |
|
| ||||
| Lean body mass (LBM) (kg) | 58.6 (41.8–87.3) | 58.6 (37.4–90.4) | 55.4 (35.1–91.2) | 0.0094 b |
|
| ||||
| Percent body fat (%) | 37.2 (21.0–51.9) | 39.2 (20.2–52.7) | 37.5 (13.8–49.4) | 0.1993 |
|
| ||||
| Total cholesterol (mg/dL) | 157 (94–254) | 157 (89–239) | 159 (82–335) | 0.4033 |
|
| ||||
| Triglycerides (mg/dL) | 105 (26–1783) | 107 (35–548) | 107.0 (44–1112) | 0.2411 |
|
| ||||
| Systolic BP (mmHg) | 117 (90–153) | 112 (84–151) | 112 (94–149) | 0.3378 |
|
| ||||
| % with hypertension | 32 (41%) | 22 (28%) | 15 (20%) | -- |
|
| ||||
| Cigarette use | 9 (11%) | 9 (11%) | 10 (14%) | -- |
|
| ||||
| Insulin use | 28 (24%) | 27 (17%) | 33 (23%) | -- |
|
| ||||
| PWC-170 (kg/min) | 691 (225–1884) | 716 (328–1958) | 658 (317–1978) | 0.2556 |
|
| ||||
| LV mass (g)/height (m)2.7 | 35.6 (22.3–60.8) | 34.7 (19.1–57.4) | 32.9 (18.1–73.8) | 0.8259 |
|
| ||||
| LV ejection fraction (%) | 69.3 (47.5–83.1) | 66.1 (52.9–78.1) | 68.5 (47.1–83.0) | 0.0045 c |
|
| ||||
| TAPSE (cm) | 2.1 (1.3–2.8) | 2.2 (1.4–3.4) | 2.1 (1.1–3.4) | 0.1617 |
|
| ||||
| Wall thickness | 0.32 (0.24–0.56) | 0.32 (0.22–0.58) | 0.32 (0.25–0.60) | 0.2173 |
|
| ||||
| E:Em Ratio | 5.8 (2.8–12.5) | 5.1 (2.1–9.2) | 6.2 (2.6–12.4) | 0.0079 c |
Data are median (min-max). P-values adjusted for sex, baseline age, hypertension, number cigarettes/day, time in study, and insulin use. Thus, p-values not given for age, time in study, % with hypertension, cigarette use, or insulin use.
M+L significantly different from M+R,
M+L significantly different from M and M+R.
M+R is significantly different from M and M+L
Model fit is questionable; no p-value obtained.
Abbreviations: M = metformin only; M+R = metformin + rosiglitazone; M+L = metformin + lifestyle program; NHB = non-Hispanic Black; NHW = non-Hispanic White; BMI = body mass index; BP = blood pressure; LV = left ventricular; TAPSE = tricuspid annular plane systolic excursion
Treatment was related to several important study variables in this analysis. When treatment groups were analyzed by ANOVA, BMI was lower in the M+L group compared with the M+R group [31.2 (22.9–47.6) kg/m2 in M+L vs. 34.7 (23.8–53.1)kg/m2 in M+R and vs. 33.5 (23.4–47.0) kg/m2 in M, ANOVA p=0.008]. FM and LBM were 16% and 5% lower in the M+L group compared with the M+R group (11% and 5% lower in the M+L group compared with the M group), respectively, indicating possible effect of the lifestyle arm on body composition with relatively higher percentage of lean mass at a lower BMI in the M+L group.
Echocardiography outcomes
Echocardiography outcome data are provided in Table I by treatment group and further described by treatment group and sex in Table II. Median LV mass in this cohort was 139.0 g; indexed for height 34.8 g/m2.7; ~90th percentile for the sex-specific population mean, and not significantly different across treatment groups (4, 14). LV ejection fraction was in the normal range. Although ejection fraction was slightly lower in the M+R group, it was within the normal range in all three treatment arms. Sensitivity analysis in the larger TODAY cohort did not reveal a relationship between treatment modality and ejection fraction. LA internal diameter (LAID) was not significantly different among the three groups. LV diastolic function (E/Em) was slightly lower in the M+R group compared with the other two groups (p=0.007). Median TAPSE was 2.1(1.1–3.4) cm, within normal limits and not significantly different among the three treatment groups.
Table 2.
Echocardiography Measures [Median (Min-Max) or Mean (SD)] by Treatment Group and Gender
| Outcome | Female | Male | ||||
|---|---|---|---|---|---|---|
| M | M+R | M+L | M | M+R | M+L | |
| LV mass (g) * | 134 (76–205) | 129 (74–204) | 117 (79–182) | 178 (102–269) | 166 (91–314) | 158 (105–364) |
| LV mass/height2.7 (g/m2.7) * | 34.5 (22.3–49.8) | 33.5 (19.1–52.8) | 32.4 (18.1–50.2) | 39.4 (27.7–60.8) | 37.7 (23.2–57.4) | 36.1 (24.8–73.8) |
| LV relative wall thickness | 0.31 (0.24–0.50) | 0.32 (0.22–0.58) | 0.31 (0.25–0.54) | 0.34 (0.27–0.56) | 0.32 (0.25–0.45) | 0.33 (0.25–0.60) |
| LV fractional shortening (%) | 38.9 (4.7) | 36.6 (4.7) | 39.5 (5.0) | 40.2 (6.2) | 37.2 (4.4) | 36.60 (5.8) |
| LA internal dimension (cm) * | 3.5 (0.4) | 3.6 (0.5) | 3.37 (0.46) | 3.6 (0.40) | 3.7 (0.4) | 3.6 (0.5) |
| LA internal dimension/height (cm/m) | 2.1 (0.2) | 2.2 (0.3) | 2.1 (0.3) | 2.1 (0.2) | 2.1 (0.2) | 2.1 (0.3) |
| TAPSE (cm) | 2.1 (0.3) | 2.2 (0.4) | 2.1 (0.4) | 2.1 (0.4) | 2.2 (0.3) | 2.1 (0.4) |
| Doppler diastology | ||||||
| LV E (cm/sec) * | 96.8 (20.6) | 90.6 (14.9) | 99.3 (20.6) | 88.8 (19.1) | 87.9 (18.0) | 89.5 (14.7) |
| LV Em (cm/sec) | 16.6 (4.1) | 17.7 (4.1) | 16.3 (3.7) | 16.6 (5.0) | 17.1 (4.5) | 17.39 (6.5) |
| E/Em ratio | 6.2 (1.9) | 5.4 (1.4) | 6.4 (1.8) | 5.8 (1.8) | 5.3 (1.2) | 5.8 (2.4) |
Abbreviations: M = metformin only; M+R = metformin + rosiglitazone; M+L = metformin + lifestyle program; LV = left ventricular; LA = left atrial; TAPSE = tricuspid annular plane systolic excursion
Females significantly different from males, p<.05.
Relationship of cardiac structure and function measures to body composition and CRF
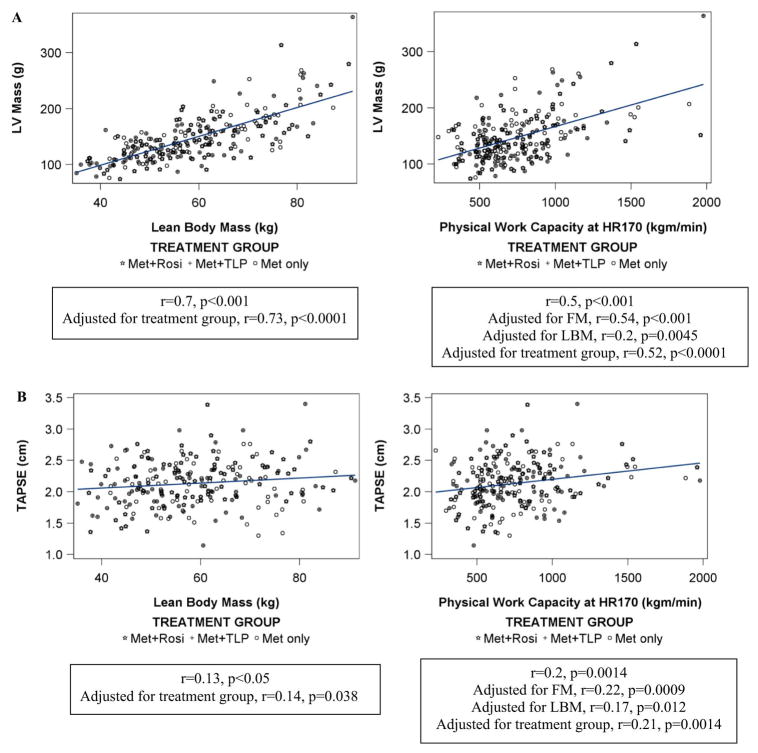
LV mass was positively related to LBM (r=0.7, p<0.001) and less strongly to FM (r=0.2, p=0.0072). LV mass positively correlated with CRF (r=0.5, p<0.001). This relationship persisted after adjusting for FM (r=0.54, p<0.001), but weakened after adjusting for LBM (r=0.2, p=0.0045) (Figure 1, A). These relationships persisted after adjustment for treatment group effect (Figure 1, A). Similar relationships of left ventricular internal dimension (LVID) with CRF and body composition measures were observed. LV relative wall thickness was positively related to LBM (r=0.2, p=0.003), but not to FM or CRF despite a trend towards lower relative wall thickness with higher fitness (p < 0.10). LAID correlated positively with LBM (r=0.3, p<0.001), FM (r=0.4, p<0.0001), and CRF (r=0.2, p=0.0033).
Figure 1.
Relationship of lean body mass and cardio-respiratory fitness to A) LV mass; and B) RV function (TAPSE).
LV ejection fraction was not related to body composition measures or to CRF. LV (E/Em), however, was positively related to FM (r=0.14, p=0.03) and % body fat (r=0.18, p=0.007) and negatively related to % lean body mass (r=−0.18, p=0.007), but not total lean mass or CRF (r=−0.117, p=0.08).
TAPSE was positively related to LBM (r=0.13, p<0.05) and to CRF (r=0.2, p=0.0014), but not to FM (Figure 1, B). The relationship between TAPSE and CRF weakened after adjusting for LBM (r=0.17, p=0.012), but not after adjusting for FM (r=0.22, p=0.0009). The relationship between TAPSE, LBM and CRF persist after adjustment for treatment group (Figure 1, B).
LV mass, E/Em ratio, and RV functional outcomes and cardiovascular disease risk factors
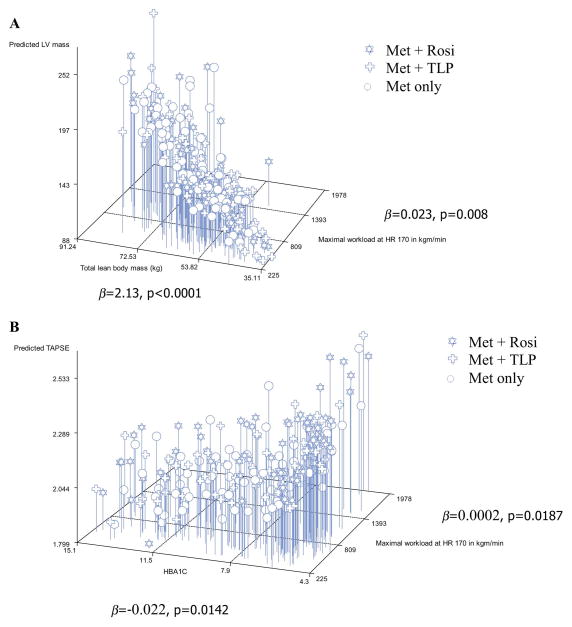
Because of the strong relationships of LV mass and TAPSE to LBM and physical fitness, regression models were run with LV mass and TAPSE as the dependent variables and as covariates: treatment group, race-ethnicity, sex, age, LBM, maximum workload at HR170, HbA1c, hypertension, number of cigarettes per day, and time participation in the study. For LV mass, LBM and work load at HR170 (p<0.0001 and p=0.0081, respectively) remained significant in the fully adjusted models. The slope estimate for LBM was 2.13 (95% CI 1.66–2.60), indicating that, for every 1 kg increase in LBM, LV mass increased by 2.13 units on average. The slope estimate for workload at HR170 was 0.023 (95% CI 0.006–0.040) (Figure 2, A). Workload at HR170 and HbA1c (p=0.0187 and p=0.0142, respectively) were significant predictors of TAPSE. The slope estimate for workload at HR170 was 0.0002 (95% CO 0.00004–0.0005). The slope estimate for HbA1c was −0.022 (95% CI −0.039 to −0.004), indicating that lower HbA1c was associated with better RV function (Figure 2, B). With E/Em as the dependent variable, and FM instead of LBM as an independent variable in the above regression model, treatment group (p=0.0016) and FM (p=0.023) contributed significantly to the variance in E/Em.
Figure 2.
A) 3D Plot of the joint distribution of lean body mass, cardio-respiratory fitness, and LV mass; and B) 3D Plot of the joint distribution of HbA1c, cardio-respiratory fitness, and RV function. The β values represent the estimate of the slope of the regression equation.
DISCUSSION
We evaluated the relationship of CRF and body composition to cardiac structure and function in older adolescents with T2D from the TODAY study. We demonstrate that, in this group of obese youth with T2D, LV mass is positively related to cardio-respiratory fitness and to lean body mass, LV diastolic function is higher (worse) with higher FM, RV function (TAPSE) is positively related to CRF and negatively related to HbA1c, and ejection fraction is unrelated to body composition or cardio-respiratory fitness.
Studies of the relationship between physical fitness and cardiac structure and function have been previously performed in athletes. These generally have been performed to show the impact of training on heart size and function, or to demonstrate differences between athletes and non-athletes with regard to cardiac function to better characterize the athlete’s heart (15–17). Generally these studies show that physical training or higher fitness is associated with a LV mass increase of about 10% and normal cardiac systolic function without adverse changes in cardiac geometry. Diastolic function is often enhanced with training. FFM and not FM predicts LVM and LV end-diastolic dimension in endurance athletes and age-matched adult controls (18). TAPSE has been studied in women playing recreational soccer and improved with training (19). This is consistent with our results showing an association of TAPSE with higher fitness. However, a study of 8–12 year old obese children did not show an association of improvement in conditioning with TAPSE (20).
In contrast, there are limited data linking measures of physical performance and body composition to measures of cardiac structure and function in overweight/obese youth and very limited information is available in youth with T2D. Our findings of a positive relationship between LV mass and CRF and LBM suggest that part of the increase in LV mass in obese youth with T2D is a healthy adaptation to allow adequate heart function that meets the demands of a heavier individual for physical performance, while maintaining a normal ejection fraction. Our results are consistent with observations from Gidding et al who studied a cohort of obese adolescents with BMI > 40 kg/m2 and assessed physical fitness by oxygen consumption at maximal effort (21). In these adolescents, a strong and independent relationship of fitness to LV mass was noted (unpublished data). Body composition was not assessed in that study. Mitchell et al studied cardiac structure and function in relation to visceral fat and FM, and showed a positive relationship of FM to LV mass and a positive correlation of fitness to LV mass/height2.7 (22). However, these investigators did not report LBM, did not directly examine the independent effects of fitness and LBM in their cohort, and, by indexing LV mass, obscured the relationship to LBM. Rosiglitazone increases fat mass as a consequence of treatment, this was observed in the TODAY cohort (1). We adjusted for treatment assignment in this analysis and an independent effect of rosiglitazone independent of fat mass assessed by DXA on outcomes was not observed.
Our findings are highly supportive of the findings of Daniels et al showing a stronger relationship of LBM than FM to LV mass (6) and by the findings from the Strong Heart study in adults showing that LBM is a stronger determinant of cardiac output than FM after adjusting for several cardiovascular disease risk factors (23). It is important to remember that to achieve a given level of fitness, obese individuals do more physical work than lean counterparts because of the need to support greater physical weight (24). This added effort naturally varies depending on the type of exercise and the need for weight support. It is likely that the increased expenditure of energy to perform a specific amount of work in an obese individual requires greater cardiac work and thus leads to cardiac adaptation at lower levels of fitness than for lean individuals.
Though LV mass is strongly related to cardiovascular outcomes (including cardiovascular death, ischemic heart disease, heart failure, peripheral arterial disease, and stroke), the relationship may not be linear (25, 26). In the CARDIA study, a longitudinal study of cardiovascular risk evolution in young adults, both LV mass and Framingham risk score predicted cardiovascular outcomes (26). However, the prevalence of adverse CV outcomes did not increase until LV mass was above the 85th percentile. Further, risk reclassification based on adding LV mass measurement to the Framingham risk score was significantly better for CV event risk prediction in lean than obese individuals.
There is limited information on the relationship between left atrial size and fitness. In general, higher fitness is associated with higher LA size both in the general population and in fit handball athletes (27, 28). Interestingly, increases in LA size are only associated with poorer diastolic function in non-athletes (28). Our data suggest that, in obese adolescents with T2D, FM is the strongest correlate of LA size, but lean mass and fitness also contribute. Because FM was associated with poorer diastolic function, it can be hypothesized that LA size is increased by either worse diastolic function associated with obesity or by a larger heart size required for physical performance. Increase in left atrial size has been reported in obese American Indian adolescents with a high prevalence of the metabolic syndrome (50%) in the Strong Heart study (29). The obese group also exhibited increased left atrial systolic force with normal LV filling pressure, interpreted to reflect some diastolic dysfunction. Relationship to body composition to LA size and function was not evaluated in that study.
In the current study, E/Em ratio from tissue Doppler imaging was used as the measure of diastolic function. In contrast to LV mass, diastolic function was most strongly associated with FM and this relationship was adverse in that higher FM was associated with a higher E/Em ratio. There was a trend suggesting higher fitness or lean mass was associated with a lower ratio, but these relationships did not reach statistical significance. Also, the effect is small and values are well within the normal range therefore the clinical significance of this difference is uncertain. There is limited information on the impact of fitness or training on this measure. In obese adolescents, one study showed no change in E/Em ratio with short-term exercise training (30) whereas another small study reported improvement in systolic and diastolic function after 3 months of aerobic training, which was more evident when evaluated by an exercise stress echocardiogram (31). Nadeau et al did not find a difference in E/Em in a relatively small group of T2D youth compared with obese and normal weight controls despite reduced maximal exercise capacity in T2D youth compared with the other two groups (32). An effect of treatment group on E/Em ratio was noted. However, there was no consistent effect of treatment on E/Em ratio and other measures of heart function in the larger cohort as assessed by sensitivity analyses looking at duration of exposure to treatment and echocardiographic outcomes (4).
There are some limitations to this study. The subset of TODAY participants evaluated for this study was younger and had lower BMI than the remaining TODAY cohort, mainly because of the technical difficulties of obtaining DXA scans in larger individuals. These adolescents may, therefore, have better overall body composition and CRF than the rest of the T2D cohort. There was a time interval of as much as 6 months (median 56 days) between echocardiograms and other measures in the study. This may have a small impact on results but in general TODAY participants did not have significant changes in BMI or physical activity level fitness during the course of the study. Because of the severe obesity of the cohort, 2D echo measures such as left atrial area and volume had low reproducibility and thus were not analyzed. More adverse cardiac structural and functional abnormalities may thus have been missed in the youth with severe obesity. Some correlations are small suggesting a limited effect, particularly for RV function and LV diastolic function. However, participants in this study were adolescents with a relatively short duration of diabetes which may explain the lack of a more pronounced effect of dysglycemia. We did not adjust for multiple comparisons. Nevertheless, our findings are still relevant to the majority of youth with obesity and T2D. The availability of a normal weight and a more fit control group would have been desirable and may have strengthened our findings. The study is cross-sectional, but planned follow-up of the cohort, including an echocardiogram, will further evaluate the long-term implications of our findings.
In conclusion, despite a high prevalence of obesity and hypertension and an overall low level of fitness in obese youth with T2D, cardiac adaptations to fitness and LBM explain substantial variability in LV mass and RV function. For LV mass, this positive association suggests that part of the increase in LV mass may be adaptive to greater body size needs. For RV function, higher fitness may counteract adverse effects of poor glycemic control.
Longitudinal follow-up of these youth will help us understand the implications of the observed increase in LV mass and the long-term effect on LV function and RV function, and the effects of glycemic control and cardiovascular disease risk factors on these variables. Our findings are supportive of promoting measures to improve CRF in individuals with T2D as it may offset some of the adverse effects of diabetes on cardiac function.
Acknowledgments
We gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service. Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
The writing group was: Fida Bacha (chair), MD, Children’s Nutrition Research Center, Baylor College of Medicine; Samuel S. Gidding, MD, Nemours Cardiac Center, A. I. DuPont Hospital for Children; Laura Pyle, PhD, Biostatistics Center, George Washington University; Lorraine Katz, MD, Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine; S; Kristen Nadeau, MD, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus; Andrea Kriska, PhD, University of Pittsburgh; Joao A.C. Lima, MD, Johns Hopkins University.
Abbreviations
- BMI
Body Mass Index
- BP
Blood pressure
- CRF
Cardio-respiratory Fitness
- FM
Fat Mass
- ID
Internal Dimension
- LA
Left atrium/atrial
- LAID
Left atrium internal diameter
- LBM
Lean Body Mass
- LV
Left ventricle/ventricular
- LVM
Left ventricular mass
- PWC-170
physical work capacity at a heart rate of 170 beats per minute
- RV
Right Ventricle/ventricular
- TAPSE
tricuspid annular plane systolic excursion (measure of right ventricular function)
Appendix 1
Additional members and institutes of the TODAY Study Group include:
Clinical Centers--Baylor College of Medicine: S. McKay*, M. Haymond*, B. Anderson, C. Bush, S. Gunn, H. Holden, S.M. Jones, G. Jeha, S. McGirk, S. Thamotharan; Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan; Children’s Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda; Children’s Hospital of Philadelphia: R. Berkowitz, S. Boyd, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi; Children’s Hospital of Pittsburgh: S. Arslanian*, S. Foster, B. Galvin, T. Hannon, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti; Columbia University Medical Center: R. Goland*, D. Gallagher, P. Kringas, N. Leibel, D. Ng, M. Ovalles, D. Seidman; Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, J. Keady, M. Malloy, K. Milaszewski, L. Rasbach; Massachusetts General Hospital: D.M. Nathan*, A. Angelescu, L. Bissett, C. Ciccarelli, L. Delahanty, V. Goldman, O. Hardy, M. Larkin, L. Levitsky, R. McEachern, D. Norman, D. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner; Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, T. Whelan, B. Wolff; State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief; University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, M.M. Kelsey, G. Klingensmith, T. Witten; University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, A. Hebensperger, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, S. Sternlof; University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters; Washington University in St Louis: N. White*, A. Arbeláez, D. Flomo, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch; Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, W. Tamborlane.
Coordinating Center-- George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long.
Project Office--National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
Central Units--Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S.M. Marcovina*, J. Harting; DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang; Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, Y. Liu; Echocardiogram Reading Center (Johns Hopkins University): J. Puccella, E. Ricketts; Fundus Photography Reading Center (University of Wisconsin): R. Danis*, A. Domalpally, A. Goulding, S. Neill, P. Vargo; Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, C. Massmann, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren.
Other--Hospital for Sick Children, Toronto: M. Palmert; Medstar Research Institute, Washington DC: R. Ratner; Texas Tech University Health Sciences Center: D. Dremaine; University of Florida: J. Silverstein.
Appendix 2
Funded by The National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health Office of the Director (U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254); the National Center for Research Resources General Clinical Research Centers (M01-RR00036 [to Washington University School of Medicine], M01-RR00043-45 [to Children’s Hospital Los Angeles], M01-RR00069 [to University of Colorado Denver], M01-RR00084 [to Children’s Hospital of Pittsburgh], M01-RR01066 [to Massachusetts General Hospital], M01-RR00125 [to Yale University], and M01-RR14467 [to University of Oklahoma Health Sciences Center]); and the National Center for Research Resources Clinical and Translational Science Awards (UL1-RR024134 [to Children’s Hospital of Philadelphia], UL1-RR024139 [to Yale University], UL1-RR024153 [to Children’s Hospital of Pittsburgh], UL1-RR024989 [to Case Western Reserve University], UL1-RR024992 [to Washington University in St Louis], UL1-RR025758 [to Massachusetts General Hospital], and UL1-RR025780 [to University of Colorado Denver]).
Footnotes
indicates principal investigator or director
Portions of the study were presented as an oral abstract at the meeting of the American Diabetes Association, <city, state>, <month, day>, 2014.
Trial registration ClinicalTrials.gov: NCT00081328
Funding information is available at www.jpeds.com (Appendix 2). The other authors declare no conflicts of interest. Donations received from the following, but none participated in study design, conduct, data analysis, or report: Becton, Dickinson and Company, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, LifeScan, Inc, Pfizer, and Sanofi Aventis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.. L.K. is a consultant for Takeda and Janssen pharmaceutical. S.G. served as an Editorial Board member of The Journal of Pediatrics (2010–2015). The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fida Bacha, Email: fbacha@bcm.edu, Children’s Nutrition Research Center, Baylor College of Medicine, Houston TX 77030 USA.
Samuel S. Gidding, Email: sgidding@nemours.org, Nemours Cardiac Center, A.I. DuPont Hospital for Children, Wilmington DE 19803 USA.
Laura Pyle, Email: laura.pyle@ucdenver.edu, Department of Pediatrics, School of Medicine, University of Colorado Denver, Aurora CO 80045 USA.
Lorraine Levitt Katz, Email: katzl@email.chop.edu, Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA 19104 USA.
Andrea Kriska, Email: aky@pitt.edu, Graduate School of Public Health, University of Pittsburgh, Pittsburgh PA 15260 USA.
Kristen J. Nadeau, Email: Kristen.nadeau@childrenscolorado.org, Childrens Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora CO 80045 USA.
Joao A. C. Lima, Departments of Medicine, Radiology and Epidemiology, Johns Hopkins University, Baltimore MD 21287 USA.
References
- 1.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366(24):2247–56. doi: 10.1056/NEJMoa1109333. Epub 2012/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group TS. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes care. 2013;36(6):1758–64. doi: 10.2337/dc12-2388. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes care. 2013;36(6):1735–41. doi: 10.2337/dc12-2420. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitt Katz L, Gidding SS, Bacha F, Hirst K, McKay S, Pyle L, et al. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatric diabetes. 2015;16(1):39–47. doi: 10.1111/pedi.12119. Epub 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kriska A, Delahanty L, Edelstein S, Amodei N, Chadwick J, Copeland K, et al. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131(3):e850–6. doi: 10.1542/peds.2012-0620. Epub 2013/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels SR, Kimball TR, Morrison JA, Khoury P, Witt S, Meyer RA. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation. 1995;92(11):3249–54. doi: 10.1161/01.cir.92.11.3249. Epub 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 7.Sivanandam S, Sinaiko AR, Jacobs DR, Jr, Steffen L, Moran A, Steinberger J. Relation of increase in adiposity to increase in left ventricular mass from childhood to young adulthood. The American journal of cardiology. 2006;98(3):411–5. doi: 10.1016/j.amjcard.2006.02.044. Epub 2006/07/25. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, et al. Quality Control and Reproducibility in M-Mode, Two-Dimensional, and Speckle Tracking Echocardiography Acquisition and Analysis: The CARDIA Study, Year 25 Examination Experience. Echocardiography. 2015;32(8):1233–40. doi: 10.1111/echo.12832. Epub 2014/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28(1):1–39. e14. doi: 10.1016/j.echo.2014.10.003. Epub 2015/01/07. [DOI] [PubMed] [Google Scholar]
- 10.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2011;24(3):277–313. doi: 10.1016/j.echo.2011.01.015. Epub 2011/02/23. [DOI] [PubMed] [Google Scholar]
- 11.Group TS. Treatment effects on measures of body composition in the TODAY clinical trial. Diabetes care. 2013;36(6):1742–8. doi: 10.2337/dc12-2534. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boreham CA, Paliczka VJ, Nichols AK. A comparison of the PWC170 and 20-MST tests of aerobic fitness in adolescent schoolchildren. The Journal of sports medicine and physical fitness. 1990;30(1):19–23. Epub 1990/03/01. [PubMed] [Google Scholar]
- 13.McMurray RG, Guion WK, Ainsworth BE, Harrell JS. Predicting aerobic power in children. A comparison of two methods. The Journal of sports medicine and physical fitness. 1998;38(3):227–33. Epub 1998/11/27. [PubMed] [Google Scholar]
- 14.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97(19):1907–11. doi: 10.1161/01.cir.97.19.1907. Epub 1998/06/03. [DOI] [PubMed] [Google Scholar]
- 15.Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, et al. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart. 2013;99(23):1727–33. doi: 10.1136/heartjnl-2012-303465. Epub 2013/03/12. [DOI] [PubMed] [Google Scholar]
- 16.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. Journal of applied physiology. 2008;104(4):1121–8. doi: 10.1152/japplphysiol.01170.2007. Epub 2007/12/22. [DOI] [PubMed] [Google Scholar]
- 17.Venckunas T, Mazutaitiene B. The role of echocardiography in the differential diagnosis between training induced myocardial hypertrophy versus cardiomyopathy. Journal of sports science & medicine. 2007;6(2):166–71. Epub 2007/01/01. [PMC free article] [PubMed] [Google Scholar]
- 18.Whalley GA, Doughty RN, Gamble GD, Oxenham HC, Walsh HJ, Reid IR, et al. Association of fat-free mass and training status with left ventricular size and mass in endurance-trained athletes. Journal of the American College of Cardiology. 2004;44(4):892–6. doi: 10.1016/j.jacc.2004.04.051. Epub 2004/08/18. [DOI] [PubMed] [Google Scholar]
- 19.Randers MB, Andersen LJ, Orntoft C, Bendiksen M, Johansen L, Horton J, et al. Cardiovascular health profile of elite female football players compared to untrained controls before and after short-term football training. Journal of sports sciences. 2013;31(13):1421–31. doi: 10.1080/02640414.2013.792950. Epub 2013/07/09. [DOI] [PubMed] [Google Scholar]
- 20.Hansen PR, Andersen LJ, Rebelo AN, Brito J, Hornstrup T, Schmidt JF, et al. Cardiovascular effects of 3 months of football training in overweight children examined by comprehensive echocardiography: a pilot study. Journal of sports sciences. 2013;31(13):1432–40. doi: 10.1080/02640414.2013.792951. Epub 2013/07/09. [DOI] [PubMed] [Google Scholar]
- 21.Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. The Journal of pediatrics. 2004;144(6):766–9. doi: 10.1016/j.jpeds.2004.03.043. Epub 2004/06/12. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell BM, Gutin B, Kapuku G, Barbeau P, Humphries MC, Owens S, et al. Left ventricular structure and function in obese adolescents: relations to cardiovascular fitness, percent body fat, and visceral adiposity, and effects of physical training. Pediatrics. 2002;109(5):E73–3. doi: 10.1542/peds.109.5.e73. Epub 2002/05/03. [DOI] [PubMed] [Google Scholar]
- 23.Collis T, Devereux RB, Roman MJ, de Simone G, Yeh J, Howard BV, et al. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation. 2001;103(6):820–5. doi: 10.1161/01.cir.103.6.820. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 24.Norman AC, Drinkard B, McDuffie JR, Ghorbani S, Yanoff LB, Yanovski JA. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics. 2005;115(6):e690–6. doi: 10.1542/peds.2004-1543. Epub 2005/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovascular imaging. 2012;5(8):837–48. doi: 10.1016/j.jcmg.2012.06.003. Epub 2012/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong AC, Jacobs DR, Jr, Gidding SS, Colangelo LA, Gjesdal O, Lewis CE, et al. Framingham score and LV mass predict events in young adults: CARDIA study. International journal of cardiology. 2014;172(2):350–5. doi: 10.1016/j.ijcard.2014.01.003. Epub 2014/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzudie A, Menanga A, Hamadou B, Kengne AP, Atchou G, Kingue S. Ultrasonographic study of left ventricular function at rest in a group of highly trained black African handball players. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2007;8(2):122–7. doi: 10.1016/j.euje.2006.02.006. Epub 2006/04/18. [DOI] [PubMed] [Google Scholar]
- 28.Nistri S, Galderisi M, Ballo P, Olivotto I, D’Andrea A, Pagliani L, et al. Determinants of echocardiographic left atrial volume: implications for normalcy. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12(11):826–33. doi: 10.1093/ejechocard/jer137. Epub 2011/09/02. [DOI] [PubMed] [Google Scholar]
- 29.Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. Journal of the American College of Cardiology. 2006;47(11):2267–73. doi: 10.1016/j.jacc.2006.03.004. Epub 2006/06/06. [DOI] [PubMed] [Google Scholar]
- 30.Millen A, Norton G, Avidon I, Woodiwiss A. Effects of short-term exercise training on tissue Doppler indices of left ventricular diastolic function in overweight and obese individuals. Journal of sports sciences. 2014;32(5):487–99. doi: 10.1080/02640414.2013.832356. Epub 2013/09/21. [DOI] [PubMed] [Google Scholar]
- 31.Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired cardiac function among obese adolescents: effect of aerobic interval training. Archives of pediatrics & adolescent medicine. 2010;164(9):852–9. doi: 10.1001/archpediatrics.2010.158. Epub 2010/09/08. [DOI] [PubMed] [Google Scholar]
- 32.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. The Journal of clinical endocrinology and metabolism. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. Epub 2009/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]